Abstract
We previously reported that S-nitrosoglutathione (GSNO), an endogenous nitric oxide carrier, attenuated TH17-mediated immune responses in experimental autoimmune encephalomyelitis (EAE), an animal model for multiple sclerosis (MS). Cellular GSNO homeostasis is regulated via its synthesis by reaction between nitric oxide and glutathione and its enzymatic catabolism by GSNO reductase (GSNOR). In this study, we evaluated potential of reversible inhibitor of GSNOR (N6022) in comparison with exogenous GSNO in immunopathogenesis of EAE. Daily treatment of EAE mice with N6022 or exogenous GSNO significantly attenuated the clinical disease of EAE, but N6022 treatment showed greater efficacy than GSNO. Both N6022 and exogenous GSNO treatments increased the spleen levels of GSNO, as documented by increased protein-associated S-nitrosothiols, and inhibited polarization and CNS effector function of proinflammatory TH17 cells while inducing the polarization and CNS effector function of anti-inflammatory CD4+ CD25+ FOXP3− regulatory T (Treg) cells. Moreover, N6022 further attenuated TH1 while inducing TH2 and CD4+ CD25+ FOXP3+ Treg in their polarization and CNS effector functions. Similar to GSNO, the N6022 treatment protected against the EAE disease induced demyelination. However, neither exogenous GSNO nor N6022 treatment did not cause significant systemic lymphopenic effect as compared to FTY720. Taken together, these data document that optimization of cellular GSNO homeostasis by GSNOR inhibitor (N6022) in NO metabolizing cells attenuates EAE disease via selective inhibition of pro-inflammatory subsets of CD4+ cells (TH1/TH17) while upregulating anti-inflammatory subsets of CD4+ cells (TH2/Treg) without causing lymphopenic effects and thus offers a potential treatment option for MS/EAE.
Keywords: EAE, GSNO, GSNOR inhibitor, Immue modulation, Multiple sclerosis, T helper lymphocytes
Introduction
Multiple sclerosis (MS), a neurological autoimmune disease, is driven by CD4+ cells of the T helper (TH) cells [1]. There is no curative treatment for MS and the present treatments typically focus on immunomodulation for slowing the progression of the disease. These includes interferon-β (IFN-β), a naturally occurring anti-inflammatory polypeptide [2], glatiramer acetate, a synthetic peptides inducing anti-inflammatory TH2 responses [3], teriflunomide, a cell proliferation inhibitor [4], dimethyl fumarate, an immunomodulatory agent with unknown mechanism [5], natalizumab, a monoclonal antibody against α4-integrin blocking immune cell infiltration [6], fingolimod (FTY720), an oral sphingosine 1-phosphate receptor modulator inhibiting lymphocyte egress from the lymph nodes to the CNS [7], alemtuzumab, a recombinant monoclonal antibody against CD52 on T and B lymphocytes [8], and mitoxantrone, a compound inhibiting DNA synthesis and thus lymphocyte proliferation [9]. These drugs, in general, are non-specific immune-modulating agents and thus increase the risk for infectious diseases by weakening the global immune system. In addition, some of peptide- or antibody-based drugs could develop autoantibody and thus may increase the risk for autoimmune-mediated conditions while chemotherapy based drugs may cause various adverse effects by killing healthy dividing cells [10]. Moreover, in spite of the immune modulating activities, these drugs do not protect against the CNS disease of MS as the CNS disease progression continues with the medications. These observations underscore the new class of drugs targeting MS specific proinflammatory autoimmune responses, as well as protection against the CNS disease progression.
MS and experimental autoimmune encephalomyelitis (EAE; an animal model for MS) are mediated by activation and CNS infiltration of myelin specific autoreactive CD4+ T cells. TH1 and TH17 cells are the major subsets of proinflammatory CD4+ T cells mediating the CNS inflammation and thus demyelination via production of proinflammatory cytokines, such as IFN-γ and IL-17 [11]. Development and function of TH1 and TH17 cells are regulated by TH2 and regulatory T (Treg) cells [12, 13] [14]. Accordingly, TH2 and Treg cells are considered as anti-inflammatory subset of CD4+ T cells in EAE and MS [15, 16]. MS patients are known to have decreased number of Treg cells at the site of local inflammation in the CNS [17, 18]. Treg cells are the major cellular source of IL-10 and TGF-β that play critical roles in immuno-tolerance and also suppression of proinflammatory T-cells [15]. Therefore, modulation of subset specific autoimmune responses (TH1/TH17 vs.TH2/Treg), rather than suppression of global immune system, is critical for efficient control of disease with minimizing the unnecessary inhibition of systemic immune surveillance.
There is a growing body of evidence that nitric oxide (NO) plays an important role in regulation of cellular processes involved in immune and inflammatory responses [19]. Cellular NO is synthesized by three distinct forms of NO synthases (NOS): neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS) [20]. NO is reported to play an important role in inhibition of differentiation of TH17 cells and their effector function, such as release of IL-17 [21, 22]. Accordingly, genetic ablation of iNOS, but not nNOS and eNOS, increased the severity of EAE disease via inducing TH17 cells [21]. NO was also reported to induce a specific subset of CD4+/CD25+/FOXP3− Treg cells, called NO-Treg, that attenuate EAE disease via inducing the release of IL-10 and thus inhibition of TH17 cells [23, 24].
NO exerts its biological functions by direct activation of soluble guanylyl cyclase (sGC) and thus activation of cGMP/PKG pathway or via formation of secondary redox intermediates, peroxynitrite (ONOO−) and S-nitrosoglutathione (GSNO). ONOO− is synthesized by reaction between NO and superoxide anion (O2•−) under pathological conditions. ONOO− is the most powerful oxidative/nitrosative agent and its deleterious role in MS/EAE pathology has been widely reported by various investigators [see review [25]]. GSNO is the most abundant low-molecular-weight S-nitrosothiol, a biological NO carrier, which is synthesized by reaction between NO and glutathione (GSH) [26]. GSNO does not release free NO [27], rather, it exerts its biological activity via secondary modification of protein thiols, a process termed S-nitrosylation [26]. At present, over 3,000 proteins, which regulate various cellular functions, are identified to be S-nitrosylated [28]. Similar to the role of ONOO−, over accumulations of cellular GSNO and S-nitrosylated proteins (PrSNO) under pathological conditions with the presence of transition metals or GSNO reductase (GSNOR/ADH5) deficient conditions is reported to be harmful for neural and immunological functions [29-31]. However, physiological levels of GSNO/PrSNO are implicated in regulation of various cellular processes for cardiovascular hemodynamics [32], redox balance [33, 34], and inflammatory processes [35, 36]. In addition, the reported beneficial effect of exogenous GSNO treatment in various neurological, cardiovascular, infectious, and immune disease models [22, 36-46] suggest the pathophysiological importance of GSNO-mediated cellular mechanisms.
We previously evaluated the immuno-modulatory efficacy of GSNO in different EAE models and reported prophylactic and therapeutic efficacy of GSNO against the clinical disease of EAE [22, 36]. GSNO inhibited the IL-6-induced STAT3 activation (Tyr705 phosphorylation) by S-nitrosylation of the STAT3 protein on Cys259 [47] and also downregulated the IL-6 and TGF-β induced expression of RORγt, a TH17 cell specific transcription factor [22]. Additionally, GSNO treatment also inhibited the IL-6/TGF-β and IL-23 induced TH17 cell polarization and their effector function under in vitro cell culture conditions but without affecting TH1 (IFN-γ) and TH2 (IL-4) immune responses [22]. Moreover, GSNO was reported to modulates the activities of proinflammatory transcription factors, such as NF-κB, AP-1, and STAT3 [35, 36, 48] and thus modulates gene expression for various proinflammatory effectors, such as ICAM-1, and VCAM-1 [35, 36, 43, 48, 49], as well as endothelial recruitment of peripheral immune cells for their CNS infiltration [36]. Therefore, it is important to understand the role of NO in immune and CNS disease process for MS and EAE.
Cellular GSNO homeostasis is regulated by its synthesis by reaction between NO and GSH and its catabolism by GSNO reductase (GSNOR/ADH5), an enzyme in the alcohol dehydrogenase (ADH) family [26]. In this study, we assessed the role of exogenous vs. endogenous GSNO in modulation of pro-inflammatory (TH1 and TH17) and anti-inflammatory (TH2 and Treg) CD4+ T cells by treating EAE mice with exogenous GSNO as compared to inhibitor of GSNOR (N6022) for endogenous GSNO under EAE conditions. N6022 is a first-in-class very potent, specific, and reversible inhibitor of GSNOR [50]. N6022 has been found to be beneficial in animal models of experimental asthma [51], allergic airway inflammation [52] and endothelial vasodilatory dysfunction [53]. In addition, safety of N6022 for human use is proven by Phase I and II studies for asthma and cystic fibrosis (ClinicalTrials.gov) [50]. In this study, we observed that overall effects of exogenously supplemented GSNO vs. endogenous generated GSNO by N6022-mediated inhibition of its degradation were similar in terms of attenuation of EAE disease with greater efficacy with N6022 than GSNO treatment. While both GSNO and N6022 inhibit TH17 cells for expression of IL-17 and induced CD4+ CD25+ FOXP3− Treg for expression of IL-10, N6022 also inhibits TH1 for expression of IFN-γ and induces CD4+ CD25+ FOXP3+ Treg for expression of IL-10. These data suggest that cellular GSNO homeostasis is important for differentiation and effector function of proinflammatory (TH1 and TH17) and anti-inflammatory (TH2 and Treg) CD4+ T cells. Moreover, this study documents that GSNOR inhibitor (N6022) is also a novel therapeutic approach for targeting NO metabolome in cells expressing NOS and GSNOR for selective modulation of CD4+ subsets (TH1/TH17 vs. TH2/Treg) and thus attenuation of autoimmune disease of MS/EAE without causing a deleterious lymphopenic effect.
