 A series of 3′,4′,5′-trimethoxy flavonoids with benzimidazole linked by different chain alkanes have been designed and synthesized.
A series of 3′,4′,5′-trimethoxy flavonoids with benzimidazole linked by different chain alkanes have been designed and synthesized.
Abstract
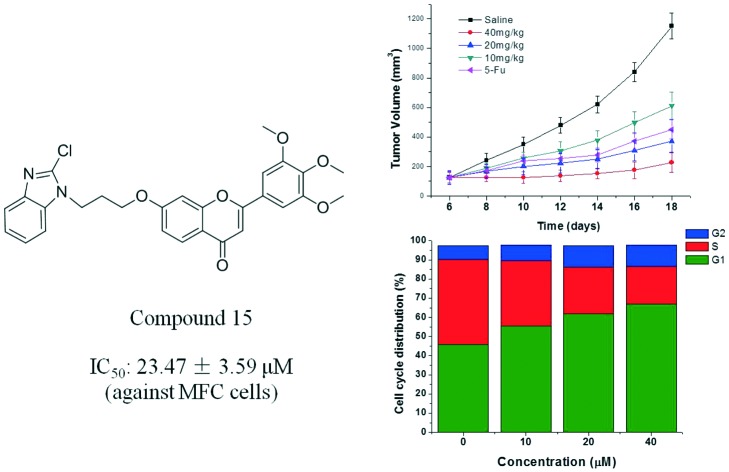
A series of 3′,4′,5′-trimethoxy flavonoids with benzimidazole linked by different chain alkanes have been designed and synthesized. The potential activity of these compounds as anti-tumor agents was evaluated by cytotoxicity assay in MGC-803 (human gastric cancer), MCF-7 (human breast cancer), HepG-2 (human hepatoma) and MFC (mouse gastric cancer) tumor cell lines. Among them, compound 15 7-(3-(2-chloro-1H-benzo[d]imidazol-1-yl)propoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one displayed the most potent antiproliferative activity, with IC50 values of 20.47 ± 2.07, 43.42 ± 3.56, 35.45 ± 2.03 μM and 23.47 ± 3.59 μM, respectively. The flow cytometry (FCM) results showed that compound 15 caused the cell cycle to be arrested in G1 phase and induced apoptosis of MFC cells in a dose-dependent manner. In addition, compound 15 exhibited a significant inhibitory effect on tumor growth in vivo. All the results outlined the great potential of compound 15 for further exploitation as anti-tumor agent.
1. Introduction
Cancer tumors represent one of the major threats to human life. There is an urgent need for safer and more effective chemotherapy drugs that will improve the survival rates of patients with various types of tumors. It is well known that many anti-tumor drugs are derived from the structure of natural products.1 Flavonoids, a class of natural products, are widely found in nature and exhibit a wide range of biological activities,2–5 and they tend to be used as a dominant parental structure in drug discovery.6 Over the past decade, a large number of natural, semi-synthetic, and synthetic flavonoids have been exploited and evaluated for a variety of therapeutic activities, such as anti-inflammatory, anti-bacterial,7 anti-allergy, anti-oxidation,8 and anti-tumor.9,10 Particularly, their potential therapeutic effects and reliable safety in the treatment of tumors have received extensive attention.11–13
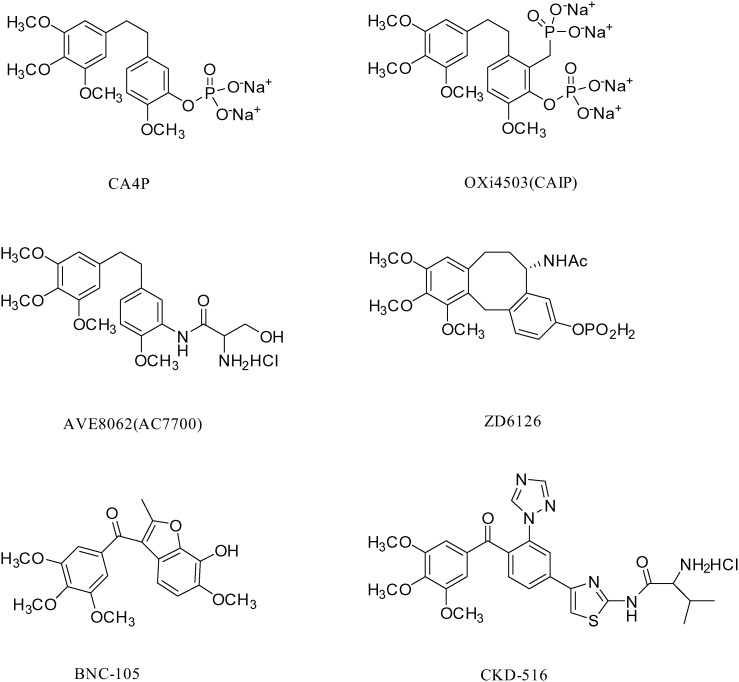
Benzene trimethoxy has special value in the design of antineoplastic drugs, particularly in the design of vascular blockers. At present, the vascular disrupting agents in the clinical research stage mainly target tubulin to inhibit the formation of microtubules; these include CA4P and its analogues OXi4503 and AVE8062,14–17 and the colchicine analogs ZD6126,18 BNC-105 (ref. 19) and CKD-516 (ref. 20) (Fig. 1). Interestingly, these compounds contain benzene trimethoxy. In addition, we also note that benzimidazoles have been widely used in the design and development of anticancer drugs;21–25 some benzimidazoles and their derivatives exhibited significant anti-tumor effect by inhibiting tumor cell mitosis and proliferation, mediating tumor cell apoptosis or inhibiting the expression of hypoxia-inducible factor (HIF).26–28
Fig. 1. The structures of CA4P, OXi4503, AVE8062, ZD6126, BNC-105 and CKD-516.
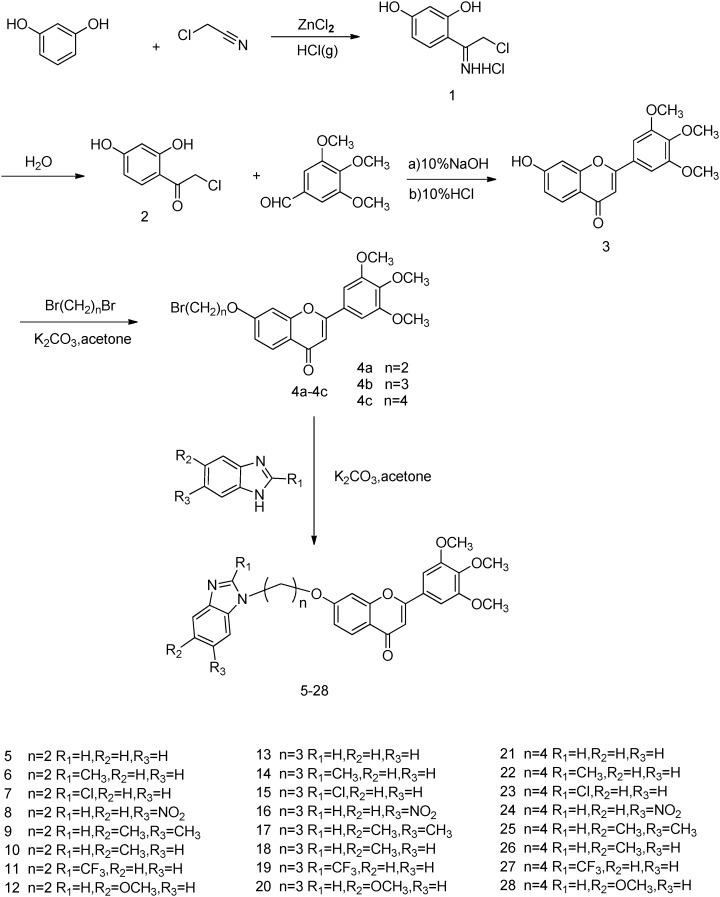
In this study, we attempted to introduce the trimethoxy group into the B-ring of the flavonoid and link benzimidazole and its derivatives to 7-OH with different chain alkanes. We are eager to discover new flavonoid derivatives with potential anti-tumor activity. In this study, we synthesized a series of 3′,4′,5′-trimethoxy flavonoid benzimidazole derivatives 5–28 and evaluated the anti-tumor activity screening results of these compounds. We successfully screened compound 15 with notable anti-tumor activity in vitro and further demonstrated its activity in vivo. The inhibitory effect of compound 15 on the expression of tubulin or hypoxia-inducible factor (HIF) requires further investigation.
2. Results and discussion
2.1. Chemistry
In this study, we synthesized twenty-four new 3′,4′,5′-trimethoxy flavonoid benzimidazole derivatives. The synthetic route is shown in Scheme 1. The key intermediate 3 was prepared in a three-step synthesis starting with resorcinol, 2-chloroacetonitrile and 3,4,5-trimethoxybenzaldehyde according to a literature procedure.29 Houben–Hoesch reaction of resorcinol under the catalysis of zinc chloride in ether at 0 °C provided 4-(2-chloro-1-iminoethyl)benzene-1,3-diol hydrochloride (1), which was then converted into 2-chloro-1-(2,4-dihydroxyphenyl)ethanone (2) by hydrolyzation. Base-catalyzed aldol condensation of 2 with 3,4,5-trimethoxybenzaldehyde afforded the chalcone. The chalcone was cyclized in the solvent of 10% hydrochloric acid and ethanol at room temperature to provide flavonoid 3 in high yield with recrystallization from 95% ethanol.
