Abstract
Purpose
Younger women (under age 45 years) diagnosed with breast cancer often face more aggressive tumors, higher treatment intensity, lower survival rates, and greater financial hardship. The purpose of this study was to estimate breast cancer costs by stage at diagnosis during the first 18 months of treatment for privately insured younger women.
Methods
We analyzed North Carolina cancer registry data linked to claims data from private insurers from 2003 to 2010. Breast cancer patients were split into two cohorts: a younger and older group aged 21–44 and 45–64 years, respectively. We conducted a cohort study and matched women with and without breast cancer using age, ZIP, and Charlson Comorbidity Index. We calculated mean excess costs between breast cancer and non-breast cancer patients at 6, 12, and 18 months.
Results
For younger women, AJCC 6th edition stage II cancer was the most common at diagnosis (40%), followed by stage I (34%). On the other hand, older women had more stage I (46%) cancer followed by stage II (34%). The excess costs for younger and older women at 12 months were $97,486 (95% confidence interval [CI] $93,631–101,341) and $75,737 (95% CI $73,962–77,512), respectively. Younger breast cancer patients had both a higher prevalence of later-stage disease and higher within-stage costs.
Conclusions
The study reports high costs of treatment for both younger and older women than a non-cancer comparison group; however, the estimated excess cost was significantly higher for younger women. The financial implications of breast cancer treatment costs for younger women need to be explored in future studies.
Keywords: Breast cancer, Medical care costs, Private insurance, Younger women, Cancer stage
Introduction
Over 22,000 women younger than 45 years of age (henceforth ‘‘younger women’’) were diagnosed with breast cancer in 2013 [1]. Although less than 10% of all breast cancers are diagnosed among women younger than age 45, the types of breast cancer younger women face are typically more aggressive, are diagnosed at more advanced stages, and result in poorer survival compared to breast cancer in older women [2–4]. For these reasons, the Breast Cancer Education and Awareness Requires Learning Young (EARLY) act and prior research have identified woman under the age of 45 years to be particularly burdened by breast cancer [5, 6]. Younger women may also experience higher treatment intensity and have cancers that are less responsive to treatment, and have distinct and more prevalent side effects from treatment than older women [3, 7–9]. These side effects can include poorer quality of life, fertility problems, and depression [9, 10].
As a result, breast cancer treatment for younger women is expensive, making them vulnerable to financial hardship [11, 12]. Recent research has shown that 31.8% of cancer survivors are likely to have cancer treatment-induced financial troubles, with higher rates among younger cancer patients [13, 14]. These financial difficulties cause some survivors to forego or delay necessary medical treatments [12, 14]. Further, younger women also face substantial productivity losses as a result of breast cancer treatment [15].
Previous work has investigated the costs of breast cancer treatment among privately insured women by age group and stage at diagnosis [11, 16–19]. Among these studies, only one addressed the costs of breast cancer treatment in younger women [11]. Two studies addressed treatment costs for women with mean age between 52 and 55 years regardless of stage at diagnosis [16, 17]. However, two other studies estimated treatment costs by stage for women under the age of 65 [18, 19]. To our knowledge, no peer-reviewed study presents the economic costs of breast cancer treatment by stage at diagnosis in younger, privately insured women.
Another limitation of the current literature on breast cancer costs in younger women is that, with the exception of Blumen et al. [19], all of these studies present prevalent, not incident, costs. Prevalent or annual costs provide a view of costs for a cross section of women across the spectrum of the treatment continuum. Incident costs, in contrast, provide cost estimates from the onset of disease until the end of the disease or a specific period of time, typically a year [20, 21]. For chronic diseases, such as cancer, incident cost estimates provide more information that can be used when evaluating prevention strategies. In their estimates of prevalent costs, Allaire et al. [11] attempt to identify annual costs for women in the active treatment phase via procedure codes but do not identify a diagnosis date. Blumen et al. [19] rely on a claim-based algorithm to infer cancer stage at diagnosis. These solutions are imperfect at capturing incident costs as they lack the data to fully identify the date of diagnosis and then follow women over the course of treatment.