Materials and Methods
Induction of active EAE and drug treatments
Female C57BL/6 mice of 8-12 weeks of age, purchased from Jackson Laboratory, were provided with food and water ad libitum and were kept in pathogen free animal care facility of Medical University of South Carolina (MUSC) throughout the study. All procedures were conducted in accordance with accepted standards of humane care as approved by the Institutional Animal Care and Use Committee (Approved number: AR#1644). EAE was induced as described previously [54]. Briefly, mice were immunized subcutaneously in the flank regions with MOG35-55 peptide (MOG; 200ug; Peptide International) emulsified (1:1) in 100ul complete Freund’s adjuvant (CFA) on day 0 and day 7. Additionally, 300 ng of Pertussis toxin (Sigma-Aldrich, St Louis, MO) was given on day 0 and day 2 by i.p. injection. Pertussis toxin used as per the standardized protocol reported by us and other investigators for the induction of EAE [54]. Similarly, healthy control group received subcutaneous injection of PBS and CFA emulsion on day 0 and day 7. Clinical signs of EAE were scored by examiners blinded to experimental treatments using the following scale: 0 = no clinical signs of disease; 1 = piloerection and sluggish; 2 = limp tail (ataxia); 2.5 = ataxia with partial hind limb paralysis; 3 = full paralysis of hind limb; 3.5 = full paralysis of hind limb with paralysis of one fore limb; 4 = full paralysis of two limbs; 4.5 = moribund stage; 5 = death. At the onset of the disease (with clinical score between 1 and 2), the animals were given daily treatment with GSNO (1 mg/kg body weight/i.p.), N6022 (1 mg/kg body weight/i.p. or 2.5mg/kg body weight/oral; Axon Medchem, Reston, VA), or FTY720 (1 mg/kg body weight; Cayman Chemical, Ann Arbor, MI). The drug treatments were continued till the termination of the study (day 41 post immunization). EAE animals without drug treatment received PBS. Likewise, healthy controls received vehicle.
Histological Analysis
After remission of EAE disease (day 41 post immunization), control mice, EAE mice, and EAE mice treated with GSNO or N6022 were anesthetized and perfused first with saline and then 4% paraformaldehyde as described previously [55]. Tissue samples were embedded in paraffin block and sectioned transversely (5 μm-thick). Haemotoxylin and Eosin (H&E) staining was performed to assess infiltration of leukocyte and inflammation. For the quantitation of infiltrates, the digital images were analyzed by Image-J. (NIH, Bethesda, MD). To assess the status of myelin, the sections were stained with antibody specific to myelin basic protein (MBP) and detected with immunofluorescent analysis. All digital images were taken using BX-60 microscope equipped with DP70 camera unit (Olympus, Tokyo, Japan).
Total Lymphocyte Count
Normal female C57BL/6 mice were treated GSNO, N6022, or FTY720 for 19 days and then sacrificed for the collection of blood. The bloods collected in EDTA blood collection tubes (BD Biosciences) were analyzed by an automated hematology analyzer for counting total lymphocytes. For counting of each subset of lymphocytes, 50uL of blood was mixed with staining buffer (20 uL) containing fluorescence labelled antibodies and red-blood-cells were lysed with FACS lysing solution (BD Biosciences) prior to fluorescence flow-cytometric analysis. For staining of CD3+, CD4+, and CD8+ cells, allophycocyanin (APC)-labeled anti-mouse CD3 (eBioscience clone 17A2), Fluorescein isothiocyanate (FITC)-labeled anti-mouse CD4 (eBioscience clone RM4-5), APC-labeled anti-mouse CD8 (eBioscience clone 53-6.7) and appropriate isotype matched controls.
Fluorescence flow cytometry analysis of TH1, TH2, TH17, and Treg cells in spinal cords and spleens:
Fluorescence flow cytometry analysis for each subset of CD4+ T cells (TH1, TH2, TH17, CD4+/CD25+/FOXP3+ Treg, and CD4+/CD25+/FOXP3− Treg) were performed as reported previously with some modification [55]. Briefly, control mice, EAE mice, EAE mice treated with GSNO or N6022 at the peak of disease (day 16 to day 19 post-immunization) were sacrificed for the collection of spinal cords and spleens. Following the preparation of single cell suspension, red blood cells were lysed with Pharma lyse buffer (BD Pharmingen) and the remaining cells were washed with RPMI 1640. CD4+ T cells were purified with CD4 microbeads (Miltenyi Biotech) then re-suspended with complete RPMI-media in 12-well plates (5 × 106 cells/2 ml per well) containing MOG peptide (25 μg/ml) for 48 hrs. Following the centrifugation, the resulting supernatants were collected for ELISA for CD4+ subset specific cytokines (see below). The cell pellets were washed with cell staining solution (ebioscience, Waltham, MA, USA) and stained with fluorescence-labeled antibody specific to IFN-γ for TH1, IL-4 for TH2, IL-17 for TH17, CD25+ for total Treg, CD25+ and FOXP3+ for FOXP3+ Treg, and CD25+ and FOXP3− for FOXP3− Tregs (ebioscience, Waltham, MA, USA). The cells were counted and analyzed using Beackman Coulter instrument (Beckman Coulter, Inc., Brea, CA, USA)
ELISA for subset specific CD4+ T cell cytokines in the spinal cords
ELISA assay was performed for analysis of CD4+ T cell subset specific cytokines released from cultured cells or spinal cord tissues. For extraction of spinal cord lysates, the spinal cords isolated from animals at the peak of the EAE disease were homogenized in PBS containing complete protease inhibitor mixture (Roche Diagnostics, Mannheim, Germany). Following centrifugation (10,000 xg), the levels of protein in the supernatant were estimated by Lowry assay using DC protein assay kit (Bio-Rad, Hercules, CA). The equal amounts of proteins were analyzed for ELISA for IFN-γ, IL-4, IL-17, and IL-10. ELISA kits for IFN-γ, IL-17, and IL-10 were purchased from R&D systems (Minneapolis, MN) and ELISA kit for IL-4 were purchased from Biolegend (San Diego, CA).
Western analysis
After remission of EAE disease (day 41 post immunization), the spinal cord tissues were homogenized in 1x SDS-PAGE sample buffer (5x: 0.25 M Tris-Cl (pH 6.8), 50% (v/v) Glycerol, 5% (w/v) SDS, 0.05% (w/v) bromophenol blue, 0.25 M DTT) by sonication. Following centrifugation (10,000 xg for 5 min), the levels of protein in the supernatant were estimated by Lowry assay using DC protein assay kit (Bio-Rad). The equal amounts of proteins were resolved in 4–20% gradient SDS-PAGE (BioRad) and transferred to nitrocellulose membranes. The membranes were then blocked with blocking buffer (5% nonfat dry milk, 20 mM Tris, 500 mM NaCl, and 0.1% Tween20, pH 7.6) and incubated in blocking buffer containing primary antibody specific to myelin basic protein (MBP; Santa Cruz Biotech, Delaware Avenue, CA), proteolipid protein (PLP; Santa Cruz Biotech), or β- actin (Cell Signaling, Danvaers, MA). Following washing, the membranes were incubated with 1:10,000 diluted horseradish peroxidase (HRP) conjugated secondary antibody (Jackson Immunoresearch Lab, West Grove, PA), washed and then incubated with ECL reagent (Amersham Life Science, Pittsbrugh, PA), and exposed to ECL film.
Analsys of protein S-nitrosylation and nitrotyrosine formation:
Protein S-Nitrosylation was detected using the biotin-switch method with slight modification as described in our previous study [49]. Spleens were homogenized in 250 mM HEPES, pH 7.7, 1 mM EDTA, 0.1 mM neocuproine, 1% Nonidet P-40, 150 mM NaCl, 1 mM PMSF, 20mM methyl methanethiosulfonate (MMTS), 80 μM carmustine, protease inhibitor mixture (Sigma), and mixed with an equal volume of 25 mM HEPES, pH 7.7, 0.1 mM EDTA, 10 μM neocuproine, 5% SDS, 20 mM MMTS and incubated at 50°C for 20 min. After acetone precipitation, the precipitates were resuspended in 25 mM HEPES, pH 7.7, 0.1 mM EDTA, 10 μM neocuproine, 1% SDS and mixed with two volumes of 20 mM HEPES, pH 7.7, 1 mM EDTA, 100 mM NaCl, 0.5% Triton X-100. The S-nitrosylated proteins were then modified with biotin in 25 mM HEPES, pH 7.7, 0.1 mM EDTA, 1% SDS, 10 μM neocuproine, 10 mM ascorbate sodium salt, and 0.2 mM N-[6-(biotinamido)hexyl]-30-(20-pyridyldithio) propionamide (biotin-HPDP, Pierce). After acetone precipitation, biotinylated proteins were resolved by SDS-PAGE and visualized by Western analysis using antibody specific to biotin (Cell Signaling).
Statistical Analysis
Clinical disease scores as average maximal scores over the treatment period (mean ± SD) are analyzed using Kruskal– Wallis test. Statistics for proliferation and cytokine responses were analyzed with one-way multiple-range analysis of variance using Graph Pad Prism 3.0 software. Significances (p value) between groups were determined using the Newman–Keul test. A value of p<0.05* and above was considered significant.
