Abstract
Objective
This study was designed to evaluate the clinical efficacy of combined traditional Chinese medicine (TCM) and conventional chemotherapy versus conventional chemotherapy in patients with stage II-IIIA non-small-cell lung cancer (NSCLC) after radical surgery.
Methods
A retrospective cohort study was conducted in patients with stage II-IIIA NSCLC from Subei People's Hospital and Yangzhou Traditional Chinese Medicine Hospital in Yangzhou City of Jiangsu Province from 2012 to 2016. Patients were divided into two groups: the TCM user group (patients receiving treatment with integrated TCM and conventional chemotherapy) and the non-TCM user group (patients receiving conventional chemotherapy only). The two groups were compared for their median disease-free survival (DFS) and median overall survival (OS).
Results
A total of 67 patients with stage II-IIIA NSCLC were enrolled between January 2012 and December 2016. The median DFS for the non-TCM user group was 601 days (95% confidence interval [CI], 375.7-826.3). The median DFS for TCM user group could not be calculated. However, log-rank analysis showed that the median survival time in the TCM user group was significantly longer than that of the non-TCM user group (P < 0.05). In addition, several significant risk factors were detected for predicting disease prognosis in patients with NSCLC, such as age, ECOG, lymphatic metastasis, and body mass index (BMI). For patients harboring these independent risk factors, the DFS of TCM user group was much longer than that of non-TCM user group (P < 0.05).
Conclusion
Adjuvant therapy with TCM may reduce the rate of tumor recurrence and metastasis and prolong DFS of patients with stage II-IIIA NSCLC.
1. Introduction
As one of the most common malignant tumors, lung cancer is a leading cause of cancer-related death worldwide [1]. Non-small-cell lung cancer (NSCLC) accounts for 85% of all cases of lung cancer. Despite advancements in therapeutic approaches, the 5-year survival rate of lung cancer remains about 10%-15% [2]. At present, the comprehensive therapy regimen for NSCLC at early stage gives priority to surgery. Multiple factors lead to the low overall survival (OS) rates in NSCLC patients, such as late diagnosis, high tumor recurrence, and metastasis. Based on current methods of therapy, tumor recurrence and metastasis remain a great challenge for a full cure despite the excellent outcomes after standard treatments.
For patients with early stage NSCLC, surgery is the first therapeutic option. After operation, platinum-based chemotherapy or combined targeted therapy and chemotherapy are recommended as standard Western medicine treatments [3]. In addition, a comprehensive therapy regimen also includes radiotherapy and Traditional Chinese medicine (TCM). TCM is considered as an important complementary therapy with beneficial effects for patients with NSCLC by reducing toxic effects, improving the quality of life, and prolonging OS [4–6]. Lots of studies on antitumor mechanism of TCM have been reported. Xiong et al. [7] have found that a novel herbal formula can induce cell cycle arrest and apoptosis by suppressing the PI3K/AKT pathway in human lung cancer A549 cells. Pang et al. [8] found that the antitumor mechanism of Bu-Fei Decoction, a classical formula of traditional Chinese medicine (TCM), was related to interruption of the link between TAMs and NSCLC cells by inhibiting the expression of IL-10 and PD-L1 in vitro and in vivo. Shen SJ et al. [9] found that Yangfei Kongliu Formula, a compound Chinese herbal medicine, combined with cisplatin, can inhibit tumor growth synergistically, mainly through the TGF-β1 signaling pathway. Chinese herbal medicines (CHMs) have been used for thousands of years as adjuvant therapy and play an indispensable role in alternative medicine and cancer therapy [10]. TCM therapy mainly consists of Chinese herbal compound, which is composed of at least two or more traditional CHMs, containing herbal decoction, Chinese patent drug, and correlative injection.
TCM exhibits advantages in preventing tumorigenesis and attenuating toxicity, enhancing the therapeutic effect, and reducing risk of recurrence and metastasis [11]. When combined with adjuvant chemotherapy on postoperative early stage NSCLC patients, TCM led to partial relief of symptoms in addition to a reduction of side effects and adverse events caused by the chemotherapy regimens [12]. Although several systematic reviews and meta-analyses have evaluated the effectiveness of TCM treatment on lung cancer [4, 5, 13], their follow-up periods were relatively short with a lack of the comparison of disease-free survival (DFS) in NSCLC of stage II-IIIA. Therefore, we conducted a retrospective cohort study to evaluate the effect of TCM combined with conventional chemotherapy on DFS of patients with stage II-IIIA NSCLC.
