Abstract
Background.
Curcuma longa (common name: turmeric) and one of its biologically active constituents, curcumin, have received increased clinical attention. Insufficient data exist on the effects of curcumin and turmeric on the gut microbiota and such studies in humans are lacking.
Methods.
Turmeric tablets with extract of piperine (Bioperine) (n = 6), curcumin with Bioperine tablets (n = 5), or placebo tablets (n = 3) were provided to healthy human subjects and subsequent changes in the gut microbiota were determined by 16S rDNA sequencing.
Results.
The number of taxa detected ranged from 172 to 325 bacterial species. The placebo group displayed an overall reduction in species by 15%, whereas turmeric-treated subjects displayed a modest 7% increase in observed species posttreatment. Subjects taking curcumin displayed an average increase of 69% in detected species. The gut microbiota response to treatment was highly personalized, thus leading to responders and nonresponders displaying response concordance. These “responsive” subjects defined a signature involving uniform increases in most Clostridium spp., Bacteroides spp., Citrobacter spp., Cronobacter spp., Enterobacter spp., Enterococcus spp., Klebsiella spp., Parabacteroides spp., and Pseudomonas spp. Common to these subjects was the reduced relative abundance of several Blautia spp. and most Ruminococcus spp.
Conclusions.
All participants’ microbiota displayed significant variation over time and individualized response to treatment. Among the responsive participants, both turmeric and curcumin altered the gut microbiota in a highly similar manner, suggesting that curcumin may drive the majority of observed changes observed in turmeric-treated subjects.
Keywords: microbiota, gastrointestinal, turmeric, curcumin, antioxidant
Curcuma longa (common name: turmeric) and one of its biologically active constituents, curcumin, are receiving increased clinical attention globally due to mounting evidence demonstrating therapeutic potential derived from outcomes that include anti-inflammatory, antioxidant, and neurotrophic effects.1 Ayurveda and other traditional systems of medicine commonly use turmeric as a medicinal herb, culinary spice, and digestive.2 Integrative health practitioners from allopathic fields have adopted turmeric and curcumin for a variety of applications in clinical practice,3 and a burgeoning interest among the lay public drives the growing global curcumin market.
Human clinical trial interventions have been heterogeneous in that various forms of curcumin, mixtures of curcuminoids, turmeric essential oil, turmeric extracts, or powdered turmeric rhizome have been used. Curcumin, for example, has demonstrated some potential in the context of chronic disease such as gastrointestinal, dermatological, and neurological disorders; however, additional human clinical trials are needed to support these initial findings.4,5 Whole turmeric rhizome has also been reported as potentially useful in gastrointestinal disease, cancer, and diabetes, and it is similarly in need of additional human clinical trial data to fully understand its therapeutic potential.6,7
Insufficient data exist on the effects of curcumin and turmeric on the gut microbiota and such studies in humans are lacking. Several animal studies suggest that these herbal medicines may affect gut microbial diversity.2 For example, in rats fed a high-fat diet, curcumin dietary supplementation shifted the gut microbiota population structure toward the lean phenotype and ameliorated high-fat, diet-induced metabolic endotoxemia and intestinal inflammation.8 In ovariectomized rats, estrogen deficiency negatively shifts the gut microbiota, and curcumin supplementation can partially restore normal gut microbial diversity.9 In a mouse model of colitis, curcumin treatment altered the gut microbiome to feature an increased abundance of butyrate producers and induced T-regulatory cells (Tregs), which are changes that may lead to improved gut barrier function and reduced systemic inflammation.10 Turmeric repressed human Ruminococcus spp. and a few Clostridium isolates but did not significantly promote the growth of Lactobacillus spp. or Bifidobacterium spp. in an in vitro culture model.11 In addition, curcumin increased the relative abundance of Lactobacillales and decreased Coriobacterales in a mouse model of colitis.12 Human studies are needed to further understand the impact of turmeric and its constituents on microbiota.
Curcumin and turmeric may promote health benefits despite low absorption by modulating intestinal barrier function, although additional investigations are needed.13,14 Curcumin may sustain high concentrations in the intestinal mucosa, modulate gut barrier function, and thereby lower circulating bacterial lipopolysaccharide levels and inflammation to promote health effects. In rats fed high-fat diet, curcumin supplementation restores intestinal barrier function and expression of tight junction proteins.8 Moreover, curcumin-treated dendritic cells can promote the differentiation of 2 types of Tregs found in the intestine.15 Curcumin-stimulated intestinal epithelial cells (Caco-2) attenuated lipopolysaccharide-induced pro-inflammatory cytokine secretion and prevented disruption of tight junction proteins.16 Such barrier effects will in turn promote changes in the composition and diversity of the gut microbiome.
