Abstract
Background.
Individuals with stroke usually show reduced muscle activities of the paretic leg and asymmetrical gait pattern during walking.
Objective.
To determine whether applying a resistance force to the nonparetic leg would enhance the muscle activities of the paretic leg and improve the symmetry of spatiotemporal gait parameters in individuals with poststroke hemiparesis.
Methods.
Fifteen individuals with chronic poststroke hemiparesis participated in this study. A controlled resistance force was applied to the nonparetic leg using a customized cable-driven robotic system while subjects walked on a treadmill. Subjects completed 2 test sections with the resistance force applied at different phases of gait (ie, early and late swing phases) and different magnitudes (10%, 20%, and 30% of maximum voluntary contraction [MVC] of nonparetic leg hip flexors). Electromyographic (EMG) activity of the muscles of the paretic leg and spatiotemporal gait parameters were collected.
Results.
Significant increases in integrated EMG of medial gastrocnemius, medial hamstrings, vastus medialis, and tibialis anterior of the paretic leg were observed when the resistance was applied during the early swing phase of the nonparetic leg, compared with baseline. Additionally, resistance with 30% of MVC induced the greatest level of muscle activity than that with 10% or 20% of MVC. The symmetry index of gait parameters also improved with resistance applied during the early swing phase.
Conclusion.
Applying a controlled resistance force to the nonparetic leg during early swing phase may induce forced use on the paretic leg and improve the spatiotemporal symmetry of gait in individuals with poststroke hemiparesis.
Keywords: stroke, locomotion, forced use, constraint force, EMG
Introduction
Stroke is the leading cause of severe disability in the United States1 and results in physical impairments that greatly compromise quality of life.2 Although the majority of people with stroke ultimately regain the ability to walk independently after rehabilitation, many stroke patients do not achieve the walking level necessary to resume all daily activities.3 In addition, individuals with stroke often show reduced paretic leg muscle activity4,5 and altered spatiotemporal characteristics during walking.6 Such spatial and temporal asymmetries may be due to the compensatory strategies adopted by either the nonparetic or the paretic leg,7 which can adversely affect walking function.8 A priority of stroke rehabilitation is to reduce hemiparesis and improve the gait symmetry.
Most functional recovery typically occurs within the first 6 months after stroke.9 Further improvements of function beyond the period of spontaneous recovery may require additional rehabilitation interventions. Constraint-induced movement therapy (CIMT) is a promising treatment for chronic stroke patients.10,11 The primary emphasis of CIMT is to overcome the learned nonuse of the paretic limb to reduce hemiparesis. CIMT emphasizes mass task-specific practice of the paretic limb while movement of the nonparetic limb is restricted.12 Several randomized controlled trials have demonstrated that 2 to 15 weeks of CIMT produce greater improvements in motor function of the paretic upper limb compared with traditional rehabilitation.10,11,13,14
The CIMT paradigm, however, has not been effectively transferred to lower limb training in individuals poststroke because both paretic and nonparetic legs must be engaged in order to practice walking, that is, it is almost impossible to completely immobilize the nonparetic leg to induce forced use of the paretic leg during locomotor training in individuals poststroke. As an alternative strategy, the nonparetic leg can be partially restrained by blocking the movement of the knee joint15 or by adding a weight to the ankle.16,17 However, blocking the movement of the knee joint of the nonparetic leg may significantly change the normal gait pattern and adding a weight to the ankle of the nonparetic leg may change the leg inertia.16,17 Thus, there is limited evidence regarding the feasibility of using CIMT to induce forced use of the paretic leg of individuals poststroke.
Previous studies of infants and healthy adults have demonstrated that perturbations given to one leg during the swing phase of gait induced enhanced muscle activity of the ankle plantarflexors and resulted in a prolonged stance phase of the contralateral leg during treadmill walking.18–20 In addition, these locomotor-related interlimb spinal reflex networks may be reserved in individuals poststroke.21 The purpose of this study was to determine whether the muscle activity of the paretic leg could be enhanced, that is, induce forced use on the paretic leg, by applying a controlled resistance force to the nonparetic leg during treadmill walking in individuals with poststroke hemiparesis. We hypothesized that applying a resistance force to the nonparetic leg during the early swing phase would enhance muscle activity of the paretic leg and improve spatiotemporal symmetry in individuals with poststroke hemiparesis.
Methods
Subjects
Fifteen subjects with chronic (> 6 months) poststroke hemiparesis participated in the study (Table 1). The inclusion criteria were (a) age 21 to 75 years; (b) unilateral, supratentorial, ischemic, or hemorrhagic stroke confirmed with radiography; (c) no prior history of stroke before the reference stroke; (d) independent ambulation with/without the use of assistive device or below knee orthoses; and (e) self-selected walking speed ≤0.80 m/s. Exclusion criteria were (a) brainstem or cerebellar stroke; (b) a score of <24 on the Mini Mental State Examination22; (c) other neurological conditions, cardiorespiratory/metabolic disorders, or orthopedic conditions affecting ambulation ability and (d) neurotoxin injection within 6 months of study enrollment visit. All subjects gave written informed consent to participate in the study, which was approved by the Northwestern University Institutional Review Board.
Table 1.
