Abstract
Persistent activity generated in the PFC during the delay period of working memory tasks represents information about stimuli held in memory and determines working memory performance. Alternative models of working memory, depending on the rhythmicity of discharges or exclusively on short-term synaptic plasticity, are inconsistent with the neurophysiological data.
Dual Perspectives Companion Paper: Working Memory: Delay Activity, Yes! Persistent Activity? Maybe Not, by Mikael Lundqvist, Pawel Herman, and Earl K. Miller
Keywords: working memory, prefrontal cortex, delay period, neurophysiology, monkey
Introduction
Working memory (WM) is the ability to maintain and manipulate information in mind, over a time span of seconds, and is a core component of many other cognitive functions, including language, problem solving, reasoning, and abstract thought (Baddeley, 2012). Neurophysiological experiments in nonhuman primates identified neurons that not only respond to sensory stimuli but remain active during a period after a stimulus was no longer present; this “persistent activity” therefore provided a neural correlate of WM (Fuster and Alexander, 1971; Funahashi et al., 1989). Here, we define persistent activity as memorandum-selective activity of single neurons that spans the delay interval of WM tasks. Persistent activity is thought to be maintained through recurrent connections in a network of neurons, although a number of architectures can give rise to persistent activity (Chaudhuri and Fiete, 2016; Zylberberg and Strowbridge, 2017).
Persistent activity has not been observed in all vertebrate species, but it appears to be present in species with larger brains and more extensively interconnected neurons, such as primates. Neurophysiological studies of WM in rodents reveal neurons that remain active for short intervals each, “tiling” the delay period between a stimulus and a response (Bolkan et al., 2017; Runyan et al., 2017). In contrast, robust persistent activity that spans the entire delay period of WM tasks has only been described in primate studies (reviewed in detail here) and in human intracranial recordings (Kamiński et al., 2017; Kornblith et al., 2017; Haller et al., 2018). Nonetheless, the role of persistent activity has been reevaluated over the past few years (Sreenivasan et al., 2014; D'Esposito and Postle, 2015; Lara and Wallis, 2015), and some neurophysiological studies have failed to demonstrate persistent activity in the PFC in some short-term memory tasks. Accordingly, other models of WM not relying on persistent discharges, but rather on the rhythmicity of spiking (Lundqvist et al., 2016, 2018), or exclusively on short-term synaptic changes (Fiebig and Lansner, 2017) have been proposed. Still, there is important evidence to support persistent activity as a neural correlate of WM.
Here, we examine the role of persistent activity in WM, with an emphasis on the lateral PFC (referred to as “PFC” henceforth, for simplicity). The role of other areas has been discussed previously (Riley and Constantinidis, 2016; Leavitt et al., 2017a). We review multiple types of information encoded in persistent activity, how it subserves behavior in WM tasks, what evidence exists against this model, and the alternative models that have been proposed.
Information maintained in persistent activity
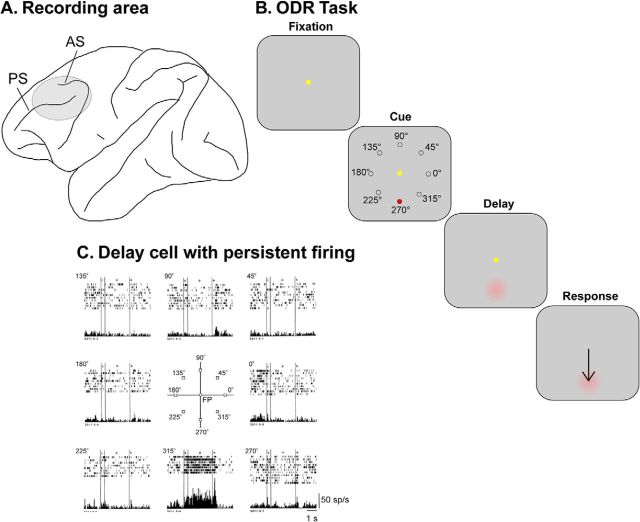
The task most extensively used to study visuospatial WM is the oculomotor delayed response task (ODR task; Fig. 1), which presents subjects with a brief stimulus and, after a delay period, requires an eye movement to the remembered location of the stimulus (Hikosaka and Wurtz, 1983; Funahashi et al., 1989). Neuronal activity recorded during execution of this task with eight targets presented at 10–15 degree eccentricity reveals that ∼30% of all neurons sampled in the region of the dlPFC in and around the posterior half of the principal sulcus exhibit persistent activity (Funahashi et al., 1989). Larger percentages, as high as 70%, of all prefrontal neurons exhibit delay period activity when tested with denser grids of stimuli, encompassing 16 locations (Leavitt et al., 2018). For many neurons, the persistent firing was not tied to the onset of the stimulus but started 300–400 ms after the visual cue presentation, which is longer than the mean of the visual response latency observed in the dlPFC. And the persistent firing often stopped abruptly around the start of the saccade. Furthermore, the majority of these neurons are selective for the spatial location of the 8 possible targets typically used in the ODR task, so that approximately one-fourth of all dorsolateral prefrontal neurons exhibit spatially tuned elevated persistent firing across the delay period. One can clearly discern persistent firing on single trials in Figure 1C, during the delay for the neuron's preferred direction compared with the low levels of firing before the cue, or other locations. Very similar percentages of spatially tuned neurons with persistent activity have been obtained from the dlPFC when tested with other spatial WM tasks, such as a spatial match/nonmatch task (Meyer et al., 2011). Proportions of active neurons vary, however, between the anterior/posterior and dorsal/ventral subdivisions (Qi and Constantinidis, 2013; Riley et al., 2017). This enrichment of neurons in caudal principal sulcal dlPFC likely results from the anatomical connectivity of this subregion and their relationship to task demands (i.e., this region receives highly processed visuospatial information from the posterior parietal association cortex and projects to the frontal eye fields to regulate the oculomotor response) (Selemon and Goldman-Rakic, 1988). In addition, the persistent firing depends on correct performance; it is diminished or absent in error trials (Fuster, 1973; Funahashi et al., 1989; Zhou et al., 2013). Thus, this pattern of persistent firing is not simply the result of increased excitability of a single neuron but, rather, the product of a circuit in an appropriate state of excitability for achieving some specific purpose.
Figure 1.
A, Schematic diagram of the monkey brain with caudal area of the principal sulcus highlighted. AS, Arcuate sulcus; PS, principal sulcus. B, Sequence of events in the oculomotor delayed response task. C, Example neuron with persistent activity. From Funahashi et al. (1989).