Turmeric is estimated to contain 2% to 6% (w/w) curcuminoids, which contain 80% curcumin, 18% demethoxycurcumin, and 2% bisdemethoxycurcumin.6 Human gut microbiota biotransform curcumin in a variety of ways including sequential reduction of the heptadienone backbone and demethylation by Blautia spp. and others to produce active metabolites that may exert local or perhaps even systemic effects.17,18 Curcumin and other curcuminoids may exhibit a variety of pharmacological activities; however, the full impact of curcumin on gut microbiota and the microbial metabolism of turmeric and related compounds is incompletely understood.
In the current human clinical pilot trial, turmeric tablets with Bioperine, curcumin with black pepper extract (Bioperine) tablets, or placebo tablets were provided to healthy human subjects and subsequent changes in the gut microbiota community architecture were determined at baseline and after 4 and 8 weeks of treatment.
Methods
Study Participants and Sample Collection
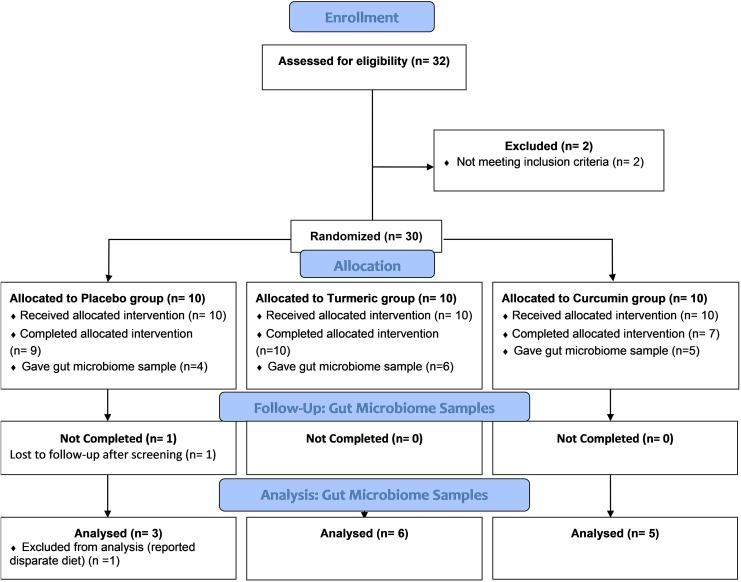
The University of California, Davis Institutional Review Board (IRB #850932-8) approved this study (http://ClinicalTrials.gov Identifier: NCT03066791), and written informed consent was obtained from all subjects prior to enrollment. A total of 32 adult subjects aged 19 to 58 years were screened, and all participants received financial compensation. Eligible subjects could not have a smoking history in the past year, chronic steroid use, history of diabetes, metabolic syndrome, cardiovascular disease, known electrocardiogram changes, malignancy, or kidney disease. Subjects were also excluded if they had used systemic antibiotics within 1 month or topical medications or any oral turmeric or curcumin products within 2 weeks prior to the start of the study. Subjects could not be included if they had a known allergy to black pepper or if they were taking angiotensin converting enzyme inhibitor medications or angiotensin receptor blocker medications for any reason. Women that were pregnant or breastfeeding were excluded. Thirty subjects meeting enrollment criteria were enrolled and randomized into 3 groups: 10 subjects each in placebo, turmeric, and curcumin tablet groups (see Figure 1). Subjects reported an omnivorous diet prior to and during the study.
Figure 1.
CONSORT clinical trials flow diagram for the gut microbiome arm of the pilot study.
Study Interventions
Turmeric tablets, curcumin tablets, and placebo tablets were provided by Sabinsa Corporation (East Windsor, NJ). The turmeric tablets contained 1000 mg turmeric root (Curcuma longa) plus 1.25 mg black pepper–derived extract of piperine alkaloid (BioPerine). The curcumin tablets contained 1000 mg of curcumin (Curcumin C3 Complex) plus 1.25 mg black pepper BioPerine. The placebo tablets were similar in size, shape, and color to the other 2 groups. Subjects were each given a bottle of 1000 mg tablets and were instructed to take 3 tablets orally with food, twice a day (total of 6 tablets daily; 6000 mg).