2. Materials and Methods
2.1. Study Population
A total of 67 NSCLC patients were retrospectively enrolled, who underwent relevant treatments at Subei People's Hospital and Yangzhou Traditional Chinese Medicine Hospital between January 2012 and December 2016. The inclusion criteria for the study population were as follows: (i) patients were pathologically diagnosed with stage II-IIIA NSCLC after radical resection of pulmonary carcinoma; and the clinical TNM staging of II-IIIA NSCLC was based on 2009 Union for International Cancer Control (UICC,2009) staging of lung cancer; (ii) patients received integrated traditional Chinese and conventional chemotherapy or only conventional chemotherapy; (iii) the ages of patients were between 18 and 75 years old; and (iv) patients had good compliance. Clinical trials were excluded if they failed to meet the above criteria. In addition, the exclusion criteria were as follows: (i) NSCLC patients without clear pathological results; (ii) severe disease or dysfunction of heart, hepatic, kidney, or hematopoietic system; (iii) second primary malignancy; (iv) children, pregnant or lactating women, and psychiatric patients. All patients had complete clinicopathological data, including age, sex, smoking status, histological type, tumor size, and clinical stage. The enrolled patients were placed into either TCM user group (receiving treatment with integrated traditional Chinese and conventional chemotherapy) or non-TCM user group (receiving conventional chemotherapy only). The present study was approved by the Ethics Committees of Yangzhou University and both involved hospitals. In view of the retrospective nature of the study, a collection of informed consent was abandoned.
2.2. Medical Treatment
All patients in the non-TCM user group received only standardized chemotherapy according to the National Comprehensive Cancer Network (NCCN,2011) Clinical Practice Guidelines of NSCLC. All patients in the TCM user group received Chinese medicine therapy consisting of Chinese herbal decoction, Chinese patent drug, and correlative injection, together with standardized chemotherapy. Notably, the traditional Chinese medicine should be used for more than six months in the TCM user group. Standardized chemotherapy should be used for four cycles in two groups.
Based on the Chinese Medicine New Medicine Clinical Practice Guideline (Trial Implementation) (published by China Medical Science Press in 2002) and TCM theory of combination of disease and syndrome, combined with years of clinical observation and experience in our department, we summarized the main syndrome differentiation types at the postoperative stage of NSCLC as follows: pulmonary Qi deficiency, Qi and Yin deficiency, and stagnation of phlegm and blood stasis. They were determined by two senior physician. Pulmonary Qi deficiency manifests as the following: cough, shortness of breath, fatigue and weakness, spontaneous sweating, pale tongue, thin coating, and a weak pulse. Qi and Yin deficiency manifests as the following: cough, small amount of sputum, fatigue and weakness, dried mouth without polydipsia, spontaneous sweat, night sweat, reddish tongue or tongue with teeth imprints, and thready and weak pulse. Stagnation of phlegm and blood stasis manifests as the following: cough, constant phlegm, dark tongue, white and greasy coating, and an uneven pulse. Above all, the core pathogenesis (the pathological basis of tumor recurrence and metastasis) was the deficiency of vital Qi and internal toxin of cancer. On this basis, a therapeutic regimen was formulated based on the real diagnosis and treatment: the postoperative patients with pulmonary Qi deficiency syndrome adopted Shen Yi capsule (SDA approval number: Z20030044, Ginsenoside Rg3, orally administered of 20mg, two times each day (BID)); patients with Qi and Yin deficiency syndrome were treated with Yifei Qinghua granules (SDA approval number: Z20050851, whose ingredients included Radix Astragali seu Hedysari, Radix Codonopsis, Radix Glehniae, Radix Ophiopogonis, Semen Armeniacae Amarum, Bulbus Fritillariae Unibracteatae, and Herba Hedyotidis Diffusae; orally administered of 20g, two times each day (BID)); and Fufang Banmao capsule (SDA approval number: Z52020238, whose ingredients included Radix Astragali seu Hedysari, Panax ginseng, Aesculus wilsonii Rehd, and Chinese blister beetle; orally administered 0.75 g, two times each day (BID)), was used to treat patients with stagnation of phlegm and blood stasis syndrome. Additionally, all patients could choose to use oral Chinese medicine decoction added and subtracted from the standardized agent based on their core pathogenesis. The herbal treatment was suitable for the specific condition of each patient in accordance with syndrome differentiation [14]. There are three matched types of CHM decoction for the TCM user group in this study: patients with pulmonary Qi deficiency syndrome adopted LiuJunZi decoction, patients with Qi and Yin deficiency syndrome were treated with ShaShenMaiDong decoction, and patients with stagnation of phlegm and blood stasis syndrome were treated with SiWu decoction together with HuaTan decoction. The general outline of the TCM syndrome differentiation used and fundamental prescriptions given to the patients were described in Table 1. The major therapeutic principle was to strengthen the body resistance to eliminate pathogenic factors.
