Abstract
The costs of predation may exert significant pressure on the mode of communication used by an animal, and many species balance the benefits of communication (e.g. mate attraction) against the potential risk of predation. Four groups of toothed whales have independently evolved narrowband high-frequency (NBHF) echolocation signals. These signals help NBHF species avoid predation through acoustic crypsis by echolocating and communicating at frequencies inaudible to predators such as mammal-eating killer whales. Heaviside's dolphins (Cephalorhynchus heavisidii) are thought to exclusively produce NBHF echolocation clicks with a centroid frequency around 125 kHz and little to no energy below 100 kHz. To test this, we recorded wild Heaviside's dolphins in a sheltered bay in Namibia. We demonstrate that Heaviside's dolphins produce a second type of click with lower frequency and broader bandwidth in a frequency range that is audible to killer whales. These clicks are used in burst-pulses and occasional click series but not foraging buzzes. We evaluate three different hypotheses and conclude that the most likely benefit of these clicks is to decrease transmission directivity and increase conspecific communication range. The expected increase in active space depends on background noise but ranges from 2.5 (Wenz Sea State 6) to 5 times (Wenz Sea State 1) the active space of NBHF signals. This dual click strategy therefore allows these social dolphins to maintain acoustic crypsis during navigation and foraging, and to selectively relax their crypsis to facilitate communication with conspecifics.
Keywords: acoustic crypsis, active space, communication, echolocation, Heaviside's dolphin, narrowband high-frequency clicks
1. Introduction
Social animals inevitably need to balance effective communication with conspecifics against the costs associated with communication, including eavesdropping and potential detection by predators and prey [1]. Trade-offs to decrease predator detection often involve shifting communication to periods or locations with lowered predation risk [2], but such acoustic avoidance can be costly if the social or ecological functions of communication are not fulfilled [3]. Alternatively, animals may use quiet, low-amplitude or high-frequency signals with short detection ranges for social interactions [4], which can be difficult for predators to locate [5].
In the aquatic environment, where light diminishes quickly, cetaceans (whales, dolphins and porpoises) rely on sound as the primary medium for orientation, foraging and communication [6]. In water, sound travels faster and attenuates less than in air [7], increasing the necessity of balancing communication with the associated risk of distant eavesdroppers. Mammal-eating killer whales (Orcinus orca) have been shown to fall silent as they hunt so as not to alert their acoustically sensitive prey [8]. Antipredator strategies that decrease the risk of passive detection by predators have potentially large benefits because echolocation used by all toothed whales puts them at heightened risk of detection by eavesdroppers [9]. For example, Blainville's beaked whales (Mesoplodon densirostris) only produce sound at depth and remain silent within several hundred metres of the surface, and this has been proposed to represent a strategy to reduce risk of detection by killer whales, which tend not to dive deeper than a few tens of metres [10]. Additionally, delphinids [11] and seals [12] seem to suppress vocal activity in the presence of killer whales.
Toothed whales are grouped into four acoustic categories by the type of biosonar pulses they emit [13,14]. While most delphinids produce broadband, extremely short biosonar clicks, 13 species from four separate clades (Kogiidae, Phocoenidae, Pontoporiidae and 6 delphinid species from the genera Cephalorhynchus and Lagenorhynchus) have evolved a narrowband, high-frequency (NBHF) click type [15,16] with energy almost exclusively above 100 kHz [17]. These four independent cases of convergent evolution have spurred several hypotheses regarding the evolution of NBHF signals [16]. Some authors have argued that NBHF signals exploit a natural low noise window occurring at frequencies above 100 kHz to favour detection in an otherwise noisy environment [18]. Other authors propose that the evolution of NBHF signals and the concurrent loss of producing lower-frequency whistles is evidence for an ‘acoustic crypsis' strategy [15,19] where NBHF species have shifted their acoustic signals to frequencies above the hearing limit of killer whales which cuts off around 100 kHz [20]. The ‘acoustic crypsis' hypothesis has become a commonly accepted explanation for the evolution of NBHF signals [14,21,22].
This cryptic biosonar strategy has had consequences for communication and social behaviour in NBHF species. Many broadband delphinid species produce a wide variety of communication signals [23,24] including low-frequency calls and whistles that can travel several kilometres underwater [25,26] and are easily distinguished from foraging sounds. By contrast, NBHF species seem to have lost the ability to whistle [15] and communication is therefore limited to clicks. Both harbour porpoises (P. phocoena) [21,27] and Hector's dolphins (C. hectori) [28] are NBHF species which have been shown to communicate acoustically with short, isolated burst-pulses during social and aggressive encounters. However, there are socio-ecological drawbacks for species constrained to producing NBHF signals for both echolocation and communication. First, the signal repertoire and thus communication complexity [29] are limited, potentially reducing options for resolving and differentiating social interactions with sound. Second, communicating with signals that are also used for echolocation and foraging may increase signal ambiguity for a receiver [30] which then needs to differentiate communication from foraging signals. Finally, as NBHF clicks are highly directional and attenuate rapidly with distance due to high-frequency-dependent absorption [22], the detection range for nearby conspecifics is typically short (less than 1 km) and dependent on the relative orientation of the source and the receiver [21].
Heaviside's dolphins (Cephalorhynchus heavisidii) are small (less than 1.7 m) delphinids endemic to the west coast of southern Africa. They are typically found in shallow coastal waters to approximately 100 m depth [31] in small groups; however, group sizes tend to be slightly larger, with more socializing activity than described for other NBHF species [32]. Heaviside's dolphins have only been reported to produce NBHF clicks with little to no energy below 100 kHz, like other NBHF species [33]. Here we present evidence that Heaviside's dolphins produce lower-frequency broadband signals, despite residing in an area with killer whale predation risk. We show that burst-pulses are generally composed of these lower-frequency broadband signals, and thus present evidence of a NBHF species with a dual click type strategy. We discuss three possible theories to explain how the production of these lower-frequency broadband signals may help this species compensate for the socio-ecological trade-offs imposed by communicating with NBHF signals. We use an acoustic model to show that a major advantage of communicating using lower-frequency clicks is that transmission directivity is lower and active space is larger over a wide range of noise levels, thus facilitating social interactions over a greater area.