Demographic Information for the Study Subjects.
| Subject | Gender | Age (y) | Weight (kg) | Postinjury (y) | Paretic Side | Assistive Device | Self-Selected Comfortable Speed (m/s) | Resistance Force (N) |
|---|---|---|---|---|---|---|---|---|
| S1 | F | 65 | 66.7 | 9 | R | Rollator/AFO | 0.27 | 20 |
| S2 | M | 40 | 90.7 | 8 | L | AFO | 0.62 | 30 |
| S3 | F | 67 | 64.9 | 23 | L | AFO | 0.37 | 18 |
| S4 | F | 54 | 68.0 | 5 | R | AFO | 0.59 | 21 |
| S5 | M | 48 | 85.7 | 16 | R | AFO | 0.46 | 27 |
| S6 | M | 46 | 67.1 | 11 | L | AFO | 0.74 | 30 |
| S7 | M | 66 | 78.5 | 3 | R | SPC/AFO | 0.43 | 18 |
| S8 | F | 46 | 92.1 | 9 | L | None | 0.53 | 36 |
| S9 | M | 74 | 83.5 | 6 | L | None | 0.41 | 20 |
| S10 | M | 67 | 62.6 | 2 | L | SBQC/AFO | 0.33 | 17 |
| S11 | F | 46 | 51.3 | 2 | L | AFO | 0.47 | 16 |
| S12 | F | 56 | 77.1 | 2 | L | AFO | 0.80 | 29 |
| S13 | F | 61 | 75.8 | 3 | L | SBQC/AFO | 0.34 | 22 |
| S14 | F | 65 | 67.6 | 1 | L | None | 0.37 | 22 |
| S15 | F | 46 | 78.7 | 8 | L | SPC/AFO | 0.31 | 21 |
Abbreviations: F, female; M, male; L, left; R, right; AFO, ankle foot orthosis; SPC, single point cane; SBQC, small based quad cane.
Procedures
Each subject completed 2 sessions of treadmill walking with the treadmill speed was set at self-selected comfortable speed, which was the same across all test sessions for each subject. In the first session, subjects walked on a tread-mill without load (baseline) for 30 strides, and then at a maximum walking speed for 30 strides. Subjects then walked on a treadmill with a controlled resistance force applied to the nonparetic leg at the early swing phase (EARLY, ie, starting from toe off to mid-swing, which was determined based on ankle position signals) or late swing phase (LATE, ie, starting from mid-swing to heel strike) for 1 minute with a 1-minute standing rest between conditions. The magnitude of the resistance force was set at ~20% of maximal voluntary contraction (MVC) of nonparetic leg hip flexors, which was determined while the subjects were standing on the treadmill.23 In the second session, subjects walked on a treadmill without load (baseline) for 30 strides, and then walked on the treadmill with different resistance force magnitudes (10%, 20%, and 30% of MVC of the nonparetic leg hip flexors) applied at the early swing phase for 1 minute with a 1-minute standing rest between conditions. The order of resistance force and phase conditions was randomized for both sessions.
The resistance force was applied through a customized cable-driven robotic system. The robotic system consisted of 2 nylon-coated stainless-steel cables, driven by 2 motors (AKM 33H, Kollmorgen, Radford, VA). The cables were affixed to a custom brace that was strapped around the subject’s ankle to provide a resistance force while subjects walked on the treadmill.24 A 4-N pretension force was applied to the cable in order to avoid slacking and no cable was attached during baseline. Two custom-designed 3-dimensional position sensors were attached at the subject’s ankles to record ankle position signals,25 which were used to trigger the resistance force. During all conditions, subjects were allowed to use the front handrail for safety and wore an overhead harness for protection only (not for body weight support).
Measurements
Surface electrodes (Delsys DE 2.1, Delsys Inc, Boston, MA) were used to record the electromyograms (EMG) from paretic leg muscles: hip abductor (gluteus medius), medial hamstrings, medial gastrocnemius, soleus, rectus femoris, vastus medialis, and tibialis anterior. EMG signals were amplified (gain 1,000) and band-pass filtered (20–450 Hz) in hardware. All signals were then sampled at 500 Hz via a 12-bit analog-to-digital converter (National Instruments, Austin, TX) on a PC running custom-written LabVIEW program.
Data Processing and Analysis
Custom software written in Matlab (The Mathworks, Natick, MA) was used for all data analyses. The EMG data was first low-pass filtered at 200 Hz, high-pass filtered at 10 Hz and band-stop filtered at 55 to 65 Hz using a second-order Butterworth filter. All EMG data were rectified and smoothed using a second order Butterworth filter with a low-pass cutoff frequency at 20 Hz. The smoothed EMG signals were segmented into gait cycles based on ankle positions. The EMG data were interpolated, resampled, and time-normalized to percentage of the gait cycle and then averaged over middle consecutive 20 strides. The EMG data during baseline and with the resistance force conditions were normalized to the peak EMG signal of each muscle during the condition with the maximum walking speed. The integral of the EMG activity was calculated in the intervals of stance phase of the gait cycle.
Stance time, single leg support time, and step length were computed using ankle positions.26 Stance time was calculated as the time from initial contact to toe-off, and single leg support time was calculated as the time period when only one foot was in contact with the treadmill. Both of them were normalized to the gait cycle time (% gait cycle). Step length was calculated as the anterior-posterior distance between the two legs’ ankle positions at initial contact.27 The symmetry index was quantified as follows: (paretic/nonparetic) × 100%,28 where paretic is the variable of the paretic leg, and nonparetic is the variable of the nonparetic leg. A symmetry index of 100% represented perfect symmetry. The symmetry index was averaged over the middle consecutive 20 strides for the statistical analysis.
Statistical Analysis
Statistical analyses were conducted using SPSS 20.0 software (IBM Corp, Armonk, NY). Repeated-measures analysis of variance (ANOVA) was used to compare the integrated EMG amplitudes of each muscle, symmetry index of stance time, single leg support time, and step length between baseline and resistance force conditions of each section. A post hoc analysis was conducted if ANOVA showed significant results. An alpha level of .05 was set for significance in all statistical tests.