Table 1.
The most commonly used herbs per traditional Chinese medicine syndrome.
| Chinese Name (Pinyin) | Pharmaceutical Name | English Name | Dosage (g) |
|---|---|---|---|
| Syndrome of Deficiency of pulmonary Qi(LiuJunZi decoction) | |||
| Huangqi | Radix Astragali seu Hedysari | Membranous milkvetch root/ Mongolian milkvetch root |
40 |
| Dangshen | Radix Codonopsis | Pilose Asiabell Root /Moderate Asiabell Root/Szechwon Tangshen Root | 15 |
| Baizhu | Rhizoma Atractylodis Macrocephalae | Largehead atractylodes rhizome | 10 |
| Fuling | Poria | Indian buead | 15 |
| Chenpi | Pericarpium Citri Reticulatae | Tangerine Peel | 6 |
| Xingren | Semen Armeniacae Amarum | Bitter apricot seed | 9 |
| Syndrome of Deficiency of Qi and Yin(ShaShenMaiDong decoction) | |||
| Huangqi | Radix Astragali seu Hedysari | Membranous milkvetch root/ Mongolian milkvetch root |
40 |
| Beishashen | Radix Glehniae | Coastal glehnia root | 15 |
| Maidong | Radix Ophiopogonis | Dwarf, lilyturf tuber, ophiopogon | 15 |
| Tiandong | Radix Asparagi | Cochinchinese asparagus root | 15 |
| Baihe | Bulbus Lilii | Lancelesf Lily Bulb / Greenish Lily Bulb | 15 |
| Beimu | Bulbus Fritillariae Unibracteatae | Unibract Fritillary Bulb | 15 |
| Syndrome of Stagnation of phlegm and blood stasis(SiWu decoction together with HuaTan decoction) | |||
| Danggui | Radix Angelicae Sinensis | Chinese Angelica | 15 |
| Shudi | Radix Rehmanniae | Rehmannia Root | 15 |
| Chishao | Radix Paeoniae Rubra | Red Paeony Root | 10 |
| Chuanxiong | Rhizoma Chuanxiong | Szechuan Lovage Rhizome | 10 |
| Yiyiren | Semen Coicis | Coix Seed | 20 |
| Chenpi | Pericarpium Citri Reticulatae | Tangerine Peel | 6 |
| Banxia | Rhizoma Pinelliae | Pinellia Tuber | 10 |
| Ban Zhi Lian | Herba Scutellariae Barbatae | Barbed Skullcap Herb | 15 |
| Baihuasheshecao | Herba Hedyotidis Diffusae | Spreading Hedyotis Herb | 15 |
Decocting method is as follows: soak the herbs in water for 30 min with water level 1 cm above the herbs. First, boil with strong heat and then with gentle heat for about 20–40 minutes. Then, decant the decoction, repeat the above course, combine the decoction, and concentrate to 300 mL. Dosage and administration are as follows: one set of herbs per day, 150 mL each time, twice a day, and one hour after breakfast and supper.
