Abstract
While changes in the abundance of keystone predators can have cascading effects resulting in regime shifts, the role of mesopredators in these processes remains underexplored. We conducted annual surveys of rocky reef communities that varied in the recovery of a keystone predator (sea otter, Enhydra lutris) and the mass mortality of a mesopredator (sunflower sea star, Pycnopodia helianthoides) due to an infectious wasting disease. By fitting a population model to empirical data, we show that sea otters had the greatest impact on the mortality of large sea urchins, but that Pycnopodia decline corresponded to a 311% increase in medium urchins and a 30% decline in kelp densities. Our results reveal that predator complementarity in size-selective prey consumption strengthens top-down control on urchins, affecting the resilience of alternative reef states by reinforcing the resilience of kelp forests and eroding the resilience of urchin barrens. We reveal previously underappreciated species interactions within a ‘classic’ trophic cascade and regime shift, highlighting the critical role of middle-level predators in mediating rocky reef state transitions.
Keywords: regime shift, predator diversity, trophic cascade, kelp forests, sea otter, sea star wasting disease
1. Introduction
The knowledge that ecological communities can abruptly shift between alternative states (i.e. dynamic community configurations maintained by feedbacks [1]), has made illuminating the mechanisms that induce these shifts a core focus in ecology [2,3]. Although there are diverse examples of ecosystems worldwide that exhibit multiple system states [4], empirical data elucidating processes that shape transition dynamics are less common because regime shifts occur rapidly, unexpectedly and over different scales of space and time. Similarly, it can be difficult to identify the mechanisms that confer or erode community configurations through time because the relevance of key species interactions may only be revealed following a significant disturbance event [5,6].
Shifts in top-down control of herbivore abundance and size can trigger the reorganization of entire ecosystems [7,8]. When predation is size-dependent, predators exacerbate shifts in the size structure of prey populations, which can strongly influence the net effect of herbivory [9,10]. Predator species often vary dramatically in the range of prey size they target as a result of optimizing efficiency, gape limitation and prey reaching size refugia [11–13]. As such, the same prey species may be subject to predation pressure from different predators during different life-history stages and/or different size classes [14,15]. Moreover, because individual body size is an important determinant of grazing capacity [16], for many herbivorous prey, the abundance of different size-selective predators could have differential cascading impacts on primary producers.
While food-web ecology has focused extensively on the direct and indirect effects of apex predators on community structure and stability [6,7,17,18], there has been less consideration of how co-occurring middle-level predators, or mesopredators, influence systems that exhibit regime shifts. Mesopredator release can cause unanticipated declines in lower trophic levels [19], but few studies investigate food webs where apex predators and mesopredators share the same prey species, or track cascading effects of mesopredator abundance to primary producers. More generally, it has been shown that predator diversity can alter the strength of a trophic cascade when one predator mediates the consumptive effects of another, either reducing (e.g. intraguild predation [20,21]) or enhancing (e.g. predator complementarity [22,23]) the net effect on herbivorous prey. Many of these studies focus on guilds of predators occupying similar trophic levels, but the degree to which community structure may be differentially affected by apex predators and mesopredators in a system prone to trophic cascades remains largely underexplored.
On temperate rocky reefs around the world, grazing by herbivorous sea urchins can drive regime shifts between productive macroalgae-dominated forests and unproductive ‘barrens’ dominated by encrusting corallines [24–26]. Kelp forest and urchin barren alternative states are persistent because the transitions tend to exhibit hysteresis (i.e. the threshold biomass of sea urchins initiating destructive grazing is greater than that which enables kelp recovery), and both states have associated feedback mechanisms that contribute to stabilizing that particular community configuration (reviewed in [25,26]). In one of the most well-known examples, sea otters (Enhydra lutris) in the northern Pacific exert strong top-down control on sea urchins and facilitate regime shifts from urchin barrens to kelp forests [18,27,28]. Whereas global reviews emphasize the role that apex predators play in influencing catastrophic regime shifts caused by urchin overgrazing [24,25], these reviews contain little information about other less-prominent urchin consumers (e.g. sea stars and decapods) that may influence the resilience of alternative rocky reef states [29–31].
Here, we examine the relative roles of an apex predator (sea otters) and a co-occurring mesopredator (sunflower sea star Pycnopodia helianthoides, hereafter Pycnopodia) in controlling sea urchin abundance and size, and thereby the degree of kelp abundance on high latitude rocky reefs. We focus on Pycnopodia because in 2013 an epidemic of sea star wasting disease (SSWD) spread across the northeast Pacific, causing a precipitous decline in sea stars, including Pycnopodia [32,33]. We hypothesized this could affect kelp forest dynamics as a growing body of literature has demonstrated Pycnopodia are effective urchin predators throughout their range [30,31,33,34]. We took advantage of the SSWD event co-occurring with sea otter recovery to analyse a unique time series of dominant rocky reef consumers and producers across sites that varied in sea otter occupation, both before and after the mass mortality of Pycnopodia. Using empirical data and a size-structured Bayesian model of urchin population dynamics, we examine how urchin densities and size structure, total annual urchin mortality rates, net size-specific urchin grazing capacities, and kelp density vary as a function of sea otter and Pycnopodia presence/absence. Finally, we explore how the loss of Pycnopodia influenced the resilience of alternative kelp-dominated and urchin-dominated rocky reef community configurations.
2. Methods
(a). Field surveys
We conducted annual subtidal surveys at 11 sites on the central coast of British Columbia, Canada (electronic supplementary material, figure S1), where sea otter populations have been recovering (since around 1980) from extirpation due to the fur trade [35]. We selected sites by identifying subtidal rocky reefs that shared similar physical characteristics (i.e. wave exposure, depth range, aspect and topography) but varied in sea otter occupation. Five sites had a documented history of sea otter occupation (i.e. rafts of foraging otters), whereas six sites had no recorded observations of otter rafts [35]. Shortly after our first survey in July 2013, a large raft of sea otters arrived and began foraging at three of the six ‘otters absent’ sites, changing their sea otter status from ‘absent’ to ‘present’ (figure 1a). At each site we measured Pycnopodia density and size within six 30 × 2 m transects within two depth ranges (3–6 m, 9–12 m), then calculated and summed the total Pycnopodia biomass per 10 m2 (electronic supplementary material, table S1). We measured the density of sea urchins and all adult kelps (order Laminariales, stipe length greater than 15 cm to exclude recruits) in 18 stratified random 1 m2 quadrats (depths 4–15 m). Although three urchin species are found in the study region, our analyses focus on red urchins (Mesocentrotus franciscanus) because this species constituted 88% of the regional abundance and 98% of the regional urchin biomass (electronic supplementary material, table S2). We calculated mean kelp stipe density at depths available to urchin grazing (i.e. approx. below 5 m) because wave-generated surge and ‘whiplashing kelp’ at shallower depths create a kelp refuge from urchin grazing [36]. Our time series encompasses 2 years before and 2 years after the onset of SSWD in the region (January 2015), during which Pycnopodia populations dramatically declined (figure 1b; electronic supplementary material, figure S2).
Figure 1.