2. Material and methods
Twenty-five hours of acoustic recordings of Heaviside's dolphins were collected in Shearwater Bay, Namibia (−26° 37′ S, 15° 05′ E), over 12 days during April and May 2016. Recordings were made by deploying two hydrophones (SoundTrap 300 HF; Ocean Instruments, New Zealand) mounted 1 m apart and suspended 1.5 m below an ocean kayak. Only data from a single hydrophone were analysed for this study. Sound was digitized at a sampling rate of 576 kHz with a 16-bit resolution (sensitivity: −171 dB re 1 V µPa−1, flat frequency response: 400 Hz–150 kHz ± 3 dB). Behaviour and group size information were collected concurrently with sound recordings (see electronic supplementary material). A land-based observer team stationed at a vantage point (20 m elevation) monitored the presence of cetaceans within the bay.
(a). Acoustic data extraction
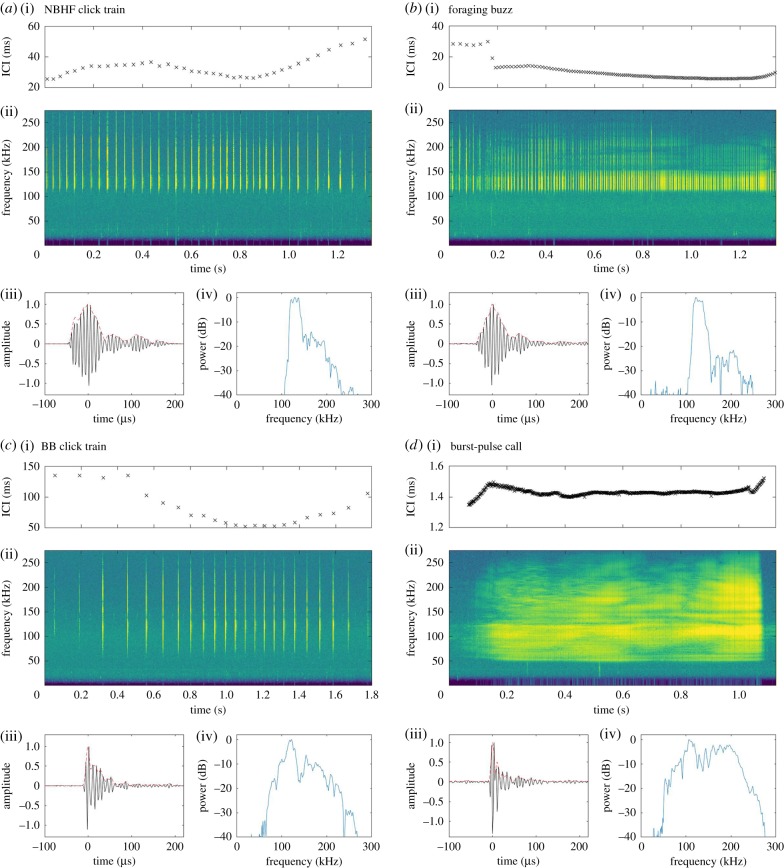
Recordings made within a visually estimated 50 m range of dolphins were selected for analysis. Acoustic signals produced by Heaviside's dolphins were identified through visual inspection of a spectrogram display in Adobe Audition CC (Adobe Systems Inc.). Heaviside's dolphin NBHF echolocation clicks have been previously described [33], and only a subset were selected for analysis. We defined three functional groups of signals based on signal context and interclick intervals (ICI, calculated as the time between subsequent clicks [9]). Click trains were defined as series of clicks with ICI exceeding 10 ms. Such click trains are likely to be echolocation signals produced by the animals. A subset of click trains were composed of lower-frequency, broader-bandwidth signals than previously described [33], and we therefore divided click trains into NBHF click trains and broadband click trains by inspecting spectrograms (figure 1). Foraging buzzes are used during prey capture by echolocating animals [34,35], including NBHF species [36]. These were defined as click series with ICIs less than 10 ms, which were preceded by a slower click train. Since buzzes occurred at the end of a click train, we defined the start of a buzz as the point when the ICI first decreased below 10 ms and the end of the buzz as the point where the click train ended or where the ICI increased to greater than 10 ms. Finally, we defined burst-pulse signals as discrete, isolated series of high repetition rate clicks that began, persisted and generally ended with interclick intervals less than 10 ms following Lammers et al. [37]. Burst-pulses are commonly considered to have an intra-specific communicative function [24,37,38], including in NBHF species [21]. Only distinguishable, high-quality pulsed signals measuring more than 10 dB above the background noise measured immediately before the signal were selected for further analysis.
Figure 1.
Examples of Heaviside's dolphin pulsed signal types. (a) Narrowband high-frequency (NBHF) click train, (b) foraging buzz, (c) broadband (BB) click train and (d) burst-pulse call. For each signal, panels represent (i) the interclick intervals throughout the signal, (ii) the spectrogram of the signal (512 pt FFT, Hamming window, 50% overlap), (iii) the normalized waveform (solid line) and envelope (dashed line) of a single click extracted from the pulsed signal shown in panel (ii) and (iv) the normalized power spectrum of the extracted click (512 pt rectangular window, 576 kHz sampling rate).
(b). Acoustic feature extraction
To quantify temporal differences in repetition rate across signals, we used a click detection algorithm developed in Matlab 2013B (MathWorks, USA). We first filtered the input signal with a six-pole Butterworth bandpass filter (20–275 kHz), calculated the signal envelope, and extracted peaks in the envelope that were separated by more than 0.5 ms. Click detections were visually inspected and manually corrected for missed detections. To compare signals with highly variable numbers of clicks, we finally calculated the 5th, 50th and 95th percentile ICI across each click series.
To quantify temporal and spectral differences of component clicks, we extracted the highest amplitude click from each click series following the methods for on-axis click analysis [39,40]. While these signals were recorded from an unknown aspect, the minute difference in the waveform and spectrum of NBHF clicks across varying off-axis angles [41] means that spectral parameters are likely reasonably close to on-axis signals. Individual signals were filtered in Matlab with a four-pole Butterworth bandpass filter between 20 and 275 kHz. Individual click power spectra were calculated with a 512-point 50% Tukey window centred on the peak envelope of each click. Spectral and temporal click parameters were calculated according to methods for measuring on-axis click parameters [9,42].