2.3. Follow-Up
All patients were followed up carefully with hospital visits, telephone, or text messaging unless they were unable to be contacted. Physical examination, physical status, imaging examination (enhanced computed tomography for head and chest, bone scanning and ultrasonography for abdomen, etc.) and the time of tumor recurrence and metastasis, death, and OS were recorded. The ending time point of follow-up period was July 2017 or in the event that further data cannot be recorded.
2.4. Statistical Analysis
SPSS version 16 was used for statistical analyses (SPSS, Inc., Chicago, IL). In all tests, P < 0.05 was considered as statistical significance. Descriptive statistics were used to analyze the baseline characteristics of all patients. The rates of tumor recurrence and metastasis were assessed using the Kaplan-Meier method, with the log-rank test for evaluating significance. The Cox proportional hazard model was used to assess the prognostic values of variables.
3. Results
3.1. Patient Characteristics
All participants were enrolled from January 2012 to July 2016. There were two patients who were excluded due to the loss of follow-up, and the other 67 patients who met the inclusion criteria were eligible for analysis. Of these patients, 32 were in the TCM group and 35 were in the non-TCM group. In accordance with 2009 Union for International Cancer Control (UICC,2009) TNM staging system, 42 (62.7%) patients were at stage IIA- IIB and 25 (37.3%) patients were at stage IIIA. The present study included 47 men and 20 women, with a mean age of 59.9 years old (ranging from 45 to 75 years old). Forty-four (65.7%) patients, 21 (31.3%) patients, and one (1.5%) patient were diagnosed with adenocarcinoma, squamous-cell carcinoma, and adenosquamous carcinoma, respectively. There was no statistical significance between the groups regarding demographic and baseline characteristics (Table 2).
Table 2.
Patient base-line characteristics.
| Characteristic | TCM user group(N=32) | non-TCM user group(N=35) | p |
|---|---|---|---|
| Sex, (%) | |||
| Male | 21 (65.62) | 26(74.29) | |
| Female | 11(34.38) | 9(25.71) | 0.439 |
| Age, yr, (%) | |||
| ≤50 | 5(15.63) | 5(14.29) | |
| 50-65 | 20(62.50) | 20(57.14) | |
| >65 | 7(21.87) | 10(28.57) | 0.820 |
| Smoking status, (%) | |||
| Never smoked | 12(37.50) | 12(34.29) | |
| <400 | 14(43.75) | 12(34.29) | |
| ≥400 | 6(18.75) | 11(31.42) | 0.474 |
| ECOG performance status score—no. (%) | |||
| 0 | 0 | 2(5.71) | |
| 1 | 27(84.38) | 27(77.14) | |
| 2 | 5(15.62) | 6(17.14) | 0.375 |
| Tumor size, (%) | |||
| <5cm | 14(43.75) | 17(48.57) | |
| ≥5cm | 18(56.25) | 18(51.43) | 0.693 |
| Histologic diagnosis, (%) | |||
| Adenocarcinoma | 23(71.88) | 21(60.0) | |
| Squamous-cell carcinoma | 9(28.12) | 12(34.28) | |
| Adenosquamous carcinoma | 0 | 1(2.86) | |
| Other | 0 | 1(2.86) | 0.496 |
| Clinical stage, (%) | |||
| IIA | 6(18.75) | 13(37.14) | |
| IIB | 14(43.75) | 9(25.71) | |
| IIIA | 12(37.50) | 13(37.14) | 0.167 |
| modus operandi, (%) | |||
| thoracotomy | 17(53.13) | 18(51.43) | |
| Thoracoscopy minimally invasive | 14(43.75) | 15(42.86) | |
| Combined | 1(3.12) | 2(5.71) | 0.877 |
3.2. Survival of Patients
Of all patients in the two groups, 35 patients suffered from tumor recurrence and metastasis. Ten patients passed away before July 2017. The median time of recurrence and metastasis for the non-TCM user group was 601 days (95% confidence interval [CI], 375.7-826.3). In comparison, the rate of recurrence and metastasis of TCM user group was 40.6%, and the median time of recurrence and metastasis could not be calculated. The log-rank analysis showed that the median survival time in the TCM user group was significantly longer than that of the non-TCM user group (P < 0.05) (Figure 1).
