Abstract
The Rho family of guanine triphosphate hydrolases controls various cellular processes, including cell migration. We describe here the demonstration of a role for a RhoA GTPase homologue during cell migration in Caenorhabditis elegans. We show that eliminating or reducing rho-1 gene function by using a dominant-negative transgene or dsRNA interference results in a severe defect in migration of hypodermal P cells to a ventral position. Biochemical and genetic data also suggest that unc-73, which encodes a Trio-like guanine nucleotide exchange factor, may act as an activator of rho-1 in the migration process. Mutations in let-502 ROCK, a homologue of a RhoA effector in mammals, also cause defects in P cell migration, suggesting that it may be one of several effectors acting downstream of rho-1 during P cell migration. Finally, we provide evidence to support the idea that other small Rac subfamily small GTPases act redundantly and in parallel to RHO-1 in this specific cell migration event.
Keywords: rho-1‖rac‖unc-73
The Rho family of small GTPases, including the Rho, Rac, and Cdc42 subfamilies, are key regulators of the actin cytoskeleton. During a variety of cellular events, including polarization, shape reorganization, and migration, Rho GTPases serve to transduce signals between extracellular ligands and cytoskeletal reorganization (1). These switch proteins cycle between an inactive GDP-bound form and an active GTP-bound form. The active state of Rho proteins is directly regulated by guanine nucleotide exchange factors (GEFs), which catalyze the release of GDP and thereby facilitate GTP binding and subsequent activation. In mammalian cells and other systems, activated Rho family members have demonstrated roles in cytoskeleton reorganization, with distinct reorganization patterns for each subfamily member. For example, in Swiss 3T3 cells, overexpression of Rho, Rac, or Cdc42 results in the assembly and organization of stress fibers, lamellipodia, or filopodia, respectively (2). How the various Rho family GTPases are spatially and temporally regulated during developmental morphogenesis is not well understood.
Rho-1 is known to be required for early events in Drosophila development, such as dorsal closure and gastrulation (3, 4). In addition, the Rho GEF Pebble is specifically required for cytokinesis (5). Another Drosophila GEF, Trio, was recently shown to regulate the Rho family effector Pak through the GTPase Rac during axon guidance (6). Mutations in the Caenorhabditis elegans Trio homologue, unc-73, also cause defects in cell migration and axon guidance (7). Both Trio and unc-73 contain two GEF domains, each of which contains successive Dbl homology (DH) and pleckstrin homology (PH) domains. In the case of Trio, the two GEF domains have been shown to specifically activate different Rho GTPases (8, 9).
Studies of mutant alleles of unc-73 have led to the hypothesis that unc-73 is generally required for cell migrations in C. elegans and can regulate the activity of Rac (7). Potential targets of unc-73 include mig-2 and ced-10/rac-1, which encode proteins similar to mammalian Rac proteins, as the roles of these two GTPases in cell migration and axon guidance have been reported previously (10, 11).
The experiments described in this report examine the molecular basis of a cell migration event during C. elegans larval development. P cells are present on the sublateral surface of the C. elegans larvae on hatching (12). During mid-L1, the P cells are loosened from neighboring cells, and their leading edges begin to intercalate along the ventral cord as the leading edge of cytoplasm moves to a ventral contralateral position (13). Soon afterward, P cell nuclei move ventrally and come to rest at the ventral midline. Once migration is complete, each P cell divides to generate five neurons and one hypodermal cell in the C. elegans hermaphrodite. Descendants from three of these P cells are later induced to form the hermaphrodite vulva. We look specifically at the role of rho-1 in the P cells and provide evidence that rho-1 is required for their ventral migration.
Materials and Methods
Site-Directed Mutagenesis.
Mutagenesis was performed by using the Stratagene QuickChange kit. Primers (Operon Technologies, Alameda, CA, or GIBCO) were designed to make two mutations in C. elegans rho-1 and ced-10/rac-1. rho-1 T19N (dominant negative) and rho-1 G14V (constitutively active or gain of function) are referred to here as rho-1(dn) and rho-1(gf), respectively. Mutant cDNAs were cloned into multiple cloning site (MCS)II of the col-10 promoter (V. Ambros, personal communication) vector by using the NheI(5′) and SacI(3′) restriction sites. A cDNA encoding the Rho toxin C3 from Clostridium botulinum was obtained by PCR and inserted into the col-10 promoter vector by using KpnI sites.
The col-10 Promoter Vector.
The col-10 promoter vector was constructed from plasmid pPD49.26 containing two MCS and the 3′ untranslated region of unc-54 (gift from A. Fire, Carnegie Institute). Into MCSI, we cloned a 1.17-kb region of genomic DNA upstream of the col-10 translational start site by using XbaI (5′) and BamHI (3′). cDNAs of interest [C3, rho-1(dn), rho-1(gf), rac-1(dn)] were then cloned into MCSII by using NheI (5′) and SacI (3′).
The unc-73(+) minigene (7) was a gift from R. Steven and J. Culotti (University of Toronto, Toronto, ON). To establish transgenic lines, 10 ng/μl of unc-73(+) was injected along with 90 ng/μl of sur-5:GFP cDNA into unc-73(e936) animals. The sur-5∷GFP was used as an injection marker and results in green fluorescent protein (GFP) expression in all somatic cell nuclei (14). Several stable lines expressing the array were established, all of which were rescued for the unc-73(e936) P cell phenotype.
Strains and Matings.
