Abstract
Exosomes are emerging as essential vehicles mediated cross-talk between different types of cells in tumor microenvironment. The extensive exploration of exosomes in hepatocellular carcinoma (HCC) enhances our comprehension of cancer biology referring to tumor growth, metastasis, immune evasion, and chemoresistance. Besides, the versatile roles of exosomes provide reasonable explanations for the propensity for liver metastasis of gastric cancer, pancreatic ductal adenocarcinoma, breast cancer, and colorectal cancer. The selective-enriched components, especially some specific proteins and noncoding RNAs in exosomes, have great potential as noninvasive biomarkers of HCC with high sensitivity and specificity. The characteristics of exosomes further inspire frontier research to interrupt intercellular malignant signals by controlling the biogenesis, release, or contents of exosomes.
1. Introduction
Hepatocellular carcinoma (HCC), which mainly develops in the context of chronic inflammation and liver fibrosis [1], is of great concern worldwide because of its high morbidity and mortality rates [2, 3]. Unresectable tumors caused by delayed diagnosis generally develop innate or acquired chemoresistance [4]. The multifocal tumors composed of heterogeneous subpopulations, with multiple dysregulated signaling pathways, limit the efficacy of targeted therapies [5].
Studies reveal that every step of tumorigenesis and development of HCC depends on the intricate interactions with the tumor microenvironment, which comprises fibroblasts, endothelial cells, cancer stem cells, myeloid cells, and the associated soluble cytokines [6]. It has emerged that exosomes serve as crucial regulator of the tumor microenvironment by promoting HCC onset and metastasis. For example, tumor-derived exosomes carry regulatory molecules and tumor antigens that are beneficial for the survival of cancer cells and the development of the malignant phenotype. Exosomes derived from cancer-associated fibroblasts (CAFs) show a synergetic effect with cancer cells in optimizing the tumor microenvironment. In contrast, modified exosomes have been demonstrated as a promising approach to cancer treatment, whether derived from human umbilical cord, bone marrow, adipose tissue mesenchymal stem cells (MSCs), or dendritic cells.
Except for the occurrence of HCC, liver occupies a pivotal position for the metastatic organotropism of gastrointestinal cancers [7]. Organ-specific metastasis theories used to put emphasis on the intrinsic properties of cancer cells, such as breast cancer cells with chemokine receptors C-X-C motif receptor 4 (CXCR4) and C-C motif receptor 7 (CCR7), prefer the metastatic destination expressing CXCL12 (lymph nodes) and CCL21 (lung) [8]. Nowadays, tumor-derived exosomes have been proved to be critical for a well-prepared premetastatic niche [9]. The exosomal compositions vary from cells of different phenotypes and status under physiological or pathological conditions. Databases of Vesiclepedia [10], EVpedia [11], and Exocarta [12] have been established to describe exosomes and their corresponding methodology.
In this review, we summarize the multifaceted roles of exosomes in the tumor microenvironment in HCC and liver metastasis. The potential utility of exosomes as noninvasive biomarkers and in therapy for HCC is also discussed.
2. Exosomal Biology: Characteristics, Biogenesis, Excretion, and Integration
According to the consensus of International Society for Extracellular Vesicles (ISEV), extracellular vesicle (EV) serves as an umbrella term for secreted vesicles existing in the extracellular space, including exosome, microvesicle (MV), dexosome, tolerosomes, oncosome, and prostasome [29]. In the present review, exosomes among these ones are subjected to summarization for its biology and functions in hepatic carcinoma.
Exosomes, the 40–100 nm, rounded extracellular vesicles with lipid bilayer membrane [30], are first discovered to transport the transferrin receptor into intercellular space during the maturation of sheep reticulocytes in 1980s [31]. Nowadays, sequential ultracentrifugation method is widely applied to isolate the exosomes from body fluids or cell culture media [32]. The morphology of isolated exosomes is then identified by transmission electron microscopy (TEM), while their size distribution can be detected by nanoparticle tracking analysis (NTA) or dynamic light scattering (DLS). Furthermore, both western blot and flow cytometry reveal the markers specific to exosomes (e.g., CD9, CD63, and TSG101) [33].
In spite of their dynamic contents, exosomes are enriched in certain common proteins, such as tetraspanins (CD9, CD63, and CD81), membrane trafficking proteins (Rab proteins, ARF GTPases, and annexins), multivesicular body (MVB) formation-related proteins (ALIX, TSG101, and clathrin), heat shock proteins (HSP60, HSP70, and HSP90), and cytoskeletal proteins (actin and tubulin) [34]. Exceptionally, dendritic cell-derived exosomes are characterized by major histocompatibility complex class I (MHC-I), class II (MHC-II), costimulatory molecules (i.e., intercellular adhesion molecule 1 (ICAM-1)), and milk fat globule EGF factor 8 (MFG-E8), which engage in docking to recipient cells [35]. Moreover, HCV-infected human hepatoma cells (Huh7.5.1) produce exosomes containing complete HCV genomes, viral proteins, and particles. These exosomes are partially resistant to neutralizing antibodies and able to transmit infection to naïve Huh7.5.1 cells [36].
Unlike microvesicles (100–1000 nm), which are directly shed from plasma membranes [37], exosomes are originated from the intracellular MVBs. Early endosomes form by inward budding of the plasma membrane. Further invagination of the endosomal membrane, mainly initiated and driven by the endosomal sorting complexes required for transport- (ESCRTs-) dependent pathway, leads to the generation of MVBs [38]. This multiprotein complex includes ESCRT-0, -I, -II, and -III, together with accessory proteins (ALG-2 interacting protein X (ALIX), vacuolar protein sorting 4 (VPS4), and vesicle trafficking 1 (VTA1)) [39]. Mechanisms underlying these processes involve recognizing ubiquitinylated proteins, recruiting deubiquitinating enzymes, and sorting specific cargos into intraluminal vesicles (ILVs) in turn. The heterogeneous cargo, containing cytosolic proteins, nucleic acids, and lipids, can be selectively incorporated into ILVs [40]. Independent of ESCRT, a variety of small RNAs, instead of 18S and 28S ribosomal RNA, enrich in the Hep3B- or PLC/PRF/5-derived exosomes in a ceramide-dependent manner [40]. Intermediated MVBs either fuse with the lysosome for degradation or fuse with the cellular membrane to form exosomes [41]. Certain Rab GTPases serving as regulators of membrane trafficking, such as RAB11 [42]/RAB35 [43] or RAB27A/RAB27B [44], facilitate the release of exosomes harboring specific cargos. Additionally, other mechanisms have been proposed for the secretion of exosomes, such as the vesicle-associated membrane protein 7- (VAMP7-) [45], negative regulator-diacylglycerol kinase α- (DGKα-) [46], and PH-dependent processes [47]. Recipient cells interact with exosomes in a stepwise manner, comprising fusion with the cell membrane, endocytosis and receptor-ligand mediated recognition, and internalization, allowing the release of the exosomal content into the cytoplasm of recipient cells [48, 49].