Figure 1.
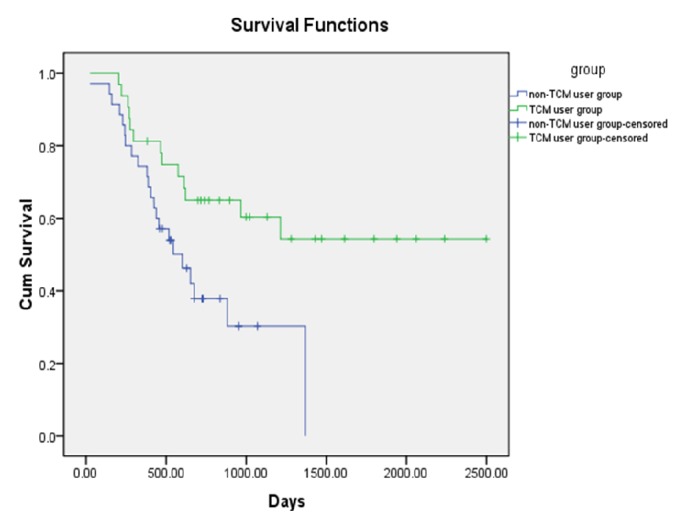
Comparison of DFS of patients according to different treatment.
3.3. Prognostic Analysis
For multivariable analysis, the following parameters were considered as risk factors: age < 50 years old, the score of ECOG being 2, more than five lymphatic metastasis, BMI < 20, and occupation being cadre, with corresponding hazard ratios (HR) of 0.272 (95% CI = 0.141-0.525), 5.161 (95% CI = 1.932-13.791), 4.101 (95% CI = 1.531-10.989), 0.259 (95% CI = 0.91-0.734), and 4.426 (95% CI = 1.274-15.381), respectively. The above variables were independent prognostic risk factors (Table 3).
Table 3.
Predictors of DFS in NSCLC patients.
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Age group (≤50 /50-65/≥65 years old) | 0.505 | 0.292-0.871 | 0.014 | 0.272 | 0.141-0.525 | 0.000 |
| ECOG (=0/1/2) | 1.936 | 0.877-4.276 | 0.102 | 5.161 | 1.932-13.791 | 0.001 |
| lymphatic metastasis(≥5/<5) | 0.043 | 0.-4536.482 | 0.595 | 4.101 | 1.531-10.989 | 0.005 |
| BMI(<20/20-24/>24) | 0.351 | 0.145-0.851 | 0.021 | 0.259 | 0.91-0.734 | 0.011 |
| Occupation (peasant/ worker/ cadre/ intellectual) | 1.483 | 0.549-4.005 | 0.437 | 4.426 | 1.274-15.381 | 0.019 |
| KPS (<80/≥80) | 0.471 | 0.235-0.943 | 0.034 | |||
| Smoke (no/yes) | 1.260 | 0.811-1.957 | 0.303 | |||
| Tumor size (≥5/<5) | 1.115 | 0.570-2.180 | 0.750 | |||
| Clinical stages (IIA /IIB/IIIA) | 1.139 | 0.748-1.735 | 0.543 | |||
| Treatment (TCM user/non-TCM user) | 0.409 | 0.200-0.837 | 0.014 | |||
3.4. NSCLC with Stage II-IIIA Subgroups
The early stage was further subdivided into several subgroups according to the presence of independent prognostic risk factors of age group, ECOG, lymphatic metastasis, BMI, and occupation as follows: no or one prognostic risk factor and two or more prognostic risk factors. Patients with no or one prognostic risk factor in the TCM user group harbored better prognostic outcome than the corresponding patients in the non-TCM user group. The median DFS time was 652 days (95% CI = 472.95-831.05) in the non-TCM user group. However, the rate of recurrence and metastasis of TCM user group was less than 50%, and the median DFS time could not be calculated. The log-rank test showed that the median survival time for the TCM user group was significantly longer than that in the non-TCM user group (P = 0.007) (Figure 2). When the prognostic risk factors reached two or more, the median DFS time was 465 days (95% CI = 199.42-730.58) in the TCM user group versus 229 days (95% CI = 196.99-261.00) in the non-TCM user group, which was not statistically significant between the two groups (P = 0.139) (Figure 3).