The wild-type strain used was N2 Bristol. The following strains were also used: let-502(sb106) (15), let-502(h392), and mel-11(it26) (16); mig-2(mu28, null); mig-2(gm103, gf) (10); unc-73(e936); unc-73(rh40) (7, 17); and dpy-5/unc-73(gm40) and unc-47∷GFP integrated line (18). Other strains were obtained from the Caenorhabditis Genetics Center at the University of Minnesota.
The rho-1(dn) transgenic lines were made by injecting dpy-20 (e1282); unc-47∷GFP animals with 10 ng/μl of rho-1(dn) cDNA in the col-10 promoter vector and 25 ng/μl of rescuing dpy-20(+) DNA. Animals propagating this simple array were therefore dpy-20(e1282); unc-47∷GFP; kuEx130[col-10:rho-1(dn); dpy-20(+)]. The extrachromosomal array was integrated into the chromosome by using γ irradiation as described previously (19) to give dpy-20(e1282); unc-47∷GFP; kuIs52[col-10:rho-1(dn); dpy-20(+)]. We refer to this strain simply as rho-1(dn). Six stable integrated lines were established, all of which displayed a phenotype similar to each other and to the rho-1(dn) line expressing the extrachromosomal array. Transgenic lines expressing a rho-1(G14V, gf) transgene under control of the col-10 promoter were established by injecting the N2 strain with 10 ng/μl of rho-1(gf) cDNA in the col-10 promoter vector and 90 ng/μl of sur-5:GFP cDNA.
We made the mig-2(mu28); unc-73(e936) double mutant as follows. Males homozygous for the null mig-2 (mu28) allele, which is on the X chromosome, were crossed into unc-73(e936); dpy-6(e14) hermaphrodites (dpy-6 maps four map units from mig-2). unc-73 F2 progeny were obtained, and those animals unable to throw Dpy progeny were mig-2(mu28); unc-73(e936) double mutants. The Unc phenotype was severely exacerbated compared with that observed in unc-73(e936).
RNAi of let-50, mig-2, and rho-1.
RNAi results in the inactivation of a gene corresponding to injected double-stranded (ds)RNA sequences and was performed essentially as described by Fire et al. (20). For let-502, PCR primers were designed to amplify a 2.9-kb region of a let-502 cDNA provided by Paul Mains (University of Calgary; ref. 16). For mig-2, T7/T3 flanked PCR primers were designed to amplify a ≈600-bp piece of genomic mig-2 DNA, including most of exon 2. dsRNAi corresponding to mig-2 sequences was injected into homozygous ced-10/rac-1(n3246) animals. After 24–48 h, F1 progeny were scored for P cell migration in the second larval stage. For rho-1, primers were designed to amplify nearly all of a 625-nt cDNA. P cell migration success was scored (see below) in let-502(RNAi) larvae 24–36 h after injection of mothers. To score P cell migration success in rho-1(RNAi) escapers, animals injected with dsRNA were moved to individual plates 5 h after injections. The next day, nonwild-type (Unc, lumpy) larvae present on plates containing arrested embryos were observed under Nomarski optics and scored as described below.
Scoring P Cell Migration Success.
To score P cell migration success, animals were picked during L2 and observed under Nomarski optics. Ventral to the nerve cord, P1.p through P11.p nuclei were counted manually in the wild-type N2 strain, mutant, and transgenic animals. That defects were caused by cell migration was confirmed by observation of ectopic lateral neurons. When fewer than 11 Pn.p cells were observed, we checked the lateral surface for, and typically found, ectopically differentiated neurons. To score let-502(sb106) animals for P cell migrations, mothers kept at the permissive temperature (15°C) were shifted to 25°C and allowed to lay eggs for 3 h. Mothers were then removed, and the larvae were scored the next day at the L2 stage. To confirm P cell migration defects in various mutant and transgenic backgrounds, we crossed or injected into a strain containing an integrated copy of an unc-47∷GFP transgene (18). In this strain, UNC-47∷GFP is expressed in 26 GABA neurons, including 13 P cell-derived neurons present in the ventral cord in wild-type animals.
GDP/GTP Exchange Assays.
We refer to the DH1/PH1 and DH2/PH2 domains of UNC-73 (7) as GEF1 and GEF2, respectively. The UNC-73 GEF1 and GEF2 proteins used for exchange assays were produced in HeLa cells by using pSO19 and pSO20, as described (21). Briefly, HeLa cells were plated at a density of 5 × 106 cells/100-mm dish and were incubated for 18 h. HeLa cells were infected with T7 RNA polymerase recombinant vaccinia virus (LO-T7) (gift from Michinori Kohara, Tokyo Metropolitan Institute of Medical Science) and then transfected with 15 mg of pSO19, pSO20 or pGEM-HA. After transfection, cells were resuspended in 0.2 ml of ice-cold suspension buffer (25 mM Tris⋅HCl at pH 7.5/1 mM DTT/0.5 mM EDTA). The lysates were prepared by using a Dounce homogenizer, and insoluble materials were removed by centrifugation in a microcentrifuge for 15 min at 4°C. RhoA and Rac1 were purified from baculovirus-infected cells (provided by Yoshimi Takai, Osaka University) (22). Exchange assays were performed by using 30 μl of HeLa cell lysates and 2 pmol of [3H]-GDP-bound form of each small GTP-binding protein, as described (22).
Results
rho-1 Activity Is Required for P Cell Migration in C. elegans.