Scheme 1. General synthesis of compounds 5–28.
New analogues 4a–4c were conveniently synthesized in high yields (83–92%) by etherification reaction of the key intermediate 3 with different chain length substituted alcohols in the presence of K2CO3 in acetone. Alkylation of the bromide intermediates 4a–4c and corresponding benzimidazoles in the presence of K2CO3 in acetone introduced basic functionalities, providing final compounds 5–28 in 50–80% yields. All of the synthetic compounds gave satisfactory analytical and spectroscopic data, which were in full accordance with their depicted structures.
2.2. Biological activity
2.2.1. In vitro antiproliferative assay
The in vitro antiproliferative activity of 3′,4′,5′-trimethoxy flavonoid benzimidazole derivatives 5–28 and the positive control 5-Fu on MGC-803, MCF-7, HepG-2 and MFC cells was studied by MTT colorimetric assay. The compounds were tested in a concentration range of 2 to 256 μM, and the calculated IC50 values are reported in Table 1.
Table 1. Anti-proliferative activity of compounds against the tumor cell lines.
| Compounds | IC50 (μM) ± SD |
|||
| MGC-803 | MCF-7 | HepG-2 | MFC | |
| 5 | >100 | >100 | >100 | >100 |
| 6 | 94.47 ± 5.21 | >100 | 84.32 ± 3.54 | 87.68 ± 5.47 |
| 7 | 75.12 ± 3.47 | 87.34 ± 4.39 | 82.53 ± 3.28 | 72.56 ± 2.83 |
| 8 | 91.54 ± 4.26 | 95.17 ± 3.21 | 87.53 ± 3.28 | 90.73 ± 5.14 |
| 9 | >100 | N.D. | N.D. | N.D. |
| 10 | 84.58 ± 3.42 | >100 | >100 | >100 |
| 11 | 75.43 ± 3.12 | 84.71 ± 4.17 | >100 | 80.37 ± 3.62 |
| 12 | >100 | N.D. | >100 | >100 |
| 13 | N.D. | N.D. | N.D. | >100 |
| 14 | 77.52 ± 3.71 | 91.46 ± 4.36 | >100 | 88.46 ± 3.16 |
| 15 | 20.47 ± 2.07 | 43.42 ± 3.56 | 35.45 ± 2.03 | 23.47 ± 3.59 |
| 16 | 83.14 ± 2.52 | 90.47 ± 5.32 | >100 | 88.43 ± 3.63 |
| 17 | N.D. | >100 | N.D. | N.D. |
| 18 | 97.38 ± 5.54 | >100 | N.D. | >100 |
| 19 | 77.27 ± 4.76 | 92.37 ± 3.62 | 86.83 ± 4.73 | 82.24 ± 3.81 |
| 20 | >100 | N.D. | N.D. | 90.32 ± 2.17 |
| 21 | 94.38 ± 4.76 | N.D. | N.D. | >100 |
| 22 | 73.62 ± 4.73 | 81.43 ± 2.57 | >100 | 77.42 ± 4.37 |
| 23 | 63.51 ± 2.64 | 78.34 ± 3.53 | 76.82 ± 5.39 | 67.15 ± 4.26 |
| 24 | 54.86 ± 4.28 | >100 | 72.48 ± 3.51 | 62.37 ± 5.14 |
| 25 | N.D. | N.D. | >100 | >100 |
| 26 | 95.62 ± 5.34 | 87.46 ± 4.17 | >100 | 90.01 ± 3.23 |
| 27 | 76.27 ± 3.74 | 62.37 ± 2.35 | >100 | 58.43 ± 4.43 |
| 28 | 84.73 ± 5.12 | >100 | N.D. | >100 |
| 5-Fu | 74.39 ± 2.03 | 57.09 ± 3.17 | 63.37 ± 2.52 | 78.52 ± 3.92 |
In general, some of the synthesized novel flavonoid derivatives exhibited excellent antiproliferative activity. Among them, compounds with substitutions on the R1-position of benzimidazole derivatives showed better antiproliferative activity than the R2- and R3-positioned ones. For the R1-position substituted ones, replacement of H atom at R1-position by electron-withdrawing groups (7, 11, 15, 19, 23, 27) resulted in their improved antiproliferative activity compared to the substitutions of electron-donating groups (6, 14, 22). For compounds 8, 16, 24 with nitro introduced on the R3-position, the antiproliferative activity was also decreased compared to the electron-donating groups on the R3-position. Furthermore, since the number of carbon atoms on the three linkages is similar, the difference in antiproliferative activity of the three chain length derivatives is not significant.
It is clear that the synthesized compounds with chlorine atom substitutions in R1-position on the benzimidazole derivatives (7, 15, 23) showed higher activity than others; especially, compound 15 displayed the most potent inhibitory activity, with IC50 of 20.47 ± 2.07 μM and 23.47 ± 3.59 μM on MGC-803 cells and MFC cells, respectively, which was better than that of 5-Fu (IC50 = 74.39 ± 2.03 and 78.52 ± 3.92, respectively). Therefore, the IC50 value suggested that compound 15 may be a promising lead for the further development of novel anti-tumor agents, and we chose the MFC cell lines to carry out cell assay, apoptosis assay and anti-tumor efficacy assay in vivo.
In addition, it is worth noting that the three benzimidazole derivatives (6-nitrobenzimidazole, 5-methylbenzimidazole and 5-methoxybenzimidazole) we selected will produce isomers during the last step of the reaction.30 Since we did not find any significant anti-tumor activity advantage among them during the previous activity screening, we did not further disassemble the isomers of these compounds (compounds 8, 10, 12, 16, 18, 20, 24, 26, 28). However, from the point of view of drug molecule synthesis design, we believe that isomers of the active compounds obtained by alkylation with 4-substituted or 5-substituted benzimidazoles may have to be isolated and purified prior to studying their further biological activity.
2.2.2. Apoptosis assay
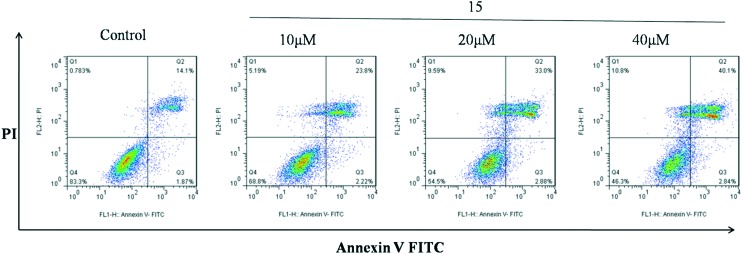
Since compound 15 exhibited excellent activity against cell growth in MGC-803 cells and MFC cells, we plan to use MFC cells to establish mouse tumor models for preliminary study of the anti-tumor effect in vivo of the candidate compounds. Prior to this, in order to further verify the antitumor activity of candidate compounds in vitro and to explore the mechanism of action, MFC cells were also selected for apoptosis study. Induction of apoptosis is an effective anti-tumor strategy. The FCM assay determined that compound 15 could induce the apoptosis of MFC cells in a dose-dependent manner. As shown in Fig. 2, MFC cells were treated with 10, 20, and 40 μM of compound 15 for 24 h. The compound increased the percentage of apoptosis detected by Annexin V-FITC/PI staining in a dose-dependent manner. The result indicated that compound 15 induced apoptosis of MFC cells.
Fig. 2. MFC cells were cultured with various concentrations of compound 15 for 24 h. Cells were stained with Annexin V-FICT/PI, and apoptosis was analyzed by flow cytometry.
2.2.3. Cell cycle assay
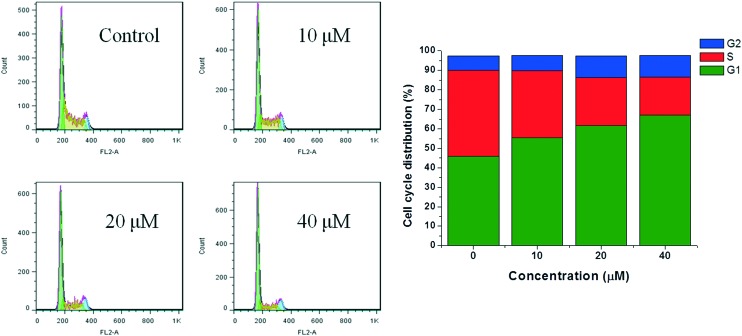
Cell cycle regulation is a necessary process to maintain cell proliferation and development. However, the main feature of tumor cells is cell cycle regulation disorders that block cell differentiation and apoptosis, causing cell proliferation to be out of control.31 We also chose MFC cell lines for cell cycle experiments. Studies were performed by flow cytometry analysis to determine the effects of compound 15 on cell cycle progression. As shown from the cell cycle detection (Fig. 3), there was significant up-regulation of G1 phase in a dose-dependent manner after treatment with three different concentrations of compound 15. Most of the tumor cells are often shortened in the G1 phase due to the abnormal expression of cyclin, CDK (cyclin-dependent kinase) and CKI (cyclin-dependent kinase inhibitor); thus, the cells rapidly enter S phase, leading to excessive proliferation.32 The experimental results showed that compound 15 could effectively upregulate G1 phase, thereby inducing apoptosis of tumor cells. However, which regulatory molecule was affected by the compound is not clear, and further study is currently in progress.
Fig. 3. Flow cytometry analysis of cell cycle distribution for MFC cells treated with compound 15 (0, 10, 20, 40 μmol L–1) for 24 h.