3. Exosomes Underlie Primary HCC: An Essential Role for Intercellular Communication
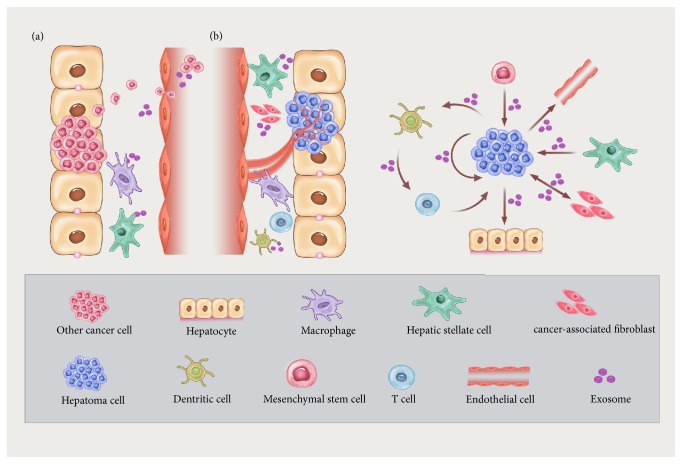
Exosomes contribute to each aspect of the interaction between intrahepatic cells, including CAFs, Kupffer cells, hepatic stellate cells (HSCs), endothelial cells, infiltrating immune cells, recruited MSCs, and cancer cells (Figure 1(b)).
Figure 1.
Exosomes exert regulatory effect on microenvironment by mediating the interaction of hepatic carcinoma cells and different types of liver cells. (a) Exosomes derived from tumors (e.g., gastric cancer, pancreatic ductal adenocarcinoma, breast cancer, and colorectal cancer) stimulate the macrophages (Kupffer cells) and hepatic stellate cells (HSCs) to facilitate a premetastatic niche in the liver. (b) Exosomes mediate the interplay between hepatocellular carcinoma (HCC) cells and different types of liver cells, including endothelial cells (EC), cancer-associated fibroblasts (CAFs), HSCs, mesenchymal stem cells (MSCs), and dendritic cells (DCs). In detail, HCC cells promote the EC-based vascularization, HSC activation, and hepatocyte malignization by exosomes. Exosomes from MSCs, HSCs, and CAFs inhibit the malignant phenotypes of HCC cells. Moreover, DCs stimulated by HCC cells-derived exosomes are involved in the T cell infiltration and activation in HCC.
3.1. HCC Cells
As is well known, HCC is composed of different heterogeneous subpopulations [5]. It is unclear how these tumor cells with diverse gene products closely connect with. Three metastatic HCC cell lines (HKCI-C3, HKCI-8, and MHCC-97L) shuttle protumorigenic molecules (i.e., MET protooncogene, S100 family member, and caveolins), both mRNA and proteins, into hepatocyte line (MIHA) via exosomes. Internalization of these exosomes activates PI3K/AKT and MAPK signaling and then promotes the migratory and invasive properties of MIHAs, which resembles the donor cells of exosomes [13]. In addition, exosomal miR-122 transferred from Huh7 to HepG2 affects the expression of miR-122-regulated genes in recipient cells. IGF-1-containing exosomes derived from HepG2 cells decrease the miR-122 level in Huh7 cells reciprocally [14]. A counteracting strategy is suggested to protect HepG2 against the exogenous miR-122 from neighboring cells, thus optimizing its microenvironment to survive and develop [14]. Furthermore, transforming growth factor-β activated kinase-1 (TAK1) has been implicated as a central target of recipient Hep3B cells in response to a set of miRNAs (i.e., miR-584, miR-517c, and miR-378) that are accumulated in Hep3B-derived exosomes [40]. TAK1, a MAP3K family member, functions as a gatekeeper for cell death and carcinogenesis of hepatocytes [50]. Exosome-mediated intercellular epigenetic modulation by miRNA resultantly trigger the loss of TAK1 with a purpose of sustaining the development of HCC [40]. Super-SILAC-based mass spectroscopy (MS) analyses have been conducted to compare the differentially expressed proteins of exosomes between nonmotile Hep3B cells and the motile MHCC-97H and LM3 cells. The glycolytic enzyme of GADPH, together with hypoxia-associated proteins caveolin-1 and calpain 1 (CAPN1), demonstrates remarkable upregulation in motile HCC cell-derived exosomes [51]. Mechanically, HCC cells under hypoxic conditions are prone to choose glycolysis as energy supply instead of mitochondrial oxidation. They acquire high motility and generate related-regulatory proteins in response to the hypoxia and related metabolic alteration [52].
3.2. Endothelial Cells
During tumor progression, neovasculature underlies the consumption of nutrients and oxygen, as well as the transportation of metabolic wastes and carbon dioxide, of cancer cells. Accordingly, it is essential to figure out the regulation mechanisms that activate the “angiogenic switch” so as to suppress tumor metastasis [53]. Exosomes have been reported to act as carrier of vasorin (VASN) transferred from HepG2 to human umbilical vein endothelial cells (HUVECs) via heparin sulfate proteoglycans- (HSPGs-) mediated endocytosis. Increased migration, but not proliferation, is observed in HUVECs after their uptake of VASN-containing exosomes [15]. Additionally, cancer stem cell-like CD90+ Huh7 cells, which express high levels of long noncoding RNA (lncRNA) H19, influence the endothelial phenotype through the release of exosomes. Compared with the effects of Huh7-secreted exosomes (Huh7-exo), HUVECs treated with exosomes from CD90+ Huh7 (CD90+ Huh7 exo) exhibit higher expression of VEGF/VEGF-R1, tubular-like structure, and increased cell adhesion [16]. Given the angiogenic effects related to VEGF/VEGF-R1 signaling, the exosome-induced VEGF/VEGF-R1 expression, and the conversion of endothelial cell phenotype seems to be a prerequisite for intravasation or extravasation of metastatic cells [53].