Results
GSNO treatment attenuates EAE disease via inhibiting the TH17 cells while inducing the CD4+ CD25+ FOXP3− Treg cells in their effector function in the CNS
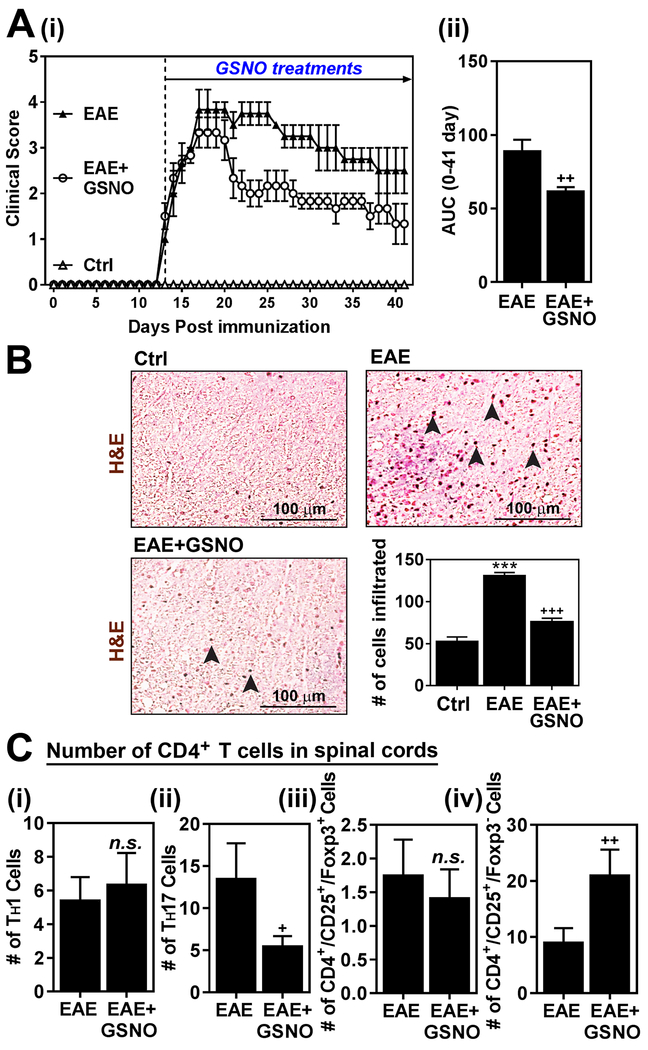
We previously reported that GSNO treatment attenuated the EAE disease by inhibiting TH17 signaling pathways (STAT3/RORγt) but without affecting on TH1 (STAT4/T-bet) and TH2 (STAT6/GATA3) signaling pathways and thus related cell types [22]. Accordingly, figure 1A shows that GSNO treatment of EAE mice significantly decreased their clinical disease as shown by clinical scores [untreated EAE mice: 3.5 ± 0.5, GSNO treated EAE mice: 3.1 ± 0.76 at the peak of disease (day 21 of post immunization); untreated EAE mice: 2.5 ± 0.5, GSNO treated EAE mice: 1.3 ± 0.76 at the remission of disease (day 41 of post immunization)]. Accordingly, GSNO treatment also reduced mononuclear cell expansion and CNS infiltration as shown by H&E saining (Fig. 1B).
Figure 1. Exogenous GSNO treatment attenuates EAE disease.
A. C57BL\6 mice (n=6) immunized with MOG peptide were treated with GSNO (1mg/kg/day) on the day of disease onset (day 14 post-immunization). Following the immunization and GSNO treatment, clinical signs of EAE disease were assessed daily as described in materials and methods (i). The area under the curve (AUC) of the overall disease severity was calculated and represented bar graph (ii). B. At the peak of EAE disease (day 20), the spinal cord infiltration of mononuclear cells was analyzed by H&E staining. The number of infiltrated cells in the H&E staining was manually counted and represented as number of cells per microscopic field (n=4). C. At the peak of EAE disease (day 20), subset specific infiltration of CD4+ cells (IFN-γ+ TH1, IL-17+ TH17, CD25+ FOXP3+ Treg, and CD25+ FOXP3+ FOXP3− Treg) in the spinal cords and brains were analyzed by fluow-cytometry analysis at the peak of disease (n=4). The graphs show mean ± standard error of the mean (SEM). *** p < 0.001 compared to control (Ctrl) group; + p < 0.05, ++ p < 0.001 compared to EAE group; n.s. = not significant.
We next investigated CNS effector functions of subset specific CD4+ T cells in EAE mice treated with GSNO. For this, total lymphocytes were isolated from spinal cords and brains and re-stimulated with MOG peptide under ex vivo conditions. Fluorescence flow cytometry analysis in figure 1C show that GSNO treatment had no obvious effect on the numbers of CD4+ IFN-γ+ (TH1) cells but significantly decreased the number of CD4+ IL-17+ (TH17) cells in the CNS of EAE animals as reported previously by our laboratory [22] and others using NO donors [23, 24]. Interestingly, the CNS of GSNO treated animals had significantly increased the number of CD4+ CD25+ FOXP3− Treg cells, which may represent NO-Treg (NO-inducible Treg) and/or Tr1 (type 1 Treg) [23, 56], without any significant differences in the number of CD4+ CD25+ FOXP3+ Treg cells, which represent natural and/or inducible Tregs (nTreg and iTreg) [57]. Accordingly, the number of total Treg cells (CD4+ CD25+) were increased in EAE mice treated with GSNO as compared to untreated EAE mice. These data describe that GSNO mediated mechanisms attenuate EAE disease via inhibiting TH17 cells while inducing CD4+ CD25+ FOXP3− Treg cells as well as their effector functions in the CNS but without affecting other subset of CD4+ T cells.
GSNO treatment attenuates subset specific polarization of TH17 and CD4+ CD25+ FOXP3− Tregs in spleen without exhibiting global lymphopenia-related effect
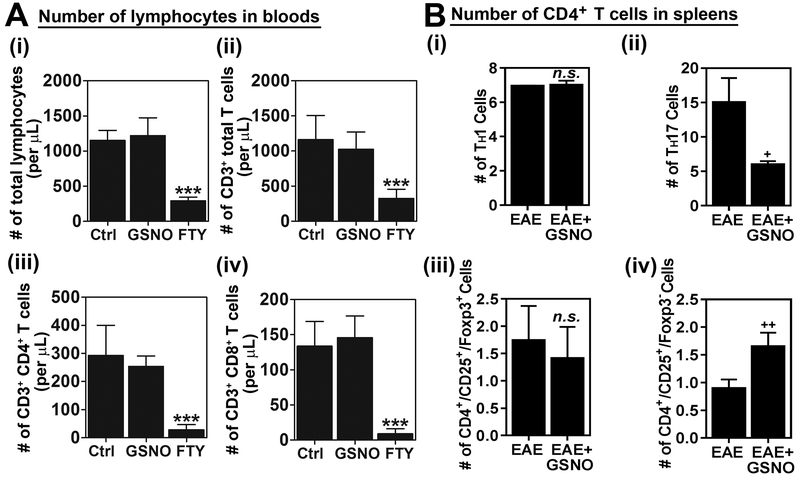
GSNOR knockout mice was previously reported to experience lymphopenia like conditions [31]. Therefore, to assess any potential lymphopenic effect of GSNO, normal C57BL\6 mice were treated with saline (Ctrl), GSNO (1 mg/kg/day.oral), or FTY720 (1 mg/kg/day/oral) as a positive drug control, for 19 days and the numbers of total lymphocytes, CD3+ T cells, CD4+ T cells, and CD8+ cells in bloods were analyzed. FTY720 (fingolimod), a FDA approved drug, provides protection against EAE disease as well as MS by inhibiting lymphocyte egress from the lymph nodes to the CNS [7]. Figure 2A shows that GSNO treatment had no obvious effect on the numbers of these cells in blood while FTY720 (FTY) significantly reduced the numbers of those lymphocytes in blood as expected, indicating that GSNO mediated reductions in CNS infiltration of mononuclear cells (Fig. 1B) and CNS effector function of TH17 cells (Fig. 1C-ii) are not associate with lymphopenia-related effects.
Figure 2. GSNO treatment differentially modulates subset specific polarization of CD4+ T cells in spleen without exhibiting lymphopenia-related effect.
A. Normal mice (without EAE) were treated with saline (Ctrl), GSNO or FTY720 (FTY) for 19 days and the numbers of total lymphocytes (i), CD3+ T cells (ii), CD4+ T cells (iii), and CD8+ cells (iv) in bloods were analyzed (n=3). B. At the peak of EAE disease (day 20), CD4+ T cells were isolated from the spleens of EAE mice treated with saline (EAE) or GSNO, re-stimulated with MOG peptide, and number of lineage specific CD4+ T cells (IFN-γ+ TH1, IL-17+ TH17, CD25+ FOXP3+ Treg, and CD25+ FOXP3+ FOXP3− Treg) were counted by fluorescence flow-cytometry analysis (n=4). The graphs show mean ± standard error of the mean (SEM): *** p < 0.001 compared to control (Ctrl) group; + p < 0.05, ++ p < 0.001 compared to EAE group; n.s. = not significant.
Next, we investigated the effect of GSNO treatment on the polarization of CD4+ T cells in the spleens of EAE mice. In accordance with the CNS data of effector function of CD4+ T cell subsets (Fig. 1C), GSNO treatment had no obvious effect on the polarization of TH1 cells but significantly decreased polarization of TH17 cells in the spleens of EAE animals (Fig. 2B-i and ii). In addition, GSNO treatment significantly increased the polarization of CD4+ CD25+ FOXP3− Treg cells without altering the number of CD4+ CD25+ FOXP3+ Treg cells (Fig. 2B-iii and iv). These data indicate that GSNO mediated mechanisms attenuate EAE disease via inhibiting TH17 and also by inducing CD4+ CD25+ FOXP3− Treg polarization and its CNS effector function.
GSNOR inhibitor (N6022) attenuates EAE disease.