The current study overcomes these limitations by estimating incident breast cancer treatment costs by stage at diagnosis for privately insured younger women. Specifically, we estimate the medical costs of treating privately insured younger women with breast cancer from the date of diagnosis. We also investigate whether treatment costs are a result of a later stage of diagnosis or higher treatment utilization within stage. Providing estimates by stage is critical because stage at diagnosis has been shown to substantially influence treatment costs [19]. The economic impact of stage at diagnosis is relevant to our understanding of how this disease affects younger women and their families during and after treatment.
Methods
Data
We analyzed 2003–2010 North Carolina Central Cancer Registry data linked to private insurers’ enrollment files for the same period as this was the most recent linked dataset available. The data were maintained by the Integrated Cancer Information and Surveillance System at the University of North Carolina and covered approximately 85% of the North Carolina population diagnosed with cancer and approximately 60% of the total North Carolina population [22]. The Institutional Review Board at RTI International and the University of North Carolina approved the research plan for this study.
The cancer registry file contains demographic characteristics including ZIP code, primary cancer site, cancer staging using the American Joint Committee on Cancer (AJCC) 6th edition definitions, date of diagnosis, and date of death. The private insurer data include the following data files: enrollment, inpatient hospital, outpatient hospital, physician office, and prescription drug. The enrollment data file contains effective date of enrollment, the enrollment termination date, demographics, and cancer registry link identifier.
Cost estimation
Our estimation proceeded in several steps. First, we set the inclusion/exclusion criteria for the breast cancer sample. Then, we set the enrollment criteria and set up the comparison population for matching. Next, we conducted an exact match to find women with the same covariates as our sample of women with breast cancer. Finally, we estimated mean excess costs for the breast cancer sample by subtracting off the mean medical costs of the comparison population.
The breast cancer sample included women who were diagnosed with invasive primary breast cancer between ages 18 and 64 years. We excluded those who died within 1 year of diagnosis so we could observe 6 months of cancer claims data while allowing for a 6-month buffer for end-of-life costs. We included cancer patients who were enrolled for at least 3 months prior to their diagnosis so we could estimate a Charlson Comorbidity Index (CCI) using pre-cancer diagnosis data. Cancers were excluded from the CCI [23].
We included breast cancer patients and non-breast cancer comparison patients in our analysis only if the patients were continuously enrolled in each of the follow-up periods analyzed (6, 12, and 18 months), known as the initial phase of care [24]. We defined continuous enrollment as being enrolled for at least 1 day in consecutive months. In addition to our younger cohort of women with breast cancer, we also estimated costs at 12 months for a cohort of women aged 45–64 years. We followed all the same sample restrictions and matching procedures as we did with the younger women with breast cancer. These were conducted as an additional comparison population to younger women with breast cancer.
We compared the costs between breast cancer patients and a comparison population of female, non-breast cancer patients who were enrolled in private insurance between 2003 and 2010. We excluded all patients in the population without breast cancer who died and who had inconsistent birth date information across enrollment periods. The final sample of younger women with breast cancer included 1106 women enrolled at 6 months, 955 enrolled at 12 months, and 805 enrolled at 18 months.
We conducted an exact match between the breast cancer patients and patients without breast cancer using categorical 5-year birth year groups (1960–1964, 1965–1969, 1970–1974, 1975–1979, 1980–1984, 1985–1989), ZIP code, and CCI (0, ≥1). The non-breast cancer sample was given a pseudo-diagnosis date, the same diagnosis date as its matched patient with breast cancer. This date was used as an index date for coverage and cost estimation. The exact match resulted in 167,681 younger women matched at 6 months, 134,427 at 12 months, and 109,218. We retained all matches and used weights to control for the varying number of non-breast cancer patients per cancer case [25].
We estimated total private insurance medical payments for three follow-up periods (6, 12, and 18 months from diagnosis) for the younger breast and non-breast cancer cohorts and for one follow-up period (12 months from diagnosis) for the older breast and non-breast cancer cohorts. For the older breast and non-breast cancer cohorts, we summed monthly payments for 12 months from diagnosis. Payments were estimated separately for expenditures related to the following services for all cohorts: physician office visits, outpatient visits, inpatient hospital stays, and prescription drugs. We calculated excess costs as the difference in mean payments between the patients with and without breast cancer by (1) type of expenditure and (2) type of expenditure by stage. Additionally, payments were estimated by stage of cancer for the younger breast cancer patients [26]. All costs were inflated to 2014 dollars using a gross domestic product deflator [27]. All estimates were generated using Stata 13.1 (College Station, TX).
