 A review focused on the synthetic methods of bioactive boron-containing compounds.
A review focused on the synthetic methods of bioactive boron-containing compounds.
Abstract
Boron-containing compounds which possess unique and attractive properties have received increasing attention from the pharmaceutical industry and academia recently. They have shown interesting and useful biological activities, including antibacterial, antifungal, antiparasitic, antiviral, and anti-inflammatory activities. In this review, the synthetic strategies for various boron-containing compounds, including peptidyl boronic acids, benzoxaboroles, benzoxaborines, benzodiazaborines, amine carboxyboranes, and amine cyanoboranes are summarized. Representative structures of each structural class and recently developed biologically active boron-containing compounds are used as examples in this review.
Introduction
Due to their unique electronic and physicochemical properties, the pharmaceutical industry and academia are paying increasing attention to boron-containing compounds in order to develop novel therapeutics. These compounds are strong Lewis acids because boron has an empty p-orbital which allows them to form dative bonds (coordinate covalent bonds) with nucleophiles. The boron center can be readily converted from neutral trigonal planar sp2 to tetrahedral sp3 hybridization under physiological conditions, rendering the unique chemical and biological properties of boron-containing compounds. Simple alkyl or aryl boron-containing compounds have been recognized as serine protease inhibitors since the 1970s.1–3 Subsequently, more boron compounds with various structures have been synthesized for the development of novel therapeutic agents.4–8
This review aims to describe the synthetic strategies toward boron-containing compounds with useful biological activities, including peptidyl boronates/boronic acids, benzoxaboroles, benzoxaborines, benzodiazaborines, amine carboxyboranes, and amine cyanoboranes. Other boron-containing compounds, such as phenylboronic acids or alkylboronic acids, are not discussed.
1. Peptidyl boronates/boronic acids
As key intermediates, α- and β-aminoboronic acids can be incorporated into peptidyl boronates/boronic acids which have shown various biological activities.9–16 In this section, the syntheses of bioactive α- and β-aminoboronic acid derivatives are summarized.
α-Aminoboronic acid derivatives
Matteson et al. developed a stereoselective homologation of chiral pinanediol boronic esters using dichloromethyllithium and applied this reaction to the synthesis of α-aminoboronic acid derivatives. The stereochemistry is controlled using (+)- or (–)-pinanediol which directs the chirality.17–21
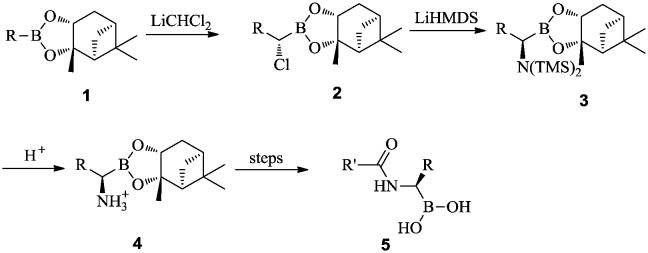
The general synthetic approach to α-aminoboronic acid derivatives is shown in Scheme 1. Substituted pinanediol boronic esters 1 are converted to α-chloroboronic esters 2 by Matteson homologation. The α-aminoboronic esters 4 are obtained through an SN2-type nucleophilic substitution reaction of α-chloroboronic esters 2 with lithium hexamethyldisilazide (LiHMDS), followed by trimethylsilyl (TMS) deprotection. The α-aminoboronic esters 4 can be coupled with different amino acids, followed by deprotection of the pinanediol through transesterification with phenylboronic acid, or under acidic conditions, to afford peptidyl α-aminoboronic acids 5.
Scheme 1. Synthesis of α-aminoboronic acids.
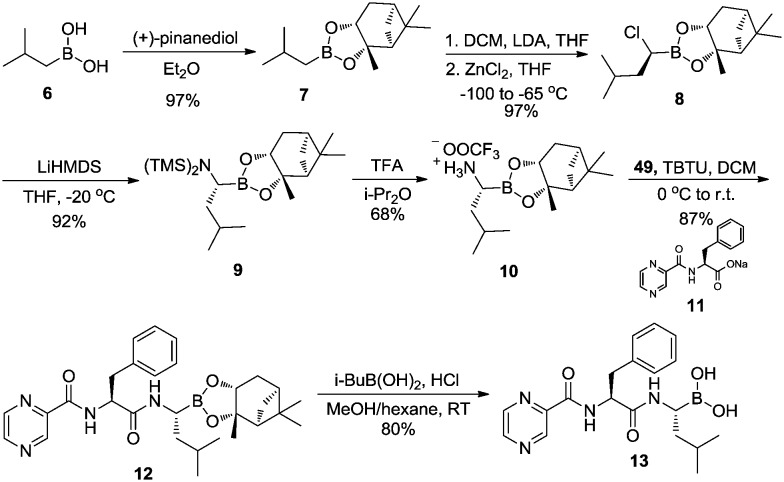
In 1998, Adams et al.22 reported that dipeptide boronic acid 13 (bortezomib) displayed potent and selective inhibition of proteasome enzymatic function. As the first boron-containing drug approved by the FDA in 2003, bortezomib has been used to treat multiple myeloma.23,24
As shown in Scheme 2, the synthesis of bortezomib (13) started with generating chiral boronic ester 7 through esterification of isobutylboronic acid 6 with (+)-pinanediol. Subsequent one-carbon homologation via Matteson rearrangement afforded α-chloroboronic ester 8 in good yield and high diastereoselectivity. Displacement of chloride with LiHMDS yielded 9. Subsequent TMS deprotection using trifluoroacetic acid (TFA) afforded the key intermediate α-aminoboronic ester 10. Coupling 10 with pyrazinoic-acid-substituted phenylalanine 11 afforded compound 12 which was reacted with isobutylboronic acid to afford the desired product 13 (bortezomib).25
Scheme 2. Synthesis of bortezomib.
