Abstract
Environmental light can exert potent effects on physiology and behaviour, including pupil size, vigilance and sleep. Previous work showed that these non-image forming effects can last long beyond discontinuation of short-wavelength light exposure. The possible functional effects after switching off long-wavelength light, however, have been insufficiently characterized. In a series of controlled experiments in healthy adult volunteers, we evaluated the effects of five minutes of intense red light on physiology and performance during subsequent darkness. As compared to prior darkness, prior red light induced a subsequent sustained pupil dilation. Prior red light also increased subsequent heart rate and heart rate variability when subjects were asked to perform a sustained vigilance task during the dark exposure. While these changes suggest an increase in the mental effort required for the task, it could not prevent a post-red slowing of response speed. The suggestion that exposure to intense red light affects vigilance during subsequent darkness, was confirmed in a controlled polysomnographic study that indeed showed a post-red facilitation of sleep onset. Our findings suggest the possibility of using red light as a nightcap.
Keywords: post-illumination effects, pupil diameter, mental effort, vigilance, sleep propensity
1. Introduction
Environmental light drives many aspects of human physiology and behaviour, including pupil diameter and both the rhythmic and acute expression of vigilance and sleep. These non-image forming effects of light critically involve retinal ganglion cells, which not only relay extrinsic input from the classical rod and cone phototransduction pathways but also possess intrinsic light sensitivity through the expression of the blue-sensitive (λmax = approximately 480 nm) photopigment melanopsin in the cellular membrane [1]. These so-called intrinsically photosensitive retinal ganglion cells (ipRGCs) spontaneously fire at a low rate [1–4] that increases with higher light intensity, with functional consequences [5] that can last long after discontinuation of the light exposure. For example, sustained post-illumination activation of the olivary pretectal nucleus (OPN) by ipRGCs leads to a persistent pupillary constriction known as the post-illumination pupil response (PIPR) [6]. The PIPR reflects a persistent photoinduced increase in the resting-state firing rate of ipRGCs beyond light exposure, which is more pronounced with increasing short-wavelength irradiance of the preceding light stimulus [1,7].
Previous studies on the sustained pupil constriction after short-wavelength light presumed no persistent post-illumination effects of long-wavelength light and considered the latter an inert control condition [8–10]. However, a closer examination of the results of some experiments suggests that after a red light stimulus is turned off, the pupil could re-dilate to diameters larger than prior to the stimulus being turned on [11,12]. The series of studies we here present is the first to systematically address whether such a post-red increase in pupil diameter can indeed be demonstrated and if so, how it relates to vigilance.
To determine the experimental approach to these aims, it should be considered that pupil diameter is not only dependent on current and past luminance involving effects on the sphincter muscle. The pupil diameter also reflects the vigilance state of the brain, involving locus coeruleus activation and sympathetic pathways acting on the dilatory muscle [13]. For example, alertness and effort increase pupil diameter. If pupil diameter reflects both past and current luminance as well as the vigilance state, which of these factors could be involved if prior exposure to bright red light induces a subsequent increase in diameter, measured during complete darkness relative to baseline?
Theoretically, a first possibility would be that a pulse of red light results in a decrease in the spontaneous firing rate of ipRGCs during subsequent darkness and consequently their effect on constriction-inducing activity in the downstream OPN. Although the possibility of post-red suppression of ipRGC resting-state firing underlying the PIPR is strictly hypothetical, it has been demonstrated that a pulse of red light can suppress ipRGC firing during darkness if it is enhanced by prior exposure to blue light [14].
A second possibility would be that a prior pulse of red light would boost subsequent input to the dilatory muscle by increasing alertness or effort. An increased pupil diameter corresponding with an increase in alertness is however unlikely: subjective alertness during darkness actually decreased across red light exposure [15]. Although no objective measures were assessed in this study, a decrease in alertness would rather contribute to a decrease in resting-state pupil dilation, not an increase. However, if the research paradigm were such that participants had to fight their drop in alertness in order to keep performing well on a task, the mental effort involved could indeed increase pupil diameter [16]. A recent study indeed suggested that people might get less alert and require more effort when exposed to broadband light that is dominated by the red part of the spectrum [17].
These examples help to define the requirements of research paradigms to systematically address whether a post-red increase in pupil diameter can be demonstrated and, if so, how it relates to vigilance. The paradigms should not only include pupillometry but also objective measures of vigilance, both with and without requiring effort. Hence, we systematically evaluated post-illumination effects of intense red light on subsequent pupil diameter, on a sustained vigilance task requiring psychophysiological effort, and on sleep propensity in the absence of effort requirements. Objective measures were obtained using: (i) pupillometry [7]; (ii) response speed indices of sustained alertness [18] and electrocardiographic indices of effort [19] during the most commonly used psychomotor vigilance task (PVT); (iii) an adapted version of the multiple sleep latency test (MSLT), which is a golden standard for sleep propensity assessment [20]. Since assessments of sustained alertness and sleep propensity require measurement intervals of at least several minutes, possible post-illumination effects of brief light pulses were deemed too short-lasting [7]. Therefore, to obtain reliable outcome measures, we used longer-lasting light pulses with longer-lasting effects [21].
2. Methods
In Experiment 1, we investigated how pupil diameter and response speed during an auditory version of the PVT, assessed in complete darkness, changed from before to after red and blue light exposure. Experiment 2 addressed whether the effects found in Experiment 1 involved changes in mental effort, by simultaneous electrocardiographic assessment of the period between the peaks of two consecutive R-waves (R–R interval). Moreover, Experiment 2 systematically evaluated whether the observed effects were consistent across the day and across supine and upright postures. This was in preparation of Experiment 3, in which we used an extended all-day adaptation of the MSLT to assess whether prior red, green or blue light exposure affected sleep propensity during subsequent darkness.