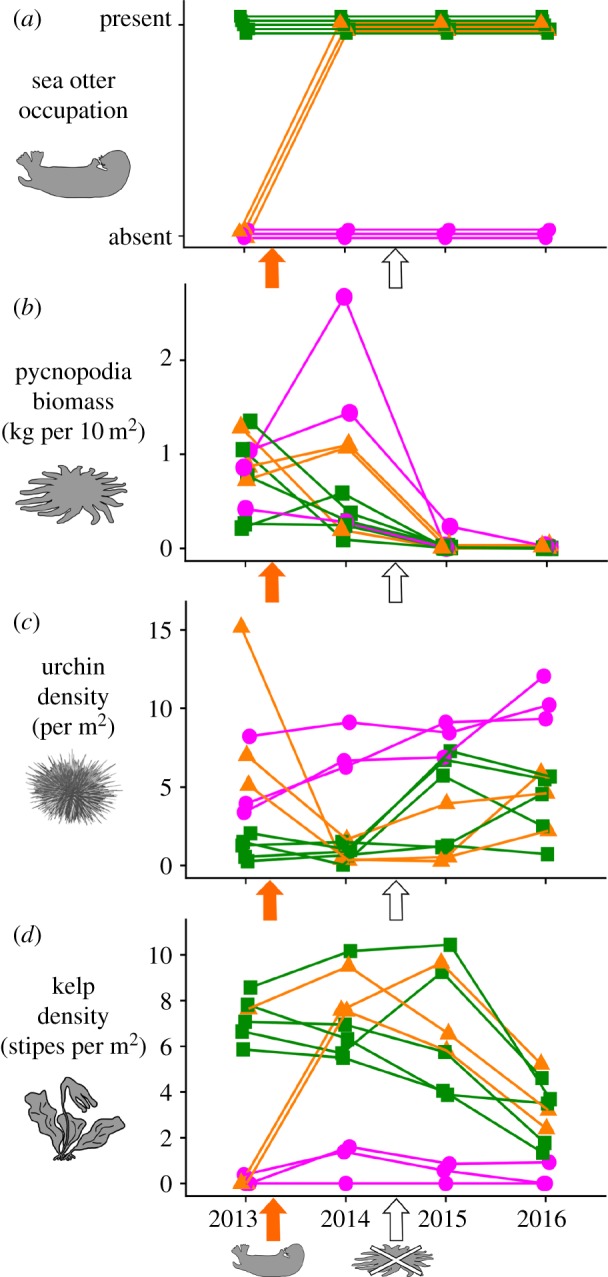
Annual changes in (a) sea otter presence, (b) total Pycnopodia biomass, (c) mean red sea urchin density and (d) mean adult kelp stipe density across subtidal rocky reef sites varying in sea otter occupation status: otters continuously present (green squares), continuously absent (pink circles) and ‘newly arrived’ during summer 2013 (orange triangles). Orange arrows indicate the timing of sea otter arrival at three sites; white arrows indicate the onset of sea star wasting disease.
Urchins were measured to the nearest centimetre and grouped into three size categories (large, medium, small) based on demarcations evident in our size distributions (electronic supplementary material, figure S3) and sea urchin natural history. ‘Large’ red urchins (≥8 cm) generally attain a size-escape from sea star predation [30,34], but are the preferred size range for foraging sea otters [16,37]. ‘Medium’ urchins (4–7 cm) are easily handled and consumed by otters and Pycnopodia, but are generally too large to seek refuge under larger urchin spine canopies [30,34]. ‘Small’ urchins (1–3 cm) represent the population recruits—individuals that settle successfully and can avoid predation by sheltering under the spines of large adults [38].
(b). Bayesian modelling framework
Mortality rates were not directly observed in our surveys; rather, they are latent unobserved processes that we estimated by fitting our survey observations to an ecological process model. We fitted a size-structured population dynamics model in a hierarchical Bayesian state-space framework to estimate instantaneous mortality rates for small, medium, and large urchins due to sea otters and Pycnopodia, while accounting for stochastic variation in baseline mortality rates across sites and years (a table describing all model elements is provided in electronic supplementary material, appendix A1.1). The Bayesian state-space framework allows us to (i) estimate and account for both process and observation error inherent in survey data that may otherwise bias parameter estimates; (ii) leverage prior information on, and incorporate uncertainty in parameters that impact model fit but are not directly of interest; and (iii) develop a mechanistic understanding of key demographic processes in this system, specifically the role of size-specific predation in driving urchin population dynamics and thus shifts in community state.
(c). Population state dynamics
The demographic process of interest, instantaneous mortality (γ) was assumed to represent the combined effects of sea otter and Pycnopodia predation, plus all other sources of mortality (hereafter ‘baseline mortality’). We combined recruitment (R), growth (i.e. size-class transition probabilities, Gi), and instantaneous mortality to calculate the dynamics of each urchin size class (small, medium, large) in discrete annual time steps:
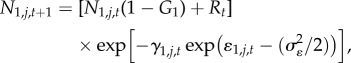 |
2.1 |
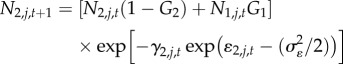 |
2.2 |
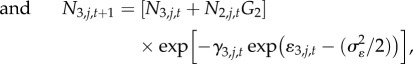 |
2.3 |
where Ni,j,t represents abundance of urchins in size class i at site j and time t (where urchins in Ni=1 = 1–3 cm, Ni=2 = 4–7 cm and Ni=3 ≥ 8 cm), γi,j,t represents instantaneous mortality rate for size class i at site j and time t, and ɛi,j,t represents Gaussian error in log-scale mortality due to annual environmental stochasticity,  . The estimated recruitment parameter (Rt) represents the combined effects of successful larval settlement and subsequent survival to 1 cm, while size class transition probabilities (Gi) were specified parameters with fixed values derived from a published red urchin growth model (electronic supplementary material, appendix M1). At all sites, we initialize the population at time 0 (1 year before observations) at the equilibrium abundance and size distributions conditional on estimated parameters.
. The estimated recruitment parameter (Rt) represents the combined effects of successful larval settlement and subsequent survival to 1 cm, while size class transition probabilities (Gi) were specified parameters with fixed values derived from a published red urchin growth model (electronic supplementary material, appendix M1). At all sites, we initialize the population at time 0 (1 year before observations) at the equilibrium abundance and size distributions conditional on estimated parameters.
We estimated independent size-specific parameters for urchin mortality due to sea otters and Pycnopodia. From equations 2.1–2.3 above, the instantaneous mortality rate (γ) for an individual urchin of size class i at site j during time step t was estimated by:
| 2.4 |
where αO,i and αP,i are fitted parameters that represent the instantaneous mortality rate of size class i attributable to predation by sea otters and Pycnopodia, respectively. We did not fit a mortality rate parameter for sea otters consuming small urchins (αO,i=1) based on extensive foraging observations showing otters rarely consume this size class [37]. Oj,t and Pj,t are variables that come from the survey data; otter presence/absence (1 or 0) and the measured biomass of Pycnopodia (kg per 10 m2). Baseline mortality (δi,j) was estimated as a hierarchical random effect to account for unexplained differences in mortality across sites associated with variation in habitat quality and/or other environmental factors. We assumed that conditions at a site would tend to be more/less favourable for urchins of all sizes, and thus deviations from average mortality rates would be correlated across size classes. Accordingly, mortality for small urchins at site j (δ1,j) was drawn from a normal distribution with mean 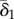 and standard deviation σδ (a fitted parameter determining the magnitude of variation across sites) and mortality values for medium and large urchins were then scaled relative to small urchin morality
and standard deviation σδ (a fitted parameter determining the magnitude of variation across sites) and mortality values for medium and large urchins were then scaled relative to small urchin morality 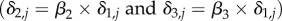 , where β2 and β3 are fitted parameters.