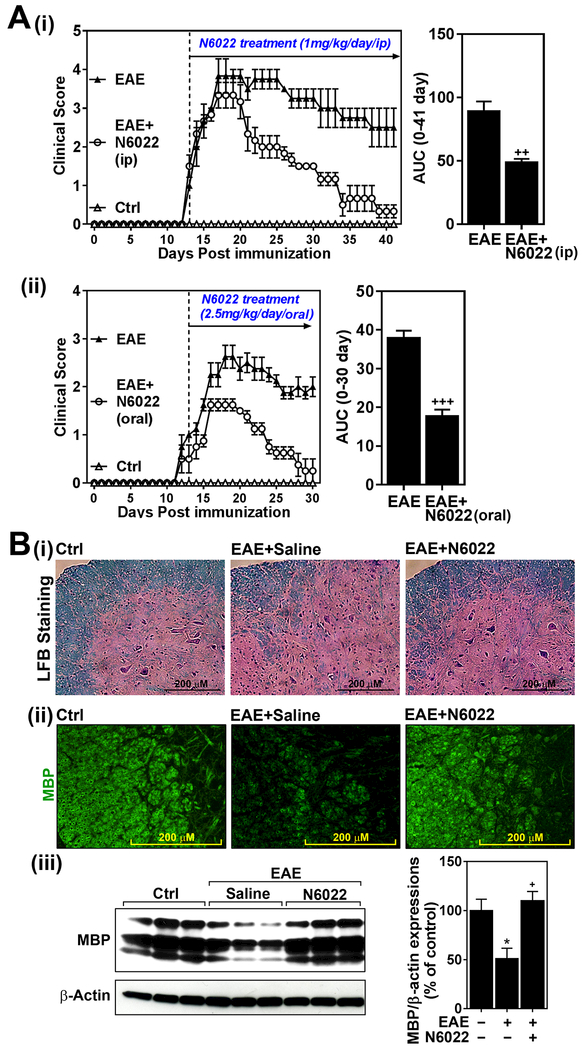
Cellular homeostasis of GSNO is maintained by its redox based synthesis from GSH and NO and its degradation by GSNOR [26]. Inhibition of GSNOR is expected to increase cellular levels of GSNO and thus potentially effective in animal model of EAE disease. However, demyelination and neurodegeneration [29], and lymphopenia [31] observed in GSNOR knockout mice suggest that inhibition of GSNOR with its inhibitors may result in neurodegeneration rather than beneficial effects in EAE. However, in previous toxicology studies with rats, N6022 was also well tolerated without any visible adverse effects even at higher doses upto 10 mg/kg/day/i.v. [2]. In addition, safety of N6022 was also proven by phase I and II human studies [50]. To address this dilemma, we evaluated the efficacy of N6022 (1mg/kg/day/i.p.) on EAE disease. We did not observe any visible illness or body weight loss in mice treated with 1mg/kg/day N6022. However, figure 3A shows that N6022 treatment of EAE mice significantly decreased EAE disease severity as shown by the clinical score [untreated EAE mice: 3.5 ± 0.5, N6022 treated EAE mice: 2.67 ± 0.29 at the peak of disease (day 21 of post immunization); untreated EAE mice: 2.5 ± 0.5, N022 treated EAE mice: 0.3 ± 0.29 at the remission of disease (day 41 of post immunization)]. We also evaluated efficacy of N6022 (2.5 mg/kg/day) by oral route and observed that N6022 was also effective by oral route on EAE disease (Fig. 3A-ii).
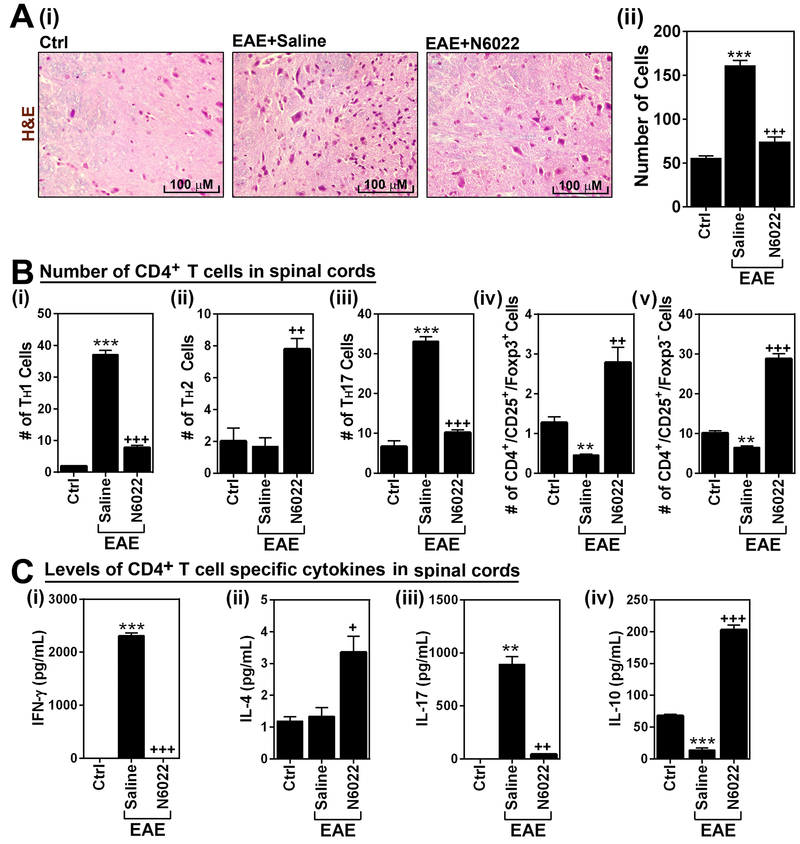
Figure 3. GSNOR inhibitor (N6022) attenuates EAE disease.
A.C57BL\6 mice immunized with MOG peptide were treated with N6022 (1mg/kg/day) via intraperitoneal (i.p.) (i) or orall routes (2.5mg/kg) (ii) on the day of disease onset (day 14 post-immunization) (n=6). Following the immunization and drug treatments, clinical signs of EAE disease were assessed daily as described in materials and methods. The area under the curve (AUC) of the overall disease severity was calculated and represented bar graph. B. At the remission of EAE, the status of myelin in spinal cords of N6022 treated mice (1mg/kg/day/i.p.) was assessed by LFB staining (i) and immunofluorescent staining with antibody specific to myelin basic protein (MBP) (ii). In addition, expression of MBP was also analyzed by Western analysis (iii) and the bands were quantified by ImageJ software and represented by bar graph (n=3). The graphs show mean ± standard error of the mean (SEM): * p < 0.05 compared to control (Ctrl) group; + p < 0.05, ++ p < 0.001, +++ p < 0.0001 compared to EAE group.
In addition to immune dysfunctions in GSNOR knockout mice [31], these mutant mice were also reported to suffer from demyelination, neurodegeneration, and neuropathic pain [29]. On the other hand, treatment of EAE with GSNO or NO-donors provide protection against EAE disease induced to demyelination and neurodegeneration [22-24, 58]. Next, we examined the status of myelin, as an index of neurodegeneration, in EAE mice treated with N6022. As expected, the LFB (Fig. 3B-i) and immunochemical staining (Fig. 3B-ii) and Western (Fig. 3-iii) for myelin basic protein (MBP) analyses document reduced amount of myelin in EAE. On the other hand, EAE animals treated with N6022 have practically normal levels of myelin (LFB), and myelin proteins (MBP). These studies document that inhibition of GSNOR with N6022 protects against EAE disease induced demyelination.
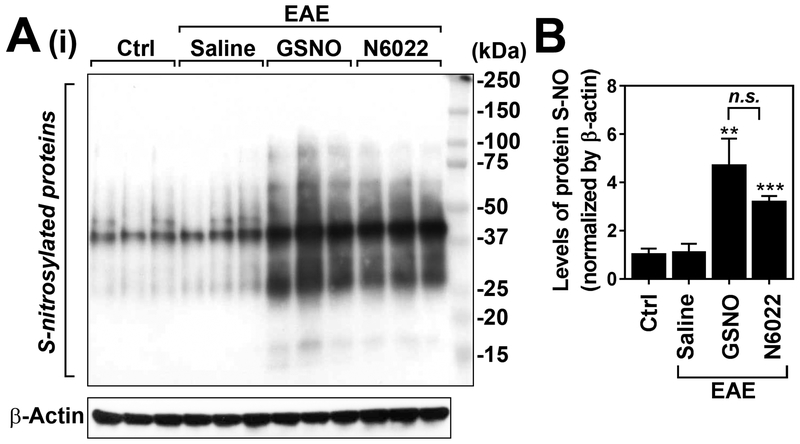
Next, we investigated the degree of protein-associated S-nitrosothiols (PrSNOs), an index of cellular GSNO levels, in spleen of GSNO or N6022 treated animals. Cellular PrSNOs are known to be in transnitrosation equilibrium with GSNO and thus can be a measurement of cellular GSNO levels [59]. N6022 is a very potent, specific, and reversible inhibitor of GSNOR. Figures 4A and B shows that induction of EAE disease had no obvious effect on the levels of PrSNOs in the spleens. As expected, treatment of EAE mice with GSNO increased spleen levels of PrSNOs as compared to untreated control and EAE mice. In addition, treatment of EAE mice with N6022 also increased spleen levels of PrSNOs to the levels comparable to GSNO treated EAE mice. These data indicate that both GSNO and N6022 are effective in induction of protein S-nitrosylation in spleen cells under EAE disease condition.
Figure 4. Treatment of EAE mice with exogenous GSNO or GSNOR inhibitor (N6022) increases S-nitrosylation of protein thiols in spleens.