(a). Participants
In total, 40 young adults participated (Experiment 1: six males, six females, mean age ± s.d.: 24.3 ± 2.1 years; Experiment 2: six males, six females, mean age ± s.d. = 26.1 ± 3.6 years; Experiment 3: six males, 10 females, mean age ± s.d. = 24.1 ± 4.2 years). All participants were in good health, free of medication, and had neither sleep complaints nor a history of ocular pathology, as indicated by the www.sleepregistry.nl [22] implementation of the Duke clinical Structured Interview for Sleep Disorders [23]. According to Nagel anomaloscope tests (Model I, Schmidt and Haensch GmbH & Co., Berlin, Germany), none of the participants suffered from red–green colour vision defects, which are the most common type of colour vision deficiency [24]. The Munich Chronotype Questionnaire showed that none of the participants was an extreme chronotype (mean mid-sleep on free days ± s.d.: 05.18 ± 00.53) [25]. All participants worked regular office hours and did not travel across time-zones for at least a month prior to participation. The participants were instructed to refrain from caffeine and alcohol intake from 16.00 on the day preceding each assessment. Daily sleep diaries and actigraphy (Geneactiv, ActivInsights Ltd., Kimbolton, UK) were assessed to verify that participants kept to the sleep timing instructions [26]. The protocol was approved by the Medical Ethical Committee of the VU University Medical Center Amsterdam (protocol NL43319.029.13) and all participants gave their written informed consent.
(b). Experiment 1
(i). Light exposure
We illuminated the right eye with a dilated pupil (tropicamide 0.5%) in free view using red and blue light-emitting diodes (red: C503B-RAS, blue: C503B-BAN; Cree, Durham, NC, USA) that were integrated in a light panel (16 × 10 cm) at 5 cm from the illuminated eye (figure 1a,c). The red (peak wavelength [full width half maximum]: 635 [20] nm) and blue (465 [20] nm) lights were matched in photon flux (14.7 ± 0.1 log10 photons/cm2/s).
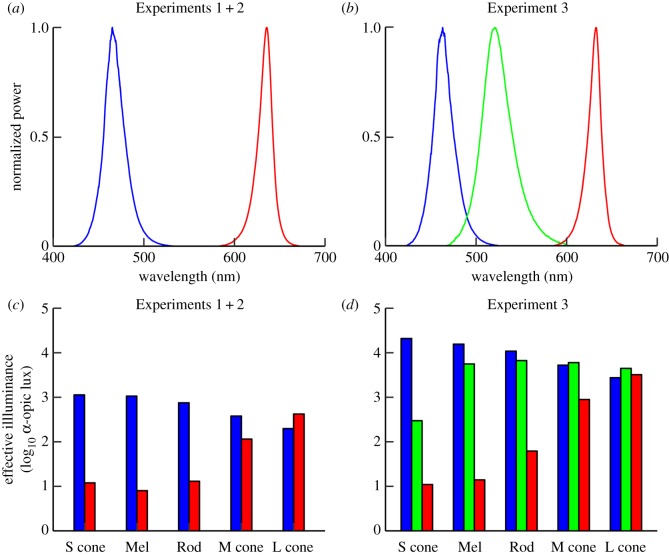
Figure 1.
Illumination characteristics. The top row shows the spectral power distribution of the red (irradiance: 178 µW cm−2) and blue (159 µW cm−2) light stimulus from Experiments 1 + 2 (a) and the red (1286 µW cm−2), green (1108 µW cm−2) and blue (2501 µW cm−2) stimuli from Experiment 3 (b). The bar plots in the bottom row indicate the activation of each of the photoreceptors in the human eye by the two stimuli from Experiments 1 + 2 (c) and the three stimuli from Experiment 3 (d). Illumination characteristics were estimated from the spectral irradiance at eye level (AvaSpec-3648-USB2 spectrometer, Avantes, Apeldoorn, The Netherlands) and described according to standard procedures (electronic supplementary material S4, table S1) [27]. S cone, short-wavelength cone; Mel, melanopsin; M cone, mid-wavelength cone; L cone, long-wavelength cone.
(ii). Pupil diameter
The pupil diameter of the left eye, which was not targeted by the light stimuli, was continuously measured at 25 Hz during the entire light exposure paradigm using a custom-made infrared pupillometry set-up that was validated and previously described in more detail [28]. The participants were asked to fixate on a target projected at infinity in front of their left eye in order to limit eye movements and prevent pupillary changes resulting from accommodation and convergence. Missing data points (e.g. due to eye blinks) were interpolated using nearest neighbour interpolation. All analyses used the pupil diameter averaged over the middle three minutes for each of the 5-min blocks of darkness, overlapping with the interval of PVT performance.
(iii). Psychomotor vigilance
The participants performed a 3-min auditory PVT implemented on an Arduino Mega microcontroller (https://www.arduino.cc/). The PVT commenced at the onset of the second minute of each 5-min non-illumination block. The participants had to press a response button as fast as possible when a continuous 880-Hz tone was played. When the button was pressed, the tone stopped and the response latency was recorded by the microcontroller with a millisecond temporal resolution. The inter-stimulus interval quasi-randomly varied between 1 and 10 s, resulting in an average of 31 observations per block. Means were calculated over the inverse of the response latency to obtain a normally distributed measure of response speed without compromising statistical power [29].
(iv). Procedures
The participants underwent two light exposure paradigms, each consisting of three consecutive 5-min blocks: (i) darkness, red light, darkness; and (ii) darkness, blue light, darkness (figure 2a,b). The participants were exposed to each light exposure paradigm once, in a randomized order, separated by 3 days. Laboratory visits were between 08.30 and 17.00, with the timing of the two visits being kept constant within each participant. The participants received the instruction to maintain regular and sufficient sleep from 3 days prior to each visit. To standardize prior light exposure, each visit commenced with a period of 30 min of dim white light (less than 3 lux), where an experimenter present in the room verified that the participants kept their eyes open and remained awake. Subsequently, participants were exposed to the light exposure paradigm.
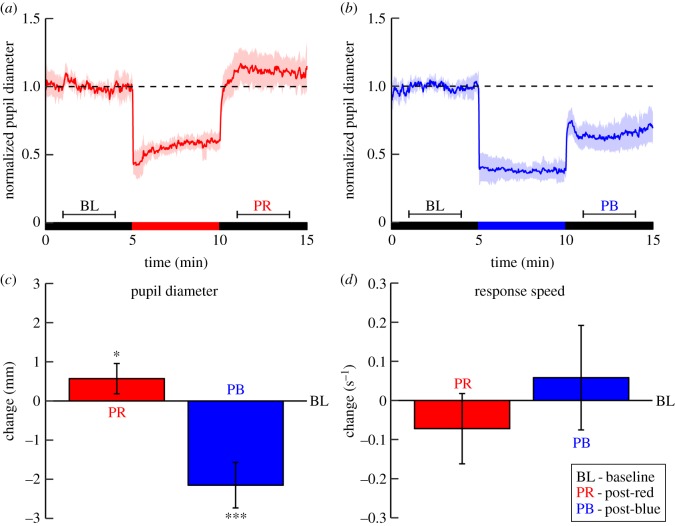
Figure 2.