Results
Different Phases of Resistance Force
Electromyography.
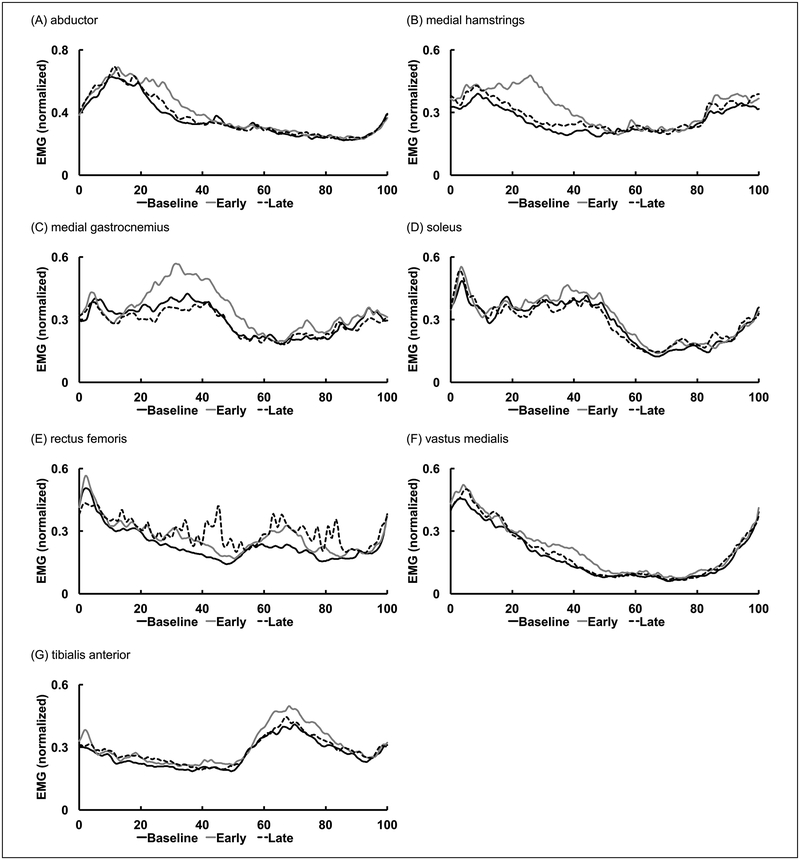
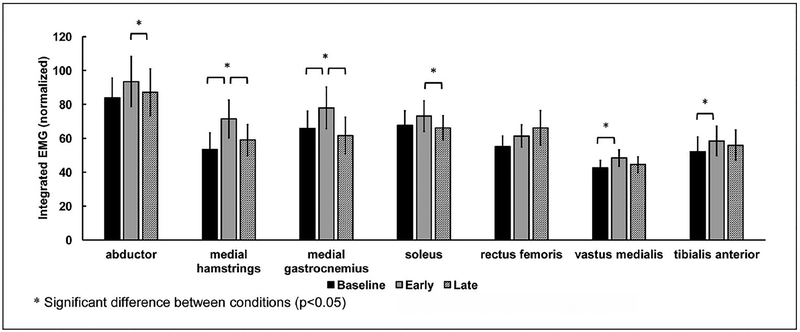
The EMG data from 14 subjects were analyzed. Data from 1 subject was excluded due to artifact. Mean muscle activity patterns across all subjects are shown in Figure 1. In general, resistance force applied to the nonparetic leg at the early swing phase induced greater level of increases in EMG amplitude of the medial hamstrings, medial gastrocnemius, abductors of the paretic leg. Average integrated EMG in each condition is shown in Figure 2. The EARLY condition increased the integrated EMG of the medial hamstrings by 33.6% (P = .001) and medial gastrocnemius by 18.5% (P = .036) compared with baseline. Similarly, the EARLY condition increased the integrated EMG of the vastus medialis by 13.9% (P = .025) and tibialis anterior by 12.1% (P = .002), compared with baseline. In addition, resistance force applied during the EARLY condition resulted in a higher EMG amplitude compared with the resistance force applied during the LATE condition. The integrated EMG of the medial hamstrings and medial gastrocnemius during the EARLY condition increased by 21% (P = .006) and 26.4% (P = .009), compared with the LATE condition. Similarly, the integrated EMG of abductor and soleus increased by 7.3% (P = .044) and 10.6% (P = .044), compared with the LATE condition.
Figure 1.
Mean electromyographic (EMG) data from all subjects for baseline and different phase of resistance force conditions. Data were averaged across 20 consecutive gait cycles and normalized to % gait cycle.
Figure 2.
Average integrated EMG during stance phase in baseline and different phases of resistance force conditions. Error bars, ±1 SE.
Stance Time.
Stance time of the paretic leg in the EARLY condition tended to be greater than baseline, although this was not significant (P = .061), and was greater than that in the LATE condition (P = .034). Stance time of the nonparetic leg had no significant change in both the EARLY (P = .956) and LATE (P = .358) conditions compared with baseline (Table 2). The symmetry index of the stance time was significantly greater in the EARLY condition compared with baseline (P < .001) and compared with the LATE condition (P < .001; Table 2).
Table 2.