At the peak of disease (day 20), the levels of S-nitroylated proteins (S-NO) were analyzed by biotin-switch assay and followed Western analysis (A) and the bands were quantified by ImageJ software and represented by bar graph (n=3) (B). The graphs show mean ± standard error of the mean (SEM): ** p < 0.001, *** p < 0.0001, compared to control (Ctrl) group; n.s. = not significant.
N6022 reduced CNS infiltration of mononuclear cells by subset specific modulation of CD4+ T cell effector function.
We next analyzed spinal cord infiltration and expansion of peripheral mononuclear cells by H&E staining. Figures 5A shows that treatment of EAE mice with N6022 significantly reduced the number of mononuclear cells in the spinal cords. To assess the effect of N6022 treatment on the subset specific modulation of CD4+ T cell effector functions, total lymphocytes were isolated from the CNS of control and EAE animals, re-stimulated with MOG peptide under ex vivo conditions, and fluorescence flow-cytometry analysis was performed to analyze the cell numbers of CD4+ IFN-γ+ (TH1), CD4+ IL-4+ (TH2), CD4+ IL-17+ (TH17), CD4+ CD25+ FOXP3+ Treg, and CD4+ CD25+ FOXP3− Treg cell types. Figures 5B-i and iii show that N6022 treatment reduced EAE-induced increases in the number of TH1 and TH17 cells in the CNS. Accordingly, N6022 treatment decreased effector functions of TH1 and TH17 cells in the CNS of EAE animals as shown by decreased levels of IFN-γ and IL-17 in the culture media of CNS derived lymphocytes (Figs. 5C-i and iii). EAE mice had no obvious alteration in the number of TH2 cells in the CNS as compared to control mice but N6022 treatment increased the number of TH2 cells (Fig. 5B-ii) as well as their effector function (IL-4 release in Fig. 5C-ii) in EAE mice. EAE mice showed a reduction in numbers of CD4+ CD25+ FOXP3+ Treg cells and CD4+ CD25+ FOXP3− Treg cells in the CNS (Fig. 5B-iv and v) as well as reduction in their expression of IL-10 (Fig. 5B-iv). However, treatment of the mice with N6022 increased the numbers of both Treg cells as well as their expression of IL-10 over the control levels. These data indicate that N6022 treatment of EAE mice inhibits infiltration and effector function of proinflammatory subsets of CD4+ T cells (TH1 and TH17) while restoring/inducing the infiltration and effector function of anti-inflammatory subsets of CD4+ T cells (TH2 and Tregs).
Figure 5. N6022 reduced CNS infiltration of peripheral mononuclear cells.
A. At the peak of disease (day 20), spinal cord infiltration of mononuclear cells was analyzed by histological staining of spinal cord section by H&E method (i). The number of infiltrated cells in the H&E staining was manually counted and represented as number of cells per microscopic field (n=4). (ii). B. Next, total lymphocytes were isolated from spinal cords of control (ctrl), EAE mice, and EAE mice treated with N6022 and cultured under ex vivo conditions. Following the activation with MOG peptide, the number of CD4+ cell subsets, such as IFN-γ+ TH1 (i), IL-4+ TH2 (ii), IL-17+ TH17 (iii), CD25+ FOXP3+ cells (iv), and CD25+ FOXP3− cells (v) were analyzed by fluorescence flow-cytometry analysis (n=4). C. From the culture media, the levels of CD4+ T cell subset specific cytokines, such as IFN-γ (i), IL-4 (ii), IL-17 (iii), and IL-10 (iv), were analyzed by ELISA (n=4). The graphs show mean ± standard error of the mean (SEM): ** p < 0.001, *** p < 0.0001, compared to control (Ctrl) group; + p < 0.05, ++ p < 0.001, +++ p < 0.0001 compared to EAE group.
N6022 treatment differentially modulates subset specific polarization of CD4+ T cells in spleen without exhibiting lymphopenia-related effect.
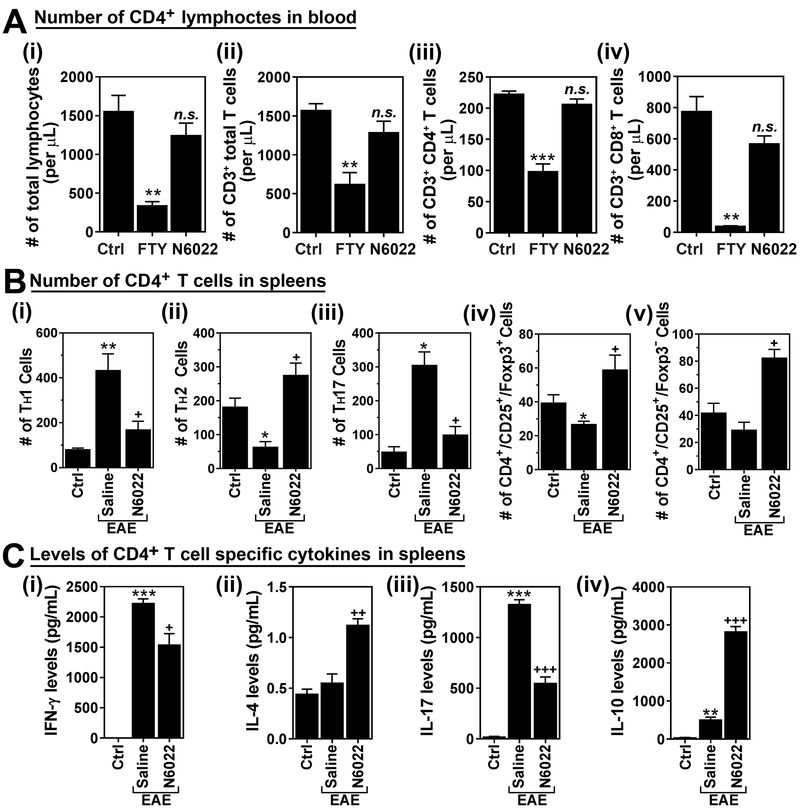
Exogenous GSNO treatment attenuated EAE disease without affecting the numbers of circulating lymphocytes (Fig. 2). Accordingly, N6022 treatment also attenuated EAE disease without affecting the numbers of circulating total lymphocytes (Fig. 6A-i) as well as CD3+ total T lymphocytes (Fig. 6A-ii), CD3+/CD4+ TH cells (Fig. 6A-iii), and CD3+/CD8+ cytotoxic T cells (Fig. 6A-iv), which play pivotal role in normal immune surveillance. These data indicate that N6022 treatment and thus increased endogenous GSNO levels selectively inhibited infiltration and effector function of TH1 and TH17 in the CNS (Fig. 5) without causing any obvious lymphopenic effect which was observed in FTY720 treated mice (Fig. 6A).
Figure 6. N6022 treatment differentially modulates subset specific polarization of CD4+ T cells in spleen without exhibiting lymphopenia-related effect.
A. Normal mice (without EAE) were treated with saline (Ctrl), GSNO or FTY720 (FTY) for 19 days and the numbers of total lymphocytes (i), CD3+ T cells (ii), CD4+ T cells (iii), and CD8+ cells (iv) in bloods were analyzed. B. At the peak of EAE disease, CD4+ T cells were isolated from the spleens of EAE mice treated with saline (EAE) or GSNO, re-stimulated with MOG peptide, and number of lineage specific CD4+ T cells , such as TH1 (i), TH17 (ii), total Treg (iii), CD25+ FOXP3+ (iv), and CD25+ FOXP3− (v), were counted by fluorescence flow-cytometry analysis. The graphs show mean ± standard error of the mean (SEM): * p < 0.05, ** p < 0.001, *** p < 0.0001, compared to control (Ctrl) group; + p < 0.05, ++ p < 0.001, +++ p < 0.0001 compared to EAE group; n.s. = not significant.
Next, we examined the effects of N6022 on the polarization of CD4+ T cells in spleens of EAE mice. In accordance with the patterns of CNS infiltrated cells and their effector functions (Fig. 5), N6022 treatment reduced the EAE-induced polarization of spleen derived TH1 and TH17 cells in response to ex vivo MOG re-stimulation (Fig. 6B-i and –iii). Similar to GSNO, N6022 treatment decreased the production of IFN-γ and IL-17 from these cells (Fig. 6C-i and –iii). In addition, N6022 treatment fully restored the EAE-induced decrease in TH2 polarization (Fig. 6B-ii) as well as increased the IL-4 production over the control levels (Fig. 6C-ii). EAE mice had decreased polarization of CD4+ CD25+ FOXP3+ Treg and CD4+ CD25+ FOXP3− Treg cells and N6022 treatment increased the polarization of both subsets of Tregs over the control levels (Figs. 6B-iv and v). Accordingly, N6022 treatment enhanced IL-10 production from these cells (Fig. 6C-iv). These data document that N6022 treatment attenuates the CNS infiltration and effector function of the proinflammatory TH1 and TH17 cells but elevates the anti-inflammatory TH2 and Treg cells via modulation of their polarization in the spleen without producing any obvious lymphopenic effect which was observed in GSNOR knockout mice [31]. Overall, the above studies document that treatment of EAE animals with inhibitor of GSNOR down regulate proinflammatory T cell response while upregulating the anti-inflammatory T cell responses as well as protection against the CNS disease of EAE.
Discussion
Current drugs approved for MS treatment are generally non-specific immune-modulating agents that can cause a global immune suppression as well as various adverse effects [10]. Therefore, development of new approach that modulates of disease specific autoimmune responses while minimizing the global immune suppression is critical for MS therapeutics. This study reports the efficacy of a drug targeting GSNO mediated mechanisms (GSNOR inhibitor N6022) on subset specific modulation of pro-inflammatory (TH1/TH17) vs. anti-inflammatory CD4+ T cells (TH2/Treg) as well as neuroprotection in EAE animal model and thus its therapeutic potential for MS.