3.3. Stromal Cells
Most cases of HCC arise in desmoplastic context; therefore, it is vital to seek effective approaches to disrupt the symbiotic cancer cell-CAF environment. Studies show that CAFs are conducive to tumor metastasis by remodeling the extracellular matrix (ECM) through secreting matrix metalloproteinases (MMPs) or by enhancing vascular mimicry formation of HCC cells through producing TGF-β and stromal cell-derived factor 1 (SDF1) [54, 55]. Except for the ECM remodeling by cytokines, CAFs play beneficial roles in HCC tumorigenesis and development through exosomes-based delivery of miRNAs. miR-320 is distinctly downregulated in HCC cells, CAFs, and para-cancer fibroblasts (PAFs) upon activation with TGF-β. Consistently, RNA sequencing indicates the lowered miR-320a level in CAF-derived exosomes in comparison to that of PAFs-derived ones [17]. Given the ameliorative effect of miR-320a against tumor phenotype by targeting PBX3, limited introduction of exogenous miR-320a in HCC might be involved in tumorigenesis [17].
By contrast, exosomal miR-1247-3p from high-metastatic HCC cells could convert normal fibroblasts into CAFs by suppressing β-1, 4-galactosyltransferases III (B4GALT3). The activation of β1-integrin-NF-κB signaling in fibroblasts enhances exosomal secretion of IL-6 and IL-8, promoting tumor stemness, epithelial-mesenchymal transition (EMT), and chemoresistance in liver cancer [18]. Besides, high serum level of exosomal miR-1247-3p positively correlates to lung metastasis in HCC patients [18]. Among the stromal cells, human hepatic stellate cells (LX2) have been revealed to load tumor suppressor-miR-335 into HCC-targeting exosomes. miR-335-5p-containing exosomes lead to the repression of HCC proliferation and invasion in vitro, and the shrinkage of HCC tumors in vivo [19].
3.4. Immune Cells
HCC is an intractable challenge because of the absence of potent immune response. However, tumor cell-derived exosomes (TEXs), which carry multiply antigens (i.e., AFP, glypican 3, and HSP-70), trigger a dendritic cells- (DCs-) mediated immune response much stronger than that of cell lysates [20]. The tumor immune microenvironment is also significantly improved in orthotopic HCC mice, manifested by increased CD8+ T lymphocytes infiltration, elevated levels of interferon-γ (IFN-γ), and decreased levels of interleukin-10 (IL-10) and TGF-β [20]. Therefore, TEXs-pulsed DC-based immunotherapy might boost the antigen presenting ability and activate cellular immunity sufficiently to counteract the immunotolerance.
4. Exosomes Precede Metastatic Hepatic Carcinoma: Creation of a Malignant Niche
Metastasis is responsible for a large majority of cancer-related death [56] and reflects a succession of biological events, involving local invasion, intravasation, extravasation, and colonization [53]. Accumulating evidences suggest that tumor-derived exosomes are pioneers that prepare the premetastatic niche, which is created prior to the arrival of disseminating tumor cells, providing a “favorable soil” for the seeding of tumor cells in host tissue [57] (Figure 1(a)).
4.1. Gastric Cancer
Investigating the liver metastasis of gastric cancer, Zhang et al. show that exosomes are capable of transporting membrane receptor-epidermal growth factor receptor (EGFR) into liver stromal cells, such as Kupffer cells and HSCs. This translocation of EGFR promotes the upregulation of hepatic growth factor (HGF) in stromal cells, mainly by suppressing miR-26a/b. As a result, the activation of HGF receptor c-MET results in favorable conditions for organ-specific metastasis [21]. EGFR-containing exosomes, therefore, determine the liver-specific metastasis of gastric cancer, based on microenvironment remodeling.
4.2. Pancreatic Ductal Adenocarcinoma (PDAC)
Integration of PDACs-derived exosomes and Kupffer cells (KCs) has been proven to induce a proinflammatory and profibrotic microenvironment in the liver. Notably, macrophage migration inhibitory factor (MIF), which is enriched in PDAC-derived exosomes, causes the secretion of TGF-β, which, in turn, leads to the activation of HSCs and ECM remodeling. Ultimately, fibronectin deposition promotes the recruitment of bone marrow-derived macrophages to the liver, producing a favorable niche for the liver metastasis of PDACs [22].
4.3. Breast Cancer
Consistent with the “seed and soil” hypothesis [58], exosomes from liver-tropic breast and pancreatic cancer cells have been found to preferentially assemble in predictive metastatic sites. The specific integrin expression pattern of tumor-derived exosomes has already been shown to govern the future colonization by tumor cells [23]. In detail, integrin subtypes of α6β4 and α6β1 in these exosomes exhibit a close association with lung metastasis by targeting fibroblasts and epithelial cells. Accordingly, exosomal integrin αvβ5 determines the formation of premetastatic niche in the liver through binding to KCs, accompanied by the upregulated expression of proinflammatory gene-S100P and S100A8 of KCs [23].
4.4. Colorectal Cancer (CRC)
After the injection of exosomes collected from a colorectal cancer cell line (HT-29) with liver-targeted metastatic properties, the mice implanted with Caco-2 cells (low metastatic colorectal cancer cell line) show an increase in the hepatic burden of metastasis via the SDF-1α/CXCR4 axis [59]. Hence, considering the distinct functional components within exosomes in colorectal cancer cells with different metastatic properties, specific exosomal miRNAs may serve as indicator of the liver metastasis of colorectal cancer. Although the expression level of miR-203 in tumor tissues is negatively correlated with metastatic potential [60], high expression of serum exosomal miR-203 in patients with CRC predicts poor prognosis and liver metastasis. Exosomal miR-203 from CRC cells could induce the formation of M2 tumor-associated macrophages (TAMs) in the host organ, which constitute a premetastatic niche and promote liver metastasis [24]. In contrast to exosomes isolated from naïve colon tissue and primary colon tumors, exosomes originated from liver metastasis of colon tumors display higher levels of tumor-suppressive miRNAs. In primary colon tumor and liver metastasis of colon tumor, tumor-suppressive miRNAs (miR-18a and miR-193a) are enriched in exosomes rather than their parent cells. Selective sorting protein-major vault protein (MVP), which binds to miR-193a, might induce miR-193a accumulation in exosomes instead of in their donor cells [61]. Interestingly, oncogenic miR-21 shows more enrichment in the primary colon tumor tissue and metastatic colon tumor in the liver as compared to their exosomes [61]. These studies indicate that colon cancer may discharge those miRNAs, which have adverse effects on its progression and liver metastasis, into serum via exosomes.