, where β2 and β3 are fitted parameters.
We linked observations (Ci,j,t,k = counts of urchins of size class i at site j during year t in quadrat k) to the hidden state dynamics using a negative binomial (NB) likelihood, with a mean value corresponding to the estimated ‘true’ abundance of urchins at a given site (Ni,j,t) and dispersion parameter (r) that controls variance relative to the mean:
| 2.5 |
For small urchins (i = 1), we included a fitted ‘observability’ parameter (θ, where 0 < θ < 1) to account for imperfect detection of this size class (i.e. patchy distribution and cryptic individuals). For medium and large urchins (i = 2 or 3) we assume θ = 1.
We estimated model parameter posteriors using Gibbs sampling. Details on prior specification, posterior distribution sampling, prior-posterior comparisons, output parameter estimates, Gelman–Rubin diagnostics and posterior predictive checks are provided in the electronic supplementary material, appendix A1. To examine the model's ability to reproduce observed urchin dynamics, we ran iterated deterministic simulations over the 4-year study period to project the density of urchins in each size class through time under four scenarios of predator status: (i) both predators absent; (ii) Pycnopodia present but otters absent; (iii) otters present but Pycnopodia absent; and (iv) both otters and Pycnopodia present (electronic supplementary material, appendix A1.5).
(d). Relative predator impacts on urchins and kelp at the reef scale
To further explore the cascading effects of sea otters and Pycnopodia, we used the four predator scenarios (described above) to examine the relative differences in size-specific rates of urchin mortality, urchin grazing, and empirical density estimates of urchins and kelp. We calculated total annual urchin mortality as 1 − e−γ by using equation 2.4 to yield (γ) for the four different combinations of predators along with the size-specific parameters and baseline mortality estimates sampled from our model posteriors. To examine how predator scenarios influenced urchin size structure, we summarized mean urchin densities (±s.e.) for site and year combinations where the appropriate predators were present or absent. To evaluate the kelp grazing capacity of different urchin densities and size structures, we calculated the maximum potential kelp consumption (kg m−2 yr−1) by multiplying mean urchin densities by size-specific per capita feeding rates for red urchins [16]. Finally, for each predator scenario we plotted the distribution of mean kelp density.
(e). Documenting kelp forest regime shifts at a regional scale
We used aerial imagery to quantify changes in the spatial extent and density of kelp canopy cover in the region where sea otters arrived in 2013 (approx. 6 km2 and over 40 km of shoreline; electronic supplementary material, figure S1). We obtained two high-resolution orthophotos captured prior to sea otter occupation and generated three additional orthophotos using aerial surveys (details in electronic supplementary material, appendix M2) in the 3 years following otter arrival. We compared the total aerial coverage (km2) of canopy kelp (mostly Nereocystis luetkeana) of high and low-density (≥ or less than 10 plants per 10 m2, respectively) across 5 years that encompass pre- and post-otter arrival and the onset of SSWD. We also show the mean density (±s.e.) of perennial understorey species (P. californica, L. setchellii, S. groenlandica, S. latissima) from three reef sites within the ‘new otter arrival’ region.
Lastly, to examine how the decline in Pycnopodia influenced rocky reef community states across the B.C. central coast region, we plotted mean urchin density versus kelp density for all sites in the years before and after the onset of SSWD to examine shifts in alternative reef states (as in [25,39]).
Statistical parameters calculated directly from empirical data are reported ±1 s.e., while Bayesian-estimated parameters are reported with 95% credible intervals. All analyses were conducted using JAGS [40] and R [41].
3. Results
(a). Trends in dominant reef consumers
Sea urchin densities decreased at sites where sea otters returned (n = 3), but also increased at 10 out of 11 sites following SSWD (figure 1a–c) with concurrent declines in kelp stipe density at macroalgae-dominated sites (n = 8; figure 1d). Notably, sea otter presence and Pycnopodia biomass influenced the density and biomass of different size classes of urchins (figure 2a–f). Sea otters had the greatest impact on large urchins; otter arrival at three unoccupied sites resulted in an 89–98% decrease in the mean density of large urchins within 1 year (from 4.4 ± 1.3 to 0.2 ± 0.3 urchins m−2), to similar densities recorded at previously otter-occupied sites (0.2 ± 0.3 urchins m−2). Conversely, Pycnopodia decline corresponded to increases in the density of medium and small sized urchins. Following 2 years of SSWD, the average density of medium urchins across all sites increased from 0.9 ± 0.9 to 3.7 ± 1.7 urchins m−2, representing a 311% increase irrespective of sea otter occupation (figure 2b). Compared to abundance, the increase in medium urchin biomass was less dramatic, increasing by 73% from 0.08 ± 0.08 to 0.3 ± 0.1 kg m−2 (figure 2e). While the densities of small urchins were variable across sites and years (figure 2c), 10 out of 11 sites showed a small net increase (+0.5 urchins m−2 on average) following 2 years of SSWD.
Figure 2.
Size-specific effects of sea otters and Pycnopodia predation on (a–c) the mean red sea urchin density and (d–f) the mean biomass for large, medium and small urchin size classes at sites with different sea otter status. Otters continuously absent (pink circles), continuously present (green squares), and ‘newly arrived’ (orange triangles). Orange arrows indicate sea otter arrival at three sites; white arrows indicate the onset of sea star wasting disease. (g–i) Additive increase in instantaneous mortality rate (mean posterior parameter estimate ± 95% CI) within each urchin size class due to the presence of sea otters or a 1 kg per 10 m2 increase in Pycnopodia biomass. Violin plots show the smoothed posterior distributions for the predator- and size-specific instantaneous mortality rate estimates.
(b). Predator-specific size-structured sea urchin mortality rates
Our model converged well (all psrf values ≤ 1.01), satisfied posterior predictive checks (met model assumptions), resulted in posterior distributions dramatically different from priors (sufficient data to inform the model), and was able to reproduce through simulations comparable urchin densities to those observed under various scenarios of predator abundance (electronic supplementary material, appendices A1.2–A1.5).
Model results suggest that Pycnopodia have a substantial size-specific predatory effect on urchins that is complementary to that of sea otters. Specifically, instantaneous urchin mortality rates attributed to sea otters were estimated as 1.46 (0.76–2.82) and 0.53 (0.07–1.23), for large and medium size classes, respectively (figure 2g,h), whereas Pycnopodia imposed instantaneous mortality rates of 0.070 (0.0–0.16), 1.27 (0.32–2.65) and 0.83 (0.03–2.52) on large, medium and small urchins, respectively (figure 2g,h,i)—having more than double the effect on medium urchins compared to sea otters (figure 2h).