Mean Values (SD) of Stance Time, Single Leg Support Time, and Step Length During Different Phases (EARLY and LATE Swing Phase of Gait), and Different Intensities (10%, 20%, and 30% of Maximum Voluntary Contraction).
| Different Phases | Different Intensities | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | EARLY | LATE | Baseline | 10% | 20% | 30% | |
| Stance time | |||||||
| Paretic leg, s | 1.16 (0.32) | 1.23 (0.36) | 1.17 (0.29) | 1.18 (0.33) | 1.23 (0.34) | 1.25 (0.37) | 1.27 (0.36) |
| Nonparetic leg, s | 1.18 (0.30) | 1.18 (0.31) | 1.20 (0.28) | 1.21 (0.32) | 1.22 (0.32) | 1.20 (0.32) | 1.19 (0.30) |
| Symmetry index, % | 98.03 (5.72) | 103.50 (6.25) | 97.19 (4.94) | 97.64 (5.78) | 100.80 (6.04) | 104.13 (6.89) | 106.12 (7.21) |
| Single leg support time | |||||||
| Paretic leg, s | 0.50 (0.08) | 0.56 (0.14) | 0.48 (0.09) | 0.49 (0.08) | 0.55 (0.11) | 0.58 (0.13) | 0.61 (0.17) |
| Nonparetic leg, s | 0.51 (0.08) | 0.52 (0.08) | 0.51 (0.08) | 0.52 (0.09) | 0.54 (0.10) | 0.52 (0.09) | 0.53 (0.10) |
| Symmetry index, % | 97.19 (12.67) | 107.90 (15.03) | 93.16 (8.86) | 95.80 (12.89) | 102.5 (13.6) | 110.56 (17.37) | 114.02 (15.88) |
| Step length | |||||||
| Paretic leg, cm | 37.56 (9.51) | 38.38 (8.55) | 39.39 (9.53) | 36.26 (9.16) | 38.67 (9.20) | 38.89 (9.35) | 38.98 (8.37) |
| Nonparetic leg, cm | 35.40 (8.60) | 35.32 (9.75) | 32.48 (7.71) | 36.84 (10.99) | 36.42 (9.82) | 37.12 (9.36) | 37.39 (11.38) |
| Symmetry index, % | 108.10 (23.34) | 113.53 (28.24) | 124.61 (31.93) | 103.78 (28.54) | 110.40 (26.73) | 108.19 (25.58) | 110.47 (28.64) |
Single Leg Support Time.
For the paretic leg, single leg support time significantly increased (P = .001) in the EARLY condition compared with baseline (P = .008) and compared with the LATE condition (P = .001) (Table 2). In addition, single leg support time significantly decreased in the LATE condition compared with baseline (P = .010). For the nonparetic leg, single leg support time had no significant change in the EARLY (P = .595) and LATE conditions (P = .949) compared with baseline. The symmetry index of the single leg support time was greater in the EARLY condition compared with baseline (P = .002) and compared with the LATE condition (P = .001; Table 2).
Step length.
For the paretic leg, step length significantly increased in the LATE condition (P = .003; Table 2), compared with baseline. For the nonparetic leg, step length significantly decreased in the LATE condition (P = .004; Table 2), compared with baseline. The symmetry index of the step length was greater in the LATE condition (P = .007; Table 2), compared with baseline. No significant changes were observed in the EARLY condition (P > .05; Table 2).
Different Intensities of Resistance Force
Electromyography.
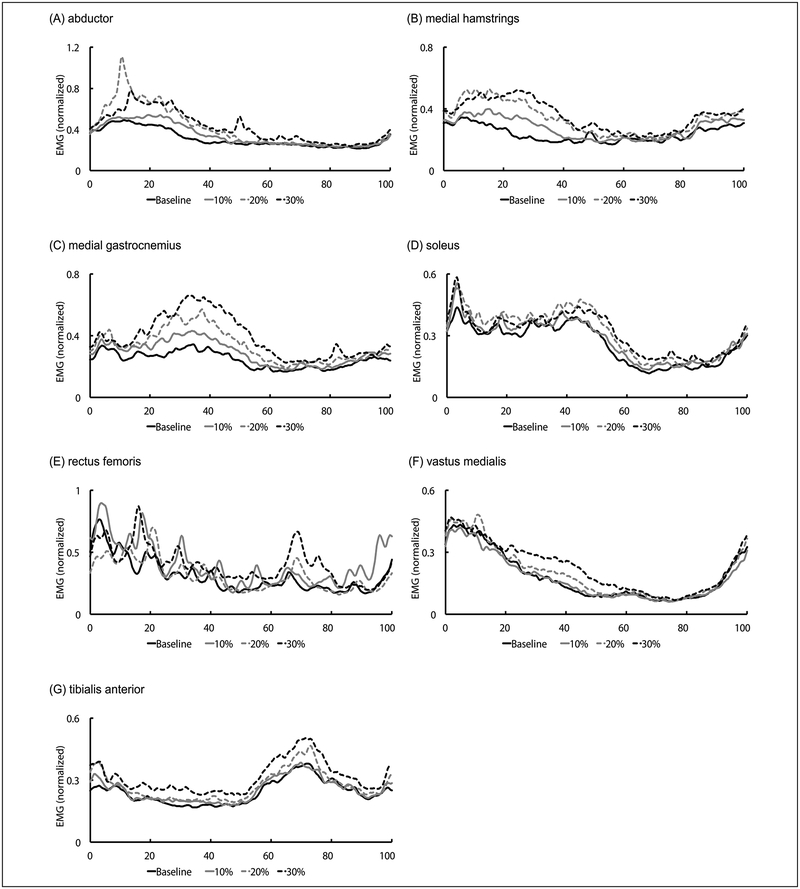
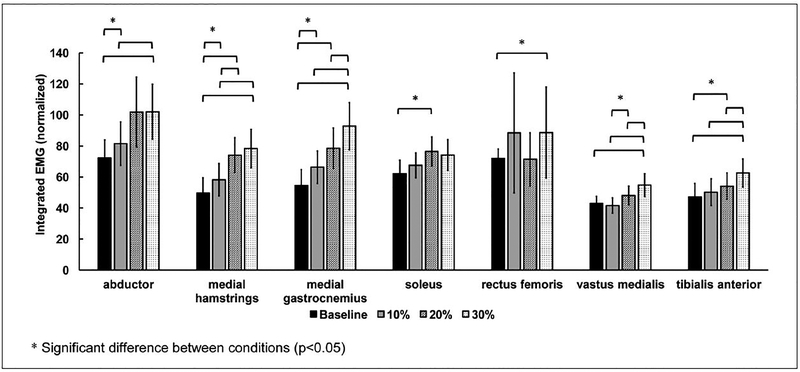
Mean muscle activation patterns of all subjects for different resistance intensities are shown in Figure 3. In general, the intensity of the resistance force exerted an impact on the EMG amplitude of the hip and ankle extensors of the paretic leg during the stance phase of gait, with 30% of the MVC resistance force induced greater changes in EMG amplitude in comparison with other amounts of resistance. Average integrated EMG in each condition is shown in Figure 4. The resistance forces significantly increased the integrated EMG of the medial ham-strings (10%:17.3%, P = .037; 20%:49.1%, P = .001; 30%:58.0%, P = .001) and medial gastrocnemius (10%:21.7%, P = .003; 20%:43.9%, P = .020; 30%:70.0%, P = .012) compared with baseline. Similarly, 30% of MVC resistance force significantly increased the integrated EMG of vastus medialis (baseline, P = .003; 10%, P = .003; 20%, P = .021) and tibialis anterior (baseline, P = .003; 10%, P = .003; 20%, P = .021) compared with baseline, or the 10% or 20% of MVC conditions. Integrated EMG of the hip abductor (41.2%, P = .004) and rectus femoris (23.1%, P = .013) also significantly increased with the resistance force at 30% of MVC compared with baseline. For the soleus muscle, significant differences in integrated EMG were observed only between the resistance condition with 20% of MVC and baseline (22.9%, P = .038).