2.2.4. In vivo anti-tumor efficacy
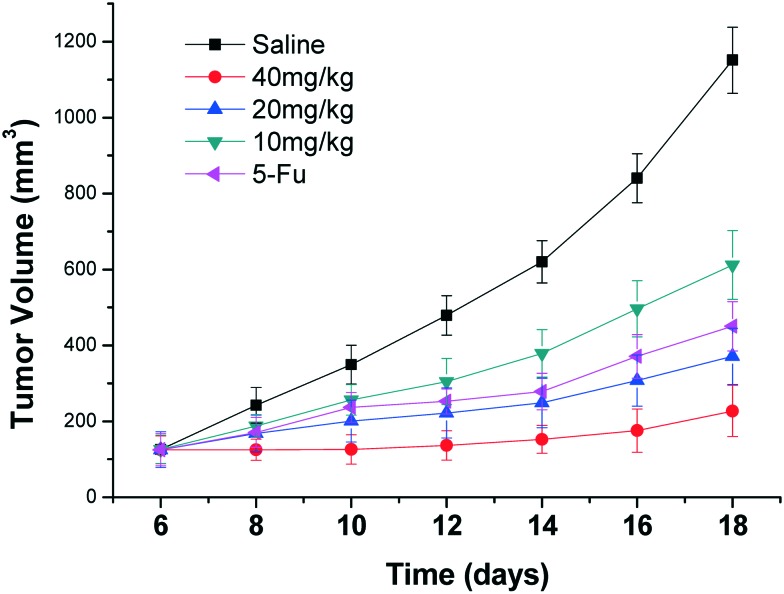
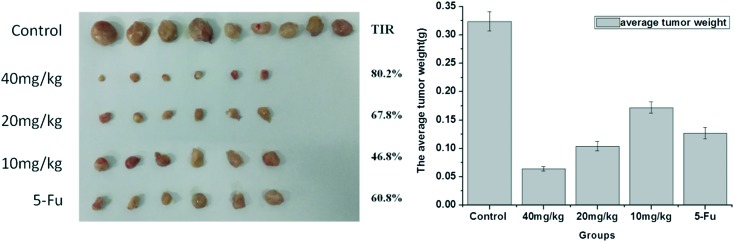
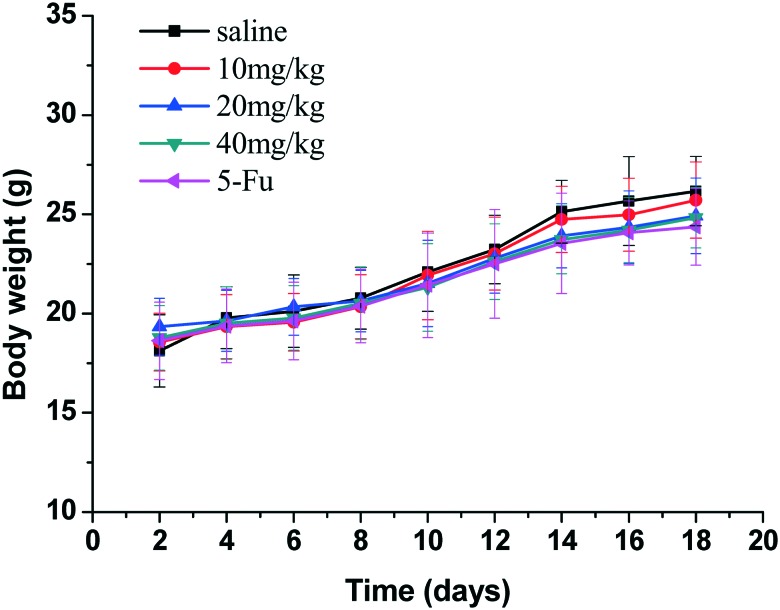
Gastric carcinoma was chosen as a model cancer to investigate the anti-tumor efficacy of the three different doses of compound 15. The anti-tumor growth effect in vivo was evaluated by measuring tumor volume following treatment with the different doses of compound 15 (10 mg kg–1, 20 mg kg–1, 40 mg kg–1), 5-Fu (25 mg kg–1) and saline. As illustrated in Fig. 4, different doses of compound 15 and the positive control 5-Fu inhibited tumor growth remarkably as compared with saline. The tumor growth inhibition of compound 15 was dose-dependent. The middle-dose group and high-dose group exhibited stronger tumor growth inhibition, with TIR of 67.8% and 80.2%, respectively, compared with that of 5-Fu (TIR of 60.8%) (Fig. 5). Fig. 6 illustrated the growth in body weight during the 18-day experimental period. The gradual increase in body weight among all the treatment groups was consistent with that of the control group, which represented the natural growth in body weight of tumor-bearing mice. The animal experiment in vivo showed that compound 15 was associated with stronger efficiency in suppressing tumor growth compared with 5-Fu and exhibited less toxic side effects. Based on the results of preliminary pharmacodynamic studies in mice above, we will conduct pharmacodynamic studies in nude mice in order to further validate the anti-tumor activity of this compound.
Fig. 4. Tumor volume growth curves of the mice after treatment with saline, different concentrations of compound 15 (10, 20, 40 mg kg–1) and 5-Fu. Data are shown as mean ± SD (n = 6). The difference in tumor volume growth among the five groups was statistically significant (P < 0.05).
Fig. 5. Photographs of tumors harvested from the mice after the 18 day experimental period (left); average tumor weight of the mice after 18 days (right).
Fig. 6. Body weight evolution curves of the mice after treatment with saline, different concentrations of compound 15 (10, 20, 40 mg kg–1) and 5-Fu. Data are shown as mean ± SD (n = 6). No significant body weight loss was observed compared with the saline group.
3. Conclusions
In summary, a series of 3′,4′,5′-trimethoxy flavonoid benzimidazole derivatives were firstly synthesized and evaluated for their antiproliferative activity against MGC-803, MCF-7, HepG-2 and MFC tumor cell lines. It is worth noting that compound 15 displayed the most outstanding antiproliferative activity in vitro, with IC50 values of 20.47 ± 2.07, 43.42 ± 3.56, 35.45 ± 2.03 μM and 23.47 ± 3.59 μM, respectively. In addition, the preliminary mechanism of compound 15 inhibition was detected by flow cytometry, revealing that the compound exerted antiproliferative activity via inducing the apoptosis of tumor cells in a dose-dependent manner. Flow cytometry analysis also revealed that compound 15 arrested the cell cycle of MFC cells in the G1 phase with a concentration-dependent effect. Furthermore, the animal experiment in vivo showed that compound 15 exhibited stronger efficiency in suppressing tumor growth compared with 5-Fu. All the results outlined the great potential of compound 15 for further exploitation as anti-tumor drug.
4. Experimental section
4.1. Chemistry
All chemicals (reagent grade) were purchased from commercial sources and used without further purification unless otherwise stated. Separation of the compounds by column chromatography was carried out with silica gel (100–200 mesh size). TLC was run on the silica gel 60 F254 plates and visualized under UV light at 254 nm or iodine vapour. Melting points (uncorrected) were determined on a Thermo Scientific electrothermal digital melting point apparatus. ESI mass spectra were obtained on a Waters GCT mass spectrometer, and 1H NMR spectra were measured on a Bruker AV-400 model spectrometer with TMS and solvent signals slotted as internal standards. Chemical shifts are reported in ppm (δ).
4.1.1. 7-Hydroxy-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (3)
To a solution of resorcinol (2.5 g, 0.023 mol) and 2-chloroacetonitrile (2 mL, 0.032 mol) in absolute ether (30 mL) with catalytic amount of ZnCl2 at 0 °C, freshly prepared hydrogen chloride gas was bubbled slowly and continuously for 2 h. After standing the reaction flask at low temperature overnight, a large amount of yellow precipitate was prepared after filtrating, washing and drying. The yellow precipitate was refluxed in water solvent, and a milky-white precipitate was produced. This precipitate, 2-chloro-1-(2,4-dihydroxyphenyl)ethanone, was filtered off, washed with water, and dried in vacuo. 3,4,5-Trimethoxybenzaldehyde (2.0 g, 0.01 mol) and 2-chloro-1-(2,4-dihydroxyphenyl)ethanone (2.2 g, 0.012 mol) were added in one portion to a stirred solution of 5 mL ethanol, then 10% NaOH aqueous solution was added dropwise into the solution. The reaction mixture was stirred at room temperature for 24 h, and acidification with 10% HCl (aq) gave a crude product, which was filtered off and purified by recrystallization from 95% ethanol to give compound 3 as yellow powder.
4.1.2. General method of synthesis of 4a–4c
To a stirred solution of compound 3 (1.0 mol) in acetone (30 ml), a certain amount of K2CO3 at room temperature and different chain length substituted alcohols (4.0 mol) were added to the reaction mixture. The mixture was refluxed for 5 h (the reaction was monitored by TLC). After cooling, the crude product was filtered off, washed with water and acetone, and dried in vacuo. The crude product was purified by column chromatography on silica gel, eluting with dichloromethane/methanol (50 : 1) to give a viridescent powder.