Post-illumination changes in pupil diameter and vigilance measured during darkness after exposure to red and blue light (Experiment 1). (a,b) The participants underwent two exposure paradigms on separate days, each consisting of three consecutive 5-min blocks, as indicated by the bars: baseline darkness (BL); red or blue illumination; post-red (PR) or post-blue (PB) darkness. The plots show the pupil diameter trace, individually normalized to the BL-block and averaged over the 12 participants, for the red light (a) and blue light (b) exposure paradigm. Semi-transparent areas represent the 95% confidence intervals. The dashed lines indicate the average pupil diameter during the BL-block prior to red or blue light exposure. The horizontal brackets indicate the intervals from which the average pupil diameter was calculated (i.e. the middle 3 min of each dark block). Note that in comparison with the BL-block, there is not only the well-known smaller pupil diameter in the PB-block, but also a larger diameter in the PR-block. Pupil constriction during light exposure (i.e. minutes 2–4 after light onset) was stronger during blue than during red light (mixed-effect regression: p < 0.001). (c,d) The mean difference in pupil diameter (c) and response speed (d) of the post-illumination versus BL-blocks. The red bars indicate the difference induced by red light exposure and the blue bars the difference induced by blue light exposure. Error bars represent the within-subject 95% confidence interval. Asterisks indicate within-subject differences in post-illumination changes induced after red versus BL and after blue versus BL (*p < 0.05, ***p < 0.001).
(c). Experiment 2
(i). Heart rate
The set-up was identical to Experiment 1, except for the addition of electrocardiography (Shield-ECG/EMG, Olimex, Plovdiv, Bulgaria), which was co-recorded during PVT performance in order to obtain the most reliable electrocardiographic marker for mental effort from the R–R interval [19]. The R–R interval was automatically detected in the electrocardiogram (sampling frequency: 512 Hz) using the Pan–Tompkins algorithm [30] implemented in MATLAB (Version 2014a, The Mathworks, Inc., Natick, MA, USA). The detection results were visually verified, and manually corrected when necessary (e.g. during noisy data segments). R–R intervals that were more than 3.5 standard deviations from the mean R–R interval of each recording were rejected in order to exclude ectopic and arrhythmic events [31], after which an average was calculated as primary index of mental effort [19]. We moreover assessed the standard deviation of the R–R intervals (SDNN) and the square root of the mean squared differences of successive R–R intervals (RMSSD). SDNN is a measure of total variability with contributions from both sympathetic as well as vagal activity, whereas RMSSD is considered as a marker of vagal tone [31]. The ratio between SDNN and RMSSD (SDNN/RMSSD) was calculated as an index of the sympathovagal balance [32]. In accordance with common procedures, R–R interval variability parameters were transformed using the natural logarithm (ln) to obtain a more normal distribution of the measures [33].
(ii). Procedures
The participants visited the laboratory twice, separated by 3 days, once at 09.00 and once at 13.00, because the effects of red light on vigilance-related measures may vary across daytime [34]. They received the instruction to maintain regular and sufficient sleep from 3 days prior to each laboratory visit. During each visit the participants underwent one light exposure paradigm in upright and one in supine position to assess the effect of body posture. Brain activity, autonomic activity and their associations with performance can differ considerably between supine and upright postures [35]. Posture moreover strongly affects arousal and vigilance [36], which in turn can tonically affect pupil diameter and its responses to light [37]. It is highly relevant to address these differences e.g. in preparation of functional magnetic resonance imaging studies. Time of day and posture were counterbalanced over the two visits and posture was moreover counterbalanced within each participant. Prior to each light exposure paradigm the participants were first put into the appropriate position, after which they remained in dim light (less than 3 lux) for 30 min. During this dimly lit period, the participants were observed by an experimenter present in the room, who was different from Experiment 1. The experimenter's interaction was standardized and limited to verifying that the participants kept their eyes open and remained awake. The first light exposure paradigm of each visit was followed by 20 min of free movement in a room light environment (200 lux).
In total, the participants underwent four light sequences, each randomly selected from three different exposure paradigms consisting of seven consecutive 5-min blocks (figure 3a). During one block the participants were exposed to red light and during a later block to blue light. Similar to another study on functional post-illumination effects [38], the red light block was always earlier in the sequence than the blue light block in order to avoid prior blue light interfering with the functional changes following red light, which form the main focus of our study. For instance, we previously showed that the pupil constriction after five minutes of blue light can remain during 30 min of subsequent darkness [28]. All other blocks were kept dark. To allow for mixed-effects model-disentangling of wavelength effects from time-on-task effects [39], the timing of the light exposure blocks was systematically shifted across the three sequences (figure 3c).
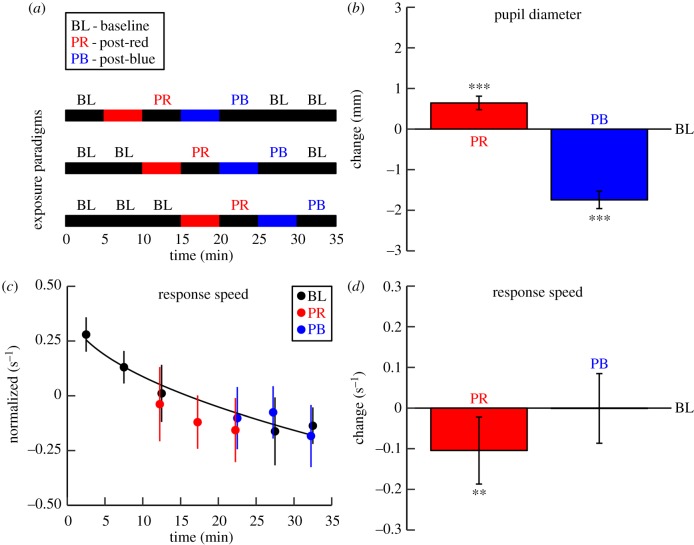
Figure 3.