(c). Statistical analysis of signal discrimination
Signal parameters, including spectral and temporal click parameters as well as interclick intervals, were compared across signal categories using a non-parametric Kruskal–Wallis test and subsequent Dunn's post-hoc tests for pairwise comparisons in R v. 3.4.2 [43,44]. We then used a random forest classifier [45] to measure prediction accuracy as a function of buzz and burst-pulse signal categories using either ICI parameters (5th, 50th and 95th ICI percentiles for each click series), spectral and temporal individual click parameters, or all signal parameters combined to test the potential benefit of spectral differences in decreasing signal ambiguity. The random forest classifier was built in Matlab 2017b using a ‘bagged trees' ensemble classifier with 30 learners [45]. Prediction accuracy was measured using 5-fold cross-validation to prevent overfitting. To measure consistency in prediction accuracy, a classifier was trained 100 times and prediction accuracy measured for each iteration.
(d). Acoustical modelling of detection range
To test the potential benefit for communication, we modelled the detection range for typical NBHF clicks and for lower-frequency clicks extracted from burst-pulses. We first filtered the input signal with a six-pole Butterworth bandpass filter (10–150 kHz), and we used a piston model [46] to estimate changes in transmission beam and empirical measurements of hearing sensitivity of a harbour porpoise [47] to estimate changes in directional hearing. We modelled the detection range (m) for a noise-limited scenario with Wenz Sea State 2 noise levels, and we accounted for changes in transmission loss due to lower frequency-specific absorption. A separate sensitivity analysis was conducted across a 25 dB variation in wind-generated ambient noise (reflecting calm sea conditions to storms) and a 25 dB variation in signal source levels (reflecting the full distribution of on-axis source levels from Heaviside's dolphins [33]) to examine how varying noise conditions and output levels affect the relative change in active space between the two signal types. The full model and sensitivity analysis are described in electronic supplementary material.
3. Results
Acoustic data were collected during recording sessions with Heaviside's dolphins during which foraging, resting, socializing, interacting with the kayak and travelling behaviours were observed. No other cetacean species were sighted visually or detected acoustically during recording sessions. A total of 90 broadband click trains, 706 buzzes and 954 burst-pulses and a subset of 33 NBHF click trains were indexed from recordings made when Heaviside's dolphins were within 50 m of the kayak.
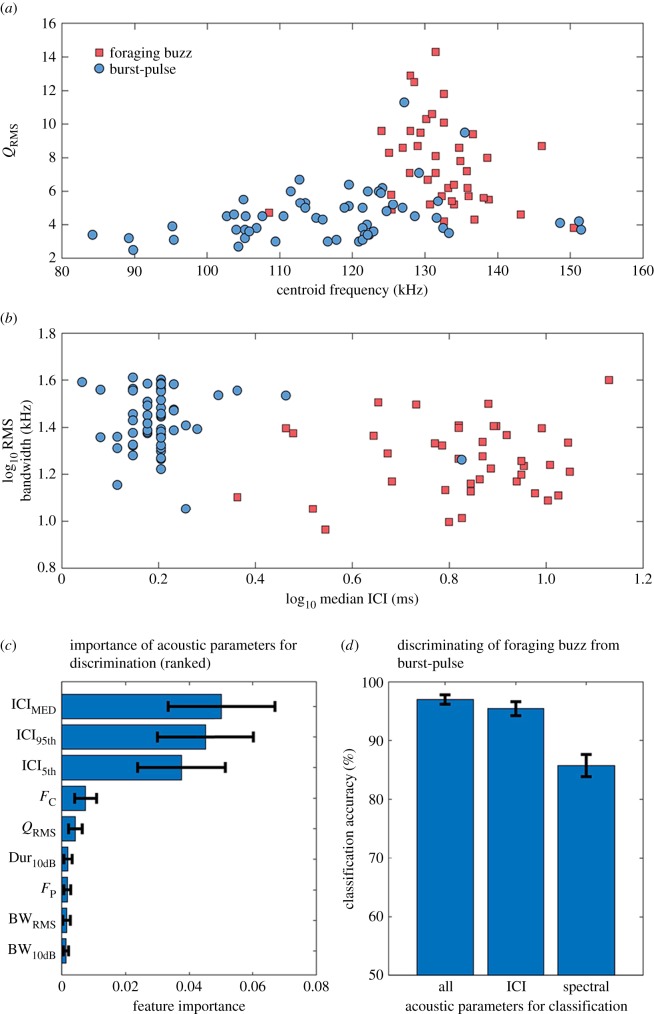
Broadband click trains and burst-pulse signals were composed of clicks with lower frequency and broader bandwidth (figure 1) compared to typical NBHF signals (table 1). Q-ratios (centroid frequency/RMS bandwidth) are an indicator of click type, and generally burst-pulse signals and broadband click trains had Q-ratios less than 5, whereas NBHF click trains and buzz signals had Q-ratios greater than 7 (table 1; electronic supplementary material, figure S1A).
Table 1.