Figure 3.
Mean electromyographic (EMG) data from all subjects for baseline and different resistance force intensity conditions. Data were averaged across 20 consecutive gait cycles and normalized to % gait cycle.
Figure 4.
Average integrated EMG during stance phase in baseline and different resistance force magnitude conditions. Error bars, ±1 SE.
Stance Time.
For the paretic leg, stance time increased in the conditions with 30%, 20%, and 10% of MVC compared with baseline (30%, P = .014; 20%, P = .012; 10%, P = .008). For the nonparetic leg, no significant change was found between the conditions with 30% (P = .471), 20% (P = .254), and 10% (P = .471) of MVC, and baseline. The symmetry index of stance time was greater in the condition with 30% of MVC, compared with baseline (P = .001) and the condition with 10% of MVC (P = .002). The symmetry index of stance time was greater in the condition with 20% of MVC compared with baseline (P < .001), and the condition with 10% of MVC (P = .001). Symmetry index of stance time also was greater in the condition with 10% of MVC compared with baseline (P = .002; Table 2).
Single Leg Support Time.
For the paretic leg, single leg support time significantly increased in the condition with 30% of MVC compared with baseline (P = .001) and compared with the conditions with 10% (P = .009) and 20% of MVC (P = .037). The single leg support time was also significantly greater in the condition with 20% of MVC compared with baseline (P = .001) and compared with the condition with 10% of MVC (P = .007). The single leg support time also was greater in the condition with 10% of MVC compared with baseline (P = .001). For the nonparetic leg, single leg support time increased in the condition with 10% of MVC compared with baseline (P = .034) and the condition with 20% of MVC (P = .016). The symmetry index of the single leg support time was significantly greater in the condition with 30% of MVC compared with baseline (P = .001) and compared with the condition with 10% of MVC (P = .004). The symmetry index was greater in the condition with 20% of MVC compared with baseline (P < .001) and the condition with 10% of MVC (P = .001). The symmetry index was also greater in the condition with 10% of MVC compared with baseline (P = .003; Table 2).
Step Length.
For the paretic leg, step length had no significant change in the conditions with 30% (P = .215), 20% (P = .185), and 10% (P = .233) of MVC compared with baseline. For the nonparetic leg, step length had no significant changes in the conditions with 30% (P = .767), 20% (P = .873), or 10% (P = .785) of MVC, compared with baseline. The symmetry index of step length had no significant changes in the conditions with 30% (P = .434), 20% (P = .558), and 10% (P = .370) of MVC, compared with baseline (Table 2).
Discussion
The results of this study indicate that applying a resistance force to the nonparetic leg during early swing phase of gait enhances muscle activities of the medial hamstrings and ankle plantarflexors of the paretic leg during stance, and induces an improved symmetry of spatiotemporal gait parameters in individuals poststroke. In addition, greater increases in the magnitude of muscle activity and duration of stance time are induced when the force is applied with a greater intensity than that with a lesser intensity. Results from this study suggest that applying a phase-dependent constraint force, that is, a resistance force, to the nonparetic leg may cause forced use of the paretic leg in individuals with poststroke hemiparesis during treadmill walking.