Some researchers have argued that the persistent firing of PFC neurons is simply a motor preparation signal rather than information held in WM. For example, the location of the preceding stimulus in the ODR task is confounded with the preparation for the motor response, leading to suggestions that persistent activity represents motor preparation (Markowitz et al., 2015). However, more complex tasks reveal that the majority of prefrontal neurons represent the stimulus properties rather than motor preparation. For example, when a task requires monkeys to make an eye movement toward a location other than the location of the visual stimulus, the majority of prefrontal neurons represent the location of the preceding stimulus rather than the location of the impeding saccade (Funahashi et al., 1993; Takeda and Funahashi, 2002).
Persistent activity tuned for the location of a stimulus appears in the PFC even in tasks where the stimulus does not immediately allow planning of a movement. In the spatial delayed-match-to-sample task, subjects are required to release a lever or press a button when a stimulus appears at a previously cued location; in the match/nonmatch task, the monkeys have to saccade to a green or blue response target depending on whether two stimuli presented in sequence appeared at the same location or not. In such tasks, prefrontal neurons generate persistent activity following the presentation of the original stimulus that is tuned for its spatial location, and not the preparation of a motor response, the direction of which is not known until later in the trial (Qi et al., 2011; Goodwin et al., 2012; Masse et al., 2017).
Prefrontal neurons generate persistent discharges that represent other types of information, in addition to spatial location. Generally, smaller populations of prefrontal neurons are tuned for object attributes, such as geometric shape, color, or complex features (e.g., specific faces), than spatial location; a regional specialization is also present, with spatial information more prevalent in the dlPFC than the vlPFC (Meyer et al., 2011). Nonetheless, robust, stimulus-selective persistent activity has been observed during WM tasks requiring subjects to remember the identity and features of stimuli. Examples include stimuli defined by simple, geometric shapes differing in color or luminance (Hoshi et al., 1998; Constantinidis et al., 2001; Sakagami et al., 2001; Inoue and Mikami, 2006; Genovesio et al., 2009); complex images, such as real objects and faces, or abstract pictures (Wilson et al., 1993; Miller et al., 1996; Rao et al., 1997; O Scalaidhe et al., 1997, 1999; Rainer et al., 1998; Rainer and Miller, 2000; Freedman et al., 2001); and the direction of motion of a random-dot stimulus that is always presented at the same location (Zaksas and Pasternak, 2006; Mendoza-Halliday et al., 2014). Persistent activity has been demonstrated not just for visual stimuli. Vibratory stimuli produce robust persistent activity, whose firing rate varies as a function of the stimulus frequency (Romo et al., 1999; Romo and Salinas, 2003). Cross-modal representations between vibratory and auditory stimuli have also been described (Fuster et al., 2000; Vergara et al., 2016).
In recent years, it has been recognized that persistent activity in the PFC also represents information beyond the physical characteristics of stimuli. Activity may represent the abstract rules of the cognitive task subjects are required to perform (White and Wise, 1999; Wallis et al., 2001), categories (Freedman et al., 2001; Shima et al., 2007; Roy et al., 2014), and numerical quantities (Nieder et al., 2002). It may be also related to perceptual decisions (Kim and Shadlen, 1999), past choices (Barraclough et al., 2004), reward expectation (Leon and Shadlen, 1999), and sequences of events or actions (Averbeck et al., 2002; Inoue and Mikami, 2006; Sigala et al., 2008; Berdyyeva and Olson, 2010). Persistent activity of single neurons may represent more information than stimulus features and task variables individually (Rigotti et al., 2013), and also play an important role for reinforcement learning by maintaining signals related to the animal's previous experience (Seo et al., 2007; Curtis and Lee, 2010).
Working memory behavior supported by persistent activity
Studies of lesions in humans and nonhuman primates first provided evidence linking the PFC with performance of even simple tasks requiring WM (Jacobsen, 1936; Milner, 1963; Rossi et al., 2007; Buckley et al., 2009; Pasternak et al., 2015). Reversible inactivation of the PFC (e.g., through cooling) impairs spatial WM and diminishes persistent firing in interconnected parts of the spatial cognition circuit (Chafee and Goldman-Rakic, 2000). Persistent activity is not merely an epiphenomenon of spatial WM but predicts behavior in WM tasks. The most straightforward evidence that persistent activity controls behavior comes from analysis of error trials in the ODR task, which are characterized by lower levels of delay period activity compared with correct trials (Funahashi et al., 1989; Zhou et al., 2013). In other words, trials in which persistent activity is diminished are more likely to result in errors. A near linear relationship between behavioral performance and persistent activity has been revealed in other tasks that modulate parametrically the difficulty of a WM judgment (Constantinidis et al., 2001), and errors in WM tasks are also seen in aged monkeys when persistent firing is insufficient to reach across the entire delay epoch (Wang et al., 2011). Choice probability analysis, comparing the distributions of firing rates in the delay period of correct and error trials, also reveals a strong relationship between prefrontal persistent activity and behavioral outcomes (Mendoza-Halliday et al., 2014).
Computational models provide a detailed picture of the relationship between behavioral performance related to WM and persistent activity. Persistent activity can be sustained in such models by virtue of recurrent connections between neurons with similar tuning for stimulus properties, so that activation outlasting afferent input is maintained in the system (Compte et al., 2000; Murray et al., 2017a). Drifts in neuronal activity across the network of prefrontal neurons have been shown to predict precisely the relationship between firing rate and the endpoint of the saccade (the spatial location being recalled by the monkey) in the ODR task (Wimmer et al., 2014). For example, persistent activity recorded from trials in which monkeys make eye movements deviating clockwise versus counterclockwise relative to the true location of the stimulus yields slightly different tuning curves, as would be expected if the location recalled was determined by the peak of activity at the end of the delay period (Fig. 2). Other, counterintuitive model predictions are confirmed experimentally; for example, negative spike-count correlations for pairs of neurons with different tuning in trials when the stimulus appears between them; and highest variability in trials when the stimulus appears at the flanks of a neuron's receptive field. Importantly, these findings cannot be predicted by alternative models assuming a gradual decay of delay period activity, nor do they hold for neurons that do not exhibit persistent discharges, even though the latter are more numerous in the PFC (Wimmer et al., 2014).
Figure 2.