Post-illumination changes in pupil diameter and vigilance measured during darkness after exposure to red and blue light (Experiment 2). (a) To allow for mixed effect model-disentangling of wavelength effects from time-on-task effects, the timing of the light exposure blocks was systematically shifted across three paradigms, which were randomly distributed over the sample. (b) The bars represent the post-red (PR) and post-blue (PB) changes in pupil diameter, relative to the baseline (BL)-blocks. Because the sustained pupil constriction after blue light remained even in the third consecutive BL-block, pupil data assessed during the BL blocks after the PB-block were omitted from this figure. (c) The dots indicate the average response speed in each dark block for each type of block. The line represents the time-on-task effect and is quantified as the optimal square-root fit through the baseline values (QR decomposition: slope =−0.26 × √Block). Note that the lower response speed after red light exposure is not merely explained as a time-on-task effect. Whereas the post-blue response speed is around the estimated baseline curve, the post-red response speed is systematically below this curve. The data were normalized to the average over all dark blocks within each participant. (d) The bars show the difference in response speed adjusted for time-on-task between the PR- and PB-block and the BL-blocks. Error bars represent the within-subject 95% confidence interval. Asterisks indicate within-subject differences between the post-illumination blocks and the baseline blocks (**p < 0.01, ***p < 0.001).
(d). Experiment 3
(i). Light exposure
Red (peak wavelength [full width half maximum]: 630 [20] nm), blue (460 [20] nm) and green (520 [31] nm) light were applied to both eyes with an undilated pupil using a light panel (53 cm × 31 cm) containing RGB light-emitting diodes (WS2812B, Luxalight B.V., Eindhoven, The Netherlands) that was placed in front of the participant at 57 cm from the eyes (figure 1b,d). The three light stimuli were matched with respect to photon flux (15.7 ± 0.2 log10 photons/cm2/s). Eye tracking (The Eye Tribe Tracker, The Eye Tribe, Copenhagen, Denmark) was performed to verify that the participants kept their eyes open and aimed their gaze at the light panel.
(ii). Sleep propensity
Using electroencephalography (Geodesic EEG system 300, Electrical Geodesics, Inc., Eugene, USA) and standardized sleep scoring procedures [40], sleep stages were scored offline in 30 s epochs by two experienced sleep technicians blind to the manipulations, with consensus reached in case of scoring differences. Sleep onset was defined as three consecutive 30 s epochs of light sleep (i.e. stage 1) or one 30 s epoch of deeper sleep (i.e. stage 2 or slow wave sleep) [20]. Given that standard sleep scoring procedures require at least half of the 30 s epoch to be spent in sleep for actually labelling this epoch as sleep [40], we estimated that sleep onset occurred at the midpoint of the first half of the sleep onset epoch. Where participants failed to reach sleep onset within 30 min, sleep onset latency was scored as 30 min [41]. Sleep onset latencies have the same skewed distribution as response latencies in the PVT, which we verified to likewise be best reciprocally transformed, resulting in a normally distributed measure of sleep propensity.
(iii). Procedures
The participants received the instruction to maintain regular and sufficient sleep from 7 days until the penultimate day prior to the sleep propensity assessment. During the night immediately preceding the laboratory visit, they were instructed to restrict their sleep: compared to their habitual sleep times, bedtime was 1 h later and wake up time was earlier (i.e. at 06.00). The adapted version of the MSLT started at 09.00 and included eight consecutive 81 min blocks. Each block started with a standardized 10 min break in dim room light (less than 1 lux), which included a mandatory bathroom visit and consumption of an isocaloric snack (150 kcal) in order to avoid of effects of postural changes [36] and food intake [42] on sleep propensity. After this break, the participants were put in bed in a semi-supine position for 30 min, while allowed to read a book of their choice. The book was illuminated by a reading light with only a limited amount of light reaching the eyes (less than 1 lux). Subsequently, the reading light was switched off and the participants were exposed to 5 min of red, blue, or green light or darkness. The participants received each exposure twice in a randomized order while they remained in a semi-supine position. After the exposure, the bed was changed to a supine position and the participants adopted their habitual sleeping posture. After this 1 min translation period in darkness, lights remained off and the participants were instructed to try to fall asleep as fast as possible within an interval of 30 min. Upon online sleep onset detection, the participants were awoken, the dim room light was switched on and the participants were asked to remain awake until the 30 min window was finished.
(e). Statistical analysis
Data from all three experiments were analysed with mixed-effect regression models (electronic supplementary material S4), which are optimally suited to account for between-subject differences (e.g. in pupil diameter [43]). All mixed-effects models were performed using the ‘lme4’ package for R (v. 3.2.3, R Foundation for Statistical Computing, Vienna, Austria).
3. Results
(a). Pupil dilation and reduced vigilance during darkness after red light
In Experiment 1, we showed that the average pupil diameter was larger during darkness after prior red light (mean ± s.e.m.: 6.24 ± 0.28 mm, p = 0.02) than the diameter during darkness preceding the light exposure (5.67 ± 0.22 mm; figure 2c). In agreement with previous work [7], the average pupil diameter was smaller after blue light exposure (3.52 ± 0.24 mm, p < 0.001). There was a non-significant trend of slowing of PVT response speed from before (4.12 ± 0.12 s−1) to after red light exposure (4.05 ± 0.16 s−1, p = 0.10), but there was no change after blue light (4.18 ± 0.13 s−1, p = 0.17; figure 2d).
The results from Experiment 2 replicated the functional changes after long-wavelength light seen in Experiment 1, with even more pronounced effects, possibly because of a lower contribution of between-day variance in Experiment 2 than in Experiment 1. Compared to baseline darkness (pupil diameter mean ± s.e.m.: 4.66 ± 0.10 mm; response speed adjusted for time-on-task: 4.11 ± 0.07 s−1), in darkness following red light we again found pupil dilation (5.34 ± 0.12 mm, p < 0.001; figure 3b) and the slowing of response speed that was marginally significant in Experiment 1 now reached significance (4.01 ± 0.12 s−1, p = 0.004; figure 3d). Response speed slowed in spite of increased mental effort as indicated by a shorter R–R interval (918 ± 20 versus 927 ± 11 ms, p = 0.04; electronic supplementary material, figure S1).