5. Exosomes Identify HCC: Biomarkers for Noninvasive Diagnosis and Follow-Up Monitoring
The characteristics of exosomes represent appealing and promising biomarkers for the diagnosis and monitoring of cancer. (1) Exosome contents reflect the real-time status of their originated cells with high sensitivity. (2) Exosomes are capable of keeping their cargos intact because of their stable structure, which is resistant to degradation by circulating ubiquitous RNases and proteases. (3) Exosomes are shed into biological fluid, thus providing a noninvasive method to identify tumor-bearing patients. (4) High-enrichment of tumor markers in exosomes compared with their low baselines in body fluids increases the signal-to-noise ratio upon analysis [62].
To select serum exosomal miRNAs as potential biomarkers, Sohn et al. report that exosomal levels of miR-18a, miR-221, miR-222, and miR-224 are higher in patients with HCC than in those with chronic hepatitis (CHB) or liver cirrhosis (LC). However, exosomal miR-101, miR-106b, miR-122, and miR-195 show the reverse tendency [63]. Microassay profiling in cases of HCC identifies that decreased miR-718 level in serum exosomes is positively correlated with recurrence following liver transplantation (LT) and poor prognosis of patients with HCC [64]. Despite miRNAs (miR-423-5p and miR-21-5p) being enriched in both cellular and exosomal compositions of SMCC-7721 (HCC cell line), disparities in the miRNA pattern between exosomes and their donor cells still exist. While highly abundant let-7d-5p, let-7b-5p, and let-7c-5p are observed in exosomes rather than cells, miR-486-5p and miR-10b-5p are elevated within cells only [65]. This implies that sequestering miRNAs in exosomes is an active biological process [65]. In addition to dysregulated miRNAs, increased lncRNA TUC339 is also detected in HCC-derived exosomes and is positively correlated with proliferation and cell adhesion of HCC [66].
Recent study analyzes proteome profiles of serum exosomes isolated from cholangiocarcinoma (CCA), primary sclerosing cholangitis (PSC), patients with HCC, and healthy individuals. A variety of differentially expressed proteomic signatures are determined. Galectin-3-binding protein (LG3BP) and polymeric immune receptor (PIGR) in serum exosomes show better diagnostic value for HCC (area under the curve (AUC): LG3BP = 0.904 and PIGR = 0.837, respectively) compared with that of alpha fetoprotein (AFP) (AUC: 0.802), which is most commonly used as a noninvasive serum indicator of HCC. However, PIGR appears to be a nonspecific, inflammation-related marker. Moreover, the high levels of exosomal pantetheinase (VNN1), C-reactive protein (CRP), fibrinogen gamma chain (FIBG), immunoglobulin heavy constant alpha 1 (IGHA1), alpha-1-acid glycoprotein 1 (A1AG1), and gamma-glutamyltransferase 1 (GGT1) in CCA might benefit the differential diagnosis of intrahepatic CCA (iCCA) versus HCC [67]. Given the complexity of tracking the origin of molecular signatures and the lack of standardization of assessment, further efforts are needed to replace traditional biomarkers with exosome-based biomarkers.
6. Exosomes Cure HCC: Therapeutic Application of Exosomal Modification
6.1. Modulation of Exosomal Excretion
Emerging evidence identifies an essential mechanism of preserving cellular homeostasis in presenescent cells by loading harmful cytoplasmic DNA fragments into exosomes under physiological conditions [68]. Likewise, the selective release of specific molecules into exosomes has a fundamental impact on their parental cells during tumor progression.
Silencing of RAB27A or RAB27B in bladder carcinoma prevents the discarding of tumor-suppressive miR-23b and miR-921 into exosomes, which affect the acquisition of metastatic properties [69]. Vps4A, which is frequently downregulated in HCC, has been proven to be a crucial regulator of exosomes biogenesis by targeting ESCRT-III [70]. Restoration of Vps4A alters the secretion and uptake of exosomal miRNAs in HCC cells. In brief, SMCC-7721 cells transfected with Vps4A tend to package oncogenic miRNAs (miR-27b-3p and miR-92a-3p) into exosomes and accumulate tumor suppressor miRNAs (miR-193a-3p, miR-320a, and miR-132-3p) inside the cells. Being compared to those in the SMCC cells coincubated with Vps4A-lacking exosomes (SMCC-ctrl-exo), five tumor-suppressive miRNAs (miR-122-5p, miR-33a-5p, miR-34a-5p, miR-193a-3p, miR-16-5p, and miR-29b-3p) are proved to be upregulated in Vps4A-overexpressing SMCCs exposure to SMCC-ctrl-exo [65]. In contrast to the Vps4A-dependent exosome-mediated interchange of specific miRNAs, inhibition of exosome secretion provokes the reactive oxygen species- (ROS-) dependent DNA damage response and then triggers senescence-like, cell-cycle arrest or apoptosis [68].
6.2. Immunotherapy
Rao et al. [20] have demonstrated that HCC-derived exosomes, which could serve as carrier of multiple antigens, trigger a DC-mediated immune response stronger than lysates. Suppression of tumor growth has also been observed in ectopic and orthotopic HCC mice treated with this kind of TEXs-pulsed DCs. In addition, these TEXs could be a source of shared antigens for tumors of different origins. DCs pulsed with TEXs from Hepa1-6 (mouse HCC cell line) resultantly provide cross protective effects against allogeneic H22 (mouse HCC cell line) and MHC-matched pancreatic cancer cells. Moreover, tumor-specific cytolysis is observed in human HCC cells (Hep3B, LM3) treated with activated DCs by TEXs from HepG2, independent of HLA types [20].
In contrast, exosomes derived from AFP-expressing DCs (DEXAFP) elicit an antigen-specific antitumor immune response, especially in the diethylnitrosamine- (DEN-) induced autochthonous HCC model. Serving as a cell-free vaccine, DEXAFP improve the immune-suppressive tumor milieu via activation of CD8+ cytotoxic T lymphocytes (CTLs) and elevated IFN-γ and IL-2 levels, but fewer CD25+/Foxp3+ regulatory T (Treg) cells and decreased IL-10 and TGF-β levels [71]. Thus, tumor-specific antigen-modified DEXs present a new pathway for HCC immunotherapy.