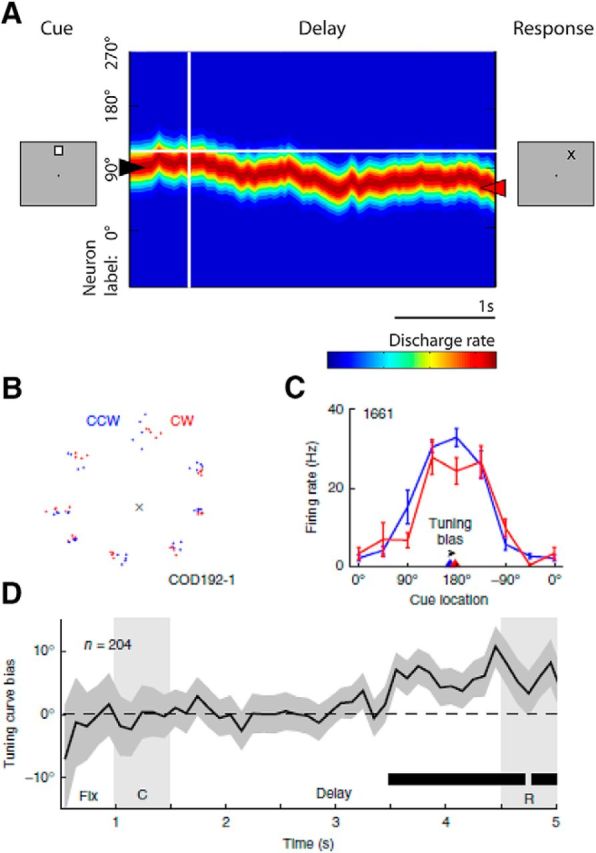
A, Illustration of the bump attractor model. Appearance of a stimulus elicits activity in a network of neurons (arranged in the y-axis based on preferred location). The bump of activity drifts during the delay period. The monkey's recall is determined by the position of the bump at the end of the delay period. From Constantinidis and Klingberg (2016). B, Representation of saccade endpoints for one session. For each cue, trials are separated in half based on their relative clockwise (red) and counterclockwise (blue) saccadic responses. C, Sample neuron delay-period responses in the clockwise and counterclockwise conditions. Triangles represent the circular mean of the responses, an estimate of the cue position for each condition. The distance between these two circular means is the tuning bias. D, Population average of the tuning bias for all neurons across time showed significantly positive values by the end of the delay. From Wimmer et al. (2014).
Dynamics of persistent activity
Part of the confusion in the literature about the role of persistent activity can be attributed to some authors equating persistent activity with perfectly stationary activity during the delay interval. Even if a firing rate is temporally modulated during the delay period and is therefore nonstationary, information can be decoded from the neuron activity through maintenance using a stable readout. Indeed, time-varying PFC activity is commonly observed in WM tasks, especially those with fixed delay duration enabling temporal expectation. For instance, the majority of neurons encoding vibrotactile information in WM have been shown to exhibit ramping, increasing or decreasing, activity while maintaining the frequency of the stimulus in a parametric fashion (Romo et al., 1999; Romo and Salinas, 2003). Temporal modulation of delay activity of anticipatory nature has been also reported in some detail by Hussar and Pasternak (2010, 2012, 2013). As we describe below, time-varying delay activity, such as ramping, is not at odds with persistent activity underlying WM maintenance. We contrast nonstationary, persistent activity, however, with transient activity of individual neurons, being active at only a short period of time during the delay period.
Fundamentally, models of persistent activity describe properties of a population code, rather than an individual neuron; that is, the representation of the memorandum is encoded as a pattern of activations across a population of neurons. For instance, in the ODR task, canonical delay activity of PFC neurons exhibits nonmonotonic, bell-shaped tuning curves to the stimulus (visuospatial angle); therefore, stimulus identity cannot be unambiguously read out from a single neuron. The population code of neuronal spiking activity based on persistent activity has the following properties. First, the memorandum is represented at a given time point in the spatial pattern of spiking intensity across the relevant population. This property is in contrast to models of purely synaptic mechanisms, and of transient activity bursts, discussed below. Second, the memorandum is represented in this code stably across time during WM maintenance. This property is in contrast to models of dynamic coding, discussed below. Theoretical and empirical analyses have shown that stable population coding of WM is consistent with time-varying neuronal activity (Machens et al., 2010; Druckmann and Chklovskii, 2012; Murray et al., 2017b). Furthermore, persistent activity does not require an overall increase of activity in the population (Murray et al., 2017b), as the population exhibits both increases and decreases in firing rate across neurons. Finally, simultaneous modulations of firing rate and resulting trial-to-trial correlation between neurons may entrain the information represented by a population of prefrontal neurons (Leavitt et al., 2017b).
Arguments against persistent activity
Some neurophysiological experiments have reported findings that appear at odds with persistent activity: only transient representation of motion information in the delay period of a WM task (Zaksas and Pasternak, 2006), achieving continuous representation of stimulus properties only through averaging of activity through multiple neurons (Hussar and Pasternak, 2012, 2013); very few prefrontal neurons with pure color information (Lara and Wallis, 2014); or encoding of only the last of a sequence of stimuli that the subject successfully recalled in its entirety (Konecky et al., 2017). Failure to detect activity in the PFC when tested with a random stimulus, without characterization of the neuron's tuning function across the dimension being tested, is not a strong argument against the role of persistent activity as the neural correlate of WM. Across the population of prefrontal neurons, only a small minority would be expected to be active during maintenance of any single stimulus in memory (Fig. 1A, vertical white line). Moreover, in contrast to earlier findings of Zaksas and Pasternak (2006), more recent experiments have revealed robust persistent activity representing direction of motion throughout the delay period of a WM task in the PFC (Mendoza-Halliday et al., 2014). Similarly, activation of only a small proportion of prefrontal neurons, in the order of 5%–15% (Lara and Wallis, 2014), may be sufficient for the faithful representation of stimulus color. It is also possible that color-selective neurons are concentrated in specific prefrontal “patches” (Lafer-Sousa and Conway, 2013), and persistent activity representing color information may be concentrated in such modules rather than diffusely distributed across the entire prefrontal surface. Representation of sequences of stimuli, or more complex displays, may also involve some transformation of the individual elements (Parthasarathy et al., 2017), without knowledge of which tuned persistent activity may not be reliably detected.