Analysis of R–R interval variability in darkness following red light showed a higher ln SDNN (4.21 ± 0.05 versus 4.16 ± 0.03 ln ms, p = 0.049). At the same time, ln RMSSD had a tendency to decrease (3.89 ± 0.07 versus 3.93 ± 0.04 ln ms, p = 0.08), indicating that the higher ln SDNN did not arise from a stronger cardiac vagal tone, but rather from an increase in cardiac sympathetic activity suggestive of increased mental effort. Accordingly, we found a larger ln SDNN/RMSSD (0.32 ± 0.05 versus 0.23 ± 0.02, p < 0.001) suggestive of a possible increased sympathovagal balance.
In darkness after blue light we found the well-documented sustained pupil constriction (2.95 ± 0.08 mm, p < 0.001), but no change in response speed (4.11 ± 0.12 s−1, p = 0.96), R–R interval (927 ± 17 ms, p = 0.93) and its variability (ln SDNN: 4.20 ± 0.05 ln ms, p = 0.15; ln RMSSD: 3.93 ± 0.51 ln ms, p = 0.81; ln SDNN/RMSSD: 0.26 ± 0.04, p = 0.16). All post-illumination effects were consistent across the day and across upright and supine posture (all p > 0.12), thus meeting the conditions for a subsequent MSLT study.
(b). Red light exposure increases subsequent sleep propensity
The results from Experiment 3 showed a stronger sleep propensity (i.e. the inverse of sleep latency) after 5 min red light (mean ± s.e.m.: 0.41 ± 0.06 min−1) than following 5 min darkness (0.33 ± 0.04 min−1, p = 0.04; figure 4). Whereas long-wavelength light facilitated the subsequent transition to sleep in darkness, no significant effect was seen after blue (0.32 ± 0.03 min−1, p = 0.95) or green light (0.27 ± 0.03 min−1, p = 0.14).
Figure 4.
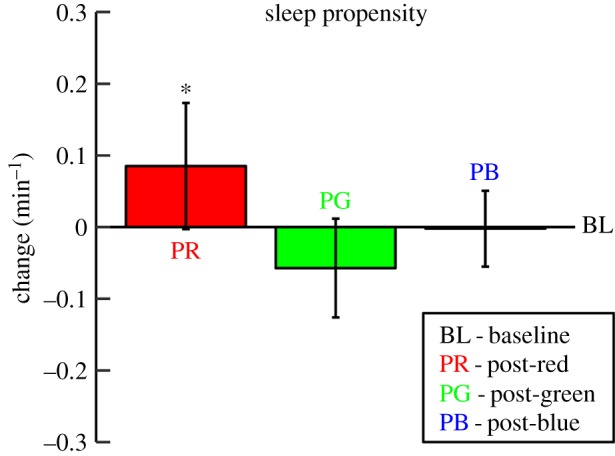
Post-illumination changes in sleep propensity (sleep latency−1) measured during darkness after exposure to red, green and blue light (Experiment 3). The bars represent sleep propensity after red (PR), green (PG) and blue (PB) light exposure relative to baseline (BL) sleep propensity (i.e. after prior exposure to darkness). The error bars represent the within-subject 95% confidence interval. Asterisk indicates within-subject differences between the post-light trials and the post-dark trials (*p < 0.05).
4. Discussion
The present study focused on late sustained effects of pre-exposure to long-wavelength light on pupil diameter and vigilance during subsequent darkness. Following bright red light exposure, we found a persistent pupil dilation. Consistent across the experiments, bright red light attenuated vigilance during subsequent darkness, as indicated by a slower response speed during vigilance tasks and by a faster transition from wakefulness to sleep during the MSLTs.
During the vigilance task performed after red light, objective cardiac indices suggested an increased mental effort that was insufficient to completely rescue task performance. We found no changes in indicators of mental effort or vigilance following blue or green (520 nm) light. This was in line with a previous study showing that subjective alertness declined from before to after red but not blue or green (515 nm) light [15]. Accordingly, electroencephalographic experiments showed a more pronounced reduction of alpha activity (e.g. a classical marker for increased mental effort [44]) during red than blue light [45,46]. Several light therapy trials used red light as a placebo treatment. Although the intensity of such red light exposures was generally 10–50 times lower than in the present study, the exposure is usually much longer than 5 min, suggesting that such a condition may not necessarily be inert. Indeed, in our own previous work, daily red light may have increased subjective sleepiness [47].
Previous reports have addressed differential post-illumination effects of broadband light in which the red part of the spectrum was differentially represented. Studies in humans have shown that complete absence of short-wavelength light before bedtime improves sleep [48], while its presence leads to the opposite effect [49] in a blue-amount-dependent manner [50]. An intriguing possibility suggested by our findings is that red light prior to bedtime might have applied practical value to facilitate sleep onset, not only as an alternative for short-wavelength light (e.g. blue-enriched light from self-luminous screens [51]) but also as actively facilitating sleep onset. It remains to be evaluated whether blue-light-depleted electronic devices with light-emitting screens could have similar effects to the red light applied in the current studies, because the typical intensity of light emitted from such screens is 10–30 times lower, and the spectrum is broader, than applied in the present study [52]. It would be interesting for future studies to determine to what extent our findings depend on the intensity of the light exposure, for instance by constructing a dose–response curve of sleep propensity as a function of intensity of prior red light.
Future studies should however first address whether the post-red effects on sleep propensity during daytime, as here demonstrated, can also be found prior to habitual bedtime. If so, long-wavelength light may serve as a nightcap and possibly be of use in the treatment of sleep-onset insomnia [53]. This suggestion was supported by previous work showing that, as compared to exposure to darkness, two hours of evening exposure to green light—with a considerably longer wavelength than in our study (550 nm)—appeared to accelerate the decrease of core body temperature that is known to be conductive to sleep [49]. Moreover, slow wave activity seemed to be higher during the first two hours of sleep and to decline faster during the first half of the night, which could be interpreted as sleep with a highly efficient dissipation of sleep pressure [54]. Given that the effect of prior light can endure beyond sleep onset [49], it seems moreover valuable to evaluate the effect of long-wavelength light prior to bedtime on subsequent sleep quality.