Biosonar parameters of pulsed signal types produced by Heaviside's dolphins. The median and 5th–95th percentile values are reported for each parameter. ICI5th, ICIMED and ICI95th represent 5th, median (50th) and 95th percentile interclick intervals, respectively. FP, peak frequency; FC, centroid frequency; BW3 dB, −3 dB bandwidth; BW10 dB, −10 dB bandwidth; BWRMS, root mean square bandwidth; QRMS, FC/BWRMS; Dur10 dB, −10 dB click duration.
| NBHF click train |
BB click train |
buzz |
burst-pulse |
|||||
|---|---|---|---|---|---|---|---|---|
|
n = 33 |
n = 28 |
n = 40 |
n = 58 |
|||||
| source parameters | median | (5–95%) | median | (5–95%) | median | (5–95%) | median | (5–95%) |
| ICI5th (ms)a | 23.5 | (14.9–41.2) | 24.8 | (7.8–78.9) | 6.0 | (2.1–9.9) | 1.5 | (1.2–1.9) |
| ICIMED (ms)a | 28.9 | (22.3–55.4) | 28.8 | (11.7–110.1) | 7.2 | (3.0–11.2) | 1.6 | (1.3–2.2) |
| ICI95th (ms)a | 46.1 | (29.4–104.8) | 40.9 | (17.4–215.8) | 10.0 | (5.0–13.0) | 1.7 | (1.4–3.2) |
| FP (kHz)b | 127.1 | (121.5–136.6) | 113.6 | (78.4–141.3) | 123.8 | (115.8–137.3) | 112.5 | (90.0–133.1) |
| FC (kHz)b | 131.3 | (125.3–136.9) | 110.8 | (87.2–146.8) | 132.4 | (124.9–143.3) | 119.5 | (94.4–149.0) |
| BW3 dB (kHz)b | 15.8 | (9.5–22.7) | 21.4 | (4.9–79.1) | 12.4 | (3.3–23.7) | 16.3 | (3.2–62.2) |
| BW10 dB (kHz)b | 31.5 | (22.7–69.8) | 79.9 | (38.1–142.5) | 37.1 | (21.2–86.1) | 75.4 | (31.0–137.9) |
| BWRMS (kHz)b | 12.8 | (8.2–23.2) | 27.5 | (17.1–38.6) | 18.3 | (10.2–31.6) | 26.6 | (18.1–38.7) |
| QRMSb | 10.2 | (5.9–15.6) | 4.1 | (2.8–7.1) | 7.2 | (4.3–12.5) | 4.4 | (3.0–6.8) |
| Dur10 dB (µm)b | 63.9 | (50.6–85.1) | 37.0 | (16.6–50.7) | 71.1 | (42.6–129.0) | 41.1 | (21.1–82.3) |
aParameters measured across a click series.
bParameters measured for an individual click.
Initially, buzz and burst-pulse signals were visually differentiated by the presence or absence of a preceding click train as burst-pulses occur as isolated signals. The measured signal parameters confirmed there were significant differences in both ICI parameters and spectral parameters between these two signal types (figure 2; see electronic supplementary material for a full comparison of different signal types). Based on these findings, a random forest classification algorithm was implemented to evaluate importance of different parameters and test if discrimination of communication signals (burst-pulses) from feeding signals (buzzes) benefits from spectral differences. The random forest classifier demonstrated that ICI parameters were most important for accurate classification of buzz and burst-pulse signal categories (figure 2c). Signal categories could be predicted with 97% accuracy using all available parameters (figure 2d). Classification accuracy decreased only marginally (95% prediction accuracy) when only interclick interval parameters were included in the model, whereas a larger drop in accuracy was seen when only spectral and temporal click parameters were included in the model (86% prediction accuracy).
Figure 2.
Signal parameters and discrimination of buzz and burst-pulse signal types. (a) Q-ratio (centroid frequency/RMS bandwidth) as a function of centroid frequency. (b) Log-transformed RMS bandwidth as a function of log-transformed median ICI. (c) Relative feature importance of acoustic signal parameters for classification accuracy. (d) Random forest classification accuracy following three scenarios: discrimination using all signal parameters (All); discrimination using interclick intervals (ICI); or discrimination using spectral and temporal click parameters (spectral). For plots (c,d), values are reported as mean (±s.d.) for 100 independently trained random forest models. Both feeding buzzes and burst-pulse calls can be accurately classified by interclick intervals without including frequency, bandwidth or other individual click parameters. (Online version in colour.)
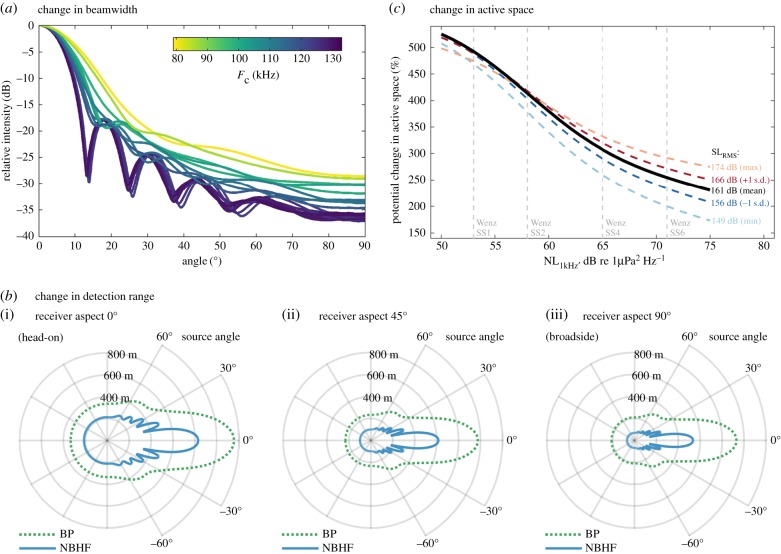
The effect of signal type on beamwidth was two-fold: first, the sidelobes seen in NBHF signals were suppressed because of the broader bandwidth of burst-pulse signals; second, the transmission directivity was lower and consequently sound intensity away from the centre of the sound beam was higher (figure 3a). The detection range for NBHF clicks and burst-pulse signals was modelled for a typical 130 kHz NBHF signal and for a burst-pulse signal with a centroid frequency of 80 kHz. While detection range depends on the modelled noise levels as well as source and receiver geometry, the estimated detection range was consistently greater for burst-pulse signals at all estimated source and receiver angle combinations (figure 3b). The potential gain in active space depended on noise level but was relatively unaffected by large changes in sound source level (figure 3c). At wind-generated noise levels corresponding to Wenz Sea State 1 (approximately 4–6 knots of wind), the active space of a burst-pulse signal would be around five times greater than the active space of a NBHF click (figure 3c). At an estimated wind-generated noise level corresponding to Wenz Sea State 6 (approximately 28–47 knots of wind), the active space would be approximately 2.5 times greater than for a NBHF click (figure 3c).