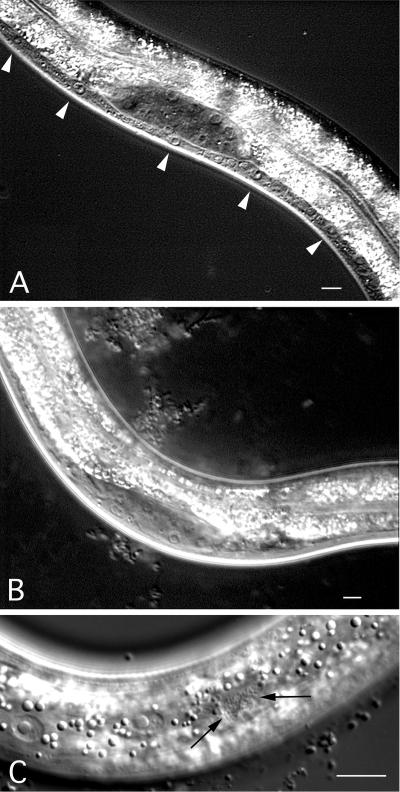
Analysis by using RNA interference indicates that the eliminating the rho-1 gene activity disrupts embryogenesis with arrested embryos, showing defects in cytokinesis (data not shown; ref. 23). To circumvent the lethality caused by rho-1(RNAi) and explore the role of rho-1 during the migration of P cells, we expressed a dominant-negative version of rho-1, rho-1(T19N) (see Discussion), under control of the col-10 promoter. The col-10 transcript is enriched during larval stages in hypodermal lineages (V. Ambros, personal communication), which include P cells. Expression of the col-10-driven rho-1(dn) from an extrachromosomal array resulted in a severe P cell migration defect (Table 1). Integration of the array into the genome had a similar and slightly stronger effect (Table 1). The P cell migration defect in rho-1(dn) animals was evident because of the lack of Pn.p cells in the ventral cord during the L2 stage (compare Fig. 1 A and B). In addition, we observed ectopic P cell-derived neurons on the lateral surface of rho-1(dn) animals, indicating that P cells were able to divide and differentiate somewhat normally in the absence of migration (Fig. 1C). The presence of ectopic neurons indicates that the rho-1(dn)-induced defect is caused by failed cell migration and not by a transformation of cell fate or nuclear migration defect, which leads to cell death (24, 25). These ectopic neurons are sometimes difficult to find but can be identified in ≈60% of rho-1(dn) animals.
Table 1.
P cell migration defects in rho-1 and unc-73 strains
| Genotype | No. of Pn.p cells migrated† | n |
|---|---|---|
| N2 | 11.0 ± 0.0 | 25 |
| Ex col-10:rho-1(T17N, dn) | 3.4 ± 1.0 | 25 |
| Is col-10:rho-1(T17N, dn) | 3.1 ± 0.7 | 25 |
| col-10:C3 | 5.9 ± 1.4 | 25 |
| rho-1(RNAi) escapers | 5.2 ± 1.7 | 22 |
| unc-73(gm40) | 7.1 ± 1.2 | 28 |
| unc-73(e936) | 7.6 ± 1.1* | 25 |
| Ex rho-1(G14V, gf) | 11.0 ± 0.0 | 25 |
| unc-73(e936);Ex rho-1(G14Vgf) | 9.8 ± 0.7* | 25 |
| unc-73(rh40) | 10.8 ± 0.4 | 25 |
Pn.p cells were scored during the L2 stage, as described in Materials and Methods. Ex, Exchromosomal array; Is, Integrated line. ±, SD.
, P value is <0.0001 when these data are compared by using Student's t test.
Data are expressed as the average number of Pn.p cells in the ventral cord during L2.
Figure 1.
Phenotype of rho-1(dn) animals. (A) Bristol N2 strain during the second larval stage with P cells (arrowheads) and a full ventral cord; (B) rho-1(dn) animals lacking P cells and many descendants thereof in the ventral cord. The ventral cord is very thin in this animal because of missing P cells. (C) Lateral surface of a rho-1(dn) animal during L2 animal showing ectopic P cell neuronal descendants (arrows). (Bar = 10 μm.)
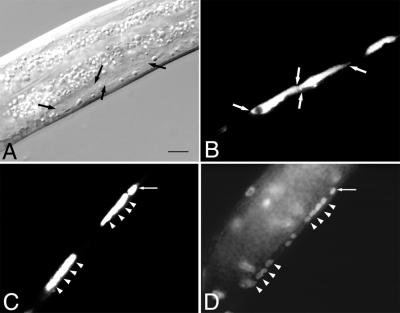
The rho-1(dn)-induced P cell migration defect resulted in Vulvaless animals unable to lay eggs (data not shown) or mate efficiently. In addition, rho-1(dn) expression caused severe uncoordination because of mislocalization and changes in the morphology of neurons normally found in the ventral cord. In rho-1(dn) animals, several ventral cord neurons, including some GABA neurons, which are marked by an unc-47∷GFP transgene (18), assume a long extended shape and appear to be significantly larger than their wild-type counterparts (Fig. 2 A and B). Staining of rho-1(dn) animals with the DNA dye 4′,6-diamidino-2-phenylindole (DAPI) revealed many, but not all, of these cells to be multinucleate (Fig. 2 C and D), suggesting rho-1(dn) can cause a cytokinesis defect in these cells (23).
Figure 2.
rho-1(dn) can cause a cytokinesis defect. (A) Abnormally large neurons (flanked by arrows) in a living rho-1(dn) animal during the second larval stage. (B) Fluorescent visualization of the unc-47∷GFP-positive cells (flanked by arrows) in the animal shown in A. (C) Fluorescent visualization of unc-47∷GFP-positive neurons in a fixed rho-1(dn) animal. (D) DAPI visualization of the multiple nuclei observed in the unc-47∷GFP-positive cells shown in C. Arrowheads in C and D denote the positions of nuclei as determined by DAPI staining. Arrows in C and D show an unc-47∷GFP-positive cell without the cytokinesis defect. (Bar = 7 μm.) In wild-type animals (see Fig. 1A), multiple neurons are clearly visible in the same region of a multinuclear cell in the mutant.