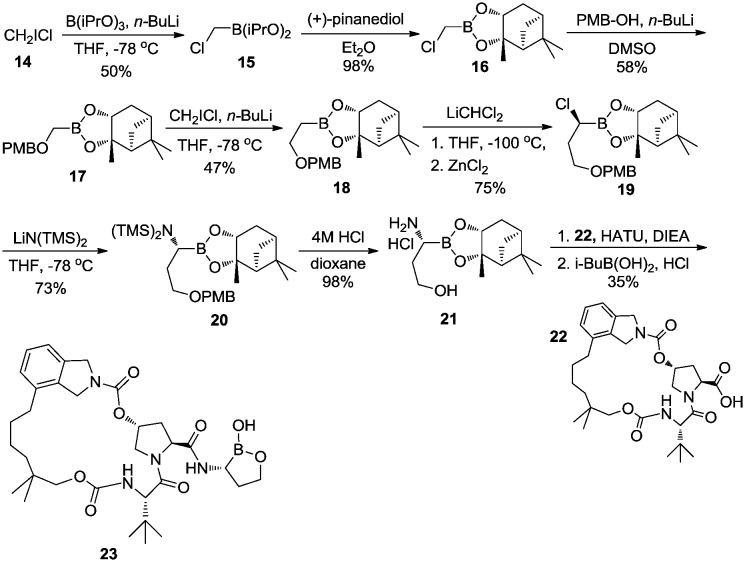
Using this Matteson rearrangement, a series of bioactive peptidyl α-aminoboronic acids have been prepared.26–29 In 2010, Li et al.30,31 reported a macrocyclic compound 23 (Scheme 3), derived from an α-amino cyclic boronate, which showed excellent inhibitory activity against HCV NS3. The synthetic route to this macrocyclic inhibitor, which contains a five-membered α-amino oxaborole, is shown in Scheme 3.31 Transmetalation of chloroiodomethane (CH2ICl) 14 with n-butyllithium (n-BuLi) in the presence of borate gave diisopropyl chloromethylborate 15 which underwent transesterification with (+)-pinanediol to afford pinanediol boronic ester 16. Reaction of 16 with p-methoxybenzyl alcohol (PMB-OH) gave PMB-protected alcohol 17. Transmetalation of 17 afforded elongated compound 18. (R)-α-Chloroboronate 19 was obtained by methylene chloride insertion into 18 using Matteson homologation. Nucleophilic displacement of 19 using LiHMDS gave TMS-protected α-aminoboronic ester 20. After treatment with anhydrous HCl, α-aminoboronic ester 21 was obtained as a salt. Subsequent coupling of 21 with macrocyclic acid 22, pinanediol removal, and spontaneous ring formation in the presence of HCl and isobutyl boronic acid yielded compound 23 in a one-pot procedure.
Scheme 3. Synthesis of the macrocyclic HCV NS3 protease inhibitor.
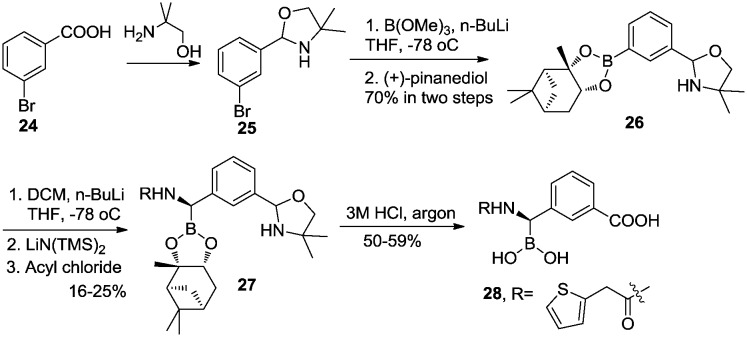
Prati et al.32,33 investigated carboxyphenyl-glycylboronic acids and identified 28 as an inhibitor of class C β-lactamase (AmpC) with a Ki value of 1 nM. The synthesis of this antibiotic is outlined in Scheme 4. Protection of the carboxy group in 24 gave oxazolidine derivative 25. Boronation of 25 at –78 °C using n-butyllithium and trimethyl borate, and subsequent transesterification with (+)-pinanediol, afforded 26. Compound 26 was then converted to 27 in a one-pot Matteson homologation to avoid epimerization of the α-chloro intermediate. Conversion of the pinanediol ester to the free boronic acid and deprotection of the carboxy moiety were achieved by hydrolysis in degassed HCl under reflux conditions.
Scheme 4. Synthesis of carboxyphenyl-glycylboronic acids as inhibitors of AmpC β-lactamase.
β-Aminoboronic acid derivatives
Lejon et al.34,35 synthesized a series of α-, β-, and α,β-disubstituted β-aminoboronic dipeptide mimetics with potent antitubercular activities. The stereoselective synthesis of these compounds combined Matteson homologation with nucleophilic substitution. The synthesis approaches are summarized in this section.