We previously reported that GSNO treatment attenuated the EAE disease by inhibiting TH17 signaling pathways (STAT3/RORγt) [22]. In this study, we evaluated the role of GSNO mediated mechanism in induction or inhibition of subset specific CD4+ T cells under EAE conditions. Consistent with earlier study [22], treatment of progressive EAE mice (immunized with MOG peptide) with exogenous GSNO attenuated the clinical disease of EAE (Fig. 1A). In addition, GSNO treatment selectively inhibited EAE-induced polarization and CNS effector function of proinflammatory TH17 cells but without affecting polarization and CNS effector function of TH1 and TH2 (Figs. 1C and 2B) [22]. Similar observation was also made earlier by Yang et al. [21] that S-nitroso-N-acetylpenicillamine (SNAP), another S-NO donor, selectively inhibited TH17 without affecting TH1 and TH2. At present, the mechanism underlying GSNO and SNAP mediated inhibition of TH17 is not well understood but we reproted that GSNO or S-nitroso-N-acetylcysteine (SNAC, a S-NO donor) inhibits IL-6-induced activation of STAT3 (phosphorylation on tyrosine705) via S-nitrosylation of cysteine259 of STAT3 [49, 60, 61]. Activated STAT3 by IL-6 and IL-23 plays a pivotal role in polarization and effector function of TH17 cells [62], and thus documenting that GSNO mediated mechanism inhibit polarization and effector function of TH17 via inactivation of STAT3.
Decreased ratio of Treg/TH17 is reported to correlate with MS disease severity[63]. Treg cells are the major anti-inflammatory subset of T cells which suppress proinflammatory T-cells by producing anti-inflammatory cytokines, such as IL-10 and TGF-β, as well as play a critical role in maintaining tolerance to self-antigens and thus preventing autoimmune disease [15, 64, 65]. CD4+ CD25+Treg cells are divided into two subgroups beased on their expression of FOXP3; FOXP3+ Tregs (nTreg and iTreg) and FOXP3− Tregs (NO-Treg and Tr1) [23, 56, 57]. In this study, we observed that GSNO treatment had no effect on polarization and CNS effector function of FOXP3+Tregs under EAE conditions (Figs. 1C and 2B), however, it sginificantly increased the polarization and CNS effector functions of FOXP3− Tregs (Figs. 1C and 2B). In consistent with our observation, previous studies reported that treatment of CD4+CD25− T cells with diazeniumdiolate (NONOate, a NO donor) induces polarization and effector function (IL-10 production) of CD4+/CD25+/FOXP3− Tregs, named, ‘NO-Treg’ [23]. Subsequently, the same research group reported that these NO-inducible CD4+/CD25+/FOXP3− Tregs inhibits TH17, but not TH1, while CD4+/CD25+/FOXP3+Tregs (nTreg and iTreg) inhibit TH1 [24]. Taken together with our previous studies documenting the role of GSNO in inhibition of STAT3 [22, 49, 60, 66], these studies document the role of GSNO mediated mechanisms in inhibition of TH17-mediated proinflammatory processes directly by inhibiting STAT3 as well as via GSNO mediated induction of CD4+/CD25+/FOXP3− Tregs (NO-Tregs).
GSNO exerts its biological effects by protein modifications via S-nitrosylation, a reversible and specific post-translational modification that regulates activities of large number of target proteins and thus various cellular activities and functions [26]. The cellular synthesis of GSNO is mediated by reaction between NOS and GSH in the presence of electron acceptors or formation of transition metal adduct [67]. Therefore, cell/tissue specific expression and activity of NOS and maintenance of redox potential (GSH) are important for cellular GSNO homeostasis. GSNO is catabolized by GSNOR, a class III alcohol dehydrogenase (ADH), and thus cell/tissue specific regulation of GSNOR in its expression and activity is also important for cellular GSNO homeostasis. At present, the mechanisms underlying the regulation of GSNOR is not well understood, but NFκB mediated regulation of its transcription [68] and GSNO-mediated feed-forward induction of its activity [69] have been reported. Based on the importance of GSNOR in cellular GSNO homeostasis, next, we evaluated the efficacy of endogenous GSNO using N6022, a first-in-class inhibitor of GSNO catabolizing enzyme GSNOR in EAE disease. GSNOR inhibitors are being tested for clinical use for asthma and cystic fibrosis [70]. Among these, N6022 is the best characterized and its safety was proven by Phase I and II studies [50].
In this study, we observed that N6022 treatment of EAE mice attenuated the progression of EAE disease efficiently. Similar to GSNO studies, EAE mice treated with N6022 also showed decreased polarization and effector function of TH17 cells and increased polarization and effector function of CD4+/CD25+/FOXP3− Tregs (Figs. 5B, 5C, 6B, and 6C). Moreover, N6022 treatment, but not GSNO treatment, also attenuated TH1, while increasing TH2 and CD4+/CD25+/FOXP3+ Treg, in polarizations and their effector functions in EAE mice (Figs. 5B, 5C, 6B and 6C). Although GSNO and N6022 treatments produced differential effects on these polarizations in spleens of EAE mice (Figs. 5 and 6) but both treatments increased comparable levels of protein-associated S-nitrosolthiols in the spleen (Fig. 4). Cellular PrSNOs are known to be in transnitrosation equilibrium with GSNO [59], indicating that the observed increases in PrSNO levels reflect increased levels of GSNO in the spleens of GSNO and N6022 treated mice. At present, mechanisms underlyng the differential effects of GSNO and N6022 on the polarization of different subsets of CD4+ T cells is not understood. Logically, exogenous GSNO treatment is expected to distribute all over the body but cell specific accumulation of exogenous GSNO levels depends on cell specific activity of GSNOR. On the other hand, N6022 treatment is expected to elevate GSNO levels more in the cells with higher GSNO dynamics as high GSNO synthesis (NOS expressing cells) and degradation (GSNOR expressing cells). Therefore, even though exogenous GSNO and endogenous GSNO (N6022 treatment) increased near comparable levels of PrSNOs (GSNO) in the spleens of EAE mice, their cell type specific effects may be different in GSNO or GSNOR inhibitor treated mice. It should be of interest to evaluate the NO metabolome mediated regulation of different subset of CD4+ T cells.
Studies with GSNOR knockout mice reported decreased CD4+ cells and increased CD19+ cells (B-cell) in the blood [31] as well as demyelination, neurodegeneration, and neuropathic pain [29]. To understand the basis of these differences in immune function of GSNOR knockout mice and GSNO treated mice, we studied the relative efficacy of GSNO vs. sphingosine 1-phosphate receptor agonist FTY720 known to cause lymphopenia in EAE disease mechanisms. FTY720 is a first-in-class orally bioavailable compound for the treatment of MS. The proposed mechanism of action for FTY720 in MS is a reversible retention of T-cells and B-cells in lymph nodes which thereby reduces the number of inflammatory cells in circulation and thus their access to the CNS. Therefore, FTY720-mediated immuno-suppression is nonspecific and thus can cause a lymphopenia, a condition of low level of lymphocytes in the blood [71]. In addition, FTY720 is also known to inhibit Treg and thus abrogate their anti-inflammatory and immune suppressor functions [72]. Our data show that N6022 or GSNO treatment do not cause any obvious lymphopenic effects whereas FTY720 reduces the number of lymphocytes in blood (Figs. 2A and 6A). Moreover, N6022 treatment selectively inhibited EAE-induced pro-inflammatoryTH1 and TH17 immune responses while elevating the EAE-induced compromises in TH2 and Treg immune responses (Figs 5B and C and Figs 6B and C). Secondly, GSNOR knockout mice were reported to develop demyelination and neurodegeneration. For it we investigated the status of myelin in EAE animals treated with GSNOR inhibitor N6022. Our data shows that N6022 treatment provides protection rather than degeneration in EAE animals. At present, we do not fully understand the basis for opposing results from GSNOR knockout mice and GSNOR inhibitor studies. However, the different degree of inhibition of GSNOR activities under conditions with pharmacological inhibitor (N6022) vs. genetic elimination of GSNOR (GSNOR knockout mice) may account, at least in part, for these observed differences. Secondly, GSNOR degrades a number of other compounds in addition to GSNO [73] and this may also account for these observed differences.
In summary, this study describes the efficacies of exogenous GSNO and endogenous N6022 (GSNOR inhibitor) in EAE disease. Both drugs attenuated the clinical disease of EAE with inhibition of TH17 cells and induction of Treg cells in their differentiations and their effector functions but without producing any global lymphopenia effect, like FTY720. N6022 treatment was also effective on the inhibition of TH1 cells and induction of TH2 cells with greater efficacy compared to the same dose of GSNO against the clinical disease of EAE. It is of interest that both exogenous GSNO as well as endogenous GSNO by inhibition of GSNOR with N6022 induce inhibition of TH17 and expression of IL-10 in the spinal cord derived T cells, one of the potential mechanism for immunomodulation and CNS protection[74, 75] and thus neuroprotective efficacy against EAE. Overall, these studies document that cellular GSNO homeostasis plays a critical role in differentiation and effector function of proinflammatory (TH1 and TH17) and anti-inflammatory (TH2 and Treg) CD4+ T cells. Moreover, this study also reports that GSNOR inhibitor (N6022) is a novel therapeutic approach for targetting NO metabolome in NOS/GSNOR expressing cell types for selective modulation of CD4+ subsets (TH1/TH17 vs. TH2/Treg) and thus greater efficacy against autoimmune disease of EAE as compared to treatment with exogenous GSNO. Secondly, the observed increased expression of IL-10 in the CNS of EAE animals treated with GSNO as well as N6022 indicates that these drugs may potentially promote neurorepair in EAE and MS.