We used two methods to test the hypothesis that the rho-1(dn) transgene specifically reduced rho-1(+) activity, as opposed to interfering with other Rho family GTPases. First, we expressed the C. botulinum toxin C3 from the col-10 promoter. C3 ADP-ribosylates and specifically inactivates rho-1; it appears to have no toxic effect on other Rho family proteins like Rac and Cdc-42 (26). Expression of col-10:C3 in a wild-type background caused a severe P cell migration defect (Table 1). Secondly, we showed that reducing rho-1(+) activity by using RNAi results in failed P cell migrations. Injection of dsRNA corresponding to rho-1 sequences caused embryonic arrest, as reported above. However, a small percentage of early progeny from rho-1 dsRNA-injected hermaphrodites escaped the embryonic lethal phenotype and developed as uncoordinated kinky larvae. This effect is because of maternal rescue of the RNAi effect in the more proximal oocytes of dsRNA-injected hermaphrodites (27, 28). We scored P cell migration success in these rho-1(RNAi) escapers and found moderate to severe P cell migration defects similar to those observed in col-10∷C3 transgenic animals (Table 1). When rho-1(RNAi) escapers were examined in the unc-47∷GFP background, we again observed the P cell migration defect. In addition, we saw a reduction in the number and changes in the morphology of GABA neurons in the ventral cord similar to the phenotype seen in rho-1(dn) animals (data not shown; Fig. 2 A and B). The rho-1(RNAi) escapers grew to adulthood but were sterile because of defective gonad development. The combined results of C3 expression and rho-1(RNAi) escapers indicate clearly that rho-1(+) activity is required for P cell migration.
unc-73 May Act as an Exchange Factor for rho-1 During P Cell Migration.
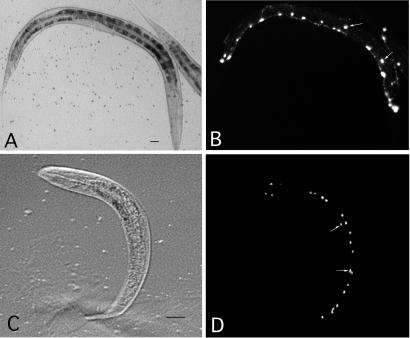
GEFs catalyze the release of GDP from RHO-1 to facilitate GTP binding and RHO-1 activation. Mutations in unc-73 cause axon guidance defects and failed migrations in some cell types, including P cells (7, 29–31). We show here that two unc-73 alleles, gm40 and e936, cause similar partial P cell migration defects (Table 1). These P cell migration defects are observed in nearly all unc-73 mutant animals, each showing a few affected cells. gm40 and e936 were previously characterized as putative null and partial loss-of-function mutation, respectively (7). unc-73(e936) and unc-73(gm40) display a similar penetrance of the cell migration phenotype. Because gm40 homozygous animals are very unhealthy due to severe but incomplete embryonic and larval lethality, they are very difficult to score for migration analysis; therefore, we used e936 for further study. To easily monitor the success of P cell migrations toward the ventral cord, we examined unc-73(e936) animals in the unc-47∷GFP background (ref. 18; see Materials and Methods). In unc-73(e936); unc-47∷GFP animals, some P cell-derived GABA neurons normally found in the ventral cord were observed on the lateral surface of animals during the second larval stage (Fig. 3 A and B). This observation confirms that some P cells fail to migrate ventrally and continue to differentiate in an ectopic position in unc-73(e936) animals. The P cell defect of unc-73(e936) animals was partially rescued by the col-10 promoter-driven expression of rho-1(gf) (Table 1). Although the non-null nature of the unc-73(e936) allele makes such genetic interaction open to other interpretations, this result is consistent with rho-1 acting downstream of unc-73. The rho-1(gf) transgene alone displayed normal P cell migrations.
Figure 3.
unc-47∷GFP visualization of P cell-derived neurons in strains with P cell migration defects. (A) Nomarski and (B) fluorescent image of unc-73(e936) in the unc-47∷GFP background. (C) Nomarski and (D) fluorescent image of a let-502(RNAi) larva in the unc-47∷GFP background. Mislocalized GABA neurons are indicated with white arrows.
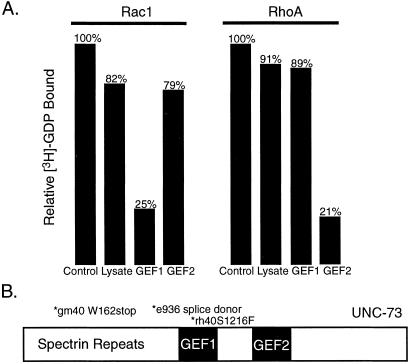
We sought biochemical evidence that UNC-73 could act as an exchange factor for RHO-1. The exchange activities of GEFs are conferred by consecutive DH/PH domains (1, 32). Two such DH/PH domains are present in UNC-73 (Fig. 4B; ref. 7) and are referred to here as GEF1 and GEF2. Previously, an UNC-73 fusion protein containing only GEF1 was shown to exhibit exchange activity toward mammalian RAC1 (7). To directly determine whether either of the UNC-73 GEFs could catalyze GDP exchange from RHO, we expressed the GEF1 and GEF2 domains of UNC-73 separately in HeLa cells and measured their ability to catalyze the release of [3H]-GDP from mammalian RHOA and RAC1. UNC-73 GEF1 was able to efficiently release [3H]-GDP from RAC1 but not from RHOA (Fig. 4A). In contrast, UNC-73 GEF2 exhibited significant exchange activity only toward RHOA (Fig. 4A). These results indicate that GEF1 and GEF2 act specifically on RAC and RHO, respectively, in vitro. Given the high degree of homology between RHOA and C. elegans RHO-1 protein (see Discussion), it is reasonable to suggest that the UNC-73 GEF2 domain has the exchange activity on RHO-1 in vivo.