While individuals with poststroke hemiparesis often show reduced muscle activation of the paretic leg during walking,5 partially constraining the nonparetic leg by applying a resistance force to the nonparetic leg during the early swing instead of late swing phase enhances muscle activity of the paretic leg during stance. The early swing phase of the nonparetic leg corresponds to the period from loading response to mid-stance of the paretic leg when the body weight is shifted over to the paretic leg. The weightbearing of the paretic leg is high during this time period. Thus, applying a backward resistance force to the nonparetic leg during this time period may force subjects to increase the muscle activity of extensors of the paretic leg in order to counteract the external perturbation force, and prolong the stance phase of the paretic leg. This is consistent with previous animal studies, which indicated that load afferents from extensor muscles29,30 or cutaneous afferents from the plantar surface of the foot31,32 could enhance muscle activity of the leg extensors during the stance phase, and delay the onset of leg flexor activity associated with the swing phase. This is also partially consistent with results from a human infant study, which found a prolonged duration of stance phase when the leg load was increased,19 and results from a healthy control study, which indicated enhanced muscle activity of leg extensors with increased leg load.33 In addition, applying a resistance force to the nonparetic leg during this time period may also force subjects to increase muscle activation of hip abductors and medial hamstrings on the paretic side to stabilize the pelvis during single leg stance.34,35 In contrast, the late swing phase of the nonparetic leg corresponds to the period from mid-stance to unloading phase of the paretic leg when the body weight is shifting away from the paretic leg. The weightbearing of the paretic leg is low during this time period. Thus, the modulation effects of load afferents from extensor muscles or cutaneous afferents from the plantar surface of the foot of the paretic leg may be muted. Thus, we observed no significant change in muscle activity of the extensors of the paretic leg in the LATE condition. These phase-dependent muscle responses in the paretic leg might be induced through crossed-spinal reflex mechanisms.36–38
The intensity of the resistance force applied to the nonparetic leg also exerted impact on the level of muscle activity of the extensors of the paretic leg and the duration of the single leg support time of the paretic leg. When a greater resistance force was applied to the nonparetic leg, the force that was transferred to the paretic leg (ie, the supporting leg) through the pelvis also increased, which might cause subjects to generate greater extension moments from the hip, knee, and ankle joints of the paretic leg, in order to counteract the resistance force to maintain equilibrium of the paretic leg. With the increase in loading to the paretic leg, the load related afferent inputs from ankle plantarflexor muscles29,30,39 and cutaneous receptors on the foot40 of the paretic leg will increase, resulting in greater muscle activities of the medial hamstrings and ankle plantarflexors, which will prolong stance time on the paretic leg. In addition, the resistance force applied to the nonparetic leg might induce a disturbance to the body’s angular momentum, which needs to be tightly regulated to maintain dynamic balance during walking.41 Thus, individuals poststroke might have to generate an additional moment through the standing leg (ie, the paretic leg) for the regulation of body’s angular momentum, which might be achieved by recruiting additional muscle activation of ankle plantarflexors and medial hamstrings of the paretic leg.42 Alternatively, applying manual resistance to the pelvis of the paretic side during gait based on the proprioceptive neuromuscular facilitation principle may facilitate muscle activation of the paretic leg and has been widely used in clinics.43 However, while the theoretical basis of this paradigm is solid, recent studies indicated limited functional gains on walking function of individuals poststroke using the proprioceptive neuromuscular facilitation principle.44,45
Significant changes in stance time and single leg support time were observed in the paretic leg when the resistance force was applied to the nonparetic leg. Individuals with poststroke hemiparesis may still retain the capability to adjust interlimb coordination during walking in response to an external force perturbation.21 The improvements in temporal symmetry were mainly achieved by an increase in temporal measures on the paretic leg. It should be noted that the magnitude of temporal asymmetry was less than 5% at baseline. As the same percentage of asymmetry below or above 100% represents the same magnitude of absolute asymmetry, applying 10% of resistance force at the early swing phase appeared to be sufficient to improve temporal symmetry of the subjects we tested. In addition, 4 subjects had longer temporal gait parameter measures on the paretic leg than on the nonparetic leg at baseline. These subjects had even longer temporal measures on the paretic leg and greater temporal asymmetry in the resistance conditions. The application of a resistance force to the nonparetic leg may not be appropriate for improving temporal symmetry for individuals with longer temporal measures on the paretic leg at baseline.
While individuals with poststroke hemiparesis may walk with relatively longer paretic or nonparetic steps,46–48 our subjects walked with longer nonparetic steps at baseline, which may be a result of various compensatory strategies developed in chronic stroke patients.48 Both stance time and step length of the paretic leg increased with the application of the resistance force, indicating a relative decrease in swing time and an increase in leg swing velocity. The increase in swing velocity of the paretic leg may be due to an enhanced forward propulsion of the paretic leg, which could be manifested as an enhanced muscle activity of ankle plantarflexors of the paretic leg,49 and/or due to enhanced afferents from hip flexors of the paretic leg,50 which may facilitate leg swing.51
This study provides evidence for the application of CIMT for lower limb training in individuals poststroke and may have important applications for chronic stroke rehabilitation. In clinical settings, locomotor training has been widely used to improve walking function in patients with stroke, but patients may rely more on their nonparetic leg for locomotion using compensatory strategies, resulting in limited improvements in motor function of the paretic leg. Our results suggest that partially restraining the nonparetic leg during locomotor training, may enhance the muscle activity of the paretic leg, that is, inducing a forced use of the paretic leg and improving spatiotemporal symmetry. It should be noted that providing greater resistance force to the nonparetic leg may deteriorate spatial symmetry for subjects with a longer step length on the paretic leg at baseline. Evaluating baseline walking pattern to identify individuals who may benefit from this training strategy is needed to maximize rehabilitation outcomes. In addition, subjects tested in this study were limited community ambulators52(ie, average overground walking speed was 0.67 ± 0.16 m/s), we do not know whether this paradigm could be applied to subjects with lower walking function.