4.1.2.1. 7-(2-Bromoethoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (4a)33
Yellow powder, yield: 95%. Mp: 142–145 °C; 1H NMR (400 MHz, CDCl3) δ 7.62 (dd, J = 20.0, 9.8 Hz, 1H), 7.07 (s, 2H), 6.84–6.55 (m, 3H), 4.30 (dt, J = 20.6, 4.8 Hz, 2H), 3.87 (t, J = 9.4 Hz, 9H), 3.65 (dt, J = 11.1, 8.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 182.76 (s), 168.05 (s), 165.61 (s), 153.32 (s), 147.21 (s), 127.71 (s), 126.08 (s), 115.51 (s), 112.49 (s), 112.18 (s), 108.81 (s), 97.62 (s), 68.43 (s), 61.03 (s), 56.25 (s), 29.71 (s), 28.21 (s). MS (ESI): 434.0 (C20H19BrO6, [M + H+]).
4.1.2.2. 7-(3-Bromopropoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (4b)33
Yellow powder, yield: 94%. Mp: 168–170 °C; 1H NMR (400 MHz, CDCl3) δ 7.72 (d, J = 8.3 Hz, 1H), 7.16 (s, 2H), 6.77 (d, J = 10.0 Hz, 3H), 4.25 (t, J = 5.4 Hz, 2H), 3.94 (d, J = 11.6 Hz, 9H), 3.63 (t, J = 6.0 Hz, 2H), 2.52–2.29 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 182.78 (s), 168.22 (s), 166.36 (s), 153.30 (s), 147.29 (s), 139.81 (s), 127.79 (s), 125.88 (s), 115.07 (s), 112.43 (s), 112.26 (s), 108.70 (s), 97.24 (s), 66.22 (s), 61.03 (s), 56.22 (s), 31.91 (s), 29.54 (s). MS (ESI): 448.1 (C21H21BrO6, [M + H+]).
4.1.2.3. 7-(4-Bromobutoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (4c)
Yellow powder, yield: 95%. Mp: 159–160 °C; 1H NMR (400 MHz, CDCl3) δ 7.71 (d, J = 8.5 Hz, 1H), 7.16 (s, 2H), 6.75 (t, J = 8.3 Hz, 3H), 4.13 (t, J = 5.2 Hz, 2H), 3.93 (d, J = 10.9 Hz, 9H), 3.51 (t, J = 6.0 Hz, 2H), 2.26–1.86 (m, 4H); 13C NMR (101 MHz, CDCl3) δ 182.75 (s), 168.24 (s), 166.59 (s), 153.29 (s), 147.32 (s), 139.78 (s), 127.81 (s), 125.83 (s), 114.89 (s), 112.41 (s), 112.14 (s), 108.69 (s), 97.14 (s), 67.86 (s), 61.02 (s), 56.21 (s), 33.17 (s), 29.24 (s), 27.58 (s). MS (ESI): 462.1 (C22H23BrO6, [M + H+]).
4.1.3. General method of synthesis of 5–28
Equimolar quantities of the bromide intermediates (4a–4c) and corresponding benzimidazoles were dissolved in acetone (30 mL), and certain amount of K2CO3 was also added to the solution. The solution was then refluxed for approximately 12 h. The product was extracted with dichloromethane. After evaporation of the solvent, the residue was purified by column chromatography on silica gel, eluting with dichloromethane/methanol (30 : 1) to give a yellow powder.
4.1.3.1. 7-(2-(1H-Benzo[d]imidazol-1-yl)ethoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (5)
Yellow powder, yield: 64%. Mp: 209–210 °C; 1H NMR (400 MHz, CDCl3) δ 8.03 (s, 1H), 7.82 (d, J = 7.0 Hz, 1H), 7.68 (d, J = 8.6 Hz, 1H), 7.47 (d, J = 7.3 Hz, 1H), 7.32 (dq, J = 7.2, 6.0 Hz, 2H), 7.11 (s, 2H), 6.74–6.70 (m, 2H), 6.70 (d, J = 2.1 Hz, 1H), 6.65 (d, J = 2.0 Hz, 1H), 4.64 (t, J = 5.2 Hz, 2H), 4.41 (t, J = 5.1 Hz, 2H), 3.91 (d, J = 7.6 Hz, 9H); 13C NMR (101 MHz, CDCl3) δ 182.66 (s), 167.85 (s), 165.22 (s), 153.28 (s), 147.09 (s), 143.84 (s), 143.45 (s), 139.88 (s), 133.63 (s), 127.65 (s), 126.02 (s), 123.27 (s), 122.49 (s), 120.69 (s), 115.64 (s), 112.58 (s), 111.87 (s), 109.31 (s), 108.74 (s), 97.56 (s), 66.85 (s), 61.03 (s), 56.21 (s), 44.08 (s). MS (ESI): 472.1 (C27H24N2O6, [M + H+]).
4.1.3.2. 7-(2-(2-Methyl-1H-benzo[d]imidazol-1-yl)ethoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (6)
Orange yellow powder, yield: 53%. Mp: 192–193 °C; 1H NMR (400 MHz, CDCl3) δ 7.71–7.67 (m, 1H), 7.66 (s, 1H), 7.37–7.32 (m, 1H), 7.27–7.25 (m, 2H), 7.10 (s, 1H), 6.73 (s, 1H), 6.68 (d, J = 2.0 Hz, 1H), 6.66 (d, J = 2.1 Hz, 1H), 6.60 (d, J = 2.0 Hz, 1H), 4.59 (t, J = 5.3 Hz, 2H), 4.39 (t, J = 5.2 Hz, 2H), 3.92 (t, J = 8.8 Hz, 9H), 2.72 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 182.69 (s), 167.90 (s), 165.30 (s), 153.28 (s), 152.04 (s), 147.09 (s), 142.65 (s), 139.89 (s), 134.82 (s), 127.63 (s), 126.01 (s), 122.38 (s), 122.30 (s), 119.36 (s), 115.55 (s), 112.63 (s), 111.98 (s), 108.83 (s), 108.75 (s), 97.29 (s), 66.71 (s), 61.02 (s), 56.22 (s), 42.97 (s), 14.11 (s). MS (ESI): 486.1 (C28H26N2O6, [M + H+]).
4.1.3.3. 7-(2-(2-Chloro-1H-benzo[d]imidazol-1-yl)ethoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (7)
Yellow powder, yield: 78%. Mp: 153–156 °C; 1H NMR (400 MHz, CDCl3) δ 7.70 (d, J = 7.1 Hz, 1H), 7.66 (d, J = 8.5 Hz, 1H), 7.44 (d, J = 7.4 Hz, 1H), 7.37–7.26 (m, 2H), 7.11 (s, 1H), 6.73 (s, 1H), 6.68 (d, J = 2.1 Hz, 1H), 6.66 (d, J = 2.1 Hz, 1H), 6.63 (d, J = 2.0 Hz, 1H), 4.66 (t, J = 5.4 Hz, 2H), 4.41 (dd, J = 10.6, 5.1 Hz, 2H), 3.92 (t, J = 8.7 Hz, 9H); 13C NMR (101 MHz, CDCl3) δ 182.69 (s), 167.92 (s), 165.23 (s), 153.31 (s), 147.11 (s), 141.74 (s), 140.49 (s), 140.14–139.67 (m), 135.28 (s), 127.65 (s), 126.03 (s), 123.52 (s), 123.09 (s), 119.73 (s), 115.62 (s), 112.60 (s), 112.06 (s), 109.55 (s), 108.77 (s), 97.34 (s), 66.46 (s), 61.03 (s), 56.24 (s), 43.51 (s). MS (ESI): 507.1 (C27H23ClN2O6, [M + H+]).
4.1.3.4. 7-(2-(6(5)-Nitro-1H-benzo[d]imidazol-1-yl)ethoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (8)
Orange yellow powder, yield: 74%. Mp: 207–209 °C; 1H NMR (400 MHz, CDCl3) δ 8.74 (s, 1H), 8.54 (d, J = 2.0 Hz, 1H), 8.30 (d, J = 11.2 Hz, 1H), 8.27–8.20 (m, 1H), 7.88 (d, J = 9.0 Hz, 1H), 7.69 (d, J = 8.4 Hz, 1H), 7.57 (d, J = 9.0 Hz, 1H), 7.11 (d, J = 2.1 Hz, 1H), 6.70 (dd, J = 16.3, 7.7 Hz, 2H), 4.72 (dt, J = 9.9, 4.7 Hz, 2H), 4.45 (dd, J = 9.8, 5.0 Hz, 2H), 3.92 (dd, J = 9.0, 7.4 Hz, 9H); 13C NMR (101 MHz, CDCl3) δ 182.58 (s), 167.78 (s), 164.84 (s), 153.30 (s), 147.87 (s), 147.01 (s), 146.72 (s), 144.05 (s), 143.92 (s), 127.56 (s), 126.14 (s), 120.78 (s), 119.05 (s), 118.25 (s), 117.34 (s), 115.89 (s), 112.76 (s), 111.74 (s), 109.64 (s), 108.81 (s), 108.76 (s), 107.00 (s), 97.67 (s), 67.02 (s), 66.88 (s), 61.03 (s), 56.22 (s), 44.82 (s), 44.62 (s). MS (ESI): 517.1 (C27H23N3O8, [M + H+]).