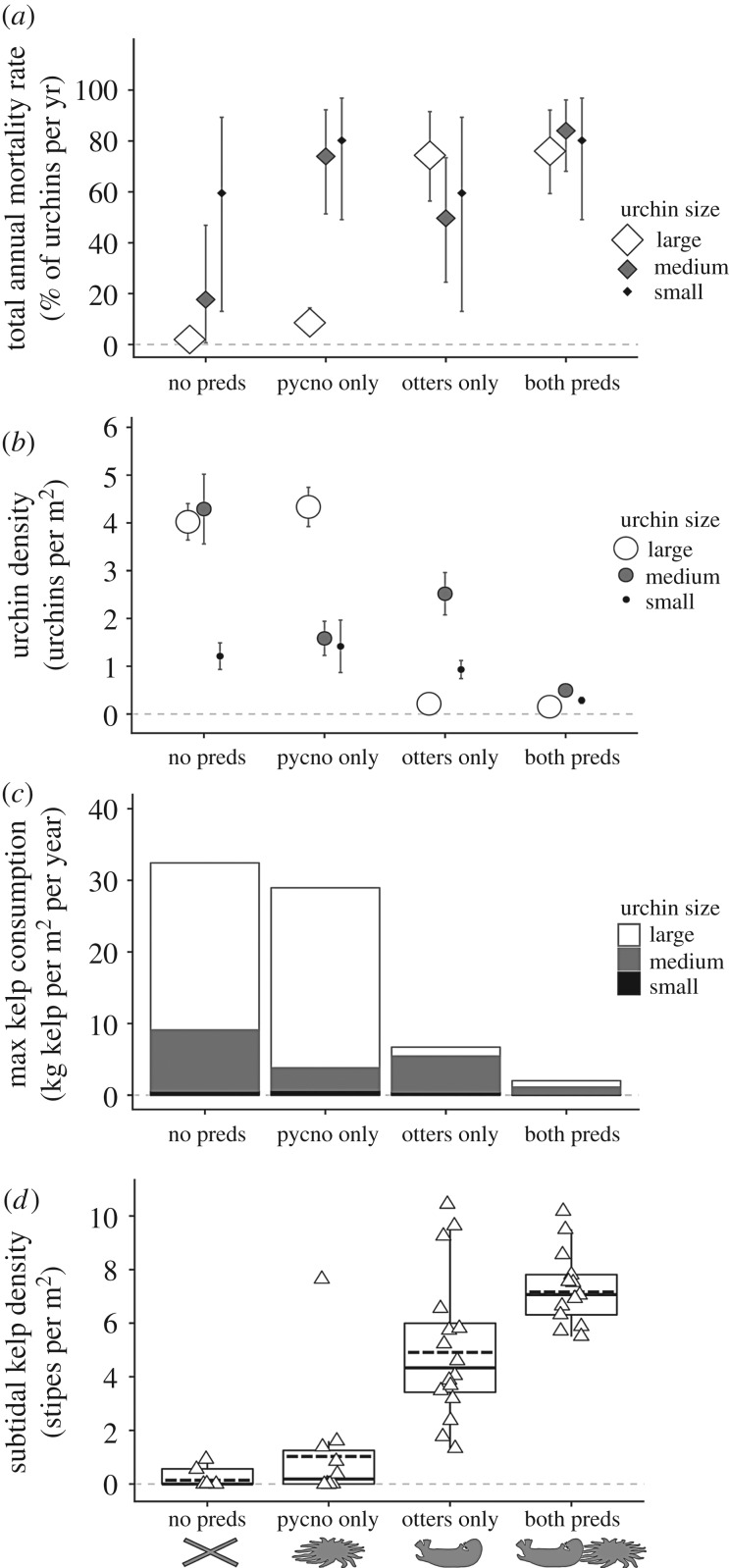
Estimates of size-specific total annual urchin mortality revealed notable differences in relative predator effects (figure 3a). The estimated total annual mortality of large urchins was low when otters were not present (2 and 9% when Pycnopodia were absent and present, respectively) and high when otters were present (74 and 76%). Medium urchin mortality depended on which predators were present in the system: total annual mortality was moderate (51%) for sites with only sea otters, was 1.5 times higher when only Pycnopodia were present (74%), and was highest when both predators co-occurred (84%). Given these rates and the average change in Pycnopodia biomass that occurred after 2 years of SSWD, we estimated that SSWD resulted in a 166% increase in the annual survival of medium urchins at a given site. Small urchins had the highest and most variable baseline mortality rate (60%), which increased in the presence of Pycnopodia (80%). Overall, estimated mortality rates reflect similar patterns to observed urchin densities (figure 3b).
Figure 3.
(a) Estimated total annual mortality (mean ± 95% CI) for small, medium and large urchins corresponding to a given predator status: no predators, only Pycnopodia predators, only sea otter predators and both predators present. (b) Observed size-specific mean urchin density (±s.e.). (c) Estimated size-specific maximum kelp consumption by urchins. (d) Observed mean density of adult kelp stipes at each survey site (n, triangles). Box plots show the median (solid line), mean (dotted line), 25th and 75th percentiles (outer box) and interquartile range.
(c). The cascading effects of sea otters and Pycnopodia
Different predator combinations had notably different kelp grazing capacities (figure 3c): estimated kelp consumption by urchins when no predators were present (32 kg kelp m−2 yr−1) was 16 times greater than when both sea otters and Pycnopodia were present (2 kg kelp m−2 yr−1; figure 3c). Owing to the higher per capita grazing rate of large urchins that are targeted by otters, estimated kelp consumption was primarily determined by sea otter presence (figure 3c). However, when otter-occupied kelp forest sites did not have Pycnopodia, there was 3.5 times more kelp consumption capacity (7 kg kelp m−2 yr−1) due to more abundant medium urchins relative to when both predators were present.
Subtidal kelp density was positively associated with the presence of Pycnopodia as well as sea otters (figures 1d and 3d). Subtidal kelp densities were high when otters were present at sites (1.3–10.4 stipes m−2) and low when otters were absent (0–1.6 stipes m−2, with the exception of one outlier; figure 3d). However, at otter-occupied sites, mean kelp stipe density was higher in the presence of Pycnopodia (7.3 ± 1.4 stipes m−2). We calculate that the loss of Pycnopodia from otter-occupied kelp forests due to SSWD corresponded to a 30% decline in the mean stipe density (to 5.1 ± 2.7 stipes m−2) and higher variation in kelp density across sites. Observations made by divers at these forested sites included sightings of medium-sized urchins consuming kelp blades and climbing up or pulling down kelp stipes (electronic supplementary material, figure S4).
A rapid and large-scale expansion in the spatial extent and density of canopy kelp occurred in the region where sea otters recovered in 2013 (figure 4; see aerial kelp maps in electronic supplementary material figure S5). The year following sea otter arrival, kelp canopy was 2.9 times greater (covering 1.0 km2) than it was the year prior. Kelp beds were also more dense in the years following sea otter return (92%, 82%, 70% of beds of ‘high density’ in 2014, 2015, 2016, respectively) compared to the years prior (45% and 46% of beds of ‘high density’ in 2006 and 2012, respectively). The aerial extent of canopy cover was reduced in 2015–2016, corresponding to the simultaneous increase in perennial understorey species and the onset of SSWD (figure 4).
Figure 4.
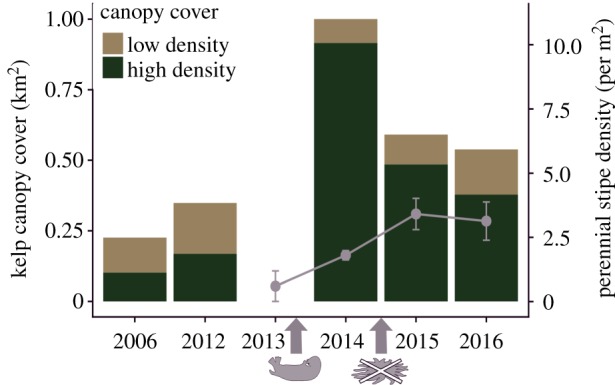
Annual changes in the spatial extent and density of kelp canopy cover in relation to sea otter recovery and the onset of sea star wasting disease (grey arrows). Points and lines show changes in mean perennial understorey kelps (±s.e.) across three subtidal survey sites within the mapping region (approx. 6 km2).