Figure 3.
Switching to lower-frequency burst-pulse signals increases beamwidth and active space. (a) Transmission beam modelled for 10 standard NBHF clicks and 9 burst-pulse clicks of varying frequency using a circular piston model. (b) Detection range modelled for a typical NBHF signal (blue solid line) and a lower-frequency (80 kHz) burst-pulse signal (green dashed line) under Wenz Sea State (SS) two noise conditions and with 161 dB RMS source level, with receivers oriented (i) towards the source, (ii) at a 45° angle to the source or (iii) at a 90° angle to the source. (c) Relative active space for burst-pulse signals compared to the active space of NBHF signals. Note that while detection range will depend on specific model parameters, the qualitative relationship between the detection range of NBHF and burst-pulse signals is consistent under a wide range of noise levels (including Wenz SS1 to Wenz SS6 wind-generated noise, with thermal noise constant) and source levels (covering full range of source levels measured for Heaviside's dolphins in [33]).
4. Discussion
Members of the genus Cephalorhynchus are thought to have evolved the exclusive use of NBHF biosonar signals to become acoustically cryptic, thereby reducing predation risk by killer whales [15]. This has consequences for the evolution and function of communication signals within the genus, because acoustic communication is thought to be limited to taking place through click series [21,28]. Here, we show that Heaviside's dolphins produce a second click type that is distinct from normal NBHF clicks by having a lower-frequency content and broader bandwidth which circumvents some of the limitations of communicating with NBHF clicks. Heaviside's dolphins produce these lower-frequency broadband signals occasionally in the form of slow click trains but predominately in the form of burst-pulses, presumably used for communication [21,24,27,28,37,38].
Communication with burst-pulses is normally achieved using clicks that are nearly indistinguishable from echolocation clicks in delphinids [28,37] and phocoenids [21,48], apart from low-frequency pulsed signals such as bottlenose dolphin (Tursiops sp.) pops [49] or jaw claps [50]. However, in Heaviside's dolphins, clicks comprising most burst-pulses appear to be a modified and clearly distinguishable version (86% classification success based only on spectral differences: figure 2d). Most of the burst-pulses analysed (63%) contained energy beginning at approximately 50 kHz, which is an octave lower than signals reported for other NBHF species [21,22,28,51]. Consequently, most of the recorded broadband signals are well within the hearing limit of killer whales (upper limit at approximately 100 kHz) [20]. This makes these signals risky to produce, especially in Namibia where killer whales are known to occur and predate on cetaceans [52], including Heaviside's dolphins in the study area (J.-P. Roux 2016, personal communication).
One explanation for the use of lower-frequency broadband signals could be to reduce signal ambiguity by allowing conspecifics to differentiate communication signals from foraging buzzes. We addressed this theory by using a cross-validated random forest classification algorithm with feature vectors containing only ICI parameters, only spectral and temporal click parameters, or containing all parameters combined. Both burst-pulses and foraging buzzes were accurately classified (95% accuracy) by interclick intervals without including spectral and temporal click parameters, so these do not seem to be necessary for accurate discrimination of burst-pulses from foraging buzzes. Rather, it seems likely that ICIs by themselves may allow animals to identify communication signals and it will be interesting to see if that is the case for other NBHF species as well.
A second, similar explanation for the use of lower-frequency broadband signals is to increase signal complexity in the repertoire, thus allowing for encoding a greater variety of messages. Repertoire complexity could be augmented either by producing non-NBHF communication signals at repetition rates that are also used for foraging signals, or by composing communication signals with different click types. However, we see only little evidence for either of these explanations: burst-pulses were composed predominantly of lower-frequency clicks, with no evidence of burst-pulses composed of different click types, and with repetition rates consistently higher than for other signal types such as click trains or foraging buzzes. However, the lower-frequency cut-off did vary between burst-pulses, and it is unclear how much of this is due to off-axis distortion [46,53] or could be used to encode information.
Finally, a third possible explanation for the use of these signals is that the lower frequency helps to increase the detection range and thus favours signal detection for nearby conspecifics. High-frequency signals suffer from increased sound absorption as they propagate through water, and thus attenuate faster than lower frequencies [7]. By reducing the predominant frequency, signals will suffer less frequency-dependent absorption and thus travel farther underwater [51]. At the same time, both transmission directivity and receiving directivity will be lower (figure 3a), and thus energy will be more equally distributed around the vocalizing animal [47,54]. The modelled detection ranges of NBHF and burst-pulse signals support this hypothesis and show that significant improvements in detection range are possible by switching to lower-frequency burst-pulse signals, especially for receivers that are oriented away from or located outside the centre of the sound beam (figure 3b). The relative change in active space is driven mostly by the change in sound radiation and partly by a lower sound absorption and thus is relatively independent of the actual source level and the absolute detection range of the animal (figure 3c). Since the noise at NBHF signal frequencies is primarily thermal noise, increasing wind-generated ambient noise decreases the potential gain in active space, but active space remains higher for burst-pulse signals across the entire range of modelled noise levels from Wenz Sea State 1 through Wenz Sea State 6 conditions (figure 3c). Furthermore, the change in active space may be greater if animals simultaneously change transmission aperture through manipulations of air sacs or soft tissue structures, such as suggested for echolocating delphinids [46] or harbour porpoises emitting foraging buzzes [55]. Thus, the most likely reason for Heaviside's dolphins to use risky, lower-frequency broadband signals is to circumvent the restrictions in communicating with a short-range, highly directional NBHF signal imposed by shifting their biosonar above the hearing range of killer whales. The estimated increase in active space achieved by the lower-frequency broadband signals is still far less than could be achieved by using whistles [26], thus this secondary click type represents a compromise between remaining acoustically cryptic (especially when foraging) and possessing the ability to communicate over a greater range when necessary.