Figure 4.
GDP/GTP exchange assays. HeLa cell extracts expressing GEF1 or GEF2 of UNC-73 were assayed for their ability to catalyze GDP release from RhoA and Rac1, as described in Materials and Methods. (A) Relative amounts of bound [3H]-GDP after incubation of GEF1 or GEF2 with Rac1 or RhoA. (B) Schematic diagram of UNC-73.
The unc-73(rh40) allele contains a missense mutation in GEF1 that eliminates its exchange activity (7). unc-73(rh40) mutants exhibit no significant P cell migration defect (Table 1, Fig. 4B), consistent with the possibility that GEF2 alone can activate RHO-1. However, there is no solid in vivo evidence to support a model that GEF1 and GEF2 of UNC-73 act specifically on Rac and Rho subfamily proteins, respectively, in C. elegans.
let-502 ROCK Activity Is Required for P Cell Migration.
let-502 encodes a Rho-kinase, and existing mutations in this gene result in larval arrest and elongation failure (16). In an attempt to identify downstream effectors of rho-1 during P cell migration, we characterized the P cell migration success rate of several let-502-deficient animals. A strong loss-of-function allele of let-502(h392) exhibits a weak but reproducible P cell defect, and a similar P cell phenotype was also observed in a temperature-sensitive hypomorphic allele, let-502(sb106) (Table 2). Further, let-502(RNAi) causes an embryonic elongation phenotype similar to the h392 allele and exhibits a comparable P cell migration defect (Table 2). When let-502(RNAi) was performed in the unc-47∷GFP background, GABA neurons normally found ventrally were observed on the lateral surface of affected animals during the second larval stage (Fig. 3 C and D). Taken together, these observations suggest that let-502 activity is required for wild-type P cell migration. Consistent with let-502 acting downstream of rho-1, let-502(sb106);rho-1(gf) animals did not exhibit rescue of the let-502(sb106) defect (Table 2).
Table 2.
P cell migration in let-502, mig-2, and ced-10 strains
| Genotype | Pn.p cells migrated‡ | n |
|---|---|---|
| N2 | 11.0 ± 0.0 | 25 |
| let-502(h392) | 8.4 ± 1.1 | 25 |
| let-502(sb106), 15°C | 11.0 ± 0.0 | 20 |
| let-502(sb106), 20°C | 8.5 ± 1.5 | 20 |
| let-502(RNAi) | 8.5 ± 1.4 | 20 |
| let-502(sb106);Ex rho-1(G14Vgf) | 8.5 ± 1.5 | 20 |
| Ex col-10:ced-10 (T17N, dn) | 11.0 ± 0.0 | 25 |
| ced-10(n3246) | 11.0 ± 0.0 | 25 |
| mig-2(mu28) | 11.0 ± 0.0 | 25 |
| ced-10(n3246); mig-2(RNAi) | 9.0 ± 1.3 | 10 |
| unc-73(e936) | 7.6 ± 1.1 | 25 |
| mig-2(mu28); unc-73(e936) | 4.8 ± 1.1* | 20 |
| mig-2(mu28); unc-73(e936), Ex rho-1 (G14V, gf) | 6.7 ± 1.1* | 10 |
| mig-2(gm103, gf) | 7.0 ± 0.7† | 25 |
| mig-2(gm103, gf); Ex rho-1(G14V, gf) | 9.3 ± 1.6† | 25 |
Pn.p cells were scored during the L2 stage. Ex, extrachromosomal array. ced-10 is also known as rac-1.
,
, P value is <0.0001 when these data are compared by using Student's t test.
Data are expressed as the average number of Pn.p cells in the ventral cord during L2.
mig-2 and ced-10/rac-1 May Act in Parallel with rho-1 During P Cell Migration.
Loss-of-function mutations in the Rac subfamily genes mig-2 and ced-10/rac-1 cause cell migration defects (10, 11). To determine whether mutations in these genes affected the P cell lineage, we examined P cell migrations in mig-2(mu28) and ced-10/rac-1(n3246), which are null and loss-of-function alleles, respectively. P cell migrations were normal in both mig-2(mu28) and ced-10/rac-1 (n3246) animals (ref. 10; Table 2). However, a weak P cell migration defect was observed in ced-10/rac-1(n3246); mig-2(RNAi) animals (Table 2). ced-10/rac-1(n3246); mig-2(RNAi) animals were rather sick, with many individuals developing slowly or not surviving past the second larval stage (data not shown; M. Sundaram, personal communication). The relatively low penetrance of the P cell migration phenotype could be because only relatively healthier animals lived long enough to be scored and a significant portion of the signal is through rho-1. The role of mig-2 in P cell migration is also suggested by the observation that mig-2(mu28) exacerbates the weak P cell migration defect observed in unc-73(e936) (Table 2; ref. 10). This effect was weakly rescued by over expression of rho-1(gf) (Table 2). A gain-of-function mutation, mig-2(gm103), results in weak P cell migration defects that are also partially rescued by rho-1(gf). These observations support the hypothesis that parallel signaling of Rho family GTPases occurs during P cell migration (10).