It has also been noted that persistent activity can be highly variable during the course of a trial, and from trial to trial, leading to the proposal that persistent activity demonstrated in earlier studies is an “artifact of averaging across trials” (Lundqvist et al., 2016). A high level of variability of persistent activity between and within trials is also not inconsistent with the persistent activity model. To the contrary, variability in discharge rate during the course of the trial would be expected, even among neurons that are highly active at some time point, as the activity might drift in the population (Fig. 2A, horizontal white line). Indeed, higher irregularity of spiking activity (quantified by the coefficient of variation of the interspike interval) has been observed in the delay period than during the fixation period of the ODR task (Compte et al., 2003). This otherwise puzzling finding is precisely predicted by the network models of persistent activity. Furthermore, if the observed persistent activity in single-neuron peristimulus time histograms were an artifact of averaging across trials, then this scenario would make strong testable predictions for higher-order statistics of spiking activity. If individual trials of WM activity were characterized by short intermittent bursts of high-rate firing (Lundqvist et al., 2016), then across-trial spike-count variability (e.g., as quantified by the Fano factor) would increase dramatically during mnemonic delays, relative to the task foreperiod with stable fixation, which is inconsistent with empirical measurements (Chang et al., 2012; Qi and Constantinidis, 2012).
As mentioned above, the concentration of neurons with domain-related persistent firing likely reflects the underlying anatomical projections (e.g., neurons with persistent firing to visual features, such as faces, are concentrated more ventrally than those with persistent activity to visual space) (Wilson et al., 1993), whereas those with persistent firing to somatosensory information are found even more ventrally in the inferior prefrontal convexity (Romo et al., 1999), all of which corresponds to the location of visual spatial, feature, and somatosensory inputs to the lateral PFC (Goldman-Rakic, 1987; Carmichael and Price, 1995). Topographical differences in neuronal response may also reflect the efferent projections to differing motor systems (e.g., subregions that project to frontal eye fields for control of eye movements compared with subregions projecting to premotor and motor cortices for a manual response) (Preuss and Goldman-Rakic, 1989). Thus, the relative concentration of neurons with persistent firing to a specific sensory/motor domain likely reflects the underlying anatomy. This topography must be respected, especially when interpreting apparent negative results, where the researcher may not have recorded from the most relevant subregion of PFC.
Alternative models of WM
Recent models have proposed mechanisms other than persistent discharges as the neural correlate of WM. We focus here on two categories of models: nonspiking models dependent exclusively on synaptic mechanisms and rhythmic-spiking models conveying information based on the frequency and phase of discharges without necessarily a persistent increase in spiking activity. We point out the limitations of these models and emphasize that researchers should distinguish between different levels of analysis and different underlying mechanisms.
First, computational models have been proposed to account for information storage via mechanisms that do not depend on spike generation, but instead involve short-term modification of synaptic properties that are not reflected in spiking activity (Mongillo et al., 2008; Sugase-Miyamoto et al., 2008; Mi et al., 2017). These models use synaptic mechanisms, such as short-term facilitation of vesicle release through availability of calcium at the presynaptic terminal, whose kinetics have time constant in the scale of seconds (Mongillo et al., 2008), or short-term potentiation of synaptic strengths (Fiebig and Lansner, 2017), which decays over minutes (Erickson et al., 2010). Beyond imprinting a passive trace of the stimulus activation pattern, it is less straightforward to consider how purely synaptic mechanisms would mediate other WM tasks, such as the ODR, delayed alternation, or free recall tasks, which can be controlled more flexibly. For example, short-term potentiation cannot underlie the updating of WM contents within the timeframe of seconds. We should note that plastic neuromodulation and other synaptic mechanisms have an important role in WM in that they can lower the threshold for the generation of spiking activity or help to shape network inputs (Arnsten et al., 2012), but lack the flexible, precise pattern and timing needed for accurate neural representations.
Second, WM may be maintained through spiking activity in a population code, but a code that is dynamic in time, rather than the stable population coding of persistent activity (Sreenivasan et al., 2014; D'Esposito and Postle, 2015; Lara and Wallis, 2015; Stokes, 2015). In dynamic coding models, population-level mnemonic representations are time-varying and do not generalize across time during maintenance. By contrast, recent population-level analyses of PFC activity have found that stimulus representations during WM delays provide a stable population code that generalizes across time, although they are distinct from transient representations present during stimulus presentation (Murray et al., 2017b; Spaak et al., 2017). As noted above, observation of time-varying neuronal activity does not imply dynamic coding and can be consistent with stable population coding and persistent activity (Machens et al., 2010; Druckmann and Chklovskii, 2012; Murray et al., 2017b).
Third, rhythmic activity has been implicated in WM (Siegel et al., 2009; Buschman et al., 2012; Liebe et al., 2012; Salazar et al., 2012; Brincat and Miller, 2015). The magnitude, frequency, and phase of oscillations within the PFC and between the PFC and other areas have been shown to be modulated depending on stimulus and task information (Buschman et al., 2012; Liebe et al., 2012). The coherence in rhythmic synchronization between neurons in prefrontal and posterior parietal cortex has also been reported to be content-dependent; prefrontal and parietal neurons synchronize their firing at specific frequencies according to stimuli held in memory (Salazar et al., 2012). In addition, spiking activity at different phases of local field potential (LFP) oscillations could also differentiate information representing two sequentially presented stimuli (Siegel et al., 2009).
Recent studies have specifically proposed that the rate of LFP bursting in the gamma frequency range, which correlates negatively with power in the beta frequency, underlies WM (Lundqvist et al., 2016, 2018; Bastos et al., 2018; Stanley et al., 2018; Wutz et al., 2018). A corollary of this model is that gamma-bursting pooled from error trials should be lower than that of correct trials. Unfortunately, no measures of behavior were shown to correlate with the purported neural basis of WM during the delay interval in any of these studies. Differences in gamma-bursting between correct and error trials were reported in one study (Lundqvist et al., 2018). Critically, no differences were reported during the delay periods following the sample presentations in the WM task used. Instead, error and correct trials were differentiated by levels of gamma-bursting only during the period when test stimuli were presented and the monkeys had to judge whether they matched stimuli held in memory, and errors were characterized by generally higher (not lower) levels of gamma-bursting (Lundqvist et al., 2018). In summary, gamma-bursting rate appears to be a poor predictor of whether information is successfully maintained during the delay period of a WM task, unlike persistent activity.
An additional concern in the Lundqvist et al., 2018 study is that LFP recordings were obtained from high impedance electrodes, which also isolated spikes. The existence of gamma power in such recordings is not a strong prediction of a model that seeks to falsify persistent activity. Multiple studies have shown remnants of spikes on LFPs, especially in high frequencies (Ray and Maunsell, 2011), even if one were to remove the spike waveforms from the LFP record (Zanos et al., 2011), which was not attempted in the aforementioned study. It is possible that the LFP measures reported are driven by spiking activity in the delay period of the task, including persistent activity.