In terms of DC-mediated immunotherapy, a phase II clinic trial using exosomes derived from IFN-γ-matured DCs (IFN-γ-DEX) is being conducted in patients with advanced nonsmall-cell lung carcinoma (NSCLC) at 4 months after platinum-based chemotherapy. Benefitting from the enhanced T cell response, DEX-based maintenance immunotherapy yields improved progression-free survival. Thirty-two percent of patients who received IFN-γ-DEX therapy have achieved stabilization for 4 months or more. The median overall survival (OS) for all patients is 15 months, with a survival rate at 6 months of 86%, at 1 year of 55%, and at 2 years of 25% [72].
6.3. Chemosensitivity
Resistance to chemotherapeutic drugs is a major issue of treatment failure of cancers. And exosomes are proved to mediate the horizontal transfer of property of chemoresistance among different populations of HCC cells. Qu et al. show that HCC cells (MHCC-97 L and MHCC-97H) secreted exosomes prompt sorafenib resistance and inhibit sorafenib-induced apoptosis of SMCC-7721 cells in vitro and in vivo [25]. Exosomes derived from highly invasive HCC cell (MHCC-97H) have greater effect than those from the less invasive cell (MHCC-97L) [25]. The intercellular delivery of HGF, and subsequently activation of HGF/c-MET/Akt signaling, might account for the acquired sorafenib resistance [25].
Except for the intercellular transfer of drug-transporters [73], genetic cargos within tumor-derived exosomes refer to another mechanism that limits the response of target cells to anticancer drugs. Takahashi et al. attribute the TGFβ-induced tumor cells dedifferentiation and acquired chemoresistance to the deregulated linc-ROR in HepG2 cells, whereas sorafenib exposure increases linc-ROR in HCC cells, HCC-derived exosomes, and exosome-treated recipient cells [26]. Similarly, chemotherapeutic stress (e.g., sorafenib, camptothecin, and doxorubicin) leads to linc-VLDLR upregulation in both HCC cells (HepG2) and exosomes. Exosomal delivery of linc-VLDLR increases the expression of ABCG2 (ATP-binding cassette, subfamily G member 2) [27], which guarantees an inadequate concentration of the toxicant via drug export [74]. Thus, a chemoresistant property is conferred on the recipient HCC cells. In addition, the intercellular delivery of HGF and subsequently activation of HGF/c-MET/Akt signaling might account for the acquired sorafenib resistance [25]. Since the EMT-dependent deprivation of chemosensitivity [75], the role of HCC-derived exosomes in driving EMT may also be one of the determining factors in chemoresistance [76].
6.4. Exosomal Therapy Based on Mesenchymal Stem Cells
Exosomes possess promising properties of vehicles, including biocompatibility, inherent stability, and selective integration at the targeted site [21, 32]. They are also able to penetrate the blood-brain barrier, endure modification, and load drugs [77, 78]. Moreover, MSCs can mobilize into the tumor microenvironment [79]. MSC-secreted exosomes, therefore, are considered a prospective research hotspot for therapeutic application with feasibility and effectiveness against tumors.
Exosomes originating from human umbilical cord MSCs have been proposed to counteract 5-fluorouracil-induced apoptosis and abrogate chemosensitivity in gastric cancer. Mechanically, MSC-derived exosomes induce the activation of the CaM-Ks/Raf/MEK/ERK pathway in gastric cancer cells and further promote the expression of multidrug resistance associated proteins, such as multidrug-resistance protein (MDR), multidrug-resistance like protein 1 (MRP), and lung resistance protein (LRP) [80]. MSC-derived exosomes also facilitate angiogenesis and stimulate tumor growth when coinjected with tumor cells in nude mice [81].
When the murine MSC line (SR4987) is loaded with Paclitaxel (PTX), the PTX-MSC-derived exosomes demonstrate the capacity to attenuate the proliferation of a pancreatic cell line (CFPAC-1) [82]. Employing the Cy5-labelled tracking method, Jessian et al. verify the anti-miR-9 delivery from bone marrow- (BM-) MSCs to glioblastoma multiforme (GBM) via exosomes. Then exogenous anti-miR-9 then reverses the expression of P-glycoprotein (P-gp) and sensitizes GBM to temozolomide (TMZ) [83]. Another HCC model is constructed in Fischer-344 rats by subcutaneous inoculation with HCC cells obtained from N1S1 rats. However, treatment with exosomes, being secreted by adipose-derived mesenchymal stem cells (ADMSCs), produce smaller, lower grade tumors that harbor an increased natural killer T (NKT) cell response [84]. Another study shows that intratumoral injection of miR-122-modified ADMSC-derived exosomes, combined with sorafenib, could significantly reduce tumor volume and weight [28]. Although 122-exo could alter the expression of target genes, such as those encoding cyclin G1 (CCNG1), disintegrin and metalloprotease 10 (ADAM10), and insulin-like growth factor receptor 1 (IGF1R) in hepatoma cells, 122-exo alone failed to inhibit tumor growth in a HepG2 cell xenograft model in nude mice [28].
7. Conclusion
The exosome-mediated intercellular transfer of biomolecules has been demonstrated in a myriad of process associated with HCC onset, progression, and poor response to chemotherapy (Table 1). The occurrence and propensity of liver metastasis may be ascribed to exosomes derived from primary cancer cells. In addition, exosomes possess promising characteristics of biomarkers, especially through monitoring their content of oncogenic and tumor-suppressive miRNAs. Importantly, exosome-based treatment holds great prospect in cancer by disrupting tumor cell homeostasis, activating immune response, and delivering chemotherapeutic agents. Despite all these achievements, further explorations are still needed to comprehensively evaluate the clinical application of exosomal functional molecules concerning the diagnosis and therapy of liver cancer.
Table 1.
Exosome-mediated cellular interaction involved in liver cancer.