This study has several limitations to consider when interpreting the results. First, all subjects held onto the front handrail during testing, it is possible that the pulling force of the hand have confounded our results. In addition, a pretension force (~ 4 N) was applied to the cable to prevent the cable from slacking. The pretension force may influence walking patterns. However, the pretension force was retained during all the resistance sessions, any potential influence of pretension force would be evenly distributed across all conditions. Finally, the application of resistance force during treadmill walking was short in this study. It is unknown whether prolonged exposure to resistance force (ie, long-term training) will have clinically significant improvements in walking function of individuals poststroke. Future research is needed to determine the long-term effects of this paradigm on walking function in individuals poststroke.
Conclusion
Applying a controlled constraint force to the nonparetic leg during treadmill walking may induce forced use on the paretic leg and improve spatiotemporal symmetry of individuals poststroke during treadmill walking. Knowledge gained from this study, including the phase and magnitude of the application of the resistance force, provides insights for developing robotic gait training protocols.
Acknowledgments
The authors would like to thank Dr Rongnian Tang for assisting with data collection, and Ms Jill Landry, PT, for editing the manuscript.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the National Institutes of Health, R01HD082216.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haghgoo HA, Pazuki ES, Hosseini AS, Rassafiani M. Depression, activities of daily living and quality of life in patients with stroke. J Neurol Sci. 2013;328:87–91. [DOI] [PubMed] [Google Scholar]
- 3.Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:27–32. [DOI] [PubMed] [Google Scholar]
- 4.Lamontagne A, Stephenson JL, Fung J. Physiological evaluation of gait disturbances post stroke. Clin Neurophysiol. 2007;118:717–729. [DOI] [PubMed] [Google Scholar]
- 5.Burridge JH, Wood DE, Taylor PN, McLellan DL. Indices to describe different muscle activation patterns, identified during treadmill walking, in people with spastic drop-foot. Med Eng Phys. 2001;23:427–434. [DOI] [PubMed] [Google Scholar]
- 6.Patterson KK, Parafianowicz I, Danells CJ, et al. Gait asymmetry in community-ambulating stroke survivors. Arch Phys Med Rehabil. 2008;89:304–310. [DOI] [PubMed] [Google Scholar]
- 7.Chen G, Patten C, Kothari DH, Zajac FE. Gait deviations associated with post-stroke hemiparesis: improvement during treadmill walking using weight support, speed, support stiffness, and handrail hold. Gait Posture. 2005;22:57–62. [DOI] [PubMed] [Google Scholar]
- 8.Olney SJ, Richards C. Hemiparetic gait following stroke. Part I: characteristics. Gait Posture. 1996;4:136–148. [Google Scholar]
- 9.Jørgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Støier M, Olsen TS. Outcome and time course of recovery in stroke. Part II: time course of recovery. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:406–412. [DOI] [PubMed] [Google Scholar]
- 10.Page SJ, Sisto S, Levine P, McGrath RE. Efficacy of modified constraint-induced movement therapy in chronic stroke: a single-blinded randomized controlled trial. Arch Phys Med Rehabil. 2004;85:14–18. [DOI] [PubMed] [Google Scholar]
- 11.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. [DOI] [PubMed] [Google Scholar]
- 12.Wolf SL, Blanton S, Baer H, Breshears J, Butler AJ. Repetitive task practice: a critical review of constraint-induced movement therapy in stroke. Neurologist. 2002;8:325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf SL, Winstein CJ, Miller JP, et al. Retention of upper limb function in stroke survivors who have received constraint-induced movement therapy: the EXCITE randomised trial. Lancet Neurol. 2008;7:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevenson T, Thalman L, Christie H, Poluha W. Constraint-induced movement therapy compared to dose-matched interventions for upper-limb dysfunction in adult survivors of stroke: a systematic review with meta-analysis. Physiother Can. 2012;64:397–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonnyaud C, Zory R, Boudarham J, Pradon D, Bensmail D, Roche N. Effect of a robotic restraint gait training versus robotic conventional gait training on gait parameters in stroke patients. Exp Brain Res. 2014;232:31–42. [DOI] [PubMed] [Google Scholar]
- 16.Regnaux JP, Pradon D, Roche N, Robertson J, Bussel B, Dobkin B. Effects of loading the unaffected limb for one session of locomotor training on laboratory measures of gait in stroke. Clin Biomech (Bristol, Avon). 2008;23:762–768. [DOI] [PubMed] [Google Scholar]
- 17.Bonnyaud C, Pradon D, Zory R, et al. Effects of a gait training session combined with a mass on the non-paretic lower limb on locomotion of hemiparetic patients: a randomized controlled clinical trial. Gait Posture. 2013;37:627–630. [DOI] [PubMed] [Google Scholar]
- 18.Dietz V, Quintern J, Boos G, Berger W. Obstruction of the swing phase during gait: phase-dependent bilateral leg muscle coordination. Brain Res. 1986;384:166–169. [DOI] [PubMed] [Google Scholar]
- 19.Yang JF, Stephens MJ, Vishram R. Transient disturbances to one limb produce coordinated, bilateral responses during infant stepping. J Neurophysiol. 1998;79:2329–2337. [DOI] [PubMed] [Google Scholar]