Discussion
The proposal that rho-1 is plays a role during P cell migration is based on the migration defect caused by rho-1(dn), expression of the rho-1 inhibitor C3, and rho-1(RNAi). The rho-1 (T19N) dominant-negative mutation is based on the homologous Ras (T17N) dominant-negative mutation and has been used to inhibit rho-1 activity in many experimental systems (33, 34). Expression of the C3 enzyme specifically inactivates RHOA by ADP ribosylation at asparagine 41 (26). Our results identify RHO-1 as an important GTPase during P cell migration in C. elegans.
In addition to the P cell migration defects caused in rho-1-deficient animals, we observed gross morphological changes in ventral cord neurons. This effect appeared to be caused by inhibition of rho-1 activity, because both rho-1(dn) animals and rho-1(RNAi) escapers displayed a similar phenotype. Staining with the DNA dye DAPI suggested the phenotype may be the result of failed cytokinesis, as many such cells were multinucleate. Multinucleate cells were also observed in early rho-1(RNAi) embryos (ref. 23; this study). These observations suggest that rho-1 is required for at least some cytokinesis events in C. elegans. A role for rho-1 in cytokinesis has also been reported in other organisms (35–37).
We also present results that are consistent with the idea that unc-73 is an exchange factor for rho-1 during P cell migration. Biochemical analysis of the UNC-73 exchange factor domains indicates that the GEF2 domain can efficiently catalyze GDP release from mammalian RhoA. It is reasonable to draw biochemical parallels between RHO-1 and RHOA because they share 85% primary structure identity including 98% identity in the GEF interaction domain (38, 39). Such biochemical parallels have been used to confirm genetic evidence in C. elegans previously (7). Genetically, mutations in both unc-73 and rho-1 cause a P cell migration defect, and a rho-1(G14Vgf) transgene can partially suppress the P cell migration defect in an unc-73 mutant. These biochemical and genetic data, although subject to other interpretations, are consistent with a model in which unc-73 and rho-1 act together during P cell migration.
The G14V mutation is known to result in some constitutive activity of the RHO protein because of reduced intrinsic GTP hydrolysis (33). That rescue of the unc-73(e936) allele with rho-1(gf) was incomplete may be explained by two possibilities. First, the rho-1(G14V) mutation may not lead to a sufficiently strong hyperactive Rho protein. Rho family member proteins may need to cycle between GDP- and GTP-bound forms to achieve maximal activity. This has been proposed as an explanation for the observation that constitutively active Rho family members are only weak oncogenes (1), whereas endogenous RHO activated by exchange factors like Dbl can be potent (40, 41). It is also possible that Rho-1 may have a higher intrinsic GTPase activity that reduces the effectiveness of the G14V mutation. Second, as suggested by the results on mig-2 and ced-10/rac-1, unc-73 may function by activating more than one GTPase during P cell migration so that the rho-1(gf) gene can suppress only part of the defect associated with the unc-73 allele.
There could also be several possible explanations for that the P cell migration phenotype in unc-73 mutants are significantly weaker than that in the rho-1(dn) transgenic strain (Table 1). For example, it is possible that rho-1 is activated by more than one exchange factor. In the Ras pathway in C. elegans, eliminating let-341/SOS function also results in a mutant phenotype that is significantly weaker than that of eliminating the let-60/ras function, promoting a proposal that there is another exchange factor for the Ras activation (42). Alternatively, the unc-73(gm40) mutants we examined may not eliminate enough unc-73 activity. The severe sickness and lethality of the gm40 mutant allowed us to examine only relatively healthy escapers among the homozygous progeny from heterozygous mothers. gm40, although a putative null from previous characterization (7), could be a non-null allele, and null mutations of the gene could be completely lethal at the embryonic or an early larval stage. Another possibility is that the RHO-1 GTPase activity may not totally depend on the activity from exchange factors. For example, repressing a Rho GDI (GDP-dissociation inhibitor) protein may also contribute to its activation (43). Therefore, rho-1 may conceivably be partially active in absence of the UNC-73 function.
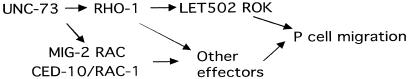
The idea of parallel GTPase signaling during P cell migration was first proposed after the observation was made that mig-2(mu28, null) exacerbates a P cell mislocalization phenotype of unc-73(e936) (10). Zipkin et al. speculated that mig-2 is likely to act redundantly with another GTPase-dependent pathway during P cell nuclear migration. Our analysis of rho-1, mig-2, and rac-1/ced-1 genes supports such prediction. A likely redundant role for mig-2 and ced-10/rac-1 in P cell migration is suggested by the observation that reducing the activity of both genes causes a defect in P cell migration, whereas single mutation in either gene does not (Table 2). Redundancy of these two gene activities has also been observed recently by others for axon guidance, cell migrations, apoptotic cell phagocytosis, and vulval morphogenesis (ref. 44; M. Sundaram, personal communication). We also confirmed that mig-2(null) exacerbates the phenotype of unc-73(e936) (Table 2) and show that such an effect is partially overcome by the rho-1(gf) transgene (Table 2). Thus, we may speculate that MIG-2 and CED-10/RAC-1 may act in parallel to RHO-1 and share UNC-73 as the exchange factor for the P cell migration function (Fig. 5). It is possible that the majority of the signal for P cell migration travels through the rho-1 pathway because a more severe mutant phenotype is observed in the rho-1(dn) mutant and rho-1(RNAi) escapers. All proteins proposed to act during P cell migration in our model (Fig. 5) have been shown to express in the P cell lineage at the appropriate time (7, 10, 16, 45).