4.1.3.5. 7-(2-(5,6-Dimethyl-1H-benzo[d]imidazol-1-yl)ethoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (9)
Yellow powder, yield: 68%. Mp: 235–237 °C; 1H NMR (400 MHz, CDCl3) δ 7.91 (s, 1H), 7.68 (d, J = 8.5 Hz, 1H), 7.57 (s, 1H), 7.21 (s, 1H), 7.11 (s, 2H), 6.73 (s, 1H), 6.70 (dd, J = 8.6, 2.0 Hz, 1H), 6.65 (d, J = 2.0 Hz, 1H), 4.58 (t, J = 5.1 Hz, 2H), 4.38 (t, J = 5.2 Hz, 2H), 3.91 (d, J = 7.8 Hz, 9H), 2.38 (d, J = 13.9 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 182.47 (s), 167.68 (s), 165.32 (s), 153.32 (s), 147.15 (s), 142.69 (s), 139.89 (s), 132.47 (s), 131.42 (s), 127.68 (s), 120.67 (s), 115.63 (s), 112.59 (s), 111.91 (s), 109.45 (s), 108.76 (s), 97.62 (s), 66.81 (s), 61.04 (s), 56.23 (s), 44.04 (s), 20.67 (s), 20.25 (s), 0.01 (s). MS (ESI): 500.1 (C29H28N2O6, [M + H+]).
4.1.3.6. 7-(2-(5(6)-Methyl-1H-benzo[d]imidazol-1-yl)ethoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (10)
Yellow powder, yield: 62%. Mp: 208–210 °C 1H NMR (400 MHz, CDCl3) δ 8.01 (d, J = 6.5 Hz, 1H), 7.73 (d, J = 8.5 Hz, 1H), 7.66 (d, J = 12.8 Hz, 1H), 7.39 (d, J = 8.5 Hz, 1H), 7.24–7.10 (m, 3H), 6.80–6.72 (m, 2H), 6.70 (s, 1H), 4.64 (s, 2H), 4.44 (s, 2H), 3.96 (t, J = 9.0 Hz, 9H), 2.54 (d, J = 13.7 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 182.68 (s), 167.87 (s), 165.27 (s), 153.28 (s), 147.11 (s), 144.20 (s), 143.39 (s), 143.03 (s), 141.95 (s), 139.88 (s), 133.79 (s), 133.29 (s), 132.21 (s), 131.71 (s), 127.65 (s), 126.02 (s), 124.73 (s), 124.08 (s), 120.41 (s), 120.17 (s), 115.61 (s), 112.57 (s), 111.89 (s), 109.18 (s), 108.72 (s), 97.56 (s), 66.82 (d, J = 9.0 Hz), 61.02 (s), 56.20 (s), 43.95 (s), 21.88 (s), 21.51 (s). MS (ESI): 486.1 (C28H26N2O6, [M + H+]).
4.1.3.7. 7-(2-(2-(Trifluoromethyl)-1H-benzo[d]imidazol-1-yl)ethoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (11)
Yellow powder, yield: 77%. Mp: 207–208 °C 1H NMR (400 MHz, CDCl3) δ 7.91 (d, J = 8.0 Hz, 1H), 7.67 (t, J = 8.3 Hz, 2H), 7.51 (t, J = 7.5 Hz, 1H), 7.42 (t, J = 7.6 Hz, 1H), 7.12 (s, 2H), 6.74 (s, 1H), 6.71–6.58 (m, 2H), 4.82 (s, 2H), 4.47 (s, 2H), 3.94 (t, J = 8.2 Hz, 9H); 13C NMR (101 MHz, CDCl3) δ 182.70 (s), 167.93 (s), 165.11 (s), 153.32 (s), 147.08 (s), 141.08 (s), 139.97 (s), 135.82 (s), 127.62 (s), 126.04 (s), 125.71 (s), 124.07 (s), 121.84 (s), 115.66 (s), 112.69 (s), 112.11 (s), 110.89 (s), 108.81 (s), 97.20 (s), 66.95 (s), 61.03 (s), 56.25 (s), 44.08 (s). MS (ESI): 540.1 (C28H23F3N2O6, [M + H+]).
4.1.3.8. 7-(2-(5(6)-Methoxy-1H-benzo[d]imidazol-1-yl)ethoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (12)
Yellow powder, yield: 71%. Mp: 204–206 °C 1H NMR (400 MHz, CDCl3) δ 7.96 (d, J = 17.8 Hz, 1H), 7.69 (dd, J = 16.8, 9.5 Hz, 2H), 7.12 (s, 2H), 6.97 (dd, J = 22.2, 11.3 Hz, 2H), 6.81–6.61 (m, 3H), 4.60 (d, J = 4.8 Hz, 2H), 4.41 (s, 2H), 4.04–3.79 (m, 12H); 13C NMR (101 MHz, CDCl3) δ 182.67 (s), 167.87 (s), 165.24 (s), 157.03 (s), 156.37 (s), 153.28 (s), 147.10 (s), 144.68 (s), 143.57 (s), 142.67 (s), 139.88 (s), 138.29 (s), 134.31 (s), 128.22 (s), 127.65 (s), 126.03 (s), 121.10 (s), 115.63 (s), 113.51 (s), 112.60 (s), 111.92 (s), 111.42 (s), 109.74 (s), 108.73 (s), 102.54 (s), 97.54 (s), 93.22 (s), 66.94 (s), 66.84 (s), 61.03 (s), 56.21 (s), 55.97 (s), 55.82 (s), 44.22 (s), 43.99 (s). MS (ESI): 502.1 (C28H26N2O7, [M + H+]).
4.1.3.9. 7-(3-(1H-benzo[d]imidazol-1-yl)propoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (13)
Yellow powder, yield: 70%. Mp: 185–187 °C; 1H NMR (400 MHz, CDCl3) δ 7.87 (d, J = 17.7 Hz, 1H), 7.82 (d, J = 3.3 Hz, 1H), 7.74 (d, J = 8.5 Hz, 1H), 7.41 (s, 1H), 7.36–7.21 (m, 4H), 6.78 (d, J = 9.3 Hz, 2H), 6.66 (s, 1H), 4.49 (t, J = 6.3 Hz, 2H), 4.00 (dd, J = 13.4, 8.4 Hz, 2H), 3.92 (d, J = 7.2 Hz, 9H), 2.52–2.32 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 182.76 (s), 168.15 (s), 165.93 (s), 153.31 (s), 147.21 (s), 143.93 (s), 143.10 (s), 139.87 (s), 133.67 (s), 127.73 (s), 126.02 (s), 123.19 (s), 122.36 (s), 120.61 (s), 115.35 (s), 112.45 (s), 112.31 (s), 109.43 (s), 108.74 (s), 97.23 (s), 64.79 (s), 61.03 (s), 56.23 (s), 41.29 (s), 29.18 (s). MS (ESI): 486.1 (C28H26N2O6, [M + H+]).
4.1.3.10. 7-(3-(2-Methyl-1H-benzo[d]imidazol-1-yl)propoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (14)
Yellow powder, yield: 57%. Mp: 169–171 °C; 1H NMR (400 MHz, CDCl3) δ 7.62 (t, J = 8.8 Hz, 2H), 7.19 (t, J = 12.3 Hz, 2H), 7.11 (td, J = 14.2, 6.7 Hz, 3H), 6.67 (d, J = 11.4 Hz, 2H), 6.56 (s, 1H), 4.30 (t, J = 6.3 Hz, 2H), 3.90 (dd, J = 12.8, 7.5 Hz, 2H), 3.83 (d, J = 3.5 Hz, 9H), 2.44 (d, J = 42.5 Hz, 2H), 2.28 (dd, J = 11.8, 5.7 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 182.74 (s), 168.14 (s), 165.86 (s), 153.29 (s), 151.52 (s), 147.19 (s), 142.44 (s), 139.89 (s), 134.88 (s), 127.69 (s), 126.02 (s), 122.32 (s), 122.15 (s), 119.12 (s), 115.32 (s), 112.50 (s), 112.32 (s), 109.01 (s), 108.76 (s), 97.19 (s), 64.76 (s), 61.00 (s), 56.21 (s), 40.07 (s), 28.84 (s). MS (ESI): 500.1 (C29H28N2O6, [M + H+]).
4.1.3.11. 7-(3-(2-Chloro-1H-benzo[d]imidazol-1-yl)propoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (15)
Yellow powder, yield: 66%. Mp: 140–142 °C; 1H NMR (400 MHz, CDCl3) δ 7.72 (t, J = 7.8 Hz, 1H), 7.56 (s, 1H), 7.37–7.29 (m, 2H), 7.14 (s, 2H), 7.07 (s, 1H), 6.76 (d, J = 6.8 Hz, 1H), 6.65 (s, 1H), 4.49 (t, J = 5.7 Hz, 2H), 4.09 (d, J = 20.6 Hz, 2H), 3.93 (d, J = 6.5 Hz, 9H), 2.40 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 182.89 (s), 168.18 (s), 165.98 (s), 153.31 (s), 147.24 (s), 141.59 (s), 140.51 (s), 139.87 (s), 135.03 (s), 128.92 (s), 127.73 (s), 126.04 (s), 123.52 (s), 123.10 (s), 123.03 (s), 119.53 (s), 115.29 (s), 112.59 (s), 112.36 (s), 109.27 (s), 108.77 (s), 97.22 (s), 64.90 (s), 61.05 (s), 56.25 (s), 41.00 (s), 29.28 (s), 28.76 (s). MS (ESI): 521.1 (C28H25ClN2O6, [M + H+]).