Acknowledgments
We acknowledge Ms. Joyce Bryan for her help in procurement of animals and supplies. This work was supported in part by U.S. Department of Veterans Affairs (BX002829) and National Institutes of Health (NS037766).
Abbreviations:
- ADH5
alcohol dehydrogenase 5
- CFA
complete Freund’s adjuvant
- CNS
central nervous system
- Cys
cysteine
- DTT
dithiothreitol
- EAE
experimental autoimmune encephalomyelitis
- GSNO
S-nitrosoglutathione
- GSNOR
S-nitrosoglutathione reductase
- H&E
Haemotoxylin and Eosin
- LFB
Luxol fast blue
- MBP
myelin basic protein
- MS
multiple sclerosis
- NO
nitric oxide
- NOS
nitric oxide synthase
- ONOO−
peroxynitrite
- PrSNO
S-nitrosylated proteins
- TH1
Type 1 T helper cell
- TH1
Type 2 T helper cell
- TH17
Type 17 T helper cell
- Treg
regulatory T cell
- Tyr
tyrosine
References:
- [1].Lovett-Racke AE, Yang Y, Racke MK, Th1 versus Th17: are T cell cytokines relevant in multiple sclerosis?, Biochim Biophys Acta 1812(2) (2011) 246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Colagiovanni DB, Drolet DW, Langlois-Forget E, Piche MP, Looker D, Rosenthal GJ, A nonclinical safety and pharmacokinetic evaluation of N6022: a first-in-class S-nitrosoglutathione reductase inhibitor for the treatment of asthma, Regulatory toxicology and pharmacology : RTP 62(1) (2012) 115–24. [DOI] [PubMed] [Google Scholar]
- [3].Racke MK, Lovett-Racke AE, Karandikar NJ, The mechanism of action of glatiramer acetate treatment in multiple sclerosis, Neurology 74 Suppl 1 (2010) S25–30. [DOI] [PubMed] [Google Scholar]
- [4].Papadopoulou A, Kappos L, Sprenger T, Teriflunomide for oral therapy in multiple sclerosis, Expert Rev Clin Pharmacol 5(6) (2012) 617–28. [DOI] [PubMed] [Google Scholar]
- [5].Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, Tornatore C, Sweetser MT, Yang M, Sheikh SI, Dawson KT, D.S. Investigators, Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis, N Engl J Med 367(12) (2012) 1098–107. [DOI] [PubMed] [Google Scholar]
- [6].Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW, A. Investigators, A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis, N Engl J Med 354(9) (2006) 899–910. [DOI] [PubMed] [Google Scholar]
- [7].Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P, F.S. Group, A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis, N Engl J Med 362(5) (2010) 387–401. [DOI] [PubMed] [Google Scholar]
- [8].Aranha AA, Amer S, Reda ES, Broadley SA, Davoren PM, Autoimmune thyroid disease in the use of alemtuzumab for multiple sclerosis: a review, Endocr Pract 19(5) (2013) 821–8. [DOI] [PubMed] [Google Scholar]
- [9].Fox EJ, Management of worsening multiple sclerosis with mitoxantrone: a review, Clin Ther 28(4) (2006) 461–74. [DOI] [PubMed] [Google Scholar]
- [10].Torkildsen O, Myhr KM, Bo L, Disease-modifying treatments for multiple sclerosis - a review of approved medications, Eur J Neurol 23 Suppl 1 (2016) 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F, C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE, Nat Immunol 10(5) (2009) 514–23. [DOI] [PubMed] [Google Scholar]
- [12].Mosmann TR, Coffman RL, TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties, Annu Rev Immunol 7 (1989) 145–73. [DOI] [PubMed] [Google Scholar]
- [13].Khoury SJ, Hancock WW, Weiner HL, Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain, J Exp Med 176(5) (1992) 1355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, Xia J, Tan TG, Sefik E, Yajnik V, Sharpe AH, Quintana FJ, Mathis D, Benoist C, Hafler DA, Kuchroo VK, Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses, Immunity 40(4) (2014) 569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Singer BD, King LS, D’Alessio FR, Regulatory T cells as immunotherapy, Frontiers in immunology 5 (2014) 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kuchroo VK, Anderson AC, Waldner H, Munder M, Bettelli E, Nicholson LB, T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire, Annu Rev Immunol 20 (2002) 101–23. [DOI] [PubMed] [Google Scholar]
- [17].Correale J, Villa A, Role of CD8+ CD25+ Foxp3+ regulatory T cells in multiple sclerosis, Ann Neurol 67(5) (2010) 625–38. [DOI] [PubMed] [Google Scholar]
- [18].Durelli L, Conti L, Clerico M, Boselli D, Contessa G, Ripellino P, Ferrero B, Eid P, Novelli F, T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-beta, Ann Neurol 65(5) (2009) 499–509. [DOI] [PubMed] [Google Scholar]
- [19].Bogdan C, Nitric oxide and the immune response, Nat Immunol 2(10) (2001) 907–16. [DOI] [PubMed] [Google Scholar]
- [20].Griffith OW, Stuehr DJ, Nitric oxide synthases: properties and catalytic mechanism, Annu Rev Physiol 57 (1995) 707–36. [DOI] [PubMed] [Google Scholar]
- [21].Jianjun Y, Zhang R, Lu G, Shen Y, Peng L, Zhu C, Cui M, Wang W, Arnaboldi P, Tang M, Gupta M, Qi CF, Jayaraman P, Zhu H, Jiang B, Chen SH, He JC, Ting AT, Zhou MM, Kuchroo VK, Morse HC 3rd, Ozato K, Sikora AG, Xiong H, T cell-derived inducible nitric oxide synthase switches off Th17 cell differentiation, J Exp Med 210(7) (2013) 1447–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nath N, Morinaga O, Singh I, S-nitrosoglutathione a physiologic nitric oxide carrier attenuates experimental autoimmune encephalomyelitis, Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 5(2) (2010) 240–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Niedbala W, Cai B, Liu H, Pitman N, Chang L, Liew FY, Nitric oxide induces CD4+CD25+ Foxp3 regulatory T cells from CD4+CD25 T cells via p53, IL-2, and OX40, Proc Natl Acad Sci U S A 104(39) (2007) 15478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Niedbala W, Besnard AG, Jiang HR, Alves-Filho JC, Fukada SY, Nascimento D, Mitani A, Pushparaj P, Alqahtani MH, Liew FY, Nitric oxide-induced regulatory T cells inhibit Th17 but not Th1 cell differentiation and function, J Immunol 191(1) (2013) 164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Witherick J, Wilkins A, Scolding N, Kemp K, Mechanisms of oxidative damage in multiple sclerosis and a cell therapy approach to treatment, Autoimmune Dis 2011 (2010) 164608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gaston BM, Carver J, Doctor A, Palmer LA, S-nitrosylation signaling in cell biology, Mol Interv 3(5) (2003) 253–63. [DOI] [PubMed] [Google Scholar]
- [27].He W, Frost MC, Direct measurement of actual levels of nitric oxide (NO) in cell culture conditions using soluble NO donors, Redox Biol 9 (2016) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nakamura T, Lipton SA, Emerging role of protein-protein transnitrosylation in cell signaling pathways, Antioxidants & redox signaling 18(3) (2013) 239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nakamura T, Lipton SA, Protein S-Nitrosylation as a Therapeutic Target for Neurodegenerative Diseases, Trends Pharmacol Sci 37(1) (2016) 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Montagna C, Di Giacomo G, Rizza S, Cardaci S, Ferraro E, Grumati P, De Zio D, Maiani E, Muscoli C, Lauro F, Ilari S, Bernardini S, Cannata S, Gargioli C, Ciriolo MR, Cecconi F, Bonaldo P, Filomeni G, S-nitrosoglutathione reductase deficiency-induced S-nitrosylation results in neuromuscular dysfunction, Antioxidants & redox signaling 21(4) (2014) 570–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yang Z, Wang ZE, Doulias PT, Wei W, Ischiropoulos H, Locksley RM, Liu L, Lymphocyte development requires S-nitrosoglutathione reductase, J Immunol 185(11) (2010) 6664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Haldar SM, Stamler JS, S-nitrosylation: integrator of cardiovascular performance and oxygen delivery, J Clin Invest 123(1) (2013) 101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chiueh CC, Neuroprotective properties of nitric oxide, Ann N Y Acad Sci 890 (1999) 301–11. [DOI] [PubMed] [Google Scholar]
- [34].Qian J, Chen F, Kovalenkov Y, Pandey D, Moseley MA, Foster MW, Black SM, Venema RC, Stepp DW, Fulton DJ, Nitric oxide reduces NADPH oxidase 5 (Nox5) activity by reversible S-nitrosylation, Free Radic Biol Med 52(9) (2012) 1806–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Won JS, Kim J, Annamalai B, Shunmugavel A, Singh I, Singh AK, Protective role of S-nitrosoglutathione (GSNO) against cognitive impairment in rat model of chronic cerebral hypoperfusion, J Alzheimers Dis 34(3) (2013) 621–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Prasad R, Giri S, Nath N, Singh I, Singh AK, GSNO attenuates EAE disease by S-nitrosylation-mediated modulation of endothelial-monocyte interactions, Glia 55(1) (2007) 65–77. [DOI] [PubMed] [Google Scholar]
- [37].Foster MW, Hess DT, Stamler JS, Protein S-nitrosylation in health and disease: a current perspective, Trends Mol Med 15(9) (2009) 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lima B, Lam GK, Xie L, Diesen DL, Villamizar N, Nienaber J, Messina E, Bowles D, Kontos CD, Hare JM, Stamler JS, Rockman HA, Endogenous S-nitrosothiols protect against myocardial injury, Proc Natl Acad Sci U S A 106(15) (2009) 6297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Khan M, Dhammu TS, Dhaindsa TS, Khan H, Singh AK, Singh I, An NO/GSNO-based Neuroregeneration Strategy for Stroke Therapy, J Neurol Neurosci 6(4) (2015). [PMC free article] [PubMed] [Google Scholar]