| Donor cells | Components | Recipient cells | Functions | References |
|---|---|---|---|---|
| HKCI-C3 HKCI-8 MHCC-97L |
MET proto-oncogene, caveolins, S100 family |
MIHA | Migration, invasion | [13] |
|
| ||||
| Huh7 | miR-122 | HepG2 | Proliferation | [14] |
|
| ||||
| HepG2 | VASN | HUVECs | Migration | [15] |
|
| ||||
| CD90+ Huh7 | lncRNA H19 | HUVECs | Angiogenesis, adhesion | [16] |
|
| ||||
| CAFs | miR-320a | MHCC97-H | Proliferation, migration, metastasis | [17] |
|
| ||||
| CSQT-2 HCC-LM3 |
miR-1247-3p | Normal fibroblasts | Conversion into CAFs | [18] |
|
| ||||
| LX-2 | miR-335 | MHCC97H, MHCC97L, HepG2 and Huh7 | Proliferation, invasion, tumor shrinkage |
[19] |
|
| ||||
| Hepa1-6 |
α-AFP, glypican 3, HSP 70 |
DCs | T cell activation | [20] |
|
| ||||
| Gastric cancer | EGFR | Kupffer cells HSCs |
Liver metastasis | [21] |
|
| ||||
| Pancreatic ductal adenocarcinoma | MIF | Kupffer cells HSCs |
Liver metastasis | [22] |
|
| ||||
| Breast cancer | integrin αvβ5 | Kupffer cells | Liver metastasis | [23] |
|
| ||||
| Colorectal cancer | miR-203 | M2-TAMs | Liver metastasis | [24] |
|
| ||||
| MHCC-97H | HGF | SMCC-7721 | Chemoresistance | [25] |
|
| ||||
| HepG2 | linc-ROR | HepG2 | Chemoresistance | [26] |
|
| ||||
| HepG2 | linc-VLDLR | HepG2 | Chemoresistance | [27] |
|
| ||||
| ADMSCs | miR-122 | HepG2 | Sorafenib sensistivity | [28] |
ADMSCs: adipose-derived mesenchymal stem cells; CAFs: cancer associated fibroblasts; DCs: dendritic cells; HSCs: hepatic stellate cells; HUVECs: human umbilical vein endothelial cells; H22: murine hepatocarcinoma cell line; LX-2: hepatic stellate cells line; MIF: macrophage migration inhibitory factor; MIHA: immortalized hepatocyte cell line; and TAMs: tumor-associated macrophages.
Acknowledgments
This work is supported by the National Key Research and Development Plan “Precision Medicine Research”, no. 2017YFC0908903; National Natural Science Foundation of China (81070346, 81270492, 81470859, 81270491, and 81470840); State Key Development Program for Basic Research of China, no. 2012CB517501; 100 Talents Program, no. XBR2011007h; and Program of the Committee of Science and Technology, no. 09140903500.
Contributor Information
Yu-Qin Wang, Email: wangyuqin@xinhuamed.com.cn.
Qin Pan, Email: panqin@xinhuamed.com.cn.
Conflicts of Interest
The authors disclose no conflicts of interest.
Authors' Contributions
Fang Sun and Jin-Zhi Wang contributed equally to this paper.
References
- 1.Ghouri Y. A., Mian I., Rowe J. H. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. Journal of Carcinogenesis. 2017;16(1):1–8. doi: 10.4103/jcar.JCar_9_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R. L., Miller K. D., Jemal A. Cancer statistics, 2017. CA: A Cancer Journal for Clinicians. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Chen W., Zheng R., Baade P. D., et al. Cancer statistics in China, 2015. CA: A Cancer Journal for Clinicians. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Nishida N., Kitano M., Sakurai T., Kudo M. Molecular Mechanism and Prediction of Sorafenib Chemoresistance in Human Hepatocellular Carcinoma. Digestive Diseases. 2015;33(6):771–779. doi: 10.1159/000439102. [DOI] [PubMed] [Google Scholar]
- 5.Lee J.-S., Thorgeirsson S. S. Genome-scale profiling of gene expression in hepatocellular carcinoma: Classification, survival prediction, and identification of therapeutic targets. Gastroenterology. 2004;127:S51–S55. doi: 10.1053/j.gastro.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Birgani M. T., Carloni V. Tumor microenvironment, a paradigm in hepatocellular carcinoma progression and therapy. International Journal of Molecular Sciences. 2017;18(2, article no. 405) doi: 10.3390/ijms18020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bacigalupo M. L., Carabias P., Troncoso M. F. Contribution of galectin-1, a glycan-binding protein, to gastrointestinal tumor progression. World Journal of Gastroenterology. 2017;23(29):5266–5281. doi: 10.3748/wjg.v23.i29.5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müller A., Homey B., Soto H., et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 9.Deng Z., Rong Y., Teng Y., et al. Exosomes miR-126a released from MDSC induced by DOX treatment promotes lung metastasis. Oncogene. 2017;36(5):639–651. doi: 10.1038/onc.2016.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalra H., Simpson R. J., Ji H., et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotatio. PLoS Biology. 2012;10(12) doi: 10.1371/journal.pbio.1001450.e1001450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim D.-K., Kang B., Kim O. Y., et al. EVpedia: An integrated database of high-throughput data for systemic analyses of extracellular vesicles. Journal of Extracellular Vesicles (JEV) 2013;2(1) doi: 10.3402/jev.v2i0.20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathivanan S., Fahner C. J., Reid G. E., Simpson R. J. Exocarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Research. 2012;40(1):D1241–D1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He M., Qin H., Poon T. C. W., et al. Hepatocellular carcinoma-derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and RNAs. Carcinogenesis. 2015;36(9):1008–1018. doi: 10.1093/carcin/bgv081. [DOI] [PubMed] [Google Scholar]
- 14.Basu S., Bhattacharyya S. N. Insulin-like growth factor-1 prevents miR-122 production in neighbouring cells to curtail its intercellular transfer to ensure proliferation of human hepatoma cells. Nucleic Acids Research. 2014;42(11):7170–7185. doi: 10.1093/nar/gku346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang A., Dong J., Li S., et al. Exosomal transfer of vasorin expressed in hepatocellular carcinoma cells promotes migration of human umbilical vein endothelial cells. International Journal of Biological Sciences. 2015;11(8):961–969. doi: 10.7150/ijbs.11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conigliaro A., Costa V., Lo Dico A., et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Molecular Cancer. 2015;14(1, article no. 155) doi: 10.1186/s12943-015-0426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z., Li X., Sun W., et al. Loss of exosomal miR-320a from cancer-associated fibroblasts contributes to HCC proliferation and metastasis. Cancer Letters. 2017;397:33–42. doi: 10.1016/j.canlet.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Fang T., Lv H., Lv G., et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nature Communications. 2018;9(1) doi: 10.1038/s41467-017-02583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang F., Li L., Piontek K., Sakaguchi M., Selaru F. M. Exosome miR-335 as a novel therapeutic strategy in hepatocellular carcinoma. Hepatology. 2018;67(3):940–954. doi: 10.1002/hep.29586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao Q., Zuo B., Lu Z., et al. Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology. 2016;64(2):456–472. doi: 10.1002/hep.28549. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H., Deng T., Liu R., et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nature Communications. 2017;8:p. 15016. doi: 10.1038/ncomms15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costa-Silva B., Aiello N. M., Ocean A. J., et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nature cell biology. 