Results
Table 1 compares the age categories, CCI, and cancer stage of younger compared to older breast cancer patients. The average age at diagnosis was 39 years (range 21–44) for younger breast cancer patients and 55 years (range 45–64) for older patients. Overall, 96 and 89% of younger and older women, respectively, had a CCI of zero in the 3 months prior to their diagnosis. For younger women at 12 months, stage II was the most common stage at diagnosis (40%), followed by stage I cancer (34%). Older women had a significantly higher percentage of stage I cancer (46%) and lower percentage of stage II (34%). Enrollment for younger breast cancer patients was stable with 73% (805/1106) of those enrolled at 6 months still enrolled at 18 months.
Table 1.
Characteristics of women diagnosed with breast cancer in North Carolina, 2003–2010, by age group
| Variable | Women aged 21–44 years at diagnosis | Women aged 45–64 years at diagnosis | ||
|---|---|---|---|---|
| Continuously enrolled 6 months from diagnosis (N = 1106) | Continuously enrolled 12 months from diagnosis (N = 955) | Continuously enrolled 18 months from diagnosis (N = 805) | Continuously enrolled 12 months from diagnosis (N = 4082) | |
| Age (years)a | 39.15 | 39.18 | 39.25 | 54.8 |
| Birth year | ||||
| 1955–1959 | 1% | 1% | 1% | |
| 1960–1964 | 31% | 33% | 36% | - |
| 1965–1969 | 41% | 41% | 40% | - |
| 1970–1974 | 19% | 19% | 17% | - |
| 1975–1979 | 6% | 5% | 4% | - |
| 1980–1984 | 2% | 2% | 2% | - |
| 1985–1989 | 0.3% | 0.1% | 0.1% | - |
| Charlson Comorbidity Index (CCI) | ||||
| CCI score = 0 | 96% | 96% | 96% | 89% |
| CCI score >0 | 4% | 4% | 4% | 11% |
| American Joint Committee on Cancer (AJCC) stage at diagnosis | ||||
| Stage I | 34% | 34% | 34% | 46% |
| Stage II | 41% | 40% | 40% | 34% |
| Stage III/IV | 16% | 16% | 15% | 12% |
| Unknown | 9% | 10% | 10% | 9% |
| No. of matched comparisons | 167,681 | 134,427 | 109,218 | 299,663 |
We matched using birth year intervals; however, we present age in this table for further information
Private insurance costs were significantly higher among younger women with breast cancer than those without breast cancer for all services at all points in time (Table 2). The excess costs at 12 months relative to no cancer were $97,486 (confidence interval [CI] $93,631–101,341). Hospital outpatient and physician services costs were the largest drivers, with excess costs of $43,453 (CI $40,256–46,650) and $47,644 (CI $45,154–50,134) at 12 months, respectively.
Table 2.