Study Design
We conducted this prospective, single-center, evaluator-blinded randomized pilot study between August 2016 and July 2017. Healthy subjects were each given one bottle (180 tablets) of placebo, turmeric, or curcumin tablets at the baseline visit (visit 1) and another bottle of 180 tablets was dispensed at the week 4 visit (visit 2). They were advised to not consume any other turmeric-containing foods or supplements for the duration of the study period. Subjects were seen at a screening visit, followed by a baseline visit (visit 1), and at week 4 (visit 2) and week 8 (visit 3) posttreatment for safety and response evaluations. The entire intervention was performed at the Department of Dermatology, University of California–Davis, Sacramento, CA.
Randomization
Subjects were randomized by a UC Davis Investigational Drug Services pharmacist prior to study recruitment using a web-based service from a list of randomly generated numbers. A pharmacist not involved in the study kept the randomization list off-site. After recruitment and assigning subject numbers in sequential order of study visits, a research team member dispensed the pill bottle labeled with the corresponding subject number. Study investigators and participants remained blinded to which study tablets they received (placebo, turmeric, or curcumin tablets) for the duration of the study. The codes were not disclosed to the study investigators until the study was complete.
Study Procedures and Measures
Subjects were evaluated at baseline, 4 weeks, and 8 weeks posttreatment by the study investigators. Participants ate their normal (omnivorous) diets and donated an optional morning fecal sample in stool hats. Not all participants provided a stool sample. The fecal samples were transferred to conical tubes and then frozen on ice and stored at −80°C until further processing.
Subjects were asked to report any adverse effects throughout the study.
Microbial DNA Isolation
A total of 4 subjects from the placebo group, 5 subjects from the curcumin-treated group, and 6 subjects from the turmeric-treated group successfully provided stool samples. One subject from the placebo group self-reported a disparate diet (vegan) and was thus excluded from further gut microbiota analysis as all subjects self-reported an omnivorous diet prior to and during the study. Genomic DNA was isolated from human subject stool samples using the procedures of the QiaAmp DNA stool kit (Qiagen) with a modification that included an additional step of bead beating using the FastPrep instrument (MP Bio) to ensure uniform lysis of bacterial cells.
Microbiota Analysis by 16S rDNA Sequencing
Multiplexed 16S rDNA libraries were prepared using standard 16S metagenomic sequencing library protocols from Illumina, which uses V3-V4 region of 16S rDNA for target amplification. We performed paired end reads (250 bp) sequencing to generate ∼200 000 sequences/sample using the Illumina MiSeq. Subsequent analysis was done in CLC Microbial Genomics Module 2.5 (Qiagen) and R.19 Paired end reads were merged (mismatch cost, 2; minimum score, 8; gap cost, 3; maximum unaligned end mismatches, 0) and trimmed to the same length. Additional quality filter steps were applied to exclude short reads, sequences with poor-quality scores, and chimeras. To ensure comparable high coverage in all samples, we excluded samples producing <35 000 reads, which was the case for one of the curcumin-treated subjects whose data were thus excluded from downstream analyses. A total of 16 participants elected to provide stool samples, and a total of 3 placebo, 5 curcumin-treated, and 6 turmeric-treated subjects passed quality control for further analysis.
We did not use OTU-based enumeration of taxa due to the overmerging that occurs. Instead, each unique 16S rDNA sequence was subjected to BLAST using the NCBI 16S rRNA database (Bacteria and Archaea) to identify best matches to taxa at the genus and species levels based on % identity.
Fecal samples were obtained at baseline and at 4 weeks and 8 weeks following the initiation of the intervention. To simplify analysis and reduce the noise of gut microbiota profiles, we compared baseline microbiota profiles to an average of the 4 weeks and 8 weeks fecal species profiles. Genomic DNAs obtained from fecal samples were used to amplify the V3-V4 region of 16S rDNA for subsequent sequence analysis.
We filtered sequences that were observed less than 10 times, resulting in a total of 2770 unique phylotypes present in this cohort. We define a phylotype as a unique 16S rDNA sequence distinguished by at least one base difference. The abundance range of these phylotypes ranged from 10 to 67 324 sequences. Phylotypes were merged based on BLAST analysis that defined 443 unique bacterial species present in the cohort.
Statistical Analysis
As part of exploratory analyses, 2-way (group by time) ANOVAs using 16S rDNA data were performed in SPSS (Version 23).