4.1.3.12. 7-(3-(6(5)-Nitro-1H-benzo[d]imidazol-1-yl)propoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (16)
Yellow powder, yield: 54%. Mp: 225–228 °C; 1H NMR (400 MHz, CDCl3) δ 8.50 (d, J = 113.5 Hz, 1H), 8.23–8.01 (m, 2H), 7.62 (ddd, J = 88.9, 65.5, 8.9 Hz, 2H), 7.05 (s, 2H), 6.76–6.54 (m, 3H), 4.61–4.38 (m, 2H), 4.01 (dd, J = 11.2, 5.5 Hz, 2H), 3.86 (t, J = 10.2 Hz, 9H), 2.40 (dt, J = 11.4, 5.6 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 182.66 (s), 168.06 (s), 165.60 (s), 153.31 (s), 148.18 (s), 147.54 (s), 147.13 (s), 146.46 (s), 143.96 (s), 143.85 (s), 143.23 (s), 139.95 (s), 137.82 (s), 133.07 (s), 127.65 (s), 126.11 (s), 120.78 (s), 118.99 (s), 118.16 (s), 117.34 (s), 115.55 (s), 112.60 (s), 112.53 (s), 112.08 (s), 109.48 (s), 108.79 (s), 106.62 (s), 97.28 (s), 64.68 (s), 61.03 (s), 56.23 (s), 53.47 (s), 41.97 (s), 29.23 (s). MS (ESI): 531.1 (C28H25N3O8, [M + H+]).
4.1.3.13. 7-(3-(5,6-Dimethyl-1H-benzo[d]imidazol-1-yl)propoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (17)
Yellow powder, yield: 63%. Mp: 222–224 °C; 1H NMR (400 MHz, CDCl3) δ 7.73 (dd, J = 23.4, 14.8 Hz, 2H), 7.56 (s, 1H), 7.14 (s, 3H), 6.78 (d, J = 12.1 Hz, 2H), 6.65 (s, 1H), 4.42 (s, 2H), 3.99 (s, 2H), 3.92 (d, J = 6.9 Hz, 9H), 2.38 (d, J = 14.7 Hz, 6H), 2.31 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 182.74 (s), 168.17 (s), 166.01 (s), 153.30 (s), 147.22 (s), 142.35 (s), 139.84 (s), 132.36 (s), 132.25 (s), 131.23 (s), 127.73 (s), 125.96 (s), 120.47 (s), 115.25 (s), 112.41 (s), 109.66 (s), 108.71 (s), 97.21 (s), 64.77 (s), 61.02 (s), 56.21 (s), 41.12 (s), 29.26 (s), 20.52 (s), 20.24 (s). MS (ESI): 514.1 (C30H30N2O6, [M + H+]).
4.1.3.14. 7-(3-(5(6)-Methyl-1H-benzo[d]imidazol-1-yl)propoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (18)
Yellow powder, yield: 58%. Mp: 154–156 °C; 1H NMR (400 MHz, CDCl3) δ 7.85 (d, J = 6.4 Hz, 1H), 7.74 (t, J = 9.0 Hz, 1H), 7.70 (d, J = 8.3 Hz, 1H), 7.62 (s, 1H), 7.22–7.09 (m, 3H), 6.85–6.75 (m, 2H), 6.67 (s, 1H), 4.46 (s, 2H), 4.01 (d, J = 4.7 Hz, 2H), 3.94 (d, J = 7.3 Hz, 9H), 2.50 (s, 2H), 2.46–2.38 (m, 3H); 13C NMR (101 MHz, CDCl3) δ 182.78 (s), 168.17 (s), 165.98 (s), 153.30 (s), 147.22 (s), 144.21 (s), 143.03 (s), 142.63 (s), 141.91 (s), 139.85 (s), 133.92 (s), 133.22 (s), 132.10 (s), 131.74 (s), 127.73 (s), 126.01 (s), 124.69 (s), 123.97 (s), 120.26 (s), 120.00 (s), 115.28 (s), 112.70–112.18 (m), 109.36 (s), 108.96 (s), 108.73 (s), 97.21 (s), 64.78 (d, J = 3.4 Hz), 61.02 (s), 56.22 (s), 41.29 (s), 41.09 (s), 29.24 (s), 21.76 (s), 21.50 (s). MS (ESI): 500.1 (C29H28N2O6, [M + H+]).
4.1.3.15. 7-(3-(2-(Trifluoromethyl)-1H-benzo[d]imidazol-1-yl)propoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (19)
Yellow powder, yield: 72%. Mp: 175–178 °C; 1H NMR (400 MHz, CDCl3) δ 7.91 (d, J = 6.4 Hz, 1H), 7.75 (d, J = 8.4 Hz, 1H), 7.40 (dd, J = 15.9, 8.3 Hz, 3H), 7.15 (s, 2H), 6.78 (d, J = 6.8 Hz, 2H), 6.68 (s, 1H), 4.62 (t, J = 6.8 Hz, 2H), 4.14 (s, 2H), 3.95 (t, J = 10.9 Hz, 9H), 2.45 (d, J = 5.6 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 182.89 (s), 168.17 (s), 165.94 (s), 153.31 (s), 147.23 (s), 141.00 (s), 139.88 (s), 135.45 (s), 127.72 (s), 126.03 (s), 125.69 (s), 123.99 (s), 123.64 (s), 121.72 (s), 115.32 (s), 112.64 (s), 112.31 (s), 110.30 (s), 108.78 (s), 97.22 (s), 65.17 (s), 61.04 (s), 56.21 (s), 41.80 (s), 29.57 (s). MS (ESI): 554.1 (C29H25F3N2O6, [M + H+]).
4.1.3.16. 7-(3-(5(6)-Methoxy-1H-benzo[d]imidazol-1-yl)propoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (20)
Yellow powder, yield: 67%. Mp: 129–131 °C; 1H NMR (400 MHz, CDCl3) δ 7.81 (d, J = 15.8 Hz, 1H), 7.74 (d, J = 8.4 Hz, 1H), 7.69 (d, J = 8.9 Hz, 1H), 7.28 (s, 1H), 7.14 (s, 2H), 6.93 (t, J = 8.6 Hz, 1H), 6.86–6.74 (m, 2H), 6.67 (s, 1H), 4.43 (d, J = 6.0 Hz, 2H), 4.07–3.98 (m, 2H), 3.98–3.69 (m, 12H), 2.51–2.29 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 182.77 (s), 168.17 (s), 165.95 (s), 156.98 (s), 156.30 (s), 153.31 (s), 147.20 (s), 142.27 (s), 139.90 (s), 138.30 (s), 134.39 (s), 127.70 (s), 126.05 (s), 121.04 (s), 115.34 (s), 113.51 (s), 112.57 (s), 112.38 (s), 111.64 (s), 109.82 (s), 108.76 (s), 102.42 (s), 97.21 (s), 92.91 (s), 64.73 (s), 61.03 (s), 56.24 (s), 55.82 (s), 55.77 (s), 41.40 (s), 41.05 (s), 29.24 (s). MS (ESI): 516.1 (C29H28N2O7, [M + H+]).
4.1.3.17. 7-(4-(1H-benzo[d]imidazol-1-yl)butoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (21)
Yellow powder, yield: 59%. Mp: 177–179 °C; 1H NMR (400 MHz, CDCl3) δ 7.95 (s, 1H), 7.83 (d, J = 6.8 Hz, 1H), 7.70 (t, J = 9.8 Hz, 1H), 7.43 (d, J = 7.1 Hz, 1H), 7.31 (dd, J = 19.0, 13.9 Hz, 4H), 6.72 (dd, J = 19.2, 10.4 Hz, 3H), 4.31 (t, J = 6.7 Hz, 2H), 4.09 (t, J = 5.5 Hz, 2H), 4.02–3.84 (m, 9H), 2.22–2.07 (m, 2H), 2.00–1.83 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 182.77 (s), 168.19 (s), 166.38 (s), 153.30 (s), 147.28 (s), 139.83 (s), 127.78 (s), 125.92 (s), 123.02 (s), 122.24 (s), 120.56 (s), 115.04 (s), 112.26 (s), 109.58 (s), 108.73 (s), 97.20 (s), 68.07 (s), 61.03 (s), 56.23 (s), 44.75 (s), 26.70 (s), 26.33 (s). MS (ESI): 500.1 (C29H28N2O6, [M + H+]).
4.1.3.18. 7-(4-(2-Methyl-1H-benzo[d]imidazol-1-yl)butoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (22)
Yellow powder, yield: 61%. Mp: 160–163 °C; 1H NMR (400 MHz, CDCl3) δ 7.71 (d, J = 8.2 Hz, 2H), 7.31 (s, 1H), 7.25 (d, J = 3.2 Hz, 2H), 7.15 (s, 2H), 6.77–6.65 (m, 3H), 4.23 (t, J = 6.9 Hz, 2H), 4.10 (dd, J = 14.1, 8.6 Hz, 2H), 3.94 (t, J = 10.4 Hz, 9H), 2.64 (s, 3H), 2.15–1.99 (m, 2H), 1.89 (d, J = 6.3 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 182.77 (s), 168.20 (s), 166.39 (s), 153.32 (s), 151.32 (s), 147.29 (s), 142.71 (s), 135.06 (s), 127.78 (s), 125.95 (s), 122.10 (s), 121.93 (s), 119.23 (s), 115.07 (s), 112.27 (s), 112.20 (s), 109.04 (s), 108.76 (s), 97.21 (s), 68.17 (s), 61.04 (s), 56.25 (s), 43.49 (s), 26.60 (s), 26.45 (s), 14.05 (s). MS (ESI): 514.1 (C30H30N2O6, [M + H+]).