Costs for patients with and without breast cancer, younger than age 45, by service type, North Carolina, 2003–2010
| Patients with breast cancer (N = 1106) | Patients without breast cancer (N = 167,681) | Excess cost | |
| 6 months from diagnosis | |||
| All service types | $66,797 ($65,214 to $68,381) | $1609 ($25 to $3192) | $65,189* ($62,950 to $67,428) |
| Physician office | $33,894 ($32,805 to $34,982) | $667 (-$421-$1756) | $33,226* ($31,687 to $34,766) |
| Inpatient hospital | $2928 ($2583 to $3273) | $235 (−109 to $580) | $2693* ($2205 to $3180) |
| Outpatient hospital | $27,629 ($26,264 to $28,995) | $402 (−964 to $1768) | $27,227* ($25,296 to $29,159) |
| Prescription drugs | $2346 ($2137 to $2556) | $304 ($94 to $513) | $2043* ($1746 to $2339) |
| Breast cancer (N = 955) | Comparison (N = 134,427) | Excess cost | |
| 12 months from diagnosis | |||
| All service types | $100,705 ($97,980 to $103,430) | $3219 ($493 to $5946) | $97,486* ($93,631 to $101,341) |
| Physician office | $48,985 ($47,224 to $50,745) | $1340 (-$421 to $3101) | $47,644* ($45,154 to $50,134) |
| Inpatient hospital | $4296 ($3821 to $4771) | $452 (-$24 to $927) | $3844* ($3172 to $4516) |
| Outpatient hospital | $44,250 ($41,990 to $46,510) | $797 (-$1464 to $3058) | $43,453* ($40,256 to $46,650) |
| Prescription drugs | $3174 ($2886 to $3463) | $630 ($341 to $919) | $2544* ($2136 to $2953) |
| Breast cancer (N = 805) | Comparison (N = 109,218) | Excess cost | |
| 18 months from diagnosis | |||
| All service types | $113,683 ($110,104 to $117,262) | $4826 ($1248 to $8405) | $108,857* ($103,796 to $113,918) |
| Physician office | $55,315 ($53,012 to $57,618) | $2001 (-$302 to $4304) | $53,313* ($50,056 to $56,570) |
| Inpatient hospital | $5410 ($4720 to $6101) | $681 (-$10 to $1371) | $4729* ($3753 to $5706) |
| Outpatient hospital | $49,035 ($46,287 to $51,784) | $1175 (-$1573 to $3924) | $47,860* ($43,973 to $51,747) |
| Prescription drugs | $3923 ($3543 to $4303) | $969 ($589 to $1349) | $2954* ($2416 to $3492) |
Excess cost is significantly different among breast cancer and comparison samples at the 1% level
Table 3 presents the treatment cost of older women with and without breast cancer among those continuously enrolled for 12 months. In this older population, the estimated excess cost of $75,737 (CI $73,962–77,512) at 12 months was significantly higher among older women compared to those without breast cancer. Additionally, in the 12 months after diagnosis, the estimated excess cost among younger women with breast cancer was $97,486 (Table 2), which was significantly higher than that of older women estimated to be $75,737 (Table 3).
Table 3.
Treatment costs for breast cancer in women aged 45–64 years and comparison patients, by service type, North Carolina, 2003–2010
| Breast cancer (N = 4082) | Comparison (N = 299,663) | Excess cost | |
|---|---|---|---|
| 12 months from diagnosis | |||
| All service types | $80,578 ($79,322 to $81,833) | $4841 ($3586 to $6095) | $75,737a, b ($73,962 to $77,512) |
| Physician office | $37,580 ($36,817 to $38,344) | $1836 ($1073 to $2599) | $35,744a, b ($34,665 to $36,824) |
| Inpatient hospital | $3480 ($3163 to $3798) | $750 ($433 to $1068) | $2730a, b ($2281 to $3179) |
| Outpatient hospital | $36,631 ($35,706 to $37,556) | $1140 ($215 to $2064) | $35,492a, b ($34,184 to $36,799) |
| Prescription drugs | $2886 ($2771 to $3001) | $1115 ($1000 to $1230) | $1771a, b ($1609 to $1934) |
Excess cost is significantly different among breast cancer and comparison samples at the 1% level
Significantly different from women aged 18–44 years at the 1% level
Figure 1 presents the average monthly medical costs for younger non-breast cancer controls and breast cancer patients by stage at diagnosis. The overall pattern in average monthly costs is similar among each cancer stage: highest costs during the first year and a downward trend that tapers off at about 1 year after diagnosis. Generally, costs that occur after the first 12 months of care are known as ‘‘continuing’’ phase of care. [28] The comparison population experienced stable costs over the whole time period. Stage II patients and patients with an unknown stage converge closely toward the end of the 18 months, at around $2000 per month. Stage III/IV breast cancer patients maintained an average monthly cost of about $5000 1 year after diagnosis with a spike in costs at 15 months.
Fig. 1.