It is possible that other NBHF species may take advantage of selectively increasing their active space. Neonatal phocoenids have been reported to produce pulsed signals with a strong low-frequency (approx. 1–3 kHz) content just after birth and begin to exclusively produce NBHF clicks between four [56] and 20 [57] days postnatal. It is not yet understood if this is related to morphological changes or learned call behaviour. Regardless, calves' ability to produce lower-frequency signals with greater active space may be useful for mother–offspring cohesion during the first days of life. Additionally, sporadic broadband clicks and low-frequency (4–16 kHz) whistle sounds have been recorded in the presence of mother and calf pairs of Commerson's dolphins (C. commersonii) [58]. Thus, we should not unequivocally dismiss the possibility of finding lower-frequency communication signals in species that are considered acoustically cryptic NBHF species.
Supplementary Material
Supplementary Material
Acknowledgements
The authors gratefully acknowledge Heiko Metzger, Dr J.-P. Roux, Jeff Slater, Melissa Nel, Robert Sparg, the leaders of the SNAK Acoustic Communication Course, Aarhus University, and four anonymous reviewers for providing comments and helpful feedback to improve this manuscript.
Ethics
This research was conducted by the ‘Namibian Dolphin Project' with permission from the Namibian Ministry of Fisheries and Marine Resources and with ethics 252 clearance from the University of Pretoria Animal Use and Care Committee (Reference: ec061-09 AUCC).
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material and in Dryad Digital Repository (doi:10.5061/dryad.64048p0) [59].
Authors' contributions
M.J.M., T.G. and S.H.E. conceived and designed the experiments. M.J.M. performed the experiments and collected data. M.J.M. and F.H.J analysed the data. M.J.M., T.G., S.H.E. and F.H.J. contributed materials and analysis tools. F.H.J. contributed to acoustic modelling. M.J.M., T.G., S.H.E. and F.H.J. wrote the paper and approved final submission.
Funding
This research was supported by a Fulbright U.S. Research Fellowship, the National Geographic Society's Emerging Explorers Grant in conjunction with the Waitt Foundation (38115) and the University of Pretoria's Zoology Department. T.G. was funded by the Claude Leon Foundation, and S.H.E. was funded by the South African National Research Foundation. F.H.J. acknowledges funding from the Office of Naval Research (N00014-1410410) and an AIAS-COFUND fellowship from Aarhus Institute of Advanced Studies.
Competing interests
We declare we have no competing interests.
References
- 1.Bradbury J, Vehrencamp S. 1998. Signal design rules. In Principles of animal communication, pp. 571–618. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 2.Curio E. 1976. The ethology of predation. Berlin, Germany: Springer-Verlag. [Google Scholar]
- 3.Ruxton GD. 2009. Non-visual crypsis: a review of the empirical evidence for camouflage to senses other than vision. Phil. Trans. R. Soc. B 364, 549–557. ( 10.1098/rstb.2008.0228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson DR, Hare JF. 2006. The adaptive utility of Richardson's ground squirrel (Spermophilus richardsonii) short-range ultrasonic alarm signals. Can. J. Zool. 84, 1322–1330. ( 10.1139/Z06-120) [DOI] [Google Scholar]
- 5.Jones KJ, Hill WL. 2001. Auditory perception of hawks and owls for passerine alarm calls. Ethology 107, 717–726. ( 10.1046/j.1439-0310.2001.00698.x) [DOI] [Google Scholar]
- 6.Tyack P. 1998. Acoustic communication under the sea. In Animal acoustic communication (eds Hopp SL, Owren MJ, Evans CS), pp. 163–220. Berlin, Germany: Springer; ( 10.1007/978-3-642-76220-8_6) [DOI] [Google Scholar]
- 7.Clay CS, Medwin H. 1977. Acoustical oceanography: principles and applications. New York, NY: Wiley-Interscience. [Google Scholar]
- 8.Deecke VB, Ford JKB, Slater PJB. 2005. The vocal behaviour of mammal-eating killer whales: communicating with costly calls. Anim. Behav. 69, 395–405. ( 10.1016/j.anbehav.2004.04.014) [DOI] [Google Scholar]
- 9.Au W. 1993. The sonar of dolphins. New York, NY: Springer-Verlag; ( 10.1007/978-1-4612-4356-4) [DOI] [Google Scholar]
- 10.Aguilar de Soto N, Madsen PT, Tyack P, Arranz P, Marrero J, Fais A, Revelli E, Johnson M. 2012. No shallow talk: cryptic strategy in the vocal communication of Blainville's beaked whales. Mar. Mamm. Sci. 28, 75–92. ( 10.1111/j.1748-7692.2011.00495.x) [DOI] [Google Scholar]
- 11.Rankin S, Archer F, Barlow J. 2013. Vocal activity of tropical dolphins is inhibited by the presence of killer whales, Orcinus orca. Mar. Mamm. Sci. 29, 679–690. ( 10.1111/j.1748-7692.2012.00613.x) [DOI] [Google Scholar]
- 12.Thomas JA, Ferm LM, Kuechle VB. 1989. Silence as an antipredation strategy by weddell seals. San Diego, CA: Naval Ocean Systems Center. [Google Scholar]
- 13.Wahlberg M, Beedholm K, Heerfordt A, Møhl B. 2011. Characteristics of biosonar signals from the northern bottlenose whale, Hyperoodon ampullatus. J. Acoust. Soc. Am. 130, 3077–3084. ( 10.1121/1.3641434) [DOI] [PubMed] [Google Scholar]
- 14.Fenton BM, Jensen FH, Kalko EK, Tyack PL. 2014. Sonar signals of bats and toothed whales. In Biosonar (eds Surlykke A, Nachtigall P, Fay R, Popper A), pp. 11–59. New York, NY: Springer; ( 10.1007/978-1-4614-9146-0_2) [DOI] [Google Scholar]
- 15.Morisaka T, Connor RC. 2007. Predation by killer whales (Orcinus orca) and the evolution of whistle loss and narrow-band high frequency clicks in odontocetes. J. Evol. Biol. 20, 1439–1458. ( 10.1111/j.1420-9101.2007.01336.x) [DOI] [PubMed] [Google Scholar]
- 16.Morisaka T. 2012. Evolution of communication sounds in odontocetes: a review. Int. J. Comp. Psychol. 25, 1–20. [Google Scholar]
- 17.Au WWL. 1997. Echolocation in dolphins with a dolphin-bat comparison. Bioacoustics 8, 137–162. ( 10.1080/09524622.1997.9753357) [DOI] [Google Scholar]
- 18.Møhl B, Andersen S. 1973. Echolocation: high-frequency component in the click of the harbour porpoise (Phocoena ph.). J. Acoust. Soc. Am. 54, 1368–1372. ( 10.1121/1.1914435) [DOI] [PubMed] [Google Scholar]