4.1.3.19. 7-(4-(2-Chloro-1H-benzo[d]imidazol-1-yl)butoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (23)
Yellow powder, yield: 60%. Mp: 163–166 °C; 1H NMR (400 MHz, CDCl3) δ 7.61 (q, J = 8.0 Hz, 3H), 7.33–7.12 (m, 4H), 6.75–6.50 (m, 3H), 4.24 (t, J = 6.7 Hz, 2H), 4.01 (t, J = 5.5 Hz, 2H), 3.85 (t, J = 9.3 Hz, 9H), 2.13–1.92 (m, 2H), 1.89–1.74 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 182.78 (s), 168.19 (s), 166.40 (s), 153.31 (s), 147.29 (s), 141.79 (s), 140.45 (s), 139.85 (s), 134.94 (s), 127.78 (s), 125.93 (s), 123.27 (s), 122.80 (s), 119.63 (s), 115.04 (s), 112.26 (s), 112.23 (s), 109.35 (s), 108.75 (s), 97.21 (s), 69.53 (s), 68.06 (s), 61.03 (s), 56.23 (s), 53.80 (s), 53.47 (s), 44.11 (s), 31.76 (s), 29.28 (s), 26.24 (s), 26.18 (s). MS (ESI): 534.1 (C29H27ClN2O6, [M + H+]).
4.1.3.20. 7-(4-(6(5)-Nitro-1H-benzo[d]imidazol-1-yl)butoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (24)
Yellow powder, yield: 62%. Mp: 140–142 °C; 1H NMR (400 MHz, CDCl3) δ 8.56 (d, J = 113.5 Hz, 1H), 8.21 (dd, J = 23.4, 12.4 Hz, 2H), 7.98–7.38 (m, 2H), 7.12 (s, 2H), 6.70 (dd, J = 8.7, 5.1 Hz, 3H), 4.39 (dt, J = 14.0, 7.1 Hz, 2H), 4.22–4.03 (m, 2H), 3.92 (d, J = 6.2 Hz, 9H), 2.36–2.06 (m, 2H), 1.91 (dd, J = 14.0, 7.0 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 182.69 (s), 168.12 (s), 166.22 (s), 153.28 (s), 148.18 (s), 147.37 (s), 147.20 (s), 146.31 (s), 143.86 (s), 143.73 (s), 143.17 (s), 139.85 (s), 137.83 (s), 133.06 (s), 127.70 (s), 125.91 (s), 120.66 (s), 118.84 (s), 118.05 (s), 117.23 (s), 115.08 (s), 112.33 (s), 112.18 (s), 112.11 (s), 109.66 (s), 108.73 (s), 106.74 (s), 97.28 (s), 97.17 (s), 67.93 (s), 67.87 (s), 61.01 (s), 56.21 (s), 45.27 (s), 26.84 (s), 26.25 (s), 26.19 (s). MS (ESI): 545.2 (C29H27N3O8, [M + H+]).
4.1.3.21. 7-(4-(5,6-Dimethyl-1H-benzo[d]imidazol-1-yl)butoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (25)
Yellow powder, yield: 73%. Mp: 204–207 °C; 1H NMR (400 MHz, CDCl3) δ 7.82 (s, 1H), 7.69 (t, J = 8.7 Hz, 1H), 7.57 (s, 1H), 7.16 (d, J = 11.1 Hz, 3H), 6.82–6.61 (m, 3H), 4.25 (t, J = 6.7 Hz, 2H), 4.12–3.99 (m, 2H), 3.94 (t, J = 10.1 Hz, 9H), 2.38 (d, J = 7.1 Hz, 6H), 2.22–2.02 (m, 2H), 1.83 (d, J = 19.3 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 182.74 (s), 168.18 (s), 166.42 (s), 153.29 (s), 147.28 (s), 142.58 (s), 142.17 (s), 139.80 (s), 132.15 (s), 131.10 (s), 127.78 (s), 125.85 (s), 120.45 (s), 114.96 (s), 112.21 (s), 109.77 (s), 108.70 (s), 97.16 (s), 68.08 (s), 61.02 (s), 56.21 (s), 44.67 (s), 26.54 (s), 26.29 (s), 20.64 (s), 20.26 (s). MS (ESI): 528.1 (C31H32N2O6, [M + H+]).
4.1.3.22. 7-(4-(5(6)-Methyl-1H-benzo[d]imidazol-1-yl)butoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (26)
Yellow powder, yield: 54%. Mp: 159–163 °C; 1H NMR (400 MHz, CDCl3) δ 7.88 (d, J = 7.1 Hz, 1H), 7.75–7.58 (m, 2H), 7.30 (d, J = 8.3 Hz, 1H), 7.22–7.08 (m, 3H), 6.78–6.64 (m, 3H), 4.27 (d, J = 3.1 Hz, 2H), 4.08 (d, J = 5.2 Hz, 2H), 3.93 (d, J = 9.0 Hz, 9H), 2.50 (d, J = 6.4 Hz, 3H), 2.11 (dd, J = 23.2, 16.2 Hz, 2H), 1.84 (t, J = 19.2 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 182.76 (s), 168.19 (s), 166.40 (s), 153.30 (s), 147.29 (s), 142.84 (s), 142.47 (s), 127.79 (s), 125.92 (s), 124.51 (s), 123.85 (s), 120.27 (s), 115.02 (s), 112.24 (s), 109.45 (s), 109.09 (s), 108.73 (s), 97.19 (s), 68.08 (s), 61.03 (s), 56.23 (s), 44.76 (s), 44.62 (s), 26.65 (s), 26.32 (s), 21.88 (s), 21.53 (s). MS (ESI): 514.1 (C30H30N2O6, [M + H+]).
4.1.3.23. 7-(4-(2-(Trifluoromethyl)-1H-benzo[d]imidazol-1-yl)butoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (27)
Yellow powder, yield: 63%. Mp: 153–156 °C; 1H NMR (400 MHz, CDCl3) δ 7.92 (d, J = 7.8 Hz, 1H), 7.72 (t, J = 8.4 Hz, 1H), 7.48 (q, J = 8.0 Hz, 2H), 7.42 (dd, J = 14.8, 7.5 Hz, 1H), 7.17 (s, 2H), 6.81–6.68 (m, 2H), 5.32 (s, 1H), 4.47 (t, J = 7.3 Hz, 2H), 4.15 (t, J = 5.1 Hz, 2H), 3.96 (t, J = 10.3 Hz, 9H), 2.25–2.09 (m, 2H), 1.98 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 182.79 (s), 168.20 (s), 166.33 (s), 153.32 (s), 147.28 (s), 141.21 (s), 139.87 (s), 135.31 (s), 127.78 (s), 125.96 (s), 125.46 (s), 123.78 (s), 121.87 (s), 115.13 (s), 112.29 (s), 112.22 (s), 110.42 (s), 108.76 (s), 97.22 (s), 68.03 (s), 61.03 (s), 56.23 (s), 53.45 (s), 44.77 (s), 26.86 (s), 26.26 (s). MS (ESI): 568.2 (C30H27F3N2O6, [M + H+]).
4.1.3.24. 7-(4-(5(6)-Methoxy-1H-benzo[d]imidazol-1-yl)butoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (28)
Yellow powder, yield: 56%. Mp: 87–90 °C; 1H NMR (400 MHz, CDCl3) δ 7.86 (d, J = 17.0 Hz, 1H), 7.76–7.64 (m, 2H), 7.15 (s, 2H), 6.95 (t, J = 10.7 Hz, 1H), 6.85 (s, 1H), 6.79–6.63 (m, 3H), 4.25 (t, J = 6.7 Hz, 2H), 4.08 (d, J = 4.7 Hz, 2H), 3.93 (dd, J = 23.4, 14.4 Hz, 12H), 2.20–2.04 (m, 2H), 1.88 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 182.79 (s), 168.19 (s), 166.39 (s), 156.83 (s), 153.31 (s), 147.28 (s), 144.72 (s), 142.99 (s), 142.16 (s), 139.85 (s), 138.41 (s), 134.32 (s), 127.78 (s), 125.95 (s), 120.96 (s), 115.04 (s), 113.35 (s), 112.31 (s), 112.22 (s), 111.27 (s), 109.97 (s), 108.75 (s), 108.52 (s), 102.37 (s), 97.20 (s), 96.29 (s), 93.35 (s), 68.09 (s), 61.03 (s), 56.24 (s), 55.94 (s), 55.82 (s), 44.89 (s), 44.64 (s), 26.67 (s), 26.49 (s), 26.30 (s). MS (ESI): 530.1 (C30H30N2O7, [M + H+]).
4.2. Biological assay
4.2.1. Cell culture
Human gastric tumor cell line MGC-803, breast tumor cell line MCF-7, liver tumor cell line HepG-2 and murine gastric tumor cell line MFC were obtained from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China, and cells were cultured in DMEM medium (Gibco, NY, USA), which was supplemented with 10% fetal bovine serum (FBS) (Sigma). The cultures were incubated at 37 °C in a humidified 5% CO2 incubator. For experiments performed in the absence of PBS, PBS was eliminated 24 h before initiating the experiments.
4.2.2. In vitro antiproliferative assay
The antiproliferative activity of compounds 5–28 was determined using a standard (MTT)-based colorimetric assay (Sigma). The cells were seeded at a density of approximately 8 × 103 cells per well in 96-well microtiter plates (Costar) and maintained at 37 °C in 95% humidity and 5% CO2 for 48 h. Different concentrations (256, 128, 64, 32, 16, 8, 4, 2 μM) of compounds were used in treatments. After 48 h, the cells were washed twice with PBS, 20 μL of MTT solution (5 mg mL–1 in PBS) was added to each well, and the plate was incubated at 37 °C. After 4 h, 100 μL of DMSO was added to each well to dissolve the formazan crystals, and absorbance was measured at 570 nm on a Wellscan MK-2 microplate reader. The results were compared with the standard drug 5-Fu as positive control drug against all tested cell lines. IC50 values were determined for replicates of 96-well plates from three independent experiments.