2015;17(8):816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoshino A., Costa-Silva B., Shen T.-L., et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takano Y., Masuda T., Iinuma H., et al. Circulating exosomal microRNA-203 is associated with metastasis possibly via inducing tumor-associated macrophages in colorectal cancer. Oncotarget . 2017;8(45):78598–78613. doi: 10.18632/oncotarget.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qu Z., Wu J., Wu J., Luo D., Jiang C., Ding Y. Exosomes derived from HCC cells induce sorafenib resistance in hepatocellular carcinoma both in vivo and in vitro. Journal of experimental & clinical cancer research : CR. 2016;35(1):p. 159. doi: 10.1186/s13046-016-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi K., Yan I. K., Kogure T., Haga H., Patel T. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio. 2014;4:458–467. doi: 10.1016/j.fob.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi K., Yan I. K., Wood J., Haga H., Patel T. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Molecular Cancer Research. 2014;12(10):1377–1387. doi: 10.1158/1541-7786.MCR-13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lou G., Song X., Yang F., et al. Exosomes derived from MIR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. Journal of Hematology & Oncology. 2015;8(1, article no. 122) doi: 10.1186/s13045-015-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gould S. J., Raposo G. As we wait: Coping with an imperfect nomenclature for extracellular vesicles. Journal of Extracellular Vesicles (JEV) 2013;2(1) doi: 10.3402/jev.v2i0.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conde-Vancells J., Rodriguez-Suarez E., Embade N., et al. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. Journal of Proteome Research. 2008;7(12):5157–5166. doi: 10.1021/pr8004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan B. T., Johnstone R. M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–977. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 32.Kamerkar S., Lebleu V. S., Sugimoto H., et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu R., Greening D. W., Zhu H.-J., Takahashi N., Simpson R. J. Extracellular vesicle isolation and characterization: toward clinical application. The Journal of Clinical Investigation. 2016;126:1152–1162. doi: 10.1172/jci81129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roma-Rodrigues C., Raposo L. R., Cabral R., Paradinha F., Baptista P. V., Fernandes A. R. Tumor microenvironment modulation via gold nanoparticles targeting malicious exosomes: Implications for cancer diagnostics and therapy. International Journal of Molecular Sciences. 2017;18(1, article no. 162) doi: 10.3390/ijms18010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitt J. M., André F., Amigorena S., et al. Dendritic cell-derived exosomes for cancer therapy. The Journal of Clinical Investigation. 2016;126(4):1224–1232. doi: 10.1172/JCI81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramakrishnaiah V., Thumann C., Fofana I., et al. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proceedings of the National Acadamy of Sciences of the United States of America. 2013;110(32):13109–13113. doi: 10.1073/pnas.1221899110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Théry C. Exosomes: secreted vesicles and intercellular communications. F1000 Biology Reports. 2011;3(1, article 15) doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu X., Odenthal M., Fries J. Exosomes as miRNA Carriers: Formation–Function–Future. International Journal of Molecular Sciences. 2016;17(12):p. 2028. doi: 10.3390/ijms17122028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abels E. R., Breakefield X. O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cellular and Molecular Neurobiology. 2016;36(3):301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kogure T., Lin W. L., Yan I. K., Braconi C., Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54(4):1237–1248. doi: 10.1002/hep.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi R.-U., Prieto-Vila M., Hironaka A., Ochiya T. The role of extracellular vesicle microRNAs in cancer biology. Clinical Chemistry and Laboratory Medicine. 2017;55(5):648–656. doi: 10.1515/cclm-2016-0708. [DOI] [PubMed] [Google Scholar]
- 42.Savina A., Fader C. M., Damiani M. T., Colombo M. I. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6(2):131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 43.Hsu C., Morohashi Y., Yoshimura S.-I., et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. The Journal of Cell Biology. 2010;189(2):223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ostrowski M., Carmo N. B., Krumeich S., et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nature Cell Biology. 2010;12(1):19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 45.Fader C. M., Sánchez D. G., Mestre M. B., Colombo M. I. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2009;1793(12):1901–1916. doi: 10.1016/j.bbamcr.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Alonso R., Mazzeo C., Rodriguez M. C., et al. Diacylglycerol kinase α regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death & Differentiation. 2011;18(7):1161–1173. doi: 10.1038/cdd.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ban J. J., Lee M., Im W., Kim M. Low pH increases the yield of exosome isolation. Biochemical and Biophysical Research Communications. 2015;461(1):76–79. doi: 10.1016/j.bbrc.2015.03.172. [DOI] [PubMed] [Google Scholar]
- 48.Muller L., Simms P., Hong C.-S., et al. Human tumor-derived exosomes (TEX) regulate Treg functions via cell surface signaling rather than uptake mechanisms. OncoImmunology. 2017;6(8) doi: 10.1080/2162402X.2016.1261243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mulcahy L. A., Pink R. C., Carter D. R. F. Routes and mechanisms of extracellular vesicle uptake. Journal of Extracellular Vesicles (JEV) 2014;3(1) doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inokuchi S., Aoyama T., Miura K., et al. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proceedings of the National Acadamy of Sciences of the United States of America. 2010;107(2):844–849. doi: 10.1073/pnas.0909781107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J., Lu S., Zhou Y., et al. Motile hepatocellular carcinoma cells preferentially secret sugar metabolism regulatory proteins via exosomes. Proteomics. 2017;17(13-14) doi: 10.1002/pmic.201700103.1700103 [DOI] [PubMed] [Google Scholar]
- 52.Yang J., Wang C., Zhao F., et al. Loss of FBP1 facilitates aggressive features of hepatocellular carcinoma cells through the Warburg effect. Carcinogenesis. 2017;38(2):134–143. doi: 10.1093/carcin/bgw109.bgw109 [DOI] [PubMed] [Google Scholar]