The loss of Pycnopodia due to SSWD was associated with less distinct ‘kelp dominated’ reef states (figure 5). Following the near elimination of Pycnopodia, sea otter-occupied kelp forest sites had higher and wider ranging urchin densities, slightly increased urchin biomass, and lower kelp densities with greater variation within and among sites (figure 5b). During SSWD years, the coefficient of variation for kelp density across sites increased from 20 to 54%.
Figure 5.

Mean red urchin and adult kelp stipe density (±s.e.) for all rocky reef survey sites (a) 2 years before SSWD (2013–2014) and (b) 2 years after SSWD (2015–2016). Point sizes are scaled to the mean biomass of urchins at each site. Dark green circles indicate sites occupied by sea otters; red symbols indicate sites where sea otters are absent. The trophic interactions between species are illustrated for each scenario; solid lines show direct negative interactions, and dashed lines show indirect positive interactions.
4. Discussion
Our results show that complementarity between a mesopredator and an apex predator in their prey size selection can enhance top-down regulation of a strongly interacting herbivore, with cascading impacts on primary producers. In this study, the unpredictable events of SSWD in conjunction with sea otter range expansion provided a unique opportunity to disentangle the responses of sea urchin prey to both a keystone predator and mesopredator. Overall, our findings provide strong evidence that Pycnopodia predation on small and medium urchins helps reinforce kelp-dominated reef states, and may facilitate the rapid transitions from urchin barrens to kelp forests that follow sea otter reintroduction.
(a). Complementary size-structured predation
There are several mechanisms by which multiple predators can exhibit complementarity to successfully exploit the same prey species. Predators can target the same prey species in different habitats [42], life-history stages [15], via different feeding strategies [43] or by consuming different size-classes [44]. In this study, sea otters and Pycnopodia differentially impact prey sizes based on unique feeding strategies and constraints. Sea otters' high metabolic rate and inability to store energy necessitate that they maximize energy intake per unit of foraging time [12]. Conversely, sea stars are slower acting invertebrate predators constrained by the size of prey they can physically digest [30,45]. Sea otters select large urchins when they are highly abundant and easily obtainable, but then switch prey species rather than consume smaller-sized urchins that require increased foraging effort for lower caloric gain [12]. Our data support this foraging theory, showing that large red urchins were only abundant at sites unoccupied by sea otters, and that upon otter arrival, large urchins suffered the most dramatic declines (figure 2). Our estimates of urchin mortality from sea otter predation are consistent with published reports of high attack rates by sea otters on the largest size class of urchins in the years following sea otter recolonization [37] and another study from this region showing the median size of red urchins dropped by 63% following sea otter arrival [16]. While Pycnopodia are also capable of consuming large red urchin prey [45], they typically avoid larger individuals because their long spines render them difficult to consume [30,46]. Similar to other invertebrates that exhibit size escape from sea star predation [13], our data show large red urchins can form extensive barrens on rocky reefs despite high abundance and biomass of Pycnopodia.
Building on other studies, our results demonstrate that Pycnopodia are effective consumers of small and medium-sized (0–7 cm) urchins [30,31,33]. When Pycnopodia are present at a given site, the estimated annual mortality rate for medium urchins was 4.1 times higher than the natural mortality rate in the absence of predators, and 1.5 times higher compared with sites with only sea otter predators (figure 3a). These results are supported by several laboratory studies showing that Pycnopodia actively consume smaller urchin prey [46–48], and even target small red urchins over other urchin species [47]. Correspondingly, the behavioural tendency for small red urchins to seek refuge under the spines of larger conspecifics is suggested to be a direct adaptation to minimize predation risk [47–49].
(b). Indirect effects on kelp forests
Consistent with previous studies on northern Pacific temperate reefs, we found that sea otters had a large positive indirect influence on subtidal kelp abundance [16,18,27,28,50,51]. Unlike those studies however, we show that complementary predation by Pycnopodia contributes to these indirect effects: mean kelp densities at otter-occupied sites were 1.4 times higher in the presence of Pycnopodia (figure 3d). Sea otters have dramatic indirect effects on kelp due to their rapid consumption of large high biomass urchins, which due to metabolic scaling laws, have almost three times the kelp consumptive capacity of medium urchins (figure 3c) [16]. Nonetheless, we show the increased abundance of medium urchins following Pycnopodia decline had sufficient consumptive capacity to reduce standing kelp abundance. This is consistent with studies where Pycnopodia are the primary controlling force on smaller-sized S. purpuratus and S. droebachiensis urchin species, and where Pycnopodia declines were correlated with increased kelp abundance [31,33]. Because Pycnopodia are reported to suppress the grazing rates in urchins [23,48], we note that the observed kelp declines following SSWD could reflect both a numerical (more urchins) and behavioural (increased per capita grazing rates) response.
Our data are unique in demonstrating that the indirect effects of two key predators on kelp abundance scale up from individual reefs to regional-scale patterns in aerial canopy kelp extent. However, kelp abundance and distribution are also well known to be influenced by environmental factors [52,53] and successional processes [28,54]. For example, water temperature is negatively correlated with the nutrients required for kelp growth [55]. While SSWD was first observed in southern British Columbia in 2013 [33], its sudden appearance on British Columbia's central coast may be in part due to an anomalous marine heatwave that affected the region in 2015 and 2016 [56]. Although our data and diver observations suggest destructive grazing by medium urchins was a key factor, it is possible the co-occurrence of warmer ocean temperatures contributed directly to the observed kelp decline. While summer average sea surface temperatures in this region (electronic supplementary material, figure S6) during the ‘heatwave’ years did not exceed levels where nutrient limitation affects kelp sporophytes (greater than 16°C [55]), winter temperatures were 1–2°C warmer than historical averages which may have negatively affected kelp reproduction and gametophyte stages [57].
At sites where sea otters returned, post-disturbance successional processes that occur within kelp forests probably additionally contributed to canopy kelp declines following SSWD. The patterns we observed are similar to other studies that have shown a large regional expansion in N. luetkeana kelp canopy following rapid sea urchin removal, followed by a decline in canopy cover concordant with an increase in subtidal perennial species that suppress annuals by dominating light and space resources [28,50,54].
(c). Mechanisms of species interactions
While natural removal experiments are a powerful way to demonstrate the effect one species has on others in the system, a limitation is that they do not reveal specific mechanisms. Our population model is framed in terms of lethal consumptive effects of sea otters and Pycnopodia on urchins; however, both predators are known to elicit behavioural responses from their herbivore prey [30,46,58]. As such, it is likely some of the increases in medium urchins we observed following SSWD reflected vulnerable individuals emerging from hiding or immigrating into surveyed habitat in the absence of Pycnopodia [33]. This would not change the visible trends in our empirical data, and indeed is consistent with our model formulation as we assume that the alpha parameters represent ‘apparent mortality’, including both consumptive and behavioural effects.