From our experiments we were unable to discriminate between the relative contributions of the different phototransduction pathways to the functional effects of red light. Long-wavelength cones possess the highest sensitivity to red light and their involvement therefore seems most probable, particularly because previous experiments indicated that cones contribute to various non-image forming effects of red light [55]. The post-illumination effects of red light in our study may also involve intrinsic ipRGC phototransduction, even if acute ipRGC-mediated effects during long-wavelength exposure can be marginal in some paradigms [1], but not in all. For example, patch clamp experiments on in vitro mouse retinas showed that, after pharmacological blockage of input from rods and cones while preserving the intrinsic phototransduction circuitry, the sustained ipRGC photoresponse induced after short-wavelength light is acutely suppressed after subsequent exposure to long-wavelength (orange) light [14]. Moreover, rod photoreceptors may also add to the effects we observed, given their important contributions to the pupillary light reflex [56] and circadian photoentrainment [57]. Given that the red light stimuli, like the other stimuli in our study, activated each of the known photoreceptors in the human eye [27], it would be most interesting to add complementary pupillometry paradigms that can disentangle the contributions from the different photoreceptors to the observed functional effects [58]. Moreover, it may prove fruitful for future studies to apply light with additional wavelengths (e.g. green light with a longer wavelength) in order to add to the multivariate finger print of rod, cone and melanopsin involvement in functional post-illumination effects.
A limitation of Experiment 2 was that the order of the two light stimuli was fixed: red light was always earlier in the light exposure paradigm than blue. We cannot completely exclude the possibility that prior red light exposure has affected the size of post-illumination effects following blue light, although we previously showed that prior red light exposure did not affect the pupil constriction following blue light [28]. A limitation of Experiment 3 was that the light stimuli were applied to an undilated pupil, preventing us from standardizing the amount of light reaching the retina, which may have added variance to the post-illumination effects on sleep propensity. The physiological and possible (pre-) clinical significance of our findings remains to be investigated, because the size of the effects of prior red light was rather small with considerable variability.
In conclusion, the present study identified lasting effects of five minutes of exposure to bright red light. Such exposure increased pupil diameter and slowed response speed despite cardiac indices suggesting increased mental effort during a vigilance task. It also facilitated the transition between wake and sleep. Underlying mechanisms including the possibility of sustained suppression of ipRGC firing rate and functional consequences thereof remain to be investigated in animal studies. Our findings suggest that it should carefully be considered whether red light is an appropriate inert control condition, and may act as nightcap.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Brit Giesbertz, Jessica Bruijel, Liz Vink, Jesminne Castricum, Jeske Hendriks and Ruud van der Blom (Netherlands Institute for Neuroscience) for their contribution to the realization of this study.
Data accessibility
The data supporting this article can be found in the electronic supplementary material, which comprises one Excel spreadsheet for each of the three experiments (S1 Data.xlsx, S2 Data.xlsx and S3 Data.xlsx).
Authors' contributions
This study was designed by W.P.v.d.M., C.C., P.B. and E.J.W.V.S. The data were collected by W.P.v.d.M., B.H.W.t.L. and J.R.R., and analysed by W.P.v.d.M., Y.W., J.E.C. and E.J.W.V.S. The results were interpreted by W.P.v.d.M., M.K. and E.J.W.V.S. All authors contributed to the preparation of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This project has been funded with support from the NeuroTime Erasmus+: Erasmus Mundus (awarded to C.C., P.B. and E.J.W.V.S.) and the European Research Council (ERC-ADG-2014-671084 INSOMNIA; awarded to E.J.W.V.S.) programs of the European Commission. This publication/communication reflects the views only of the authors, and the Commission cannot be held responsible for any use that may be made of the information contained therein. This work was supported by Project NeuroSIPE 10738 (awarded to E.J.W.V.S.), of the Dutch Technology Foundation STW, which is part of the Netherlands Organization for Scientific Research (NWO) and partly funded by the Ministry of Economic Affairs, Agriculture and Innovation of The Netherlands.
References
- 1.Berson DM, Dunn FA, Takao M. 2002. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073. ( 10.1126/science.1067262) [DOI] [PubMed] [Google Scholar]
- 2.Zhao X, Stafford BK, Godin AL, King WM, Wong KY. 2014. Photoresponse diversity among the five types of intrinsically photosensitive retinal ganglion cells. J. Physiol. 592, 1619–1636. ( 10.1113/jphysiol.2013.262782) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reifler AN, et al. 2015. The rat retina has five types of ganglion-cell photoreceptors. Exp. Eye Res. 130, 17–28. ( 10.1016/j.exer.2014.11.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Do MT, Kang SH, Xue T, Zhong H, Liao HW, Bergles DE, Yau KW. 2009. Photon capture and signalling by melanopsin retinal ganglion cells. Nature 457, 281–287. ( 10.1038/nature07682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. 2006. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 497, 326–349. ( 10.1002/cne.20970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. 2005. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 433, 749–754. ( 10.1038/nature03387) [DOI] [PubMed] [Google Scholar]