Adjusted average monthly cost per person from month of diagnosis to18 months for women younger than age 45 by AJCC stage of diagnosis
Figure 2 shows excess costs at 12 months by service type and stage of cancer across younger and older breast cancer patients. For younger women, the excess costs at 12 months after diagnosis ranged from $72,177 for stage I to $131,812 for stages III/IV, whereas for older women, the excess costs ranged from $53,288 for stage I to $124,237 for stages III/IV. Treatment costs increased with cancer stage within each service category, with physician and outpatient services having the highest excess costs for each stage.
Fig. 2.
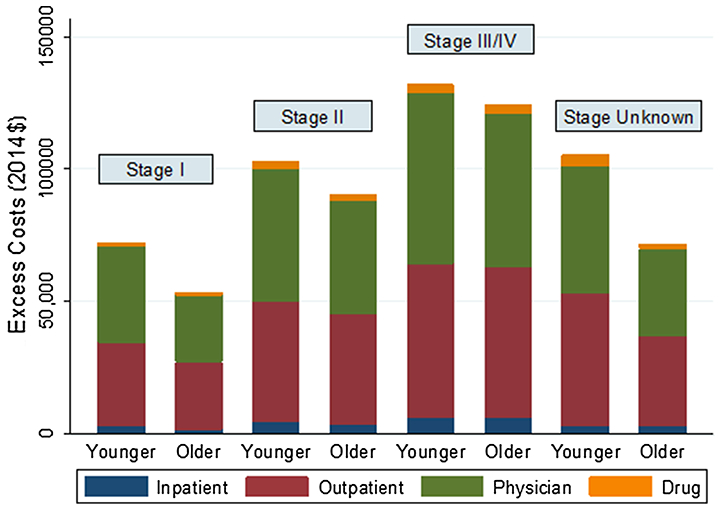
Direct medical costs of breast cancer 12 months from date of diagnosis by AJCC stage, age group, and type of service. Younger, age ≤45; older, age 45–64
Discussion
Younger breast cancer patients not only have a higher proportion of later-stage cancer than older women, but also have higher within-stage excess costs. Breast cancer treatment costs for younger women are substantial; estimated excess costs at 6, 12, and 18 months were $65,189, $97,486, and $108,857, respectively. Younger women with stage I cancer have the largest difference with an excess cost (relative to older women) of $18,889, whereas stages II and III/IV have smaller differences of $12,790 and $7575, respectively.
These differences in costs could reflect the more intensive treatment offered to younger patients diagnosed with breast cancer even at an early stage; 5 years of adjuvant tamoxifen is considered endocrine treatment for premenopausal women with estrogen [29, 30]. Higher excess costs may also be the result of younger breast cancer patients diagnosed with more aggressive forms of breast cancers that tend to progress faster [31]. Treating more aggressive cancer requires more intensive combination chemotherapies, which may underlie the higher costs reported in this study and previous studies [18, 19]. This evidence dovetails with recent research indicating that tumor histology has shown to be more predictive of effective treatment than age [29, 32].
Breast cancer patients incurred markedly higher costs for the initial 12-month period after diagnosis after which the costs tend to stabilize, but remain higher compared to individuals without a breast cancer diagnosis regardless of age. The largest cost components by type of service are physician office and hospital outpatient costs, which accounted for almost 90% of the total cost difference between those with breast cancer and the comparison cohort, regardless of age. Costs also vary by stage at diagnosis for younger women, with treatment costs estimated to be much lower for stage I ($72,177) than for stage III/IV ($105,254) at 12 months. The difference in breast cancer treatment costs by stage at diagnosis has been reported in prior studies, but these studies did not estimate costs for younger women [19, 33, 34].
One of the key strengths of this study was the ability to report costs for younger women diagnosed with breast cancer based on linked administrative and cancer registry data. This allowed us to capture incident breast cancer costs occurring from the date of diagnosis, rather than rely on an algorithm to detect the stage at diagnosis [19] or prevalent-based costs reported in previous work [11]. Using cancer registry data by stage at diagnosis also enabled us to examine whether costs for younger women were higher because of a later stage at diagnosis or greater treatment utilization within stage. Finally, we leveraged the size of our claims data to construct two separate cost comparisons for younger women: a sample of women without breast cancer to properly capture the attributable costs, and a group of older women diagnosed with breast cancer.