In any case, oscillatory activity is not incompatible with persistent activity but, rather, might reflect the underlying persistent firing and its ramifications from a distance. For example, both robust persistent activity and gamma-band rhythmicity have been reported during the delay period of the ODR task (Pesaran et al., 2002; Lundqvist et al., 2016), as well as the two-item sequential WM task (Siegel et al., 2009). Furthermore, concurrent persistent activity and gamma-band rhythmicity are observed when recordings are performed in the cortical site that corresponds to task demands: both increased persistent firing (Warden and Miller, 2007) and gamma-band activity (Lundqvist et al., 2018) were captured from more ventral recording sites during an object feature WM task. Similarly, the classic ODR spatial WM task that generates persistent firing in dlPFC was associated with pronounced gamma bursts from the same region (Lundqvist et al., 2016). Thus, although measures of oscillatory activity allow the researcher to sample a broader range of neuronal activity than can be performed with single- or multiple-unit recordings, persistent firing appears to underlie the oscillatory events captured during WM.
In conclusion, the evidence reviewed here demonstrates that stimulus-tuned, persistent activity is readily observed in PFC during the execution of any type of WM task. Proportions of neurons active during the delay period may vary between prefrontal subdivisions depending on task. Overall levels of task performance and more specific behavioral outcomes that reflect the accuracy of recall are predicted by persistent activity. In the presence of such evidence, negative results of individual studies should be treated with caution. Alternative models not depending on persistent activity have been inspired by functional imaging studies, MEG studies, and the emergence of computer models that are relatively unconstrained. These may appear attractive on theoretical grounds but fall short of accounting for WM behavior. Synaptic mechanisms, including calcium availability, short-term potentiation, and neuromodulation, may play a role in WM because they facilitate the generation of spiking activity or shape its selectivity. Persistent activity appears in brains that have undergone a massive expansion of the devoted prefrontal microcircuits that readily maintain and manipulate information for abstract thought, a process that reached its apex in the human PFC (Haller et al., 2018).
Footnotes
This work was supported by National Institutes of Health, National Eye Institute Awards R01 EY017077 and R01 EY016773 to C.C., R01MH112746 to J.D.M., R01MH108643 and R01MH108629 to D.L., and DP1AG047744 to A.F.T.A.
The authors declare no competing financial interests.
References
- Arnsten AF, Wang MJ, Paspalas CD (2012) Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron 76:223–239. 10.1016/j.neuron.2012.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck BB, Chafee MV, Crowe DA, Georgopoulos AP (2002) Parallel processing of serial movements in prefrontal cortex. Proc Natl Acad Sci U S A 99:13172–13177. 10.1073/pnas.162485599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. (2012) Working memory: theories, models, and controversies. Annu Rev Psychol 63:1–29. 10.1146/annurev-psych-120710-100422 [DOI] [PubMed] [Google Scholar]
- Barraclough DJ, Conroy ML, Lee D (2004) Prefrontal cortex and decision making in a mixed-strategy game. Nat Neurosci 7:404–410. 10.1038/nn1209 [DOI] [PubMed] [Google Scholar]
- Bastos AM, Loonis R, Kornblith S, Lundqvist M, Miller EK (2018) Laminar recordings in frontal cortex suggest distinct layers for maintenance and control of working memory. Proc Natl Acad Sci U S A 115:1117–1122. 10.1073/pnas.1710323115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdyyeva TK, Olson CR (2010) Rank signals in four areas of macaque frontal cortex during selection of actions and objects in serial order. J Neurophysiol 104:141–159. 10.1152/jn.00639.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolkan SS, Stujenske JM, Parnaudeau S, Spellman TJ, Rauffenbart C, Abbas AI, Harris AZ, Gordon JA, Kellendonk C (2017) Thalamic projections sustain prefrontal activity during working memory maintenance. Nat Neurosci 20:987–996. 10.1038/nn.4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brincat SL, Miller EK (2015) Frequency-specific hippocampal-prefrontal interactions during associative learning. Nat Neurosci 18:576–581. 10.1038/nn.3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley MJ, Mansouri FA, Hoda H, Mahboubi M, Browning PG, Kwok SC, Phillips A, Tanaka K (2009) Dissociable components of rule-guided behavior depend on distinct medial and prefrontal regions. Science 325:52–58. 10.1126/science.1172377 [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Denovellis EL, Diogo C, Bullock D, Miller EK (2012) Synchronous oscillatory neural ensembles for rules in the prefrontal cortex. Neuron 76:838–846. 10.1016/j.neuron.2012.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Price JL (1995) Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol 363:642–664. 10.1002/cne.903630409 [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS (2000) Inactivation of parietal and prefrontal cortex reveals interdependence of neural activity during memory guided-saccades. J Neurophysiol 83:1550–1566. 10.1152/jn.2000.83.3.1550 [DOI] [PubMed] [Google Scholar]
- Chang MH, Armstrong KM, Moore T (2012) Dissociation of response variability from firing rate effects in frontal eye field neurons during visual stimulation, working memory, and attention. J Neurosci 32:2204–2216. 10.1523/JNEUROSCI.2967-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri R, Fiete I (2016) Computational principles of memory. Nat Neurosci 19:394–403. 10.1038/nn.4237 [DOI] [PubMed] [Google Scholar]
- Compte A, Brunel N, Goldman-Rakic PS, Wang XJ (2000) Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex 10:910–923. 10.1093/cercor/10.9.910 [DOI] [PubMed] [Google Scholar]
- Compte A, Constantinidis C, Tegner J, Raghavachari S, Chafee MV, Goldman-Rakic PS, Wang XJ (2003) Temporally irregular mnemonic persistent activity in prefrontal neurons of monkeys during a delayed response task. J Neurophysiol 28:3441–3454. 10.1152/jn.00949.2002 [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Franowicz MN, Goldman-Rakic PS (2001) The sensory nature of mnemonic representation in the primate prefrontal cortex. Nat Neurosci 4:311–316. 10.1038/85179 [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Klingberg T (2016) The neuroscience of working memory capacity and training. Nat Rev Neurosci 17:438–449. 10.1038/nrn.2016.43 [DOI] [PubMed] [Google Scholar]