Figure 2.
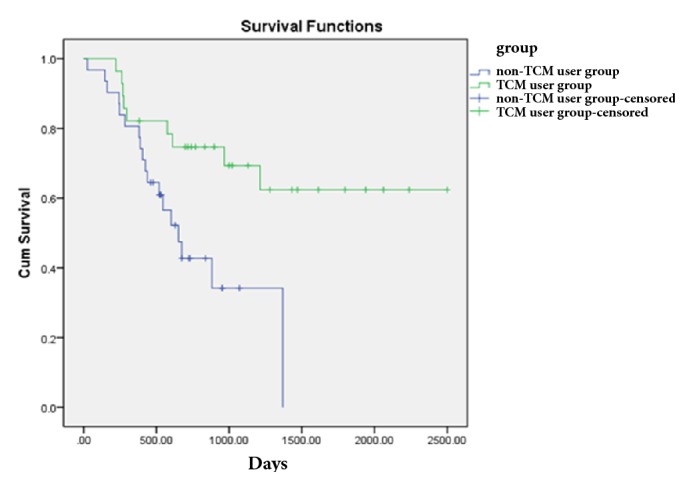
Comparison of DFS of patients with 0-1 prognostic risk factors according to treatment.
Figure 3.
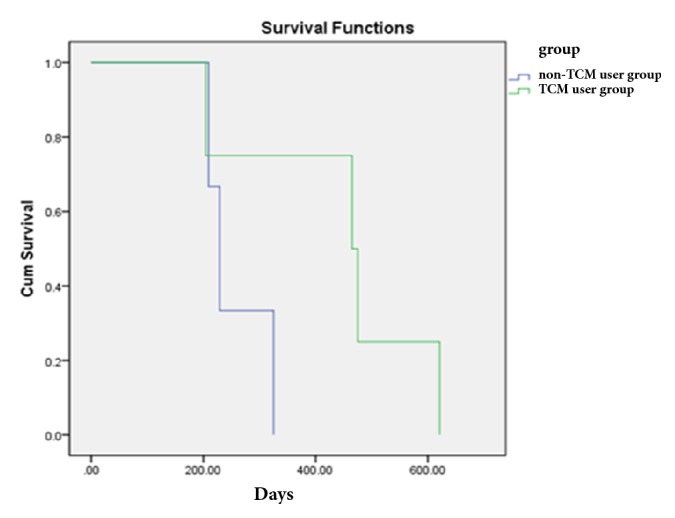
Comparison of DFS of patients with ≥2 prognostic risk factors according to treatment.
4. Discussion
NSCLC is a devastating disease with high incidence and mortality worldwide [15]. At present, surgical resection is the main treatment for early stage lung cancer. Platinum-based chemotherapy is considered to be necessary and effective after surgery for patients with early stage NSCLC, which might be able to reduce the risk of tumor recurrence [16]. In recent years, the concept of maintenance chemotherapy is questioned and revised because of the lack of survival benefit, increased toxicity, economic considerations, and poor quality of patients' life. About 50% of patients failed to complete the entire adjuvant treatment due to toxicity [17].
Therefore, whether Chinese medicine can reduce tumor recurrence and metastasis and prolong the survival of patients with NSCLC has become a hot topic in the field of Chinese medicine oncology. Recent studies have reported that combined with conventional chemotherapy, TCM showed superiority in relieving symptoms for patients with breast and gastric cancer, enhancing short-term efficacy, and improving quality of life [18, 19]. Moreover, no patients stopped TCM treatment attributed from the minimal adverse effects of herbs. In China, CHM is the most commonly used category of TCM [20]. Thus, it is worth evaluating the efficacy of TCM combined with conventional chemotherapy in the treatment of NSCLC, from a long-term perspective, to provide additional practice guidelines for treatment of lung cancer.