This study has several limitations. First, we only analyzed data from private insurers in one state; therefore, the findings reported here may not be reflective of the treatments or resources used in other settings. Second, we did not have a large enough sample of patients to assess cost of stages III and IV separately; thus, we report pooled costs for stage III and IV breast cancer patients, and the cost of treatment may differ for these two stages [19]. Third, the encounter data may not have captured all diagnoses included in the CCI. The imprecision of the CCI measurement could bias the results either up or down. Fourth, the study population displayed a surprising amount of stability in maintaining insurance coverage over time, which may limit generalization of our findings to populations that remain in a similar occupation or geographic area over time. Fifth, owing to data limitations, we could not generate estimates by race/ethnicity or by hormone receptor status. Future research should seek to diversify these estimates at these levels.
This study offers an important contribution to the literature on the incidence-based cost of breast cancer treatment for privately insured younger women by stage at diagnosis. Incidence-based cost estimates are especially important for chronic diseases such as cancer, where prevalence-based costing provides a cross section of patients along the treatment spectrum. Incidence-based costs are also critical for future research as inputs into cost-effectiveness analyses for decision making [20].
The treatment costs of breast cancer in younger women are high and increase with the stage at diagnosis. Screening by mammography can help detect breast cancer at earlier stages and is recommended for women ages 50–74 [35]; however, screening has not been shown to be effective for younger women [29]. The EARLY Act authorized the Centers for Disease Control and Prevention (CDC) to conduct research and develop initiatives to advance understanding and awareness of breast cancer among young women [5]. CDC’s digital media campaign Bring Your Brave promotes awareness of breast health and breast cancer in young women [36]. Consideration also needs to be given to research leading to a better understanding of the influence primary preventive factors (e.g., environmental exposures, genetic predisposition, etc.) on early onset disease [37].
The high cost of treatment underscores the importance obtaining and retaining insurance coverage for younger women. Psychological financial hardship, the mental distress related to meeting financial obligations, occurs much more frequently among the uninsured [38]. In addition, insurance coverage may be associated with breast cancer diagnosed at an earlier stage, meaning that having insurance coverage could reduce the public health burden of treatment considerably [39]. Researchers have found that cancer survivors with insurance are much less financially vulnerable than those without, especially among younger survivors [12].
The high costs of treatment have implications for out-of-pocket costs incurred by younger patients because most of these treatments require copayments [40]. Given the higher cost of treatment for younger versus older women, the out-of-pocket costs borne by younger women are likely to be higher. The financial implications of breast cancer treatment costs for younger women need to be explored in future studies. New research should seek to identify the drivers of those costs using detailed treatment data and the impact these costs have on quality of life for younger breast cancer survivors. Multifaceted strategies to counter these drivers and reduce costs, and primary prevention efforts that address breast cancer risk factors and family history, may be helpful in reducing the treatment costs of breast cancer among younger women.
Acknowledgements
Work on this study was supported by the Integrated Cancer Information and Surveillance System (ICISS), UNC Lineberger Comprehensive Cancer Center with funding provided by the University Cancer Research Fund (UCRF) via the State of North Carolina.