- Curtis CE, Lee D (2010) Beyond working memory: the role of persistent activity in decision making. Trends Cogn Sci 14:216–222. 10.1016/j.tics.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR (2015) The cognitive neuroscience of working memory. Annu Rev Psychol 66:115–142. 10.1146/annurev-psych-010814-015031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckmann S, Chklovskii DB (2012) Neuronal circuits underlying persistent representations despite time varying activity. Curr Biol 22:2095–2103. 10.1016/j.cub.2012.08.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MA, Maramara LA, Lisman J (2010) A single brief burst induces GluR1-dependent associative short-term potentiation: a potential mechanism for short-term memory. J Cogn Neurosci 22:2530–2540. 10.1162/jocn.2009.21375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebig F, Lansner A (2017) A spiking working memory model based on Hebbian short-term potentiation. J Neurosci 37:83–96. 10.1523/JNEUROSCI.1989-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK (2001) Categorical representation of visual stimuli in the primate prefrontal cortex. Science 291:312–316. 10.1126/science.291.5502.312 [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS (1989) Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol 61:331–349. 10.1152/jn.1989.61.2.331 [DOI] [PubMed] [Google Scholar]
- Funahashi S, Chafee MV, Goldman-Rakic PS (1993) Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature 365:753–756. 10.1038/365753a0 [DOI] [PubMed] [Google Scholar]
- Fuster JM. (1973) Unit activity in prefrontal cortex during delayed-response performance: neuronal correlates of transient memory. J Neurophysiol 36:61–78. 10.1152/jn.1973.36.1.61 [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE (1971) Neuron activity related to short-term memory. Science 173:652–654. 10.1126/science.173.3997.652 [DOI] [PubMed] [Google Scholar]
- Fuster JM, Bodner M, Kroger JK (2000) Cross-modal and cross-temporal association in neurons of frontal cortex. Nature 405:347–351. 10.1038/35012613 [DOI] [PubMed] [Google Scholar]
- Genovesio A, Tsujimoto S, Wise SP (2009) Feature- and order-based timing representations in the frontal cortex. Neuron 63:254–266. 10.1016/j.neuron.2009.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. (1987) Circuitry of the prefrontal cortex and the regulation of behavior by representational knowledge. In: Handbook of physiology (Plum F, Mountcastle VB, eds), pp 373–417. Bethesda, MD: American Physiological Society. [Google Scholar]
- Goodwin SJ, Blackman RK, Sakellaridi S, Chafee MV (2012) Executive control over cognition: stronger and earlier rule-based modulation of spatial category signals in prefrontal cortex relative to parietal cortex. J Neurosci 32:3499–3515. 10.1523/JNEUROSCI.3585-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller M, Case J, Crone NE, Chang EF, King-Stephens D, Laxer KD, Weber PB, Parvizi J, Knight RT, Shestyuk AY (2018) Persistent neuronal activity in human prefrontal cortex links perception and action. Nat Hum Behav. 2:80–91. 10.1038/s41562-017-0267-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH (1983) Visual and oculomotor functions of monkey substantia nigra pars reticulata: III. Memory-contingent visual and saccade responses. J Neurophysiol 49:1268–1284. 10.1152/jn.1983.49.5.1268 [DOI] [PubMed] [Google Scholar]
- Hoshi E, Shima K, Tanji J (1998) Task-dependent selectivity of movement-related neuronal activity in the primate prefrontal cortex. J Neurophysiol 80:3392–3397. 10.1152/jn.1998.80.6.3392 [DOI] [PubMed] [Google Scholar]
- Hussar C, Pasternak T (2010) Trial-to-trial variability of the prefrontal neurons reveals the nature of their engagement in a motion discrimination task. Proc Natl Acad Sci U S A 107:21842–21847. 10.1073/pnas.1009956107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussar CR, Pasternak T (2012) Memory-guided sensory comparisons in the prefrontal cortex: contribution of putative pyramidal cells and interneurons. J Neurosci 32:2747–2761. 10.1523/JNEUROSCI.5135-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussar CR, Pasternak T (2013) Common rules guide comparisons of speed and direction of motion in the dorsolateral prefrontal cortex. J Neurosci 33:972–986. 10.1523/JNEUROSCI.4075-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Mikami A (2006) Prefrontal activity during serial probe reproduction task: encoding, mnemonic, and retrieval processes. J Neurophysiol 95:1008–1041. 10.1152/jn.00552.2005 [DOI] [PubMed] [Google Scholar]
- Jacobsen CF. (1936) Studies of cerebral function in primates. Comp Psychol Monogr 13:1–68. [Google Scholar]
- Kamiński J, Sullivan S, Chung JM, Ross IB, Mamelak AN, Rutishauser U (2017) Persistently active neurons in human medial frontal and medial temporal lobe support working memory. Nat Neurosci 20:590–601. 10.1038/nn.4509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JN, Shadlen MN (1999) Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat Neurosci 2:176–185. 10.1038/5739 [DOI] [PubMed] [Google Scholar]
- Konecky RO, Smith MA, Olson CR (2017) Monkey prefrontal neurons during Sternberg task performance: full contents of working memory or most recent item? J Neurophysiol 117:2269–2281. 10.1152/jn.00541.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblith S, Quian Quiroga R, Koch C, Fried I, Mormann F (2017) Persistent single-neuron activity during working memory in the human medial temporal lobe. Curr Biol 27:1026–1032. 10.1016/j.cub.2017.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafer-Sousa R, Conway BR (2013) Parallel, multi-stage processing of colors, faces and shapes in macaque inferior temporal cortex. Nat Neurosci 16:1870–1878. 10.1038/nn.3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara AH, Wallis JD (2014) Executive control processes underlying multi-item working memory. Nat Neurosci 17:876–883. 10.1038/nn.3702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara AH, Wallis JD (2015) The role of prefrontal cortex in working memory: a mini review. Front Syst Neurosci 9:173. 10.3389/fnsys.2015.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt ML, Mendoza-Halliday D, Martinez-Trujillo JC (2017a) Sustained activity encoding working memories: not fully distributed. Trends Neurosci 40:328–346. 10.1016/j.tins.2017.04.004 [DOI] [PubMed] [Google Scholar]
- Leavitt ML, Pieper F, Sachs AJ, Martinez-Trujillo JC (2017b) Correlated variability modifies working memory fidelity in primate prefrontal neuronal ensembles. Proc Natl Acad Sci U S A 114:E2494–E2503. 10.1073/pnas.1619949114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt ML, Pieper F, Sachs AJ, Martinez-Trujillo JC (2018) A quadrantic bias in prefrontal representation of visual-mnemonic space. Cereb Cortex 28:2405–2421. 10.1093/cercor/bhx142 [DOI] [PubMed] [Google Scholar]