Another limitation of our model is the assumption of a linear functional response between predators and urchins, which results in an estimated urchin mortality rate by otters that may underestimate high predation rates when sea otters arrive at a new site (high urchin availability) and overestimate low urchin predation rates at sites with long sea otter occupation (reduced urchin availability). A more elaborate model, which would require longer time series to fit, could account for prey-switching with a type 3 functional response, whereby per capita urchin mortality would decline with reduced urchin availability [12]. Similarly, greater kelp habitat complexity might increase urchin survival via crypsis, and also increase the amount of drift in the system that can augment urchin growth rates via bottom-up effects [59]. Under such conditions urchins typically switch from actively grazing kelp to consumption of drift algae, thus weakening their influence on attached kelp abundance [60]. Future ecosystem models could examine the implications of multiple and interacting ecological phenomena well known to occur in kelp forest systems.
(d). Implications for the resilience of alternative states in systems prone to trophic cascades
Many different stabilizing feedback mechanisms can maintain the persistence of macro-algae dominated states: high spore production facilitates kelp recruitment, whiplashing macro-algae limits urchin grazing, abundant kelp detritus promotes passive feeding by urchins and macro-algae provides habitat for predators that limit urchin populations [25,26]. While predator-induced mortality of sea urchins has been considered a key mechanism contributing to the stability of kelp forests [25], most empirical studies are focused on the mortality of adult urchins, with less known about the role of predators suppressing smaller size classes. Notable exceptions include algal forests in the Mediterranean, where abundant ‘micropredators’ (primarily decapods) consume post-settled urchins and are thought to be an important mechanism stabilizing algae-dominated reefs [29]. Similarly, predation intensity on juvenile S. droebachiensis by crabs and fish in Atlantic Canada is considered a key factor regulating urchin populations at an important demographic bottleneck, which in turn influences kelp abundance [61]. Our findings suggest that, on the Pacific coast of Canada, complementary predation on small and medium red urchins by Pycnopodia at sea otter-occupied reefs is an important factor contributing to the resilience of kelp forests (i.e. maintaining structure and function in the face of disturbance [2]). We show that while sea otters dictate alternative community configurations, the absence of Pycnopodia leads to higher urchin grazing, reduced kelp densities and increased spatial patchiness within and among sites (figure 5).
Suppression of small-to-medium sea urchin densities by Pycnopodia probably also affects the rate of algal recovery following sea otter-induced trophic cascades. We observed that when sea otters arrived at new reefs and removed most large urchins, kelp recovery occurred rapidly (within 1 year). While equally rapid transitions are reported in southeast Alaska, slower transitions are observed in the Aleutian Islands [27], which are beyond the range limit of Pycnopodia. On Aleutian reefs, high densities of unconsumed smaller S. polycanthus urchins (less than 3 cm) are sufficient to prevent the recolonization of kelp sporophytes for years, even decades after the initial arrival of foraging otters [27]. While higher and more consistent urchin recruitment rates are considered key factors driving high densities of small urchins in the Aleutians [27], our data suggest that suppression of smaller urchins by Pycnopodia may be an important factor facilitating more rapid otter-induced regime shifts in British Columbia and southeast Alaska. By facilitating kelp recovery in the face of a sea otter ‘disturbance’, Pycnopodia presence may reduce the resilience of urchin barrens. Our current data are insufficient to fully test this hypothesis, however the arrival of sea otters to new reefs in the wake of SSWD may present this opportunity in the future.
Ecological surprises are important in ecology because they are catalysts for reformulating views of community dynamics [5,62]. The unanticipated event of SSWD helped reveal the important role of a mesopredator, Pycnopodia, in enhancing the top-down control on urchins, a functional role that would have remained underappreciated without this perturbation. Overall, this study expands our knowledge of the dominant species interactions operating within a ‘classic’ regime shift that affects one of the world's most productive ecosystems, and provides critical information helpful in managing these systems which may be subject to more compound perturbations in an increasingly unpredictable world.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We are indebted B. Keeling, O. Pontier, J. Wilson, C. Stevenson, S. Humchitt, E. Henderson, R. Whippo, J. Bergmann, K. Thorton, T. Prinzing, D. VanMaanen, K. Hall, A. McCurdy and M. Hessing-Lewis for field assistance. We deeply thank the staff of the Hakai Institute Calvert Island Field Station for logistical and field support, along with Brain Hunt and Jennifer Jackson for assistance with temperature data. We also thank the Heiltsuk Integrated Resource Management Department and the Wuikinuxv First Nation for providing their support and ongoing feedback, and for permitting this research in their traditional territories.
Ethics
Research was approved by the Heiltsuk & Wuikinuxv First Nations and under BC Parks permit no. 107262.
Data accessibility
Datasets and analysis code supporting this article are available on Dryad (http://dx.doi.org/10.5061/dryad.5r2q8t8) [63].
Authors' contributions
J.M.B., A.K.S. and K.W.D. designed the field study; J.M.B., K.W.D., K.H. and A.K.S. collected data; M.T.T., D.K.O. and J.M.B. designed and performed modelling; J.M.B., M.T.T., D.K.O., K.W.D. and A.K.S. analysed output data; J.M.B. and K.H. performed mapping and GIS analysis; J.M.B. created species icons in figures and wrote the first draft of the manuscript. All authors contributed substantially to revisions.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by an NSERC Canadian Graduate Scholarship; graduate fellowships from the Hakai Institute and Simon Fraser University to J.M.B.; and an NSERC Discovery Grant, Pew Fellowship, and Canadian Foundation for Innovation Grant to A.K.S.