Funding support This research was supported by contract number 200–2008-27958 Task Order 38 from the Centers for Disease Control and Prevention.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Publisher's Disclaimer: Disclaimer The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.US Cancer Statistics Working Group (2015) United States cancer statistics: 1999–2013 Cancer incidence and mortality data. http://www.cdc.gov/uscs
- 2.Chollet-Hinton L, Anders CK, Tse C-K, Bell MB, Yang YC, Carey LA, Olshan AF, Troester MA (2016) Breast cancer biologic and etiologic heterogeneity by young age and menopausal status in the Carolina Breast Cancer Study: a case-control study. Breast Cancer Res 18(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anders CK, Johnson R, Litton J, Phillips M, Bleyer A (2009) Breast cancer before age 40 years. Semin Oncol 36(3):237–249. doi: 10.1053/j.seminoncol.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee HB, Han W (2014) Unique features of young age breast cancer and its management. J Breast Cancer 17(4):301–307. doi: 10.4048/jbc.2014.17.4.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breast Cancer Education And Awareness Requires Learning Young (EARLY) act of 2009 (HR 1740 111th). http://www.govtrack.us/congress/bills/111/hr1740/text
- 6.Ekwueme DU, Trogdon JG (2016) The economics of breast cancer in younger women in the US. Am J Prev Med 50(2):249–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maggard MA, O’Connell JB, Lane KE, Liu JH, Etzioni DA, Ko CY (2003) Do young breast cancer patients have worse outcomes? J Surg Res 113(1):109–113 [DOI] [PubMed] [Google Scholar]
- 8.Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H (2009) Breast cancer in young women: poor survival despite intensive treatment. PLoS ONE 4(11):e7695. doi: 10.1371/journal.pone.0007695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard-Anderson J, Ganz PA, Bower JE, Stanton AL (2012) Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst 104(5):386–405. doi: 10.1093/jnci/djr541 [DOI] [PubMed] [Google Scholar]
- 10.Trivers KF, Fink AK, Partridge AH, Oktay K, Ginsburg ES, Li C, Pollack LA (2014) Estimates of young breast cancer survivors at risk for infertility in the US. Oncologist 19(8):814–822. doi: 10.1634/theoncologist.2014-0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allaire BT, Ekwueme DU, Guy GP, Li C, Tangka FK, Trivers KF, Sabatino SA, Rodriguez JL, Trogdon JG (2016) Medical care costs of breast cancer in privately insured women aged 18–44 years. Am J Prev Med 50(2):270–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banegas MP, Guy GP Jr, de Moor JS, Ekwueme DU, Virgo KS, Kent EE, Nutt S, Zheng Z, Rechis R, Yabroff KR (2016) For working-age cancer survivors, medical debt and bankruptcy create financial hardships. Health Aff (Millwood) 35(1):54–61. doi: 10.1377/hlthaff.2015.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner L, Lacey MD (2004) The hidden costs of cancer care: an overview with implications and referral resources for oncology nurses. Clin J Oncol Nurs 8(3):279–287. doi: 10.1188/04.cjon.279-287 [DOI] [PubMed] [Google Scholar]
- 14.Kent EE, Forsythe LP, Yabroff KR, Weaver KE, de Moor JS, Rodriguez JL, Rowland JH (2013) Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer 119(20):3710–3717. doi: 10.1002/cncr.28262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekwueme DU, Trogdon JG, Khavjou OA, Guy GP (2016) Productivity costs associated with breast cancer among survivors aged 18–44 years. Am J Prev Med 50(2):286–294 [DOI] [PubMed] [Google Scholar]
- 16.Fu AZ, Jhaveri M (2012) Healthcare cost attributable to recently-diagnosed breast cancer in a privately-insured population in the United States. J Med Econ 15(4):688–694. doi: 10.3111/13696998.2012.673524 [DOI] [PubMed] [Google Scholar]
- 17.Barron JJ, Quimbo R, Nikam PT, Amonkar MM (2008) Assessing the economic burden of breast cancer in a US managed care population. Breast Cancer Res Treat 109(2):367–377. doi: 10.1007/s10549-007-9650-4 [DOI] [PubMed] [Google Scholar]
- 18.Montero AJ, Eapen S, Gorin B, Adler P (2012) The economic burden of metastatic breast cancer: a US managed care perspective. Breast Cancer Res Treat 134(2):815–822. doi: 10.1007/s10549-012-2097-2 [DOI] [PubMed] [Google Scholar]