- Leon MI, Shadlen MN (1999) Effect of expected reward magnitude on the response of neurons in the dorsolateral prefrontal cortex of the macaque. Neuron 24:415–425. 10.1016/S0896-6273(00)80854-5 [DOI] [PubMed] [Google Scholar]
- Liebe S, Hoerzer GM, Logothetis NK, Rainer G (2012) Theta coupling between V4 and prefrontal cortex predicts visual short-term memory performance. Nat Neurosci 15:456–462, S1–S2. 10.1038/nn.3038 [DOI] [PubMed] [Google Scholar]
- Lundqvist M, Rose J, Herman P, Brincat SL, Buschman TJ, Miller EK (2016) Gamma and beta bursts underlie working memory. Neuron 90:152–164. 10.1016/j.neuron.2016.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist M, Herman P, Warden MR, Brincat SL, Miller EK (2018) Gamma and beta bursts during working memory readout suggest roles in its volitional control. Nat Commun 9:394. 10.1038/s41467-017-02791-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machens CK, Romo R, Brody CD (2010) Functional, but not anatomical, separation of “what” and “when” in prefrontal cortex. J Neurosci 30:350–360. 10.1523/JNEUROSCI.3276-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz DA, Curtis CE, Pesaran B (2015) Multiple component networks support working memory in prefrontal cortex. Proc Natl Acad Sci U S A 112:11084–11089. 10.1073/pnas.1504172112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse NY, Hodnefield JM, Freedman DJ (2017) Mnemonic encoding and cortical organization in parietal and prefrontal cortices. J Neurosci 37:6098–6112. 10.1523/JNEUROSCI.3903-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Halliday D, Torres S, Martinez-Trujillo JC (2014) Sharp emergence of feature-selective sustained activity along the dorsal visual pathway. Nat Neurosci 17:1255–1262. 10.1038/nn.3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Qi XL, Stanford TR, Constantinidis C (2011) Stimulus selectivity in dorsal and ventral prefrontal cortex after training in working memory tasks. J Neurosci 31:6266–6276. 10.1523/JNEUROSCI.6798-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi Y, Katkov M, Tsodyks M (2017) Synaptic correlates of working memory capacity. Neuron 93:323–330. 10.1016/j.neuron.2016.12.004 [DOI] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R (1996) Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci 16:5154–5167. 10.1523/JNEUROSCI.16-16-05154.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B. (1963) Effects of different brain lesions on card sorting. Arch Neurol 9:100–110. [Google Scholar]
- Mongillo G, Barak O, Tsodyks M (2008) Synaptic theory of working memory. Science 319:1543–1546. 10.1126/science.1150769 [DOI] [PubMed] [Google Scholar]
- Murray JD, Jaramillo J, Wang XJ (2017a) Working memory and decision making in a fronto-parietal circuit model. J Neurosci 37:12167–12186. 10.1523/JNEUROSCI.0343-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JD, Bernacchia A, Roy NA, Constantinidis C, Romo R, Wang XJ (2017b) Stable population coding for working memory coexists with heterogeneous neural dynamics in prefrontal cortex. Proc Natl Acad Sci U S A 114:394–399. 10.1073/pnas.1619449114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieder A, Freedman DJ, Miller EK (2002) Representation of the quantity of visual items in the primate prefrontal cortex. Science 297:1708–1711. 10.1126/science.1072493 [DOI] [PubMed] [Google Scholar]
- O Scalaidhe SP, Wilson FA, Goldman-Rakic PS (1997) Areal segregation of face-processing neurons in prefrontal cortex. Science 278:1135–1138. 10.1126/science.278.5340.1135 [DOI] [PubMed] [Google Scholar]
- O Scalaidhe SP, Wilson FA, Goldman-Rakic PS (1999) Face-selective neurons during passive viewing and working memory performance of rhesus monkeys: evidence for intrinsic specialization of neuronal coding. Cereb Cortex 9:459–475. 10.1093/cercor/9.5.459 [DOI] [PubMed] [Google Scholar]
- Parthasarathy A, Herikstad R, Bong JH, Medina FS, Libedinsky C, Yen SC (2017) Mixed selectivity morphs population codes in prefrontal cortex. Nat Neurosci 20:1770–1779. 10.1038/s41593-017-0003-2 [DOI] [PubMed] [Google Scholar]
- Pasternak T, Lui LL, Spinelli PM (2015) Unilateral prefrontal lesions impair memory-guided comparisons of contralateral visual motion. J Neurosci 35:7095–7105. 10.1523/JNEUROSCI.5265-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran B, Pezaris JS, Sahani M, Mitra PP, Andersen RA (2002) Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nat Neurosci 5:805–811. 10.1038/nn890 [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS (1989) Connections of the ventral granular frontal cortex of macaques with perisylvian premotor and somatosensory areas: anatomical evidence for somatic representation in primate frontal association cortex. J Comp Neurol 282:293–316. 10.1002/cne.902820210 [DOI] [PubMed] [Google Scholar]
- Qi XL, Constantinidis C (2012) Variability of prefrontal neuronal discharges before and after training in a working memory task. PLoS One 7:e41053. 10.1371/journal.pone.0041053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XL, Constantinidis C (2013) Neural changes after training to perform cognitive tasks. Behav Brain Res 241:235–243. 10.1016/j.bbr.2012.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XL, Meyer T, Stanford TR, Constantinidis C (2011) Changes in prefrontal neuronal activity after learning to perform a spatial working memory task. Cereb Cortex 21:2722–2732. 10.1093/cercor/bhr058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainer G, Miller EK (2000) Effects of visual experience on the representation of objects in the prefrontal cortex. Neuron 27:179–189. 10.1016/S0896-6273(00)00019-2 [DOI] [PubMed] [Google Scholar]
- Rainer G, Asaad WF, Miller EK (1998) Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature 393:577–579. 10.1038/31235 [DOI] [PubMed] [Google Scholar]
- Rao SC, Rainer G, Miller EK (1997) Integration of what and where in the primate prefrontal cortex. Science 276:821–824. 10.1126/science.276.5313.821 [DOI] [PubMed] [Google Scholar]
- Ray S, Maunsell JH (2011) Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol 9:e1000610. 10.1371/journal.pbio.1000610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti M, Barak O, Warden MR, Wang XJ, Daw ND, Miller EK, Fusi S (2013) The importance of mixed selectivity in complex cognitive tasks. Nature 497:585–590. 10.1038/nature12160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley MR, Constantinidis C (2016) Role of prefrontal persistent activity in working memory. Front Syst Neurosci 9:181. 10.3389/fnsys.2015.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley MR, Qi XL, Constantinidis C (2017) Functional specialization of areas along the anterior-posterior axis of the primate prefrontal cortex. Cereb Cortex 27:3683–3697. 10.1093/cercor/bhw190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R, Salinas E (2003) Flutter discrimination: neural codes, perception, memory and decision making. Nat Rev Neurosci 4:203–218. 10.1038/nrn1058 [DOI] [PubMed] [Google Scholar]