4.2.3. Apoptosis assay
The MFC cells were incubated for 48 h after different concentrations of compound 15 were added, and then stained with both Annexin V-FITC (fluorescein isothiocyanate) and propidium iodide (PI). Then, samples were analyzed using a FACSCalibur flow cytometer.
4.2.4. Cell cycle assay
The MFC cells were incubated with different concentrations of compound 15 (0, 10, 20, and 40 μM) in a six-well plate. After 48 h, the cells were harvested by trypsinization, washed in PBS, and fixed in 70% ice-cold (4 °C) ethanol for 12 h. Then, 500 μL of propidium iodide stain was added to each tube. Cells were gently vortexed and incubated for 30 min at 37 °C in the dark, then analyzed by flow cytometry.
4.2.5. In vivo anti-tumor efficacy
All experiments with animals were performed in compliance with the relevant laws and institutional guidelines in China for animal experiments, including Laboratory Animal Management Regulations and Guidance on the Treatment of Experimental Animals. The Institutional Animal Care Committee of University of South China [permit number: SYXK (Xiang) 2015-0001] has approved the experiments.
Gastric tumor cell line MFC was chosen as a model tumor to investigate the anti-tumor efficacy of the three doses of compound 15 (10 mg kg–1, 20 mg kg–1, 40 mg kg–1). Gastric tumor-bearing mice were established by subcutaneous injection of MFC cells in the ventral part, suspended in saline to 1 × 107/0.2 ml per mouse (defined as day 0). On day 1, the mice were randomly divided into five treatment groups (n = 6), treated with the three doses of compound 15, 5-Fu (positive control 25 mg kg–1) and saline (control), via a intraperitoneal injection on days 2, 5, 8, 11 and 14. From the 6th day on, tumor volumes were monitored every other day by measuring two perpendicular diameters using a Vernier caliper and calculated using the formula: Volume = 0.5 × Lenght × (Width)2. Body weights were recorded every other day. On day 18, the animals were sacrificed, and the tumor mass was dissected, weighed, and photographed. The tumor inhibition ratio (TIR) was calculated using the formula: 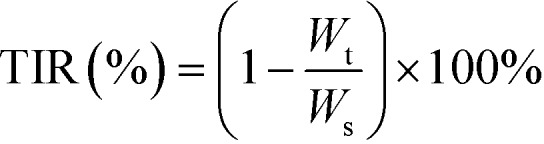 , where Wt and Ws represent the average tumor weights of the treatment and saline groups, respectively.
, where Wt and Ws represent the average tumor weights of the treatment and saline groups, respectively.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Supplementary Material
Acknowledgments
This work was financially supported by the Construct Program of the Key Discipline in Hunan province and Hunan Province Cooperative Innovation Center for Molecular Target New Drug Study.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c7md00578d
References
- Gordon P. G. G., Cragg M., Newman D. J. Chem. Rev. 2009;109:3012–3043. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- Singh M., Kaur M., Silakari O. Eur. J. Med. Chem. 2014;84:206–239. doi: 10.1016/j.ejmech.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Katiyar S. K., Athar M. Cancer Prev. Res. 2013;6:617–621. doi: 10.1158/1940-6207.CAPR-13-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzi V., Masuelli L., Tresoldi I., Sacchetti P., Modesti A., Galvano F., Bei R. Front. Biosci., Landmark Ed. 2012;17:2396–2418. doi: 10.2741/4061. [DOI] [PubMed] [Google Scholar]
- Beecher G. R. J. Nutr. 2003;133:3248–3254. doi: 10.1093/jn/133.10.3248S. [DOI] [PubMed] [Google Scholar]
- Huang W., Chen Q., Yang W. C., Yang G. F. Eur. J. Med. Chem. 2013;66:161–170. doi: 10.1016/j.ejmech.2013.05.037. [DOI] [PubMed] [Google Scholar]
- Cushnie T. P. T., Lamb A. J. Int. J. Antimicrob. Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havsteen B. Biochem. Pharmacol. 1983;32:1141–1148. doi: 10.1016/0006-2952(83)90262-9. [DOI] [PubMed] [Google Scholar]
- Lopez-Lazaro M. Curr. Med. Chem.: Anti-Cancer Agents. 2002;2:691–714. doi: 10.2174/1568011023353714. [DOI] [PubMed] [Google Scholar]
- Li Y., Fang H., Xu W. Mini-Rev. Med. Chem. 2007;7:663–678. doi: 10.2174/138955707781024463. [DOI] [PubMed] [Google Scholar]
- Ren W. Y., Qiao Z. H., Wang H. W., Zhu L., Zhang L. Med. Res. Rev. 2003;23:519–534. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- Ramos S. Mol. Nutr. Food Res. 2008;52:507–526. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- Rahmani-Nezhad S., Safavi M., Pordeli M., Ardestani S. K., Khosravani L., Pourshojaei Y., Mandavi M., Emami S., Foroumadi A., Shafiee A. Eur. J. Med. Chem. 2014;86:562–569. doi: 10.1016/j.ejmech.2014.09.017. [DOI] [PubMed] [Google Scholar]
- Pettit G. R., Lippert J. W., Herald D. L., Hamel E., Pettit R. K. J. Nat. Prod. 2000;63:969–974. doi: 10.1021/np0000623. [DOI] [PubMed] [Google Scholar]
- Rustin G. J. S., Galbraith S. M., Anderson H., Stratford M., Folkes L. K., Sena L., Gumbrell L., Price P. M. J. Clin. Oncol. 2003;21:2815–2822. doi: 10.1200/JCO.2003.05.185. [DOI] [PubMed] [Google Scholar]
- Pettit G. R., Temple Jr C., Narayanan V. L., Varma R., Simpson M. J., Boyd M. R., Rener G. A., Bansal N. Anti-Cancer Drug Des. 1995;10:299–309. [PubMed] [Google Scholar]
- Pettit G. R., Lippert J. W. Anti-Cancer Drug Des. 2000;15:203–216. [PubMed] [Google Scholar]
- Micheletti G., Poli M., Borsotti P., Martinelli M., Imberti B., Taraboletti G., Giavazzi R. Cancer Res. 2003;63:1534–1537. [PubMed] [Google Scholar]
- Kremmidiotis G., Leske A. F., Lavranos T. C., Beaumont D., Gasic J., Hall A., O'Callaghan M., Matthews C. A., Flynn B. Mol. Cancer Ther. 2010;9:1562–1573. doi: 10.1158/1535-7163.MCT-09-0815. [DOI] [PubMed] [Google Scholar]
- Lee J., Kim S. J., Choi H., Kim Y. H., Lim I. T., Yang H.-M., Lee C. S., Kang H. R., Ahn S. K., Moon S. K., Kim D.-H., Lee S., Choi N. S., Lee K. J. J. Med. Chem. 2010;53:6337–6354. doi: 10.1021/jm1002414. [DOI] [PubMed] [Google Scholar]
- Dey S., Sarkar S., Paul H., Zangrando E., Chattopadhyay P. Polyhedron. 2010;29:1583–1587. [Google Scholar]
- Devereux M., Shea D. O., Kellett A., McCann M., Walsh M., Egan D., Deegan C., Kedziora K., Rosair G., Muller-Bunz H. J. Inorg. Biochem. 2007;101:881–892. doi: 10.1016/j.jinorgbio.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Nofal Z. M., Soliman E. A., Abd El-Karim S. S., El-Zahar M. I., Srour A. M., Sethumadhavan S., Maher T. J. Acta Pol. Pharm. 2011;68:519–534. [PubMed] [Google Scholar]
- El-Gohary N. S., Shaaban M. I. Eur. J. Med. Chem. 2017;131:255–262. doi: 10.1016/j.ejmech.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Rashid M., Husain A., Mishra R., Karim S., Khan S., Ahmad M., Al-wabel N., Husain A., Ahmad A., Khan S. A. Arabian J. Chem. 2015 doi: 10.1016/j.arabjc.2015.08.019. [DOI] [Google Scholar]
- Sasaki J., Ramesh R., Chada S., Gomyo Y., Roth J. A., Mukhopadhyay T. Mol. Cancer Ther. 2002;1:1201–1209. [PubMed] [Google Scholar]
- Doudican N., Rodriguez A., Osman I., Orlow S. J. Mol. Cancer Res. 2008;6:1308–1315. doi: 10.1158/1541-7786.MCR-07-2159. [DOI] [PubMed] [Google Scholar]
- Nardinocchi L., Pantisano V., Puca R., Porru M., Aiello A., Grasselli A., Leonetti C., Safran M., Rechavi G., Givol D., Farsetti A., D'Orazi G. PLoS One. 2010;5:e15048. doi: 10.1371/journal.pone.0015048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. J., Li Y. L. Xiamen Daxue Xuebao, Ziran Kexueban. 1992;31:651–656. [Google Scholar]
- Howell J., Rasmussen M. Aust. J. Chem. 1993;46:1177–1191. [Google Scholar]
- Sherr C. J. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Sandal T. Oncologist. 2002;7:73–81. doi: 10.1634/theoncologist.7-1-73. [DOI] [PubMed] [Google Scholar]
- Pratap R., Satyanarayana M., Nath C., Raghubir R., Puri A., Chander R., Tiwari P., Tripathi B. K. and Srivastava A. K., WO 2006040621 A1, 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.