- 19.Blumen H, Fitch K, Polkus V (2016) Comparison of treatment costs for breast cancer, by tumor stage and type of service. Am Health Drug Benefits 9(1):23. [PMC free article] [PubMed] [Google Scholar]
- 20.Segel JE (2006) Cost-of-illness studies—a primer. RTI-UNC Cent Excell Health Promot Econ. http://www.rti.org/pubs/coi_primer.pdf
- 21.Barlow WE (2009) Overview of methods to estimate the medical costs of cancer. Med Care 47(7 Suppl 1):S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ICISS Data Resources. Integrated Cancer Information Center. http://iciss.unc.edu. Accessed 14 Oct 2015
- 23.Charlson M, Szatrowski TP, Peterson J, Gold J (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47(11):1245–1251 [DOI] [PubMed] [Google Scholar]
- 24.Warren JL, Yabroff KR, Meekins A, Topor M, Lamont EB, Brown ML (2008) Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst 100(12):888–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iacus SM, King G, Porro G (2009) CEM: software for coarsened exact matching. J Stat Softw 30:1–2721666874 [Google Scholar]
- 26.American Joint Committee on Cancer (2009) Breast cancer staging, 7th edn [Google Scholar]
- 27.U.S. Bureau of Economic Data - National Data (2016) Table 1.1.9. Implicit price deflators for gross domestic products. http://www.bea.gov/iTable/iTable.cfm?ReqID=9&step=1#reqid=9&step=3&isuri=1&903=13. Accessed 9 Mar 2015
- 28.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML (2011) Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 103:117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christinat A, Di Lascio S, Pagani O (2013) Hormonal therapies in young breast cancer patients: when, what and for how long? J Thorac Dis 5(1):S36–S46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aebi S, Davidson T, Gruber G, Cardoso F, Group EGW (2011) Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Anna oncol 22(suppl 6):12–24 [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg SM, Partridge AH (2015) Management of breast cancer in very young women. Breast (Edinburgh, Scotland) 24(Suppl 2):154–158. doi: 10.1016/j.breast.2015.07.036 [DOI] [PubMed] [Google Scholar]
- 32.Ribnikar D, Ribeiro J, Pinto D, Sousa B, Pinto A, Gomes E, Moser E, Cardoso M, Cardoso F (2015) Breast cancer under age 40: a different approach. Curr Treat Options Oncol 16(4):1–24 [DOI] [PubMed] [Google Scholar]
- 33.Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, Brown ML (2008) Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst 100(9):630–641. doi: 10.1093/jnci/djn103 [DOI] [PubMed] [Google Scholar]
- 34.Subramanian S, Trogdon J, Ekwueme DU, Gardner JG, Whitmire JT, Rao C (2011) Cost of breast cancer treatment in medicaid: implications for state programs providing coverage for low-income women. Med Care 49(1):89–95. doi: 10.1097/MLR.0b013e3181f81c32 [DOI] [PubMed] [Google Scholar]
- 35.US Preventative Services Task Force (2016). Final Recommendation Statement - Breast Cancer Screening Recommendations. http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-screening1. Accessed 15 Mar 2016
- 36.Centers for Disease Control and Prevention (2016) Bring Your Brave campaign. http://www.cdc.gov/cancer/breast/young_women/bringyourbrave/
- 37.Korde LA, Partridge AH, Esser M, Lewis S, Simha J, Johnson RH (2015) Breast Cancer in Young Women: Research Priorities. A report of the young survival coalition research think tank meeting. J Adolesc Young Adult Oncol 4(1):34–43 [DOI] [PubMed] [Google Scholar]
- 38.Yabroff KR, Dowling EC, Guy GP Jr, Banegas MP, Davidoff A, Han X, Virgo KS, McNeel TS, Chawla N, Blanch-Hartigan D, Kent EE, Li C, Rodriguez JL, de Moor JS, Zheng Z, Jemal A, Ekwueme DU (2016) Financial hardship associated with cancer in the United States: findings From a population-based sample of adult cancer survivors. J Clin Oncol 34(3):259–267. doi: 10.1200/jco.2015.62.0468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farooqi B, Smith B, Chowdhary M, Pavoni S, Modi A, Schnell F (2015) Racial disparities in breast cancer diagnosis in Central Georgia in the United States. J Commun Support Oncol 13(12):436–441. doi: 10.12788/jcso.0179 [DOI] [PubMed] [Google Scholar]
- 40.Short PF, Moran JR, Punekar R (2011) Medical expenditures of adult cancer survivors aged <65 years in the United States. Cancer 117(12):2791–2800. doi: 10.1002/cncr.25835 [DOI] [PMC free article] [PubMed] [Google Scholar]