In the present study, the samples were selected in accordance with uniform inclusion criteria, exclusion criteria, and strict screening, which strengthened our evidence for research results. Moreover, TCM is recommended to be used for more than six months in clinical practice. Our findings showed that combined treatment of TCM and conventional chemotherapy could significantly prolong the DFS of patients with stage II-IIIA NSCLC in comparison with those treated with chemotherapy only. In addition, our research showed that the prognosis of TCM user group was better. Therefore, TCM, as adjunctive treatment, may be considered as an option of lung cancer treatment. There were five parameters for the prognostic values: age group, ECOG, lymphatic metastasis, BMI, and occupation. Lower age, higher ECOG score, more lymph node metastasis, and higher BMI led to higher rates of recurrence and metastasis. Also, compared to farmers, the risk of recurrence and metastasis for cadres was higher.
It should be noted that there are several limitations in this study. First of all, subgroup analysis of patients with stage II to IIIA according to clinical staging has not been done due to the limit of sample size. Secondly, the specimens of tumor focus and serum were not collected uniformly, and the molecular biological indexes were not studied and explored. Thirdly, the patients treated with a longer period of Chinese medicine (such as 1, 3, and 5 years) were not observed and followed up. Finally, in consideration of the feasibility of the project and the real diagnosis and treatment, the patients were not intervened by more individualized and comprehensive treatment with TCM.
In conclusion, since there are no published prospective, double-blinded trials of a TCM plus chemotherapy regimen following surgical resection of NSCLC, standardized large-scale, multicenter, and randomized double-blind controlled study should be used to carry out the clinical study of individualized treatment of patients with lung cancer in the future. Meanwhile, an evaluation system with Chinese characteristics should be established based on the clinical curative effects in view of effectiveness, safety, health economics, and ethics, improving authenticity and objectivity of research conclusions in order to present the superiority of Chinese medicine in cancer therapy as much as possible.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of Jiangsu Province (no. BK20171290), the National Natural Science Foundation of China (Grant nos. 81403232 and 81573656), the National Science and Technology Pillar Program during the 12th Five-Year Plan Period (no. 2014BAI10B01), and the Special Subject of the State Administration of Traditional Chinese Medicine (no. JDZX2015254). The authors greatly appreciate Dr. Jinsong Wang (the associate dean of the School of Medicine of Yangzhou University) for his excellent technical assistance.
Contributor Information
Xiaochun Zhang, Email: ceiq@sina.com.
Yayun Qian, Email: yyqian@yzu.edu.cn.
Yanqing Liu, Email: liuyq@yzu.edu.cn.
Data Availability
All data come from the medical records of the hospital and subsequent statistical analysis. These data will not be released to protect patient privacy.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Xueyu Zhao and Xiaojun Dai have contributed equally to this work
References
- 1.Torre L. A., Bray F., Siegel R. L., Ferlay J., Lortet-Tieulent J. Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Miller K. D., Siegel R. L., Lin C. C. Cancer treatment and survivorship statistics, 2016. CA: A Cancer Journal for Clinicians. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 3.Xiong Y., Huang B.-Y., Yin J.-Y. Pharmacogenomics of platinum-based chemotherapy in non-small cell lung cancer: focusing on DNA repair systems. Medical Oncology. 2017;34(4, article no. 48) doi: 10.1007/s12032-017-0905-6. [DOI] [PubMed] [Google Scholar]
- 4.Chen S., Flower A., Ritchie A., et al. Oral Chinese herbal medicine (CHM) as an adjuvant treatment during chemotherapy for non-small cell lung cancer: a systematic review. Lung Cancer. 2010;68(2):137–145. doi: 10.1016/j.lungcan.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Li S. G., Chen H. Y., Ou-Yang C. S., et al. The efficacy of Chinese herbal medicine as an adjunctive therapy for advanced non-small cell lung cancer: a systematic review and meta-analysis. PLoS ONE. 2013;8(2) doi: 10.1371/journal.pone.0057604.e57604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu R., He S. L., Zhao Y. C., et al. Chinese herbal decoction based on syndrome differentiation as maintenance therapy in patients with extensive-stage small-cell lung cancer: An exploratory and small prospective cohort study. Evidence-Based Complementary and Alternative Medicine. 2015;2015 doi: 10.1155/2015/601067.601067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong F., Jiang M., Huang Z., et al. A novel herbal formula induces cell cycle arrest and apoptosis in association with suppressing the PI3K/AKT pathway in human lung cancer A549 cells. Integrative Cancer Therapies. 2014;13(2):152–160. doi: 10.1177/1534735413503544. [DOI] [PubMed] [Google Scholar]