- Romo R, Brody CD, Hernández A, Lemus L (1999) Neuronal correlates of parametric working memory in the prefrontal cortex. Nature 399:470–473. 10.1038/20939 [DOI] [PubMed] [Google Scholar]
- Rossi AF, Bichot NP, Desimone R, Ungerleider LG (2007) Top down attentional deficits in macaques with lesions of lateral prefrontal cortex. J Neurosci 27:11306–11314. 10.1523/JNEUROSCI.2939-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy JE, Buschman TJ, Miller EK (2014) PFC neurons reflect categorical decisions about ambiguous stimuli. J Cogn Neurosci 26:1283–1291. 10.1162/jocn_a_00568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan CA, Piasini E, Panzeri S, Harvey CD (2017) Distinct timescales of population coding across cortex. Nature 548:92–96. 10.1038/nature23020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami M, Tsutsui K, Lauwereyns J, Koizumi M, Kobayashi S, Hikosaka O (2001) A code for behavioral inhibition on the basis of color, but not motion, in ventrolateral prefrontal cortex of macaque monkey. J Neurosci 21:4801–4808. 10.1523/JNEUROSCI.21-13-04801.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar RF, Dotson NM, Bressler SL, Gray CM (2012) Content-specific fronto-parietal synchronization during visual working memory. Science 338:1097–1100. 10.1126/science.1224000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS (1988) Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci 8:4049–4068. 10.1523/JNEUROSCI.08-11-04049.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H, Barraclough DJ, Lee D (2007) Dynamic signals related to choices and outcomes in the dorsolateral prefrontal cortex. Cereb Cortex 17 [Suppl. 1]:i110–i117. [DOI] [PubMed] [Google Scholar]
- Shima K, Isoda M, Mushiake H, Tanji J (2007) Categorization of behavioural sequences in the prefrontal cortex. Nature 445:315–318. 10.1038/nature05470 [DOI] [PubMed] [Google Scholar]
- Siegel M, Warden MR, Miller EK (2009) Phase-dependent neuronal coding of objects in short-term memory. Proc Natl Acad Sci U S A 106:21341–21346. 10.1073/pnas.0908193106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigala N, Kusunoki M, Nimmo-Smith I, Gaffan D, Duncan J (2008) Hierarchical coding for sequential task events in the monkey prefrontal cortex. Proc Natl Acad Sci U S A 105:11969–11974. 10.1073/pnas.0802569105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaak E, Watanabe K, Funahashi S, Stokes MG (2017) Stable and dynamic coding for working memory in primate prefrontal cortex. J Neurosci 37:6503–6516. 10.1523/JNEUROSCI.3364-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasan KK, Curtis CE, D'Esposito M (2014) Revisiting the role of persistent neural activity during working memory. Trends Cogn Sci 18:82–89. 10.1016/j.tics.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley DA, Roy JE, Aoi MC, Kopell NJ, Miller EK (2018) Low-beta oscillations turn up the gain during category judgments. Cereb Cortex 28:116–130. 10.1093/cercor/bhw356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes MG. (2015) ‘Activity-silent’ working memory in prefrontal cortex: a dynamic coding framework. Trends Cogn Sci 19:394–405. 10.1016/j.tics.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugase-Miyamoto Y, Liu Z, Wiener MC, Optican LM, Richmond BJ (2008) Short-term memory trace in rapidly adapting synapses of inferior temporal cortex. PLoS Comput Biol 4:e1000073. 10.1371/journal.pcbi.1000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Funahashi S (2002) Prefrontal task-related activity representing visual cue location or saccade direction in spatial working memory tasks. J Neurophysiol 87:567–588. 10.1152/jn.00249.2001 [DOI] [PubMed] [Google Scholar]
- Vergara J, Rivera N, Rossi-Pool R, Romo R (2016) A neural parametric code for storing information of more than one sensory modality in working memory. Neuron 89:54–62. 10.1016/j.neuron.2015.11.026 [DOI] [PubMed] [Google Scholar]
- Wallis JD, Anderson KC, Miller EK (2001) Single neurons in prefrontal cortex encode abstract rules. Nature 411:953–956. 10.1038/35082081 [DOI] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D, Arnsten AF (2011) Neuronal basis of age-related working memory decline. Nature 476:210–213. 10.1038/nature10243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden MR, Miller EK (2007) The representation of multiple objects in prefrontal neuronal delay activity. Cereb Cortex 17 [Suppl 1]:i41–i50. [DOI] [PubMed] [Google Scholar]
- White IM, Wise SP (1999) Rule-dependent neuronal activity in the prefrontal cortex. Exp Brain Res 126:315–335. 10.1007/s002210050740 [DOI] [PubMed] [Google Scholar]
- Wilson FA, O Scalaidhe SP, Goldman-Rakic PS (1993) Dissociation of object and spatial processing domains in primate prefrontal cortex. Science 260:1955–1958. 10.1126/science.8316836 [DOI] [PubMed] [Google Scholar]
- Wimmer K, Nykamp DQ, Constantinidis C, Compte A (2014) Bump attractor dynamics in prefrontal cortex explains behavioral precision in spatial working memory. Nat Neurosci 17:431–439. 10.1038/nn.3645 [DOI] [PubMed] [Google Scholar]
- Wutz A, Loonis R, Roy JE, Donoghue JA, Miller EK (2018) Different levels of category abstraction by different dynamics in different prefrontal areas. Neuron 97:716–726.e8. 10.1016/j.neuron.2018.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaksas D, Pasternak T (2006) Directional signals in the prefrontal cortex and in area MT during a working memory for visual motion task. J Neurosci 26:11726–11742. 10.1523/JNEUROSCI.3420-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos TP, Mineault PJ, Pack CC (2011) Removal of spurious correlations between spikes and local field potentials. J Neurophysiol 105:474–486. 10.1152/jn.00642.2010 [DOI] [PubMed] [Google Scholar]
- Zhou X, Zhu D, Qi XL, Lees CJ, Bennett AJ, Salinas E, Stanford TR, Constantinidis C (2013) Working memory performance and neural activity in the prefrontal cortex of peri-pubertal monkeys. J Neurophysiol 110:2648–2660. 10.1152/jn.00370.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylberberg J, Strowbridge BW (2017) Mechanisms of persistent activity in cortical circuits: possible neural substrates for working memory. Annu Rev Neurosci 40:603–627. 10.1146/annurev-neuro-070815-014006 [DOI] [PMC free article] [PubMed] [Google Scholar]