Results
Gut Microbiota Species Changes Following Turmeric or Curcumin Intervention
Among the individual participants, the number of bacterial species ranged from 172 to 325 bacterial species. We compared the number of observed species present in each group pre- and posttreatment. The placebo group displayed an overall reduction in species by 15% (175 baseline vs 149 average posttreatment), whereas turmeric-treated subjects displayed a modest 7% (156 vs 167) increase in observed species. Notably, subjects taking curcumin displayed an average increase of 69% (127 vs 215) in detected species.
Alpha diversity values (Shannon entropy indices), which indicate within-group diversity, were calculated for each group to determine the effect of treatment on gut microbiota species diversity. Subjects in the curcumin-treated group had higher average alpha diversity (α = 6.31) compared with the turmeric-treated (α = 6.05) or placebo-treated (α = 6.15) groups. While treatment with curcumin tended to increase microbial diversity, ANOVA comparison of alpha diversity indices did not reveal statistically significant differences between treatment groups due to high variation within groups (P = .08).
The longitudinal sampling of stools over a 2-month period is expected to result in changes in microbiota composition, independent of treatment effects. Indeed, the placebo group displayed an increase in the relative abundance (2-fold or greater) of an average of 68 taxa compared with 88 taxa that increased in the turmeric group and 147 that increased in the curcumin group (see Supplementary Table 1; available in the online version of the article). Most of the changes in relative abundance in the placebo control group were between 2-fold and 10-fold (69%), whereas turmeric and curcumin treatment led to larger abundance alterations between 10-fold and 100-fold or greater in an average of 53% and 60% of participants, respectively.
A number of fecal taxa were also reduced in relative abundance over the course of the study. The average relative abundance of 89 taxa were reduced in the placebo group, although this was driven predominantly by one subject (subject 1) that displayed reduced abundance of a remarkably high number of taxa (181). Turmeric and curcumin treatment resulted in the reduced average relative abundance of 71 and 56 taxa, respectively.
The exploratory ANOVAs revealed group by time interactions for the following: [Clostridium] xylanolyticum (F = 4.21; P = .044), Collinsella aerofaciens (F = 7.01; P = .011), Kluyvera intermedia (F = 5.22; P = .025), and Raoultella electrica (F = 3.84; P = .054) displayed an increase in the curcumin group, a decrease in the placebo group, and no change in the turmeric group; Coprococcus catus displayed a decrease in the curcumin group and no change with placebo or turmeric (F = 4.59; P = .036); Alistipes putredinis displayed an increase in the turmeric-treated group with no change with placebo or curcumin (F = 4.95; P = .029); Eisenbergiella tayi displayed a decrease in the turmeric group with no change in the placebo or curcumin groups (F = 3.92; P = .052); and both Intestinibacillus massiliensis (F = 7.23; P = .010) and Parasutterella excrementihominis (F = 3.85; P = .054) displayed a decrease in the placebo group with no change with turmeric or curcumin.