Figure 5.
Model for rho-1-mediated P cell migration.
Our in vitro biochemical experiments also showed that UNC-73 GEF1 and GEF2 domains preferentially act on RAC and RHO proteins, respectively. Whether this preference is practiced in vivo in C. elegans is not clear. However, such a scenario has been previously indicated for UNC-73 homologues based on studies in mammalian cells (8, 9) and Drosophila (6).
Our interest in let-502 was based on its homology to a known RHOA effector in mammals, p160ROCK (46). Because the let-502 alleles examined can cause developmental arrest in the second or third larval stages, one must address whether development in these strains is generally impaired such that observations of various cell locations are subject to artifact. We do not believe that the observed P cell migration defects in let-502 alleles are artifactual because the previously reported let-502 defects appear to be restricted to embryonic elongation, and other markers of development appear to proceed normally (16). For example, the division and differentiation of P cell-derived GABA neurons marked by UNC-47∷GFP appeared normal. In each let-502 allele examined, a weak but reproducible P cell migration defect was observed. The rho-1(gf) transgene did not rescue the let-502 P cell defect. Although LET-502 is likely an effector of RHO-1 during P cell migration, other effectors are likely to exist implicated by the weak P cell defect in let-502-deficient animals.
Acknowledgments
We are indebted to the labs of Rob Steven (University of Toronto), Joe Culotti (University of Toronto), Cynthia Kenyon (University of California, San Francisco), Erik Jorgensen (University of Utah), Paul Mains, Michinor Kohara, Yoshimi Takai, and Andy Fire for providing mutant strains and vectors. We also thank Rob Steven and Joe Culotti for discussion and comments on drafts of this manuscript and Lisa Williams from the Kenyon lab for suggestions on strain constructions. A.G.S. received support from a U.S. Army Breast Cancer Postdoctoral Fellowship (DAMD17–96-1–6041) and is currently a National Institutes of Health Postdoctoral Fellow (National Research Service Award HD8653). C.J.M was a National Defense Science and Engineering Graduate Fellow. This work was supported in part by National Institutes of Health Grant GM47869 (to M.H.). M.H. is an Assistant Investigator of the Howard Hughes Medical Institute.
Abbreviations
- GEF
guanine nucleotide exchange factor
- DH
Dbl homology
- PH
pleckstrin homology
- MCS
multiple cloning site
- GFP
green fluorescent protein
- dsRNA
double-stranded RNA
- DAPI
4′,6-diamidino-2-phenylindole
References
- 1.Hall A. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 2.Ridley A J, Hall A. Cold Spring Harbor Symp Quant Biol. 1992;57:661–671. doi: 10.1101/sqb.1992.057.01.072. [DOI] [PubMed] [Google Scholar]
- 3.Magie C R, Meyer M R, Gorsuch M S, Parkhurst S M. Development (Cambridge, UK) 1999;126:5353–5364. doi: 10.1242/dev.126.23.5353. [DOI] [PubMed] [Google Scholar]
- 4.Barrett K, Leptin M, Settleman J. Cell. 1997;91:905–915. doi: 10.1016/s0092-8674(00)80482-1. [DOI] [PubMed] [Google Scholar]
- 5.Prokopenko S N, Brumby A, O'Keefe L, Prior L, He Y, Saint R, Bellen H J. Genes Dev. 1999;13:2301–2314. doi: 10.1101/gad.13.17.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newsome T P, Schmidt S, Dietzl G, Keleman K, Asling B, Debant A, Dickson B J. Cell. 2000;101:283–294. doi: 10.1016/s0092-8674(00)80838-7. [DOI] [PubMed] [Google Scholar]
- 7.Steven R, Kubiseski T J, Zheng H, Kulkarni S, Mancillas J, Ruiz-Morales A, Hogue C W, Pawson T, Culotti J. Cell. 1998;92:785–795. doi: 10.1016/s0092-8674(00)81406-3. [DOI] [PubMed] [Google Scholar]
- 8.Debant A, Serra-Pagès C, Seipel K, O'Brien S, Tang M, Park S H, Streuli M. Proc Natl Acad Sci USA. 1996;93:5466–5471. doi: 10.1073/pnas.93.11.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellanger J M, Lazaro J B, Diriong S, Fernandez A, Lamb N, Debant A. Oncogene. 1998;16:147–152. doi: 10.1038/sj.onc.1201532. [DOI] [PubMed] [Google Scholar]
- 10.Zipkin I D, Kindt R M, Kenyon C J. Cell. 1997;90:883–894. doi: 10.1016/s0092-8674(00)80353-0. [DOI] [PubMed] [Google Scholar]
- 11.Reddien P W, Horvitz H R. Nat Cell Biol. 2000;2:131–136. doi: 10.1038/35004000. [DOI] [PubMed] [Google Scholar]
- 12.Sulston J E, Horvitz H R. Dev Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- 13.Sulston J E. Philos Trans R Soc Lond Ser B Biol Sci. 1976;275:287–297. doi: 10.1098/rstb.1976.0084. [DOI] [PubMed] [Google Scholar]