- 19.Andersen S, Amundin M. 1976. Possible predator-related adaption of sound production and hearing in the harbour porpoise (Phocoena phocoena). Aquat. Mamm. 4, 56–58. [Google Scholar]
- 20.Szymanski MD, Bain DE, Kiehl K, Pennington S, Wong S, Henry KR. 1999. Killer whale (Orcinus orca) hearing: auditory brainstem response and behavioral audiograms. J. Acoust. Soc. Am. 106, 1134–1141. ( 10.1121/1.427121) [DOI] [PubMed] [Google Scholar]
- 21.Clausen KT, Wahlberg M, Beedholm K, DeRuiter SL, Madsen PT. 2010. Click communication in harbour porpoises Phocoena phocoena. Bioacoustics 20, 1–28. ( 10.1080/09524622.2011.9753630) [DOI] [Google Scholar]
- 22.Kyhn LA, Tougaard J, Beedholm K, Jensen FH, Ashe E, Williams R, Madsen PT. 2013. Clicking in a killer whale habitat: narrow-band, high-frequency biosonar clicks of harbour porpoise (Phocoena phocoena) and Dall's porpoise (Phocoenoides dalli). PLoS ONE 8, e63763 ( 10.1371/journal.pone.0063763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyack P. 1986. Population biology, social behavior and communication in whales and dolphins. Trends Ecol. Evol. 1, 144–150. ( 10.1016/0169-5347(86)90042-X) [DOI] [PubMed] [Google Scholar]
- 24.Herzing DL. 1996. Vocalizations and associated underwater behavior of free-ranging Atlantic spotted dolphins, Stenella frontalis, and bottlenose dolphins, Tursiops truncatus. Aquat. Mamm. 22, 61–79. [Google Scholar]
- 25.Miller PJ. 2006. Diversity in sound pressure levels and estimated active space of resident killer whale vocalizations. J. Comp. Physiol. A 192, 449 ( 10.1007/s00359-005-0085-2) [DOI] [PubMed] [Google Scholar]
- 26.Jensen F, Beedholm K, Wahlberg M, Bejder L, Madsen PT. 2012. Estimated communication range and energetic cost of bottlenose dolphin whistles in a tropical habitat. J. Acoust. Soc. Am. 131, 582–592. ( 10.1121/1.3662067) [DOI] [PubMed] [Google Scholar]
- 27.Amundin M. 1991. Sound production in odontocetes with emphasis on the harbour porpoise Phocoena phocoena. PhD dissertation, Stockholm, Sweden. [Google Scholar]
- 28.Dawson SM. 1991. Clicks and communication: the behavioral and social contexts of Hector's dolphin vocalizations. Ethology 88, 265–276. ( 10.1111/j.1439-0310.1991.tb00281.x) [DOI] [Google Scholar]
- 29.Fischer J, Wadewitz P, Hammerschmidt K. 2016. Structural variability and communicative complexity in acoustic communication. Anim. Behav. 134, 229–237. ( 10.1016/j.anbehav.2016.06.012) [DOI] [Google Scholar]
- 30.Wiley RH. 2013. Communication as a transfer of information: measurement, mechanism, and meaning. In Animal communication theory: information and influence (ed. Stegmann U.), pp. 113–129. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 31.Elwen S, Meÿer MA, Best PB, Kotze PGH, Thornton M, Swanson S. 2006. Range and movements of female Heaviside's dolphins (Cephalorhynchus heavisidii), as determined by satellite-linked telemetry. J. Mammal. 87, 866–877. ( 10.1644/05-MAMM-A-307R2.1) [DOI] [Google Scholar]
- 32.Behrmann CA. 2011. Occurrence and group dynamics of Heaviside's dolphins (Cephalorhynchus heavisidii) in Table Bay, Western Cape, South Africa. MSc thesis, University of Pretoria, Pretoria, South Africa. [Google Scholar]
- 33.Morisaka T, Karczmarski L, Akamatsu T, Sakai M, Dawson S, Thornton M. 2011. Echolocation signals of Heaviside's dolphins (Cephalorhynchus heavisidii). J. Acoust. Soc. Am. 129, 449–457. ( 10.1121/1.3519401) [DOI] [PubMed] [Google Scholar]
- 34.Griffin DR, Webster FA, Michael CR. 1960. The echolocation of flying insects by bats. Anim. Behav. 8, 141–154. ( 10.1016/0003-3472(60)90022-1) [DOI] [Google Scholar]
- 35.Miller LA, Pristed J, Møshl B, Surlykke A. 1995. The click-sounds of narwhals (Monodon monoceros) in Inglefield Bay, Northwest Greenland. Mar. Mamm. Sci. 11, 491–502. ( 10.1111/j.1748-7692.1995.tb00672.x) [DOI] [Google Scholar]
- 36.Wisniewska DM, Johnson M, Teilmann J, Rojano-Doñate L, Shearer J, Sveegaard S, Miller LA, Siebert U, Madsen PT. 2016. Ultra-high foraging rates of harbor porpoises make them vulnerable to anthropogenic disturbance. Curr. Biol. 26, 1441–1446. ( 10.1016/j.cub.2016.03.069) [DOI] [PubMed] [Google Scholar]
- 37.Lammers MO, Au WWL, Aubauer R, Nachtigall PE. 2004. A comparative analysis of the pulsed emissions of free-ranging Hawaiian spinner dolphins (Stenella longirostris). In Echolocation in bats and dolphins (eds Thomas JA, Moss CF, Vater M), pp. 414–419. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 38.Blomqvist C, Amundin M. 2004. High-frequency burst-pulse sounds in agonistic/aggressive interactions in bottlenose dolphins, Tursiops truncatus. In Echolocation in bats and dolphins (eds Thomas JA, Moss CF, Vater M), pp. 425–431. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 39.Madsen PT, Wahlberg M. 2007. Recording and quantification of ultrasonic echolocation clicks from free-ranging toothed whales. Deep Sea Res. 1 Oceanogr. Res. Pap. 54, 1421–1444. ( 10.1016/j.dsr.2007.04.020) [DOI] [Google Scholar]
- 40.Jensen FH, Rocco A, Mansur RM, Smith BD, Janik VM, Madsen PT. 2013. Clicking in shallow rivers: short-range echolocation of Irrawaddy and Ganges river dolphins in a shallow, acoustically complex habitat. PLoS ONE 8, e59284 ( 10.1371/journal.pone.0059284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Au WWL, Kastelein RA, Rippe T, Schooneman NM. 1999. Transmission beam pattern and echolocation signals of a harbor porpoise (Phocoena phocoena). J. Acoust. Soc. Am. 106, 3699–3705. ( 10.1121/1.428221) [DOI] [PubMed] [Google Scholar]
- 42.Madsen PT, Kerr I, Payne R. 2004. Source parameter estimates of echolocation clicks from wild pygmy killer whales (Feresa attenuata). J. Acoust. Soc. Am. 116, 1909–1912. ( 10.1121/1.1788726) [DOI] [PubMed] [Google Scholar]
- 43.Fox J, Weisberg S. 2011. An R companion to applied regression, 2nd edn. Thousand Oaks, CA: Sage Publications. [Google Scholar]
- 44.Ogle D.2017. FSA: Fisheries Stock Analysis. R package version 0.8.16.