- 7.Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. 2007. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 47, 946–954. ( 10.1016/j.visres.2006.12.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kankipati L, Girkin CA, Gamlin PD. 2010. Post-illumination pupil response in subjects without ocular disease. Invest. Ophth. Vis. Sci. 51, 2764–2769. ( 10.1167/iovs.09-4717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JC, Moura AL, Raza AS, Rhee DW, Kardon RH, Hood DC. 2011. Toward a clinical protocol for assessing rod, cone, and melanopsin contributions to the human pupil response. Invest. Ophth. Vis. Sci. 52, 6624–6635. ( 10.1167/iovs.11-7586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbst K, Sander B, Milea D, Lund-Andersen H, Kawasaki A. 2011. Test–retest repeatability of the pupil light response to blue and red light stimuli in normal human eyes using a novel pupillometer. Front. Neurol. 2, 10 ( 10.3389/fneur.2011.00010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young RS, Kimura E. 2008. Pupillary correlates of light-evoked melanopsin activity in humans. Vision Res. 48, 862–871. ( 10.1016/j.visres.2007.12.016) [DOI] [PubMed] [Google Scholar]
- 12.Joyce DS, Feigl B, Zele AJ. 2016. Melanopsin-mediated post-illumination pupil response in the peripheral retina. J. Vision 16, 5 ( 10.1167/16.8.5) [DOI] [PubMed] [Google Scholar]
- 13.Larsen RS, Waters J. 2018. Neuromodulatory correlates of pupil dilation. Front. Neural Circuits 12, 21 ( 10.3389/fncir.2018.00021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emanuel AJ, Do MT. 2015. Melanopsin tristability for sustained and broadband phototransduction. Neuron 85, 1043–1055. ( 10.1016/j.neuron.2015.02.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metz AJ, Klein SD, Scholkmann F, Wolf U. 2017. Continuous coloured light altered human brain haemodynamics and oxygenation assessed by systemic physiology augmented functional near-infrared spectroscopy. Sci. Rep. 7, 10027 ( 10.1038/s41598-017-09970-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinhauer SR, Siegle GJ, Condray R, Pless M. 2004. Sympathetic and parasympathetic innervation of pupillary dilation during sustained processing. Int. J. Psychophysiol. 52, 77–86. ( 10.1016/j.ijpsycho.2003.12.005) [DOI] [PubMed] [Google Scholar]
- 17.Lasauskaite R, Cajochen C. 2018. Influence of lighting color temperature on effort-related cardiac response. Biol. Psychol. 132, 64–70. ( 10.1016/j.biopsycho.2017.11.005) [DOI] [PubMed] [Google Scholar]
- 18.Dinges DF, Powell JW. 1985. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav. Res. Meth. Ins. C 17, 652–655. ( 10.3758/bf03200977) [DOI] [Google Scholar]
- 19.Mukherjee S, Yadav R, Yung I, Zajdel DP, Oken BS. 2011. Sensitivity to mental effort and test–retest reliability of heart rate variability measures in healthy seniors. Clin. Neurophysiol. 122, 2059–2066. ( 10.1016/j.clinph.2011.02.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. 1986. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep 9, 519–524. ( 10.1093/sleep/9.4.519) [DOI] [PubMed] [Google Scholar]
- 21.Chellappa SL, Ly JQ, Meyer C, Balteau E, Degueldre C, Luxen A, Phillips C, Cooper HM, Vandewalle G. 2014. Photic memory for executive brain responses. Proc. Natl Acad. Sci. USA 111, 6087–6091. ( 10.1073/pnas.1320005111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benjamins JS, Migliorati F, Dekker K, Wassing R, Moens S, Blanken TF, te Lindert BH, Mook JS, Van Someren EJ. 2017. Insomnia heterogeneity: characteristics to consider for data-driven multivariate subtyping. Sleep Med. Rev. 36, 71–81. ( 10.1016/j.smrv.2016.10.005) [DOI] [PubMed] [Google Scholar]
- 23.Edinger J, Kirby A, Lineberger M, Loiselle M, Wohlgemuth W, Means M. 2004. The Duke structured interview for sleep disorders. Durham, NC: Duke University Medical Center. [Google Scholar]
- 24.Birch J. 2012. Worldwide prevalence of red–green color deficiency. J. Opt. Soc. Am. A 29, 313–320. ( 10.1364/josaa.29.000313) [DOI] [PubMed] [Google Scholar]
- 25.Zavada A, Gordijn MC, Beersma DG, Daan S, Roenneberg T. 2005. Comparison of the Munich chronotype questionnaire with the Horne-Östberg's morningness–eveningness score. Chronobiol. Int. 22, 267–278. ( 10.1081/cbi-200053536) [DOI] [PubMed] [Google Scholar]
- 26.te Lindert BH, Van Someren EJ. 2013. Sleep estimates using microelectromechanical systems (MEMS). Sleep 36, 781–789. ( 10.5665/sleep.2648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucas RJ, et al. 2014. Measuring and using light in the melanopsin age. Trends Neurosci. 37, 1–9. ( 10.1016/j.tins.2013.10.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Meijden WP, et al. 2015. Post-illumination pupil response after blue light: reliability of optimized melanopsin-based phototransduction assessment. Exp. Eye Res. 139, 73–80. ( 10.1016/j.exer.2015.07.010) [DOI] [PubMed] [Google Scholar]
- 29.Whelan R. 2008. Effective analysis of reaction time data. Psychol. Rec. 58, 475 ( 10.1007/bf03395630) [DOI] [Google Scholar]
- 30.Pan J, Tompkins WJ. 1985. A real-time QRS detection algorithm. IEEE T. Biomed. Eng. 32, 230–2236. ( 10.1109/tbme.1985.325532) [DOI] [PubMed] [Google Scholar]
- 31.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. 1996. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 17, 354–381. ( 10.1093/oxfordjournals.eurheartj.a014868) [DOI] [PubMed] [Google Scholar]
- 32.Schneider U, Frank B, Fiedler A, Kaehler C, Hoyer D, Liehr M, Haueisen J, Schleussner E. 2008. Human fetal heart rate variability—characteristics of autonomic regulation in the third trimester of gestation. J. Perinat. Med. 36, 433–441. ( 10.1515/jpm.2008.059) [DOI] [PubMed] [Google Scholar]
- 33.Nunan D, Sandercock GR, Brodie DA. 2010. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pace 33, 1407–1417. ( 10.1111/j.1540-8159.2010.02841.x) [DOI] [PubMed] [Google Scholar]
- 34.Sahin L, Wood BM, Plitnick B, Figueiro MG. 2014. Daytime light exposure: effects on biomarkers, measures of alertness, and performance. Behav. Brain Res. 274, 176–185. ( 10.1016/j.bbr.2014.08.017) [DOI] [PubMed] [Google Scholar]