- 53.Hanahan D., Weinberg R. A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 54.Yang J., Lu Y., Lin Y.-Y., et al. Vascular mimicry formation is promoted by paracrine TGF-β and SDF1 of cancer-associated fibroblasts and inhibited by miR-101 in hepatocellular carcinoma. Cancer Letters. 2016;383(1):18–27. doi: 10.1016/j.canlet.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 55.Liu J., Chen S., Wang W., et al. Cancer-associated fibroblasts promote hepatocellular carcinoma metastasis through chemokine-activated hedgehog and TGF-β pathways. Cancer Letters. 2016;379(1):49–59. doi: 10.1016/j.canlet.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 56.Chang R.-M., Yang H., Fang F., Xu J.-F., Yang L.-Y. MicroRNA-331-3p promotes proliferation and metastasis of hepatocellular carcinoma by targeting PH domain and leucine-rich repeat protein phosphatase. Hepatology. 2014;60(4):1251–1263. doi: 10.1002/hep.27221. [DOI] [PubMed] [Google Scholar]
- 57.dos Anjos Pultz B., Andrés Cordero da Luz F., Socorro Faria S., et al. The multifaceted role of extracellular vesicles in metastasis: Priming the soil for seeding. International Journal of Cancer. 2017;140(11):2397–2407. doi: 10.1002/ijc.30595. [DOI] [PubMed] [Google Scholar]
- 58.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer and Metastasis Reviews. 1989;8(2):98–101. [PubMed] [Google Scholar]
- 59.Wang X., Ding X., Nan L., et al. Investigation of the roles of exosomes in colorectal cancer liver metastasis. Oncology Reports. 2015;33(5):2445–2453. doi: 10.3892/or.2015.3843. [DOI] [PubMed] [Google Scholar]
- 60.Deng B., Wang B., Fang J., et al. MiRNA-203 suppresses cell proliferation, migration and invasion in colorectal cancer via targeting of EIF5A2. Scientific Reports. 2016;6 doi: 10.1038/srep28301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teng Y., Ren Y., Hu X., et al. MVP-mediated exosomal sorting of miR-193a promotes colon cancer progression. Nature Communications. 2017;8 doi: 10.1038/ncomms14448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bach D.-H., Hong J.-Y., Park H. J., Lee S. K. The role of exosomes and miRNAs in drug-resistance of cancer cells. International Journal of Cancer. 2017;141(2):220–230. doi: 10.1002/ijc.30669. [DOI] [PubMed] [Google Scholar]
- 63.Sohn W., Kim J., Kang S. H., et al. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Experimental & Molecular Medicine. 2015;47, article e184 doi: 10.1038/emm.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sugimachi K., Matsumura T., Hirata H., et al. Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. British Journal of Cancer. 2015;112(3):532–538. doi: 10.1038/bjc.2014.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei J.-X., Lv L.-H., Wan Y.-L., et al. Vps4A functions as a tumor suppressor by regulating the secretion and uptake of exosomal microRNAs in human hepatoma cells. Hepatology. 2015;61(4):1284–1294. doi: 10.1002/hep.27660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kogure T., Yan I. K., Lin W.-L., Patel T. Extracellular vesicle-mediated transfer of a novel long noncoding RNA TUC339: a mechanism of intercellular signaling in human hepatocellular cancer. Genes & Cancer. 2013;4(7-8):261–272. doi: 10.1177/1947601913499020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arbelaiz A., Azkargorta M., Krawczyk M., et al. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2017;66(4):1125–1143. doi: 10.1002/hep.29291. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi A., Okada R., Nagao K., et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nature Communications. 2017;8:15287–15298. doi: 10.1038/ncomms15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ostenfeld M. S., Jeppesen D. K., Laurberg J. R., et al. Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Research. 2014;74(20):5758–5771. doi: 10.1158/0008-5472.CAN-13-3512. [DOI] [PubMed] [Google Scholar]
- 70.Hurley J. H., Hanson P. I. Membrane budding and scission by the ESCRT machinery: It's all in the neck. Nature Reviews Molecular Cell Biology. 2010;11(8):556–566. doi: 10.1038/nrm2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu Z., Zuo B., Jing R., et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. Journal of Hepatology. 2017;67(4):739–748. doi: 10.1016/j.jhep.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 72.Besse B., Charrier M., Lapierre V., et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. OncoImmunology. 2016;5(4) doi: 10.1080/2162402X.2015.1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jaiswal R., Gong J., Sambasivam S., et al. Microparticle-associated nucleic acids mediate trait dominance in cancer. The FASEB Journal. 2012;26(1):420–429. doi: 10.1096/fj.11-186817. [DOI] [PubMed] [Google Scholar]
- 74.Guo Y.-Y., Wu Y., Jia X.-W., An W. Augmenter of liver regeneration potentiates doxorubicin anticancer efficacy by reducing the expression of ABCB1 and ABCG2 in hepatocellular carcinoma. Laboratory Investigation. 2017;97(12):1400–1411. doi: 10.1038/labinvest.2017.72. [DOI] [PubMed] [Google Scholar]
- 75.Chen J., Jin R., Zhao J., et al. Potential molecular, cellular and microenvironmental mechanism of sorafenib resistance in hepatocellular carcinoma. Cancer Letters. 2015;367(1):1–11. doi: 10.1016/j.canlet.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 76.Chen L., Guo P., He Y., et al. HCC-derived exosomes elicit HCC progression and recurrence by epithelial-mesenchymal transition through MAPK/ERK signalling pathway. Cell Death & Disease. 2018;9(5, article 513) doi: 10.1038/s41419-018-0534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim M. S., Haney M. J., Zhao Y., et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine: Nanotechnology, Biology and Medicine. 2018;14(1):195–204. doi: 10.1016/j.nano.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 78.Yang T., Martin P., Fogarty B., et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio Rerio. Pharmaceutical Research. 2015;32(6):2003–2014. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rivera-Cruz C. M., Shearer J. J., Figueiredo Neto M., Figueiredo M. L. The Immunomodulatory Effects of Mesenchymal Stem Cell Polarization within the Tumor Microenvironment Niche. Stem Cells International. 2017;2017:1–17. doi: 10.1155/2017/4015039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ji R., Zhang B., Zhang X., et al. Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer. Cell Cycle. 2015;14(15):2473–2483. doi: 10.1080/15384101.2015.1005530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu W., Huang L., Li Y., et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Letters. 2012;315(1):28–37. doi: 10.1016/j.canlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 82.Pascucci L., Coccè V., Bonomi A., et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. Journal of Controlled Release. 2014;192:262–270. doi: 10.1016/j.jconrel.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 83.Munoz J. L., Bliss S. A., Greco S. J., Ramkissoon S. H., Ligon K. L., Rameshwar P. Delivery of functional anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Molecular Therapy—Nucleic Acids. 2013;2, article e126 doi: 10.1038/mtna.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ko S.-F., Yip H.-K., Zhen Y.-Y., et al. Adipose-derived mesenchymal stem cell exosomes suppress hepatocellular carcinoma growth in a rat model: apparent diffusion coefficient, natural killer T-cell responses, and histopathological features. Stem Cells International. 2015;2015:11. doi: 10.1155/2015/853506.853506 [DOI] [PMC free article] [PubMed] [Google Scholar]