- 20.Savin DN, Tseng SC, Morton SM. Bilateral adaptation during locomotion following a unilaterally applied resistance to swing in nondisabled adults. J Neurophysiol. 2010;104:3600–3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zehr EP, Loadman PM. Persistence of locomotor-related interlimb reflex networks during walking after stroke. Clin Neurophysiol. 2012;123:796–807. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 23.Yen SC, Landry JM, Wu M. Size of kinematic error affects retention of locomotor adaptation in human spinal cord injury. J Rehabil Res Dev. 2013;50:1187–1200. [DOI] [PubMed] [Google Scholar]
- 24.Wu M, Hornby TG, Landry JM, Roth H, Schmit BD. A cable-driven locomotor training system for restoration of gait in human SCI. Gait Posture. 2011;33:256–260. [DOI] [PubMed] [Google Scholar]
- 25.Yen SC, Schmit BD, Landry JM, Roth H, Wu M. Locomotor adaptation to resistance during treadmill training transfers to overground walking in human SCI. Exp Brain Res. 2012;216:473–482. [DOI] [PubMed] [Google Scholar]
- 26.Zeni JA Jr, Richards JG, Higginson JS. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture. 2008;27:710–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yen SC, Schmit BD, Wu M. Using swing resistance and assistance to improve gait symmetry in individuals post-stroke. Hum Mov Sci. 2015;42:212–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patterson KK, Gage WH, Brooks D, Black SE, McIlroy WE. Evaluation of gait symmetry after stroke: a comparison of current methods and recommendations for standardization. Gait Posture. 2010;31:241–246. [DOI] [PubMed] [Google Scholar]
- 29.Duysens J, Pearson KG. Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res. 1980;187:321–332. [DOI] [PubMed] [Google Scholar]
- 30.Pearson KG, Collins DF. Reversal of the influence of group Ib afferents from plantaris on activity in medial gastrocnemius muscle during locomotor activity. J Neurophysiol. 1993;70:1009–1017. [DOI] [PubMed] [Google Scholar]
- 31.Duysens J, Pearson KG. The role of cutaneous afferents from the distal hindlimb in the regulation of the step cycle of thalamic cats. Exp Brain Res. 1976;24:245–255. [DOI] [PubMed] [Google Scholar]
- 32.Guertin P, Angel MJ, Perreault MC, McCrea DA. Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. J Physiol. 1995;487:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephens MJ, Yang JF. Loading during the stance phase of walking in humans increases the extensor EMG amplitude but does not change the duration of the step cycle. Exp Brain Res. 1999;124:363–370. [DOI] [PubMed] [Google Scholar]
- 34.Winter DA, MacKinnon CD, Ruder GK, Wieman C. An integrated EMG/biomechanical model of upper body balance and posture during human gait. Prog Brain Res. 1993;97:359–367. [DOI] [PubMed] [Google Scholar]
- 35.Mercer VS, Chang SH, Williams CD, Noble K, Vance AW. Effects of an exercise program to increase hip abductor muscle strength and improve lateral stability following stroke: a single subject design. J Geriatr Phys Ther. 2009;32:50–59. [PubMed] [Google Scholar]
- 36.Ghori GM, Luckwill RG. Phase-dependent responses in locomotor muscles of walking man. J Biomed Eng. 1990;12:75–78. [DOI] [PubMed] [Google Scholar]
- 37.Rossignol S, Gauthier L. An analysis of mechanisms controlling the reversal of crossed spinal reflexes. Brain Res. 1980;182:31–45. [DOI] [PubMed] [Google Scholar]
- 38.Sherrington CS. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol. 1910;40:28–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazzaro N, Grey MJ, do Nascimento OF, Sinkjaer T. Afferent-mediated modulation of the soleus muscle activity during the stance phase of human walking. Exp Brain Res. 2006;173:713–723. [DOI] [PubMed] [Google Scholar]
- 40.Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev. 2000;80:83–133. [DOI] [PubMed] [Google Scholar]
- 41.Herr H, Popovic M. Angular momentum in human walking. J Exp Biol. 2008;211(pt 4):467–481. [DOI] [PubMed] [Google Scholar]
- 42.Neptune RR, McGowan CP. Muscle contributions to whole-body sagittal plane angular momentum during walking. J Biomech. 2011;44:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adler S, Beckers D, Buck M. PNF in Practice: An Illustrated Guide. 3rd ed. Heidelberg, Germany: Springer; 2008. [Google Scholar]
- 44.Ribeiro T, Britto H, Oliveira D, Silva E, Galvão E, Lindquist A. Effects of treadmill training with partial body weight support and the proprioceptive neuromuscular facilitation method on hemiparetic gait: a randomized controlled study. Eur J Phys Rehabil Med. 2013;49:451–461. [PubMed] [Google Scholar]
- 45.Ribeiro TS, de Sousa e Silva EM, Sousa Silva WH, et al. Effects of a training program based on the proprioceptive neuromuscular facilitation method on post-stroke motor recovery: a preliminary study. J Bodyw Mov Ther. 2014;18:526–532. [DOI] [PubMed] [Google Scholar]
- 46.Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007;88:43–49. [DOI] [PubMed] [Google Scholar]
- 47.Hsu AL, Tang PF, Jan MH. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch Phys Med Rehabil. 2003;84:1185–1193. [DOI] [PubMed] [Google Scholar]
- 48.Kim CM, Eng JJ. Symmetry in vertical ground reaction force is accompanied by symmetry in temporal but not distance variables of gait in persons with stroke. Gait Posture. 2003;18:23–28. [DOI] [PubMed] [Google Scholar]
- 49.Turns LJ, Neptune RR, Kautz SA. Relationships between muscle activity and anteroposterior ground reaction forces in hemiparetic walking. Arch Phys Med Rehabil. 2007;88:1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu M, Gordon K, Kahn JH, Schmit BD. Prolonged electrical stimulation over hip flexors increases locomotor output in human SCI. Clin Neurophysiol. 2011;122:1421–1428. [DOI] [PubMed] [Google Scholar]
- 51.Lam T, Pearson KG. Proprioceptive modulation of hip flexor activity during the swing phase of locomotion in decerebrate cats. J Neurophysiol. 2001;86:1321–1332. [DOI] [PubMed] [Google Scholar]
- 52.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995; 26:982–989. [DOI] [PubMed] [Google Scholar]