- 35.Spironelli C, Angrilli A. 2017. Posture used in fMRI-PET elicits reduced cortical activity and altered hemispheric asymmetry with respect to sitting position: an EEG resting state study. Front. Hum. Neurosci. 11, 621 ( 10.3389/fnhum.2017.00621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romeijn N, Raymann RJ, Møst E, Te Lindert B, Van Der Meijden WP, Fronczek R, Gomez-Herrero G, Van Someren EJ. 2012. Sleep, vigilance, and thermosensitivity. Pflugers Arch. 463, 169–176. ( 10.1007/s00424-011-1042-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knapen T, de Gee JW, Brascamp J, Nuiten S, Hoppenbrouwers S, Theeuwes J. 2016. Cognitive and ocular factors jointly determine pupil responses under equiluminance. PLoS ONE 11, e0155574 ( 10.1371/journal.pone.0155574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edelhäuser F, Hak F, Kleinrath U, Lühr B, Matthiessen PF, Weinzirl J, Cysarz D. 2013. Impact of colored light on cardiorespiratory coordination. Evid. Based Complement. Alternat. Med. 2013, 810876 ( 10.1155/2013/810876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raymann RJ, Van Someren EJ. 2007. Time-on-task impairment of psychomotor vigilance is affected by mild skin warming and changes with aging and insomnia. Sleep 30, 96–103. ( 10.1093/sleep/30.1.96) [DOI] [PubMed] [Google Scholar]
- 40.Iber C, Ancoli-Israel S, Chesson A, Quan SF. 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine. [Google Scholar]
- 41.Raymann RJ, Swaab DF, Van Someren EJ. 2005. Cutaneous warming promotes sleep onset. Am. J. Physiol. Reg. Integr. Comp. Physiol. 288, R1589–R1597. ( 10.1152/ajpregu.00492.2004) [DOI] [PubMed] [Google Scholar]
- 42.Wells AS, Read NW, Idzikowski C, Jones J. 1998. Effects of meals on objective and subjective measures of daytime sleepiness. J. Appl. Physiol. 84, 507–515. ( 10.1152/jappl.1998.84.2.507) [DOI] [PubMed] [Google Scholar]
- 43.Mathôt S, Fabius J, Van Heusden E, Van der Stigchel S. 2018. Safe and sensible preprocessing and baseline correction of pupil-size data. Behav. Res. Methods 50, 94–106. ( 10.3758/s13428-017-1007-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gevins A, Smith ME, McEvoy L, Yu D. 1997. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb. Cortex 7, 374–385. ( 10.1093/cercor/7.4.374) [DOI] [PubMed] [Google Scholar]
- 45.Ali MR. 1972. Pattern of EEG recovery under photic stimulation by light of different colors. Electroencephalogr. Clin. Neurophysiol. 33, 332–335. ( 10.1016/0013-4694(72)90162-9) [DOI] [PubMed] [Google Scholar]
- 46.Sahin L, Figueiro MG. 2013. Alerting effects of short-wavelength (blue) and long-wavelength (red) lights in the afternoon. Physiol. Behav. 116, 1–7. ( 10.1016/j.physbeh.2013.03.014) [DOI] [PubMed] [Google Scholar]
- 47.Lieverse R, Van Someren EJ, Nielen MM, Uitdehaag BM, Smit JH, Hoogendijk WJ. 2011. Bright light treatment in elderly patients with nonseasonal major depressive disorder: a randomized placebo-controlled trial. Arch. Gen. Psychiatry 68, 61–70. ( 10.1001/archgenpsychiatry.2010.183) [DOI] [PubMed] [Google Scholar]
- 48.Burkhart K, Phelps JR. 2009. Amber lenses to block blue light and improve sleep: a randomized trial. Chronobiol. Int. 26, 1602–1612. ( 10.3109/07420520903523719) [DOI] [PubMed] [Google Scholar]
- 49.Münch M, Kobialka S, Steiner R, Oelhafen P, Wirz-Justice A, Cajochen C. 2006. Wavelength-dependent effects of evening light exposure on sleep architecture and sleep EEG power density in men. Am. J. Physiol. Reg. Integr. Comp. Physiol. 290, R1421–R1428. ( 10.1152/ajpregu.00478.2005) [DOI] [PubMed] [Google Scholar]
- 50.Santhi N, Thorne HC, van der Veen DR, Johnsen S, Mills SL, Hommes V, Schlangen LJ, Archer SN, Dijk DJ. 2012. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J. Pineal Res. 53, 47–59. ( 10.1111/j.1600-079X.2011.00970.x) [DOI] [PubMed] [Google Scholar]
- 51.Chang AM, Aeschbach D, Duffy JF, Czeisler CA. 2015. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc. Natl Acad. Sci. USA 112, 1232–1237. ( 10.1073/pnas.1418490112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Escofet J, Bara S. 2017. Reducing the circadian input from self-luminous devices using hardware filters and software applications. Lighting Res. Technol. 49, 481–496. ( 10.1177/1477153515621946) [DOI] [Google Scholar]
- 53.Riemann D, Spiegelhalder K, Espie C, Pollmacher T, Leger D, Bassetti C, van Someren E. 2011. Chronic insomnia: clinical and research challenges—an agenda. Pharmacopsychiatry 44, 1–14. ( 10.1055/s-0030-1267978) [DOI] [PubMed] [Google Scholar]
- 54.Achermann P, Dijk DJ, Brunner DP, Borbély AA. 1993. A model of human sleep homeostasis based on EEG slow-wave activity—quantitative comparison of data and simulations. Brain. Res. Bull. 31, 97–113. ( 10.1016/0361-9230(93)90016-5) [DOI] [PubMed] [Google Scholar]
- 55.Mien IH, Chua EC, Lau P, Tan LC, Lee IT, Yeo SC, Tan SS, Gooley JJ. 2014. Effects of exposure to intermittent versus continuous red light on human circadian rhythms, melatonin suppression, and pupillary constriction. PLoS ONE 9, e96532 ( 10.1371/journal.pone.0096532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keenan WT, et al. 2016. A visual circuit uses complementary mechanisms to support transient and sustained pupil constriction. Elife 5, e15392 ( 10.7554/eLife.15392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Altimus CM, Güler AD, Alam NM, Arman AC, Prusky GT, Sampath AP, Hattar S. 2010. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat. Neurosci. 13, 1107–1112. ( 10.1038/nn.2617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spitschan M, Jain S, Brainard DH, Aguirre GK. 2014. Opponent melanopsin and S-cone signals in the human pupillary light response. Proc. Natl Acad. Sci. USA 111, 15 568–15 572. ( 10.1073/pnas.1400942111) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this article can be found in the electronic supplementary material, which comprises one Excel spreadsheet for each of the three experiments (S1 Data.xlsx, S2 Data.xlsx and S3 Data.xlsx).