Response to Turmeric and Curcumin Is Highly Personalized
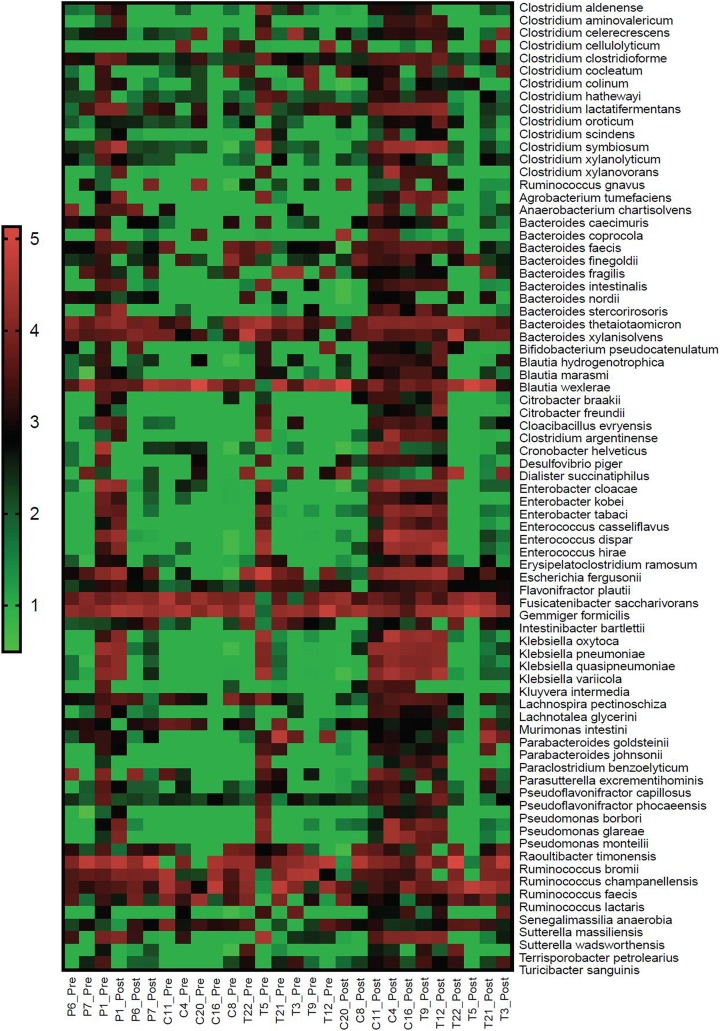
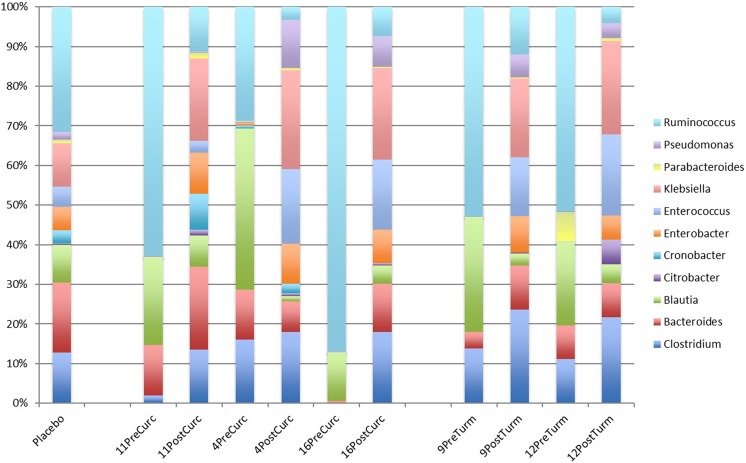
We noted that the responses of the gut microbiota to turmeric and curcumin treatment were nonuniform across individuals (Figure 2). However, the observed patterns were not random, whereas they were in the placebo group. The subject-specific response to treatments observed here are consistent with numerous similar studies including those examining the effects of resistant starch, daidzein, genistein, and polyphenols that demonstrated subject-to-subject variation in response, thus setting the precedent of defining microbiota responders and nonresponders to treatment.20–22 These personalized effects were evident in the case of turmeric and curcumin. Subjects 9 and 12 in the turmeric group displayed high response concordance (Figure 3). Similarly, subjects 4, 11, and 16 displayed high response concordance following curcumin consumption. Interestingly, the patterns of microbial species abundance changes for all of these subjects in both the turmeric and curcumin treatment groups were highly similar, which suggests that curcumin was the major driver of microbiota composition alterations (Figure 3).
Figure 2.
Heat map of relevant taxa following turmeric, curcumin, or placebo intervention. The frequency of read counts observed for each taxa were multiplied by 1 × 106 to generate values greater than 1 for each subject and then log10 transformed and depicted as a color scale. Subjects are arranged on the x-axis to highlight the congruently altered microbiota of responders compared to placebo and nonresponders.
Figure 3.
Genus-level relative abundance in treatment responsive subjects. The average relative abundance of taxa of the placebo group is compared with subjects responsive to turmeric and curcumin posttreatment.
The similarities in response to herbal intervention were broadly evident across diverse taxa. These “responsive” subjects defined a signature that involved uniform increases in most Clostridium spp., Bacteroides spp., Citrobacter spp., Cronobacter spp., Enterobacter spp., Enterococcus spp., Klebsiella spp., Parabacteroides spp., and Pseudomonas spp. Common to these subjects was the reduced relative abundance of several Blautia spp. and most Ruminococcus spp. These subjects displayed variable alterations in Eubacterium spp.
The remaining “nonresponsive” participants displayed few discernable patterns with respect to one another or uniform alterations in taxonomic groups. Overall, the only notable feature unifying the nonresponsive subjects was an overall increase in the number of taxa that were unaltered by the intervention or displaying reduced abundance in taxa across all observed taxonomic groups.
Adverse Events
Each subject reported adherence to the treatment protocol and no adverse effects were reported.