- 45.Breiman L. 2001. Random forests. Mach. Learn. 45, 5–32. ( 10.1023/A:1010933404324) [DOI] [Google Scholar]
- 46.Jensen FH, Wahlberg M, Beedholm K, Johnson M, de Soto NA, Madsen PT. 2015. Single-click beam patterns suggest dynamic changes to the field of view of echolocating Atlantic spotted dolphins (Stenella frontalis) in the wild. J. Exp. Biol. 218, 1314–1324. ( 10.1242/jeb.116285) [DOI] [PubMed] [Google Scholar]
- 47.Kastelein RA, Janssen M, Verboom WC, de Haan D. 2005. Receiving beam patterns in the horizontal plane of a harbor porpoise (Phocoena phocoena). J. Acoust. Soc. Am. 118, 1172–1179. ( 10.1121/1.1945565) [DOI] [PubMed] [Google Scholar]
- 48.Soerensen PM, Wisniewska DM, Jensen FH, Johnson M, Teilmann J, Madsen PT. 2018. Click communication in wild harbour porpoises (Phocoena phocoena). Sci. Rep. UK 8, 9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Connor RC, Smolker RA. 1996. ‘Pop’ goes the dolphin: a vocalization male bottlenose dolphins produce during consortships. Behaviour 133, 643–662. ( 10.1163/156853996X00404) [DOI] [Google Scholar]
- 50.Caldwell MC, Haugen RM, Caldwell DK. 1962. High-energy sound associated with fright in the dolphin. Science 138, 907–908. ( 10.1126/science.138.3543.907) [DOI] [PubMed] [Google Scholar]
- 51.Madsen PT, Carder D, Bedholm K, Ridgway S. 2005. Porpoise clicks from a sperm whale nose: Convergent evolution of 130 kHz pulses in toothed whale sonars? Bioacoustics 15, 195–206. ( 10.1080/09524622.2005.9753547) [DOI] [Google Scholar]
- 52.Best PB, Meÿer MA, Lockyer C. 2010. Killer whales in South African waters: a review of their biology. Afr. J. Mar. Sci. 32, 171–186. ( 10.2989/1814232x.2010.501544) [DOI] [Google Scholar]
- 53.Wahlberg M, et al. 2011. Source parameters of echolocation clicks from wild bottlenose dolphins (Tursiops aduncus and Tursiops truncatus). J. Acoust. Soc. Am. 130, 2263–2274. ( 10.1121/1.3624822) [DOI] [PubMed] [Google Scholar]
- 54.Jakobsen L, Ratcliffe JM, Surlykke A. 2013. Convergent acoustic field of view in echolocating bats. Nature 493, 93–96. ( 10.1038/nature11664) [DOI] [PubMed] [Google Scholar]
- 55.Wisniewska DM, Ratcliffe JM, Beedholm K, Christensen CB, Johnson M, Koblitz JC, Wahlberg M, Madsen PT. 2015. Range-dependent flexibility in the acoustic field of view of echolocating porpoises (Phocoena phocoena). Elife 4, e05651 ( 10.7554/eLife.05651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delgado-Garcia L. 2016. Acoustic development and behaviour of odontocete calves. PhD thesis, University of Southern Denmark, Odense, Denmark. [Google Scholar]
- 57.Li S, Wang K, Wang D, Dong S, Akamatsu T. 2008. Simultaneous production of low-and high-frequency sounds by neonatal finless porpoises. J. Acoust. Soc. Am. 124, 716–718. ( 10.1121/1.2945152) [DOI] [PubMed] [Google Scholar]
- 58.Reyes Reyes MV, Tossenberger VP, Iñiguez MA, Hildebrand JA, Melcón ML. 2016. Communication sounds of Commerson's dolphins (Cephalorhynchus commersonii) and contextual use of vocalizations. Mar. Mamm. Sci. 32, 1219–1233. ( 10.1111/mms.12321) [DOI] [Google Scholar]
- 59.Martin MJ, Gridley T, Elwen SH, Jensen FH. 2018. Data from: Heaviside's dolphins (Cephalorhynchus heavisidii) relax acoustic crypsis to increase communication range. Dryad Digital Repository ( 10.5061/dryad.64048p0) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Martin MJ, Gridley T, Elwen SH, Jensen FH. 2018. Data from: Heaviside's dolphins (Cephalorhynchus heavisidii) relax acoustic crypsis to increase communication range. Dryad Digital Repository ( 10.5061/dryad.64048p0) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material and in Dryad Digital Repository (doi:10.5061/dryad.64048p0) [59].