References
- 1.Sutherland JP. 1974. Multiple stable points in natural communities. Am. Nat. 108, 859–873. ( 10.1086/282961) [DOI] [Google Scholar]
- 2.Holling CS. 1973. Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 4, 1–23. ( 10.1146/annurev.es.04.110173.000245) [DOI] [Google Scholar]
- 3.Scheffer M, Carpenter SR, Foley JA, Folke C, Walker B. 2001. Catastrophic shifts in ecosystems. Nature 413, 591–596. ( 10.1038/35098000) [DOI] [PubMed] [Google Scholar]
- 4.Folke C, Carpenter SR, Walker B, Scheffer M, Elmqvist T, Gunderson LH, Holling CS. 2004. Regime shifts, resilience, and biodiversity in ecosystem management. Annu. Rev. Ecol. Syst. 35, 557–581. ( 10.1146/annurev.ecolsys.35.021103.105711) [DOI] [Google Scholar]
- 5.Doak DF, et al. 2008. Understanding and predicting ecological dynamics: are major surprises inevitable? Ecology 89, 952–961. ( 10.1890/07-0965.1) [DOI] [PubMed] [Google Scholar]
- 6.Paine RT. 1969. A note on trophic complexity and community stability. Am. Nat. 103, 91–93. ( 10.1086/282586) [DOI] [Google Scholar]
- 7.Estes JA, et al. 2011. Trophic downgrading of planet earth. Science 333, 301–306. ( 10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 8.Shurin JB, Borer ET, Seabloom EW, Anderson K, Blanchette CA, Broitman B, Cooper SD, Halpern BS. 2002. A cross-ecosystem comparison of the strength of trophic cascades. Ecol. Lett. 5, 785–791. ( 10.1046/j.1461-0248.2002.00381.x) [DOI] [Google Scholar]
- 9.Brooks JL, Dodson SI. 1965. Predation body size and composition of plankton. Science 150, 28–35. ( 10.1126/science.150.3692.28) [DOI] [PubMed] [Google Scholar]
- 10.Sousa WP. 1993. Size-dependent predation on the salt-marsh snail Cerithidea californica Haldeman. J. Exp. Mar. Bio. Ecol. 166, 19–37. ( 10.1016/0022-0981(93)90076-Z) [DOI] [Google Scholar]
- 11.Schoener TW. 1971. Theory of feeding strategies. Annu. Rev. Ecol. Syst. 2, 369–404. ( 10.1146/annurev.es.02.110171.002101) [DOI] [Google Scholar]
- 12.Ostfeld RS. 1982. Foraging strategies and prey switching in the California sea otter. Oecologia 53, 170–178. ( 10.1007/BF00545660) [DOI] [PubMed] [Google Scholar]
- 13.Paine RT. 1976. Size-limited predation: an observational and experimental approach with the Mytilus-Pisaster interaction. Ecology 57, 858–873. ( 10.2307/1941053) [DOI] [Google Scholar]
- 14.Werner EE, Gilliam JF. 1984. The ontogenetic niche and species interactions in size-structured populations. Annu. Rev. Ecol. Syst. 15, 393–425. ( 10.1146/annurev.es.15.110184.002141) [DOI] [Google Scholar]
- 15.De Roos AM, Schellekens T, Van Kooten T, Persson L. 2008. Stage-specific predator species help each other to persist while competing for a single prey. Proc. Natl Acad. Sci. USA 105, 13 930–13 935. ( 10.1073/pnas.0803834105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevenson CF, Demes KW, Salomon AK. 2016. Accounting for size-specific predation improves our ability to predict the strength of a trophic cascade. Ecol. Evol. 6, 1041–1053. ( 10.1002/ece3.1870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beschta RL, Ripple WJ. 2009. Large predators and trophic cascades in terrestrial ecosystems of the western United States. Biol. Conserv. 142, 2401–2414. ( 10.1016/j.biocon.2009.06.015) [DOI] [Google Scholar]
- 18.Estes JA, Palmisano JF. 1974. Sea otters: their role in structuring nearshore communities. Science 185, 1058–1060. ( 10.1126/science.185.4156.1058) [DOI] [PubMed] [Google Scholar]
- 19.Ritchie EG, Johnson CN. 2009. Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 12, 982–998. ( 10.1111/j.1461-0248.2009.01347.x) [DOI] [PubMed] [Google Scholar]
- 20.Finke DL, Denno RF. 2004. Predator diversity dampens trophic cascades. Nature 429, 407–410. ( 10.1038/nature02554) [DOI] [PubMed] [Google Scholar]
- 21.Sitvarin MI, Rypstra AL. 2014. The importance of intraguild predation in predicting emergent multiple predator effects. Ecology 95, 2936–2945. ( 10.1890/13-2347.1) [DOI] [Google Scholar]
- 22.Snyder WE, Snyder GB, Finke DL, Straub CS. 2006. Predator biodiversity strengthens herbivore suppression. Ecol. Lett. 9, 789–796. ( 10.1111/j.1461-0248.2006.00922.x) [DOI] [PubMed] [Google Scholar]
- 23.Byrnes JE, Stachowicz JJ, Hultgren KM, Randall Hughes A, Olyarnik SV, Thornber CS. 2006. Predator diversity strengthens trophic cascades in kelp forests by modifying herbivore behaviour. Ecol. Lett. 9, 61–71. [DOI] [PubMed] [Google Scholar]
- 24.Steneck RS, Graham MH, Bourque BJ, Corbett D, Erlandson JM, Estes JA, Tegner MJ. 2002. Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ. Conserv. 29, 436–459. ( 10.1017/S0376892902000322) [DOI] [Google Scholar]
- 25.Ling SD, et al. 2015. Global regime shift dynamics of catastrophic sea urchin overgrazing. Phil. Trans. R. Soc. B 370, 1–10. ( 10.1098/rstb.2013.0269) [DOI] [Google Scholar]
- 26.Filbee-Dexter K, Scheibling RE. 2014. Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Mar. Ecol. Prog. Ser. 495, 1–25. ( 10.3354/meps10573) [DOI] [Google Scholar]
- 27.Estes JA, Duggins DO. 1995. Sea otters and kelp forests in Alaska: generality and variation in a community ecological paradigm. Ecol. Monogr. 65, 75–100. ( 10.2307/2937159) [DOI] [Google Scholar]
- 28.Watson J, Estes JA. 2011. Stability, resilience, and phase shifts in rocky subtidal communities along the west coast of Vancouver Island, Canada. Ecol. Monogr. 81, 215–239. ( 10.1890/10-0262.1) [DOI] [Google Scholar]
- 29.Bonaviri C, Gianguzza P, Pipitone C, Hereu B. 2012. Micropredation on sea urchins as a potential stabilizing process for rocky reefs. J. Sea Res. 73, 18–23. ( 10.1016/j.seares.2012.06.003) [DOI] [Google Scholar]
- 30.Duggins DO. 1983. Starfish predation and the creation of mosaic patterns in a kelp-dominated community. Ecology 64, 1610–1619. ( 10.2307/1937514) [DOI] [Google Scholar]
- 31.Bonaviri C, Graham M, Gianguzza P, Shears NT. 2017. Warmer temperatures reduce the influence of an important keystone predator. J. Anim. Ecol. 86, 490–500. ( 10.1111/1365-2656.12634) [DOI] [PubMed] [Google Scholar]
- 32.Montecino-Latorre D, Eisenlord ME, Turner M, Yoshioka R, Drew Harvell C, Pattengill-Semmens CV, Nichols JD, Gaydos JK. 2016. Devastating transboundary impacts of sea starwasting disease on subtidal asteroids. PLoS ONE 11, 1–21. ( 10.1371/journal.pone.0163190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schultz JA, Cloutier RN, Côté IM. 2016. Evidence for a trophic cascade on rocky reefs following sea star mass mortality in British Columbia. PeerJ 4, 1–19. ( 10.7717/peerj.1980) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tegner MJ, Dayton PK. 1981. Population structure, recruitment and mortality of two sea urchins (Strongylocentrotus franciscanus and S. purpuratus) in a kelp forest. Mar. Ecol. Prog. Ser. 5, 255–268. ( 10.3354/meps005255) [DOI] [Google Scholar]