- 14.Yochem J, Gu T, Han M. Genetics. 1998;149:1323–1334. doi: 10.1093/genetics/149.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piekny A J, Wissmann A, Mains P. Genetics. 2000;156:1671–1689. doi: 10.1093/genetics/156.4.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wissmann A, Ingles J, McGhee J D, Mains P E. Genes Dev. 1997;11:409–422. doi: 10.1101/gad.11.4.409. [DOI] [PubMed] [Google Scholar]
- 17.McIntire S L, Garriga G, White J, Jacbson D, Horvitz H R. Neuron. 1992;8:307–322. doi: 10.1016/0896-6273(92)90297-q. [DOI] [PubMed] [Google Scholar]
- 18.McIntire S L, Reimer R J, Schuske K, Edwards R H, Jorgensen E M. Nature (London) 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- 19.Yochem J, Tuck S, Greenwald I, Han M. Development (Cambridge, UK) 1999;126:3597–3606. doi: 10.1242/dev.126.3.597. [DOI] [PubMed] [Google Scholar]
- 20.Fire A, Xu S, Montgomery M K, Kostas S A, Driver S E, Mello C C. Nature (London) 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 21.Orita S, Naito A, Sakaguchi G, Maeda M, Igarashi H, Sasaki T, Takai Y. J Biol Chem. 1997;272:16081–16084. doi: 10.1074/jbc.272.26.16081. [DOI] [PubMed] [Google Scholar]
- 22.Orita S, Kaibuchi K, Kuroda S, Shimizu K, Nakanishi H, Takai Y. J Biol Chem. 1993;268:25542–25546. [PubMed] [Google Scholar]
- 23.Jantsch-Plunger V, Gönczy P, Romano A, Schnabel H, Hamill D, Schnabel R, Hyman A A, Glotzer M. J Cell Biol. 2000;149:1391–1404. doi: 10.1083/jcb.149.7.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sulston J E, Horvitz H R. Dev Biol. 1981;82:41–55. doi: 10.1016/0012-1606(81)90427-9. [DOI] [PubMed] [Google Scholar]
- 25.Malone C J, Fixsen W D, Horvitz H R, Han M. Development (Cambridge, UK) 1999;126:3171–3181. doi: 10.1242/dev.126.14.3171. [DOI] [PubMed] [Google Scholar]
- 26.Aktories K, Hall A. Trends Pharmacol Sci. 1989;10:415–418. doi: 10.1016/0165-6147(89)90191-0. [DOI] [PubMed] [Google Scholar]
- 27.Sieburth D S, Sundaram M, Howard R M, Han M. Genes Dev. 1999;13:2562–2569. doi: 10.1101/gad.13.19.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grishok A, Tabara H, Mello C C. Science. 2000;287:2494–2497. doi: 10.1126/science.287.5462.2494. [DOI] [PubMed] [Google Scholar]
- 29.Hedgecock E M, Culotti J G, Hall D H, Stern B D. Development (Cambridge, UK) 1987;100:365–382. doi: 10.1242/dev.100.3.365. [DOI] [PubMed] [Google Scholar]
- 30.Chen E B, Branda C S, Stern M J. Dev Biol. 1997;182:88–100. doi: 10.1006/dbio.1996.8473. [DOI] [PubMed] [Google Scholar]
- 31.Forrester W C, Garriga G. Development (Cambridge, UK) 1997;124:1831–1843. doi: 10.1242/dev.124.9.1831. [DOI] [PubMed] [Google Scholar]
- 32.Habets G G, van der Kammen R A, Stam J C, Michiels F, Collard J G. Oncogene. 1995;10:1371–1376. [PubMed] [Google Scholar]
- 33.Paterson H F, Self A J, Garrett M D, Just I, Aktories K, Hall A. J Cell Biol. 1990;111:1001–1007. doi: 10.1083/jcb.111.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sander E E, ten Klooster J P, van Delft S, van der Kammen R A, Collard J G. J Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kishi K, Sasaki T, Kuroda S, Itoh T, Takai Y. J Cell Biol. 1993;120:1187–1195. doi: 10.1083/jcb.120.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mabuchi I, Hamaguchi Y, Fujimoto H, Morii N, Mishima M, Narumiya S. Zygote. 1993;1:325–331. doi: 10.1017/s0967199400001659. [DOI] [PubMed] [Google Scholar]
- 37.Drechsel D N, Hyman A A, Hall A, Glotzer M. Curr Biol. 1997;7:12–23. doi: 10.1016/s0960-9822(06)00023-6. [DOI] [PubMed] [Google Scholar]
- 38.Ihara K, Muraguchi S, Kato M, Shimizu T, Shirakawa M, Kuroda S, Kaibuchi K, Hakoshima T. J Biol Chem. 1998;273:9656–9666. doi: 10.1074/jbc.273.16.9656. [DOI] [PubMed] [Google Scholar]
- 39.Li R, Zheng Y. J Biol Chem. 1997;272:4671–4679. doi: 10.1074/jbc.272.8.4671. [DOI] [PubMed] [Google Scholar]
- 40.Srivastava S K, Wheelock R H, Aaronson S A, Eva A. Proc Natl Acad Sci USA. 1986;83:8868–8872. doi: 10.1073/pnas.83.23.8868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hart M J, Eva A, Zangrilli D, Aaronson S A, Evans T, Cerione R A, Zheng Y. J Biol Chem. 1994;269:62–65. [PubMed] [Google Scholar]
- 42.Chang C, Hopper N A, Sternberg P W. EMBO J. 2000;19:3283–3294. doi: 10.1093/emboj/19.13.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Aelst L, D'Souza-Schorey C. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 44.Lundquist, A. E., Reddien, P. W., Hartwieg, E., Horvitz, H. R. & Bargmann, C. I. (2001) Development (Cambridge, U.K.), in press. [DOI] [PubMed]
- 45.Chen W, Lim L. J Biol Chem. 1994;269:32394–32404. [PubMed] [Google Scholar]
- 46.Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. FEBS Lett. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]