Discussion
This pilot study examined the gut microbiota profiles of human subjects longitudinally from 3 groups: placebo, turmeric, and curcumin treatment. All participants’ microbiota displayed significant variation over time and individualized response to treatment. The microbiota of some participants (responders) receiving the treatment intervention were distinct from placebo controls in at least 2 important aspects. First, multiple species belonging to a given genus displayed concordant changes observed in treatment groups but not placebo controls. Second, multiple subjects derived from both treatment groups displayed highly similar responses to turmeric and curcumin. These signatures allowed us to clearly distinguish some but not all of the participants receiving treatment from the placebo group. While concordance of results is supportive of significance, given the pilot nature of the study, additional full-scale clinical trials are needed to confirm the current observations and microbial signatures.
The comparison of microbiota alterations driven by turmeric and curcumin were expected to be related, at least in part, given that curcumin is known to be a biologically important component of turmeric. Among the responsive participants, both turmeric and curcumin altered the gut microbiota in a highly similar manner. Interpretation of the turmeric response appears to reflect the catabolism of polysaccharide components present in the root involving the extensive repertoire of glycosyl hydrolases encoded by Bacteroides, Bifidobacterium, Alistipes, and Parabacteroides, all of which were elevated in responsive subjects. The liberated oligosaccharides, disaccharides, and monosaccharides provide energy for fermentative bacteria such as a large diversity of Clostridium spp. Sugar fermentation results in the production of short chain fatty acids and H2. The accumulation of H2 inhibits further sugar fermentation, unless H2 consuming bacteria are active. Known H2-consuming bacteria include some Blautia and Desulfovibrio spp., which were elevated in responsive subjects. Interestingly, Blautia spp. from human-derived gut microbiota were reported to metabolize curcumin through multiple pathways.17
While this food web serves as convincing evidence of the prebiotic effect of turmeric being driven by polysaccharide catabolism and sugar metabolism, it is unclear why microbiota profiles observed following curcumin treatment also predict the same scenario. Indeed, curcumin cannot serve as a direct energy source for commensal microbiota and thus does not fully meet the definition of prebiotic; therefore, its “prebiotic-like” effects are expected to be driven largely by indirect effects based on alterations in host physiology, which may include changes in barrier function or through selective survival of local bacteria or other microorganisms.23 Our results make clear that further study in larger cohorts is required to fully understand the effects of curcumin and turmeric on the gut microbiota and how those effects contribute to the known health benefits of these herbal medicines.
We have considered a possible explanation of our findings that suggest that the response of gut microbiota to turmeric and curcumin may be distinct and even nonoverlapping with host responsiveness. It is well known that the health benefits of turmeric and curcumin are limited by host absorption in the gut. It is conceivable that participants that efficiently absorbed turmeric or curcumin in the small intestine reduced the potential prebiotic effect of turmeric and prebiotic-like effects of curcumin in the colon (ie, site of action for prebiotic effects) as reflected in stool samples given that more substrate would be absorbed in the small intestine and thus less substrate would arrive in the colon in such circumstances. Conversely, subjects displaying poor absorption of these herbs in the small intestine may display the greatest prebiotic effects in the colon due to increased concentrations of prebiotic compounds arriving at the site of action in the colon or host-driven alterations of microbiota affecting colonic populations. These hypotheses are speculative and will require further testing and should include parallel analysis of gut and serum curcumin and tetrahydrocurcumin concentrations as a marker of local metabolism24,25 and bio-absorption.
Analysis of 16S rDNA profiles from these participants revealed a number of relevant limitations concerning human intervention studies and the current study conclusions. First, fecal microbiota profiles varied substantially over the 1-month sampling period in both placebo and experimental groups. While this variability did not prevent the identification of clear patterns associated with treatment or our ability to distinguish responders from placebo groups, a substantial number of participants in the treatment groups were not clearly distinguishable from the placebo group. These findings suggest that future studies should include not only a larger cohort and but also consider the inclusion of a controlled diet to reduce subject to subject and temporal variation in microbiota over time. However, despite these limitations, our study was still able to find patterns of change with both turmeric and curcumin, suggesting its effects in a pragmatic setting that will require further study and follow-up in larger cohorts. The addition of piperine extract has been shown to promote the growth of some gut microbiota11; thus, it is unclear the extent to which this inclusion with the curcumin and turmeric tablets added to the observed growth stimulatory effects. Indeed, we have evaluated the effects of black pepper on fecal cultures in vitro (CTP, unpublished data) and observe significant alterations in gut microbiota profiles. Future studies that use curcumin alone, turmeric alone, or include black pepper in the control group may further clarify the microbial signature of curcumin treatment in the gut. Another limitation of this study is the small sample sizes per treatment group, which was due to 16 of the study subjects not providing adequate posttreatment stool samples. We thus focused on providing a more qualitative description of the findings rather than focus on statistical analyses. We did not perform false discovery rate or Bonferroni adjustments. Pilot studies on gut microbiota represent an important starting point to inform additional confirmatory research in expanded experimental designs and new hypotheses. Therefore, the observations and interpretation of results must be tempered until larger follow-up studies are conducted. While smaller sample sizes limit conclusions, the findings presented are concordant and thus salient, especially in the context of future investigation.