- 35.Nichol LM, Boogaards MD, Abernethy R. 2009 Recent trends in the abundance and distribution of sea otters (Enhydra lutris) in British Columbia. DFO Can. Sci. Advis. Sec. Res. Doc. 2009/016. See www.dfo-mpo.gc.ca/CSAS/Csas/Publications/ResDocs-DocRech/2009/2009_016_e.htm . [Google Scholar]
- 36.Konar B, Estes JA. 2003. The stability of boundary regions between kelp beds and deforested areas. Ecology 84, 174–185. ( 10.1890/0012-9658(2003)084%5B0174:TSOBRB%5D2.0.CO;2) [DOI] [Google Scholar]
- 37.Tinker TM, Bentall G, Estes JA. 2008. Food limitation leads to behavioral diversification and dietary specialization in sea otters. Proc. Natl Acad. Sci. USA 105, 560–565. ( 10.1073/pnas.0709263105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tegner MJ, Dayton PK. 1977. Sea urchin recruitment patterns and implications of commercial fishing. Science 196, 324–326. ( 10.1126/science.847476) [DOI] [PubMed] [Google Scholar]
- 39.Estes JA, Tinker TM, Bodkin JL. 2010. Using ecological function to develop recovery criteria for depleted species: Sea otters and kelp forests in the Aleutian archipelago. Conserv. Biol. 24, 852–860. ( 10.1111/j.1523-1739.2009.01428.x) [DOI] [PubMed] [Google Scholar]
- 40.Plummer M. 2013. Just another Gibbs sampler (JAGS) v. 4.3.0 user manual. See http://mcmc-jags.sourceforge.net. [Google Scholar]
- 41.Core Team R. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 42.Vonesh JR, Osenberg CW. 2003. Multi-predator effects across life-history stages: non-additivity of egg- and larval-stage predation in an African treefrog. Ecol. Lett. 6, 503–508. ( 10.1046/j.1461-0248.2003.00470.x) [DOI] [Google Scholar]
- 43.Galasso NM, Bonaviri C, Di Trapani F, Picciotto M, Gianguzza P, Agnetta D, Badalamenti F. 2015. Fish-seastar facilitation leads to algal forest restoration on protected rocky reefs. Nat. Sci. Rep. 5, 1–9. ( 10.1038/srep12409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duplisea DE. 2005. Running the gauntlet: the predation environment of small fish in the northern Gulf of St Lawrence, Canada. ICES J. Mar. Sci. 62, 412–416. ( 10.1016/j.icesjms.2004.11.005) [DOI] [Google Scholar]
- 45.Mauzey KP, Birkeland C, Dayton PK. 1968. Feeding behavior of asteroids and escape responses of their prey in the Puget Sound region. Ecology 49, 603–619. ( 10.2307/1935526) [DOI] [Google Scholar]
- 46.Moitoza DJ, Phillips DW. 1979. Prey defense, predator preference, and nonrandom diet: the interactions between Pycnopodia helianthoides and two species of sea urchins. Mar. Biol. 53, 299–304. ( 10.1007/BF00391611) [DOI] [Google Scholar]
- 47.Nishizaki MT, Ackerman JD. 2007. Juvenile–adult associations in sea urchins (Strongylocentrotus franciscanus and S. droebachiensis): protection from predation and hydrodynamics in S. franciscanus. Mar. Biol. 151, 135–145. ( 10.1007/s00227-006-0462-6) [DOI] [Google Scholar]
- 48.Freeman A. 2005. Size-dependent trait-mediated indirect interactions among sea urchin herbivores. Behav. Ecol. 17, 182–187. ( 10.1093/beheco/arj014) [DOI] [Google Scholar]
- 49.Breen PA, Carolsfeld W, Yamanaka KL. 1985. Social behaviour of juvenile red sea urchins, Stronglyocentrotus franciscanus (Agassiz). J. Exp. Mar. Bio. Ecol. 92, 45–61. ( 10.1016/0022-0981(85)90021-8) [DOI] [Google Scholar]
- 50.Breen PA, Carson TA, Foster JB, Stewart EA. 1982. Changes in subtidal community structure associated with British Columbia sea otter transplants. Mar. Ecol. Prog. Ser. 7, 13–20. ( 10.3354/meps007013) [DOI] [Google Scholar]
- 51.Markel RW, Shurin JB. 2015. Indirect effects of sea otters on rockfish (Sebastes spp.) in giant kelp forests. Ecology 96, 2877–2890. ( 10.1890/14-0492.1) [DOI] [PubMed] [Google Scholar]
- 52.Parnell PE, Miller E, Lennert-Cody C, Dayton PK, Carter M, Stebbins T. 2010. The response of giant kelp (Macrocystis pyrifera) in southern California to low-frequency climate forcing. Limnol. Oceanogr. 55, 2686–2702. ( 10.4319/lo.2010.55.6.2686) [DOI] [Google Scholar]
- 53.Cavanaugh KC, Siegel D, Reed DC, Dennison P. 2011. Environmental controls of giant-kelp biomass in the Santa Barbara Channel, California. Mar. Ecol. Prog. Ser. 429, 1–17. ( 10.3354/meps09141) [DOI] [Google Scholar]
- 54.Duggins DO. 1980. Kelp beds and sea otters: an experimental approach. Ecology 6, 447–453. ( 10.2307/1937405) [DOI] [Google Scholar]
- 55.Dayton PK, Tegner MJ, Edwards P, Riser K. 1999. Temporal and spatial scales of kelp demography: the role of oceanographic climate. Ecol. Monogr. 69, 219–250. ( 10.1890/0012-9615(1999)069%5B0219:TASSOK%5D2.0.CO;2) [DOI] [Google Scholar]
- 56.Hunt BPV, Jackson JM, Hare AA, Wang K. 2016. Hakai oceanography program: central coast and Northern Strait of Georgia time series. In State of the physical, biological and selected fishery resources of Pacific Canadian marine ecosystems in 2015 (eds PC Chandler, SA King, Perry I), pp. 127–131. Can. Tech. Rep. Fish. Aquat. Sci. 3179 Sidney, BC: Fisheries & Oceans Canada, Institute of Ocean Sciences. [Google Scholar]
- 57.Harley CDG, Anderson KM, Demes KW, Jorve JP, Kordas RL, Coyle TA, Graham MH. 2012. Effects of climate change on global seaweed communities. J. Phycol. 48, 1064–1078. ( 10.1111/j.1529-8817.2012.01224.x) [DOI] [PubMed] [Google Scholar]
- 58.Lowry LF, Pearse JS. 1973. Abalones and sea urchins in an area inhabited by sea otters. Mar. Biol. 23, 213–219. ( 10.1007/BF00389487) [DOI] [Google Scholar]
- 59.Ebert TA. 1968. Growth rates of the sea urchin Strongylocentrotus purpuratus related to food availability and spine abrasion. Ecology 49, 1075–1091. ( 10.2307/1934491) [DOI] [Google Scholar]
- 60.Harrold C, Reed DC. 1985. Food availability, sea urchin grazing, and kelp forest community structure. Ecology 66, 1160–1169. ( 10.2307/1939168) [DOI] [Google Scholar]
- 61.Scheibling RE, Hamm J. 1991. Interactions between sea urchins (Strongylocentrotus droebachiensis) and their predators in field and laboratory experiments. Mar. Biol. 110, 105–116. ( 10.1007/BF01313097) [DOI] [Google Scholar]
- 62.Paine RT, Tegner MJ, Johnson EA. 1998. Compounded perturbations yield ecological surprises. Ecosystems 1, 535–545. ( 10.1007/s100219900049) [DOI] [Google Scholar]
- 63.Burt JM, Tinker MT, Okamoto DK, Demes KW, Holmes K, Salomon AK. 2018. Data from: Sudden collapse of a mesopredator reveals its complementary role in mediating rocky reef regime shifts Dryad Digital Repository. ( 10.5061/dryad.5r2q8t8) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Burt JM, Tinker MT, Okamoto DK, Demes KW, Holmes K, Salomon AK. 2018. Data from: Sudden collapse of a mesopredator reveals its complementary role in mediating rocky reef regime shifts Dryad Digital Repository. ( 10.5061/dryad.5r2q8t8) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Datasets and analysis code supporting this article are available on Dryad (http://dx.doi.org/10.5061/dryad.5r2q8t8) [63].