- [40].Shunmugavel A, Khan M, Hughes FM Jr., Purves JT, Singh A, Singh I, S-Nitrosoglutathione protects the spinal bladder: novel therapeutic approach to post-spinal cord injury bladder remodeling, Neurourol Urodyn 34(6) (2015) 519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chou PC, Shunmugavel A, El Sayed H, Desouki MM, Nguyen SA, Khan M, Singh I, Bilgen M, Preclinical use of longitudinal MRI for screening the efficacy of S-nitrosoglutathione in treating spinal cord injury, J Magn Reson Imaging 33(6) (2011) 1301–11. [DOI] [PubMed] [Google Scholar]
- [42].Khan M, Im YB, Shunmugavel A, Gilg AG, Dhindsa RK, Singh AK, Singh I, Administration of S-nitrosoglutathione after traumatic brain injury protects the neurovascular unit and reduces secondary injury in a rat model of controlled cortical impact, J Neuroinflammation 6 (2009) 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Khan M, Sekhon B, Giri S, Jatana M, Gilg AG, Ayasolla K, Elango C, Singh AK, Singh I, S-Nitrosoglutathione reduces inflammation and protects brain against focal cerebral ischemia in a rat model of experimental stroke, Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 25(2) (2005) 177–92. [DOI] [PubMed] [Google Scholar]
- [44].Zanini GM, Martins YC, Cabrales P, Frangos JA, Carvalho LJ, S-nitrosoglutathione prevents experimental cerebral malaria, J Neuroimmune Pharmacol 7(2) (2012) 477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Foster MW, Yang Z, Potts EN, Michael Foster W, Que LG, S-nitrosoglutathione supplementation to ovalbumin-sensitized and -challenged mice ameliorates methacholine-induced bronchoconstriction, Am J Physiol Lung Cell Mol Physiol 301(5) (2011) L739–44. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [46].Hornyak I, Pankotai E, Kiss L, Lacza Z, Current developments in the therapeutic potential of S-nitrosoglutathione, an endogenous NO-donor molecule, Curr Pharm Biotechnol 12(9) (2011) 1368–74. [DOI] [PubMed] [Google Scholar]
- [47].Kim J, Won JS, Singh AK, Sharma AK, Singh I, STAT3 Regulation by S-Nitrosylation: Implication for Inflammatory Disease, Antioxidants & redox signaling (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Corti A, Franzini M, Scataglini I, Pompella A, Mechanisms and targets of the modulatory action of S-nitrosoglutathione (GSNO) on inflammatory cytokines expression, Arch Biochem Biophys 562 (2014) 80–91. [DOI] [PubMed] [Google Scholar]
- [49].Kim J, Won JS, Singh AK, Sharma AK, Singh I, STAT3 regulation by S-nitrosylation: implication for inflammatory disease, Antioxidants & redox signaling 20(16) (2014) 2514–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Green LS, Chun LE, Patton AK, Sun X, Rosenthal GJ, Richards JP, Mechanism of inhibition for N6022, a first-in-class drug targeting S-nitrosoglutathione reductase, Biochemistry 51(10) (2012) 2157–68. [DOI] [PubMed] [Google Scholar]
- [51].Ferrini ME, Simons BJ, Bassett DJ, Bradley MO, Roberts K, Jaffar Z, S-nitrosoglutathione reductase inhibition regulates allergen-induced lung inflammation and airway hyperreactivity, PLoS One 8(7) (2013) e70351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Blonder JP, Mutka SC, Sun X, Qiu J, Green LH, Mehra NK, Boyanapalli R, Suniga M, Look K, Delany C, Richards JP, Looker D, Scoggin C, Rosenthal GJ, Pharmacologic inhibition of S-nitrosoglutathione reductase protects against experimental asthma in BALB/c mice through attenuation of both bronchoconstriction and inflammation, BMC pulmonary medicine 14(1) (2014) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chen Q, Sievers RE, Varga M, Kharait S, Haddad DJ, Patton AK, Delany CS, Mutka SC, Blonder JP, Dube GP, Rosenthal GJ, Springer ML, Pharmacological inhibition of S-nitrosoglutathione reductase improves endothelial vasodilatory function in rats in vivo, Journal of applied physiology 114(6) (2013) 752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Nath N, Khan M, Paintlia MK, Singh I, Hoda MN, Giri S, Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis, J Immunol 182(12) (2009) 8005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Nath N, Giri S, Prasad R, Singh AK, Singh I, Potential targets of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor for multiple sclerosis therapy, J Immunol 172(2) (2004) 1273–86. [DOI] [PubMed] [Google Scholar]
- [56].Groux H, Type 1 T-regulatory cells: their role in the control of immune responses, Transplantation 75(9 Suppl) (2003) 8S–12S. [DOI] [PubMed] [Google Scholar]
- [57].Curotto de Lafaille MA, Lafaille JJ, Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor?, Immunity 30(5) (2009) 626–35. [DOI] [PubMed] [Google Scholar]
- [58].Niedbala W, Alves-Filho JC, Fukada SY, Vieira SM, Mitani A, Sonego F, Mirchandani A, Nascimento DC, Cunha FQ, Liew FY, Regulation of type 17 helper T-cell function by nitric oxide during inflammation, Proc Natl Acad Sci U S A 108(22) (2011) 9220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Broniowska KA, Diers AR, Hogg N, S-nitrosoglutathione, Biochim Biophys Acta 1830(5) (2013) 3173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kaliyaperumal K, Sharma AK, McDonald DG, Dhindsa JS, Yount C, Singh AK, Won JS, Singh I, S-Nitrosoglutathione-mediated STAT3 regulation in efficacy of radiotherapy and cisplatin therapy in head and neck squamous cell carcinoma, Redox Biol 6 (2015) 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Singh I, Kim J, Singh AK, Sharma AK, Won JS, STAT3 Regulation By S-Nitrosylation: Implication In Cancer, Redox Biol 5 (2015) 416–417. [DOI] [PubMed] [Google Scholar]
- [62].Ramsay B, Radomski M, De Belder A, Martin JF, Lopez-Jaramillo P, Systemic effects of S-nitroso-glutathione in the human following intravenous infusion, British journal of clinical pharmacology 40(1) (1995) 101–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Jamshidian A, Shaygannejad V, Pourazar A, Zarkesh-Esfahani SH, Gharagozloo M, Biased Treg/Th17 balance away from regulatory toward inflammatory phenotype in relapsed multiple sclerosis and its correlation with severity of symptoms, J Neuroimmunol 262(1-2) (2013) 106–12. [DOI] [PubMed] [Google Scholar]
- [64].Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK, Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells, Nature 441(7090) (2006) 235–8. [DOI] [PubMed] [Google Scholar]
- [65].Vignali DA, Collison LW, Workman CJ, How regulatory T cells work, Nat Rev Immunol 8(7) (2008) 523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kim J, Choi S, Saxena N, Singh AK, Singh I, Won JS, Regulation of STAT3 and NF-kappaB activations by S-nitrosylation in multiple myeloma, Free radical biology & medicine 106 (2017) 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Li Q, Lancaster JR Jr., A Conspectus of Cellular Mechanisms of Nitrosothiol Formation from Nitric Oxide, For Immunopathol Dis Therap 3(2) (2012) 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wu K, Zhang Y, Wang P, Zhang L, Wang T, Chen C, Activation of GSNOR transcription by NF-kappaB negatively regulates NGF-induced PC12 differentiation, Free Radic Res 48(9) (2014) 1011–7. [DOI] [PubMed] [Google Scholar]
- [69].Brown-Steinke K, deRonde K, Yemen S, Palmer LA, Gender differences in S-nitrosoglutathione reductase activity in the lung, PLoS One 5(11) (2010) e14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sun X, Wasley JW, Qiu J, Blonder JP, Stout AM, Green LS, Strong SA, Colagiovanni DB, Richards JP, Mutka SC, Chun L, Rosenthal GJ, Discovery of s-nitrosoglutathione reductase inhibitors: potential agents for the treatment of asthma and other inflammatory diseases, ACS medicinal chemistry letters 2(5) (2011) 402–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Morris MA, Gibb DR, Picard F, Brinkmann V, Straume M, Ley K, Transient T cell accumulation in lymph nodes and sustained lymphopenia in mice treated with FTY720, Eur J Immunol 35(12) (2005) 3570–80. [DOI] [PubMed] [Google Scholar]
- [72].Wolf AM, Eller K, Zeiser R, Durr C, Gerlach UV, Sixt M, Markut L, Gastl G, Rosenkranz AR, Wolf D, The sphingosine 1-phosphate receptor agonist FTY720 potently inhibits regulatory T cell proliferation in vitro and in vivo, J Immunol 183(6) (2009) 3751–60. [DOI] [PubMed] [Google Scholar]
- [73].Barnett SD, Buxton ILO, The role of S-nitrosoglutathione reductase (GSNOR) in human disease and therapy, Crit Rev Biochem Mol Biol 52(3) (2017) 340–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Abraham KE, McMillen D, Brewer KL, The effects of endogenous interleukin-10 on gray matter damage and the development of pain behaviors following excitotoxic spinal cord injury in the mouse, Neuroscience 124(4) (2004) 945–52. [DOI] [PubMed] [Google Scholar]
- [75].Fouda AY, Pillai B, Dhandapani KM, Ergul A, Fagan SC, Role of interleukin-10 in the neuroprotective effect of the Angiotensin Type 2 Receptor agonist, compound 21, after ischemia/reperfusion injury, Eur J Pharmacol 799 (2017) 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]