This pilot study in healthy subjects has potentially raised more intriguing questions than it has fully answered and emphasizes the complexity of human intervention studies intending to study the effects of these potentially powerful herbal medicines. Future studies that include a larger human cohort will clarify whether the “responsive” microbiota we identify here are representative and whether less prevalent response signatures in our data may be clearly defined with additional participants. Detailed dietary intake accounting or full dietary control in larger scale studies will enhance the precision of identifying responsive microbial taxa and relevant signatures. Future studies should include objective measures of host absorption and whether absorption predominantly occurs in the small and/or large intestine. Future studies should also assess changes in systemic mediators to assess how changes in the gut microbiota shift chemical and lipid mediators in the bloodstream. Studies incorporating these features may allow a more precise relationship between the gut microbiota and its potential role as a mediator of the health benefits of turmeric and curcumin.
Supplemental Material
Supplementary_Table_1 for Effects of Turmeric and Curcumin Dietary Supplementation on Human Gut Microbiota: A Double-Blind, Randomized, Placebo-Controlled Pilot Study by Christine T. Peterson, Alexandra R. Vaughn, Vandana Sharma, Deepak Chopra, Paul J. Mills, Scott N. Peterson, and Raja K. Sivamani in Journal of Evidence-Based Integrative Medicine
Footnotes
Authors’ Note: This study is registered at http://ClinicalTrials.gov with identifier NCT03066791.
Author Contributions: RKS and ARV designed and led the study intervention. RKS and ARV oversaw the study, led the clinical interventions, and supervised data/sample collection. CTP designed the microbiome study. CTP wrote the manuscript, revised the manuscript, and performed biological interpretation. CTP, SNP, and VS performed genomics assays, quality control, and generated figures. CTP analyzed the microbiome data. PJM performed statistical analyses. All authors were also involved with biological interpretation and manuscript revisions. All authors reviewed the manuscript, revised the manuscript, and approved of the final version for publication.
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RKS has no relevant conflicts of interest and serves as a scientific advisor for Dermveda and as a consultant for Burt’s Bees and Dermala. PJM is Director of Research for the Chopra Foundation. DC is a founder of the Chopra Foundation and Chopra Center and a co-owner of the Chopra Center. The other authors have no conflicts of interest to declare.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by departmental funding from the Department of Dermatology at UC Davis (RKS) and a fellowship grant from the Chopra Foundation.
Ethical Approval: The University of California, Davis Institutional Review Board (IRB #850932-8) approved this study, and written informed consent was obtained from all subjects prior to enrollment.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Rahmani AH, Alsahli MA, Aly SM, Khan MA, Aldebasi YH. Role of curcumin in disease prevention and treatment. Adv Biomed Res. 2018;7:38 doi:10.4103/abr.abr_147_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shen L, Liu L, Ji HF. Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications. Food Nutr Res. 2017;61:1361780 doi:10.1080/16546628.2017.1361780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Millet JD. Progress in complementary and alternative medicine research: Yale Research Symposium on Complementary and Integrative Medicine. Yale J Biol Med. 2010;83:127–129. [PMC free article] [PubMed] [Google Scholar]
- 4. Mantzorou M, Pavlidou E, Vasios G, Tsagalioti E, Giaginis C. Effects of curcumin consumption on human chronic diseases: a narrative review of the most recent clinical data. Phytother Res. 2018;32:957–975. doi:10.1002/ptr.6037. [DOI] [PubMed] [Google Scholar]