- 8.Pang L., Han S., Jiao Y., Jiang S., He X., Li P. Bu Fei Decoction attenuates the tumor associated macrophage stimulated proliferation, migration, invasion and immunosuppression of non-small cell lung cancer, partially via IL-10 and PD-L1 regulation. International Journal of Oncology. 2017;51(1):25–38. doi: 10.3892/ijo.2017.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen SJ., Zhang YH., Jiang SJ Gu. Kongliu Formula, a compound Chinese herbal medicine, combined with cisplatin, inhibits growth of lung cancer cells through transforming growth factor-ß1 signaling pathway. Journal of Integrative Medicine. May 2017;15(3):242–251. doi: 10.1016/S2095-4964(17)60330-3. [DOI] [PubMed] [Google Scholar]
- 10.Konkimalla V. B., Efferth T. Evidence-based Chinese medicine for cancer therapy. Journal of Ethnopharmacology. 2008;116(2):207–210. doi: 10.1016/j.jep.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Ling C. Q., Yue X. Q., Ling C. Three advantages of using traditional Chinese medicine to prevent and treat tumor. Journal of Integrative Medicine. 2014;12(4):331–335. doi: 10.1016/S2095-4964(14)60038-8. [DOI] [PubMed] [Google Scholar]
- 12.Jiao L., Dong C., Liu J., et al. Effects of Chinese medicine as adjunct medication for adjuvant chemotherapy treatments of non-small cell lung cancer patients. Scientific Reports. 2017;7 doi: 10.1038/srep46524.46524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma W. Aidi Injection as an adjunct therapy for non-small cell lung cancer: a systematic review. Journal of Chinese Integrative Medicine. 2009;7(4):315–324. doi: 10.3736/jcim20090404. [DOI] [PubMed] [Google Scholar]
- 14.Zhao SM. Chinese Materia Medica. Beijing, China: Peoples Medical Publishing House; Invigorate deficiency medicine. [Google Scholar]
- 15.Zeng Q., Xue N., Dai D., et al. A Nomogram based on inflammatory factors c-reactive protein and fibrinogen to predict the prognostic value in patients with resected non-small cell lung cancer. Journal of Cancer. 2017;8(5):744–753. doi: 10.7150/jca.17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang Y., Wakelee H. A. Adjuvant chemotherapy of completely resected early stage non-small cell lung cancer (NSCLC) Translational Lung Cancer Research. 2013;2(5):403–410. doi: 10.3978/j.issn.2218-6751.2013.07.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alam N., Shepherd F. A., Winton T., et al. Compliance with post-operative adjuvant chemotherapy in non-small cell lung cancer: An analysis of National Cancer Institute of Canada and intergroup trial JBR.10 and a review of the literature. Lung Cancer. 2005;47(3):385–394. doi: 10.1016/j.lungcan.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Sun D.-L., Xie H.-B., Xia Y.-Z. A study on the inhibitory effect of polysaccharides from Radix ranunculus ternati on human breast cancer MCF-7 cell lines. African journal of traditional, complementary, and alternative medicines : AJTCAM / African Networks on Ethnomedicines. 2013;10(6):439–443. doi: 10.4314/ajtcam.v10i6.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niu L., Zhou Y., Sun B., Hu J., Kong L., Duan S. Inhibitory effect of saponins and polysaccharides from Radix ranunculi ternati on human gastric cancer BGC823 cells. African journal of traditional, complementary, and alternative medicines : AJTCAM / African Networks on Ethnomedicines. 2013;10(3):561–566. doi: 10.4314/ajtcam.v10i3.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teng L., Jin K., He K., et al. Use of complementary and alternative medicine by cancer patients at zhejiang university teaching hospital zhuji hospital, China. African Journal of Traditional, Complementary and Alternative Medicines. 2010;7(4):322–330. doi: 10.4314/ajtcam.v7i4.56699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data come from the medical records of the hospital and subsequent statistical analysis. These data will not be released to protect patient privacy.