- 5. Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. J Tradit Complement Med. 2017;7:205–233. doi:10.1016/j.jtcme.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi:10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martin RC, Aiyer HS, Malik D, Li Y. Effect on pro-inflammatory and antioxidant genes and bioavailable distribution of whole turmeric vs curcumin: similar root but different effects. Food Chem Toxicol. 2012;50:227–231. doi:10.1016/j.fct.2011.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feng W, Wang H, Zhang P, et al. Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats. Biochim Biophys Acta. 2017;1861:1801–1812. doi:10.1016/j.bbagen.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Z, Chen Y, Xiang L, Wang Z, Xiao GC, Hu J. Effect of curcumin on the diversity of gut microbiota in ovariectomized rats. Nutrients. 2017;9:E1146 doi:10.3390/nu9101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ohno M, Nishida A, Sugitani Y, et al. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS One. 2017;12:e0185999 doi:10.1371/journal.pone.0185999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu QY, Summanen PH, Lee RP, et al. Prebiotic potential and chemical composition of seven culinary spice extracts. J Food Sci. 2017;82:1807–1813. doi:10.1111/1750-3841.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McFadden RM, Larmonier CB, Shehab KW, et al. The role of curcumin in modulating colonic microbiota during colitis and colon cancer prevention. Inflamm Bowel Dis. 2015;21:2483–2494. doi:10.1097/MIB.0000000000000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghosh SS, He H, Wang J, Gehr TW, Ghosh S. Curcumin-mediated regulation of intestinal barrier function: the mechanism underlying its beneficial effects. Tissue Barriers. 2018;6:e1425085 doi:10.1080/21688370.2018.1425085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adiwidjaja J, McLachlan AJ, Boddy AV. Curcumin as a clinically-promising anti-cancer agent: pharmacokinetics and drug interactions. Expert Opin Drug Metab Toxicol. 2017;13:953–972. doi:10.1080/17425255.2017.1360279. [DOI] [PubMed] [Google Scholar]
- 15. Cong Y, Wang L, Konrad A, Schoeb T, Elson CO. Curcumin induces the tolerogenic dendritic cell that promotes differentiation of intestine-protective regulatory T cells. Eur J Immunol. 2009;39:3134–3146. doi:10.1002/eji.200939052. [DOI] [PubMed] [Google Scholar]
- 16. Wang J, Ghosh SS, Ghosh S. Curcumin improves intestinal barrier function: modulation of intracellular signaling, and organization of tight junctions. Am J Physiol Cell Physiol. 2017;312:C438–C445. doi:10.1152/ajpcell.00235.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burapan S, Kim M, Han J. Curcuminoid demethylation as an alternative metabolism by human intestinal microbiota. J Agric Food Chem. 2017;65:3305–3310. doi:10.1021/acs.jafc.7b00943. [DOI] [PubMed] [Google Scholar]
- 18. Tan S, Calani L, Bresciani L, et al. The degradation of curcuminoids in a human faecal fermentation model. Int J Food Sci Nutr. 2015;66:790–796. doi:10.3109/09637486.2015.1095865. [DOI] [PubMed] [Google Scholar]
- 19. Eglen SJ. A quick guide to teaching R programming to computational biology students. PLoS Comput Biol. 2009;5:e1000482 doi:10.1371/journal.pcbi.1000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martinez I, Kim J, Duffy PR, Schlegel VL, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS One. 2010;5:e15046 doi:10.1371/journal.pone.0015046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bolca S, Possemiers S, Herregat A, et al. Microbial and dietary factors are associated with the equol producer phenotype in healthy postmenopausal women. J Nutr. 2007;137:2242–2246. [DOI] [PubMed] [Google Scholar]
- 22. Bolca S, Van de Wiele T, Possemiers S. Gut metabotypes govern health effects of dietary polyphenols. Curr Opin Biotechnol. 2013;24:220–225. doi:10.1016/j.copbio.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 23. Peterson CT, Sharma V, Uchitel S, et al. Prebiotic potential of herbal medicines used in digestive health and disease [published online March 22, 2018]. J Altern Complement Med. doi:10.1089/acm.2017.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hassaninasab A, Hashimoto Y, Tomita-Yokotani K, Kobayashi M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc Natl Acad Sci U S A. 2011;108:6615–6620. doi:10.1073/pnas.1016217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. An CY, Sun ZZ, Shen L, Ji HF. Biotransformation of food spice curcumin by gut bacterium Bacillus megaterium DCMB-002 and its pharmacological implications. Food Nutr Res. 2017;61:1412814 doi:10.1080/16546628.2017.1412814. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Table_1 for Effects of Turmeric and Curcumin Dietary Supplementation on Human Gut Microbiota: A Double-Blind, Randomized, Placebo-Controlled Pilot Study by Christine T. Peterson, Alexandra R. Vaughn, Vandana Sharma, Deepak Chopra, Paul J. Mills, Scott N. Peterson, and Raja K. Sivamani in Journal of Evidence-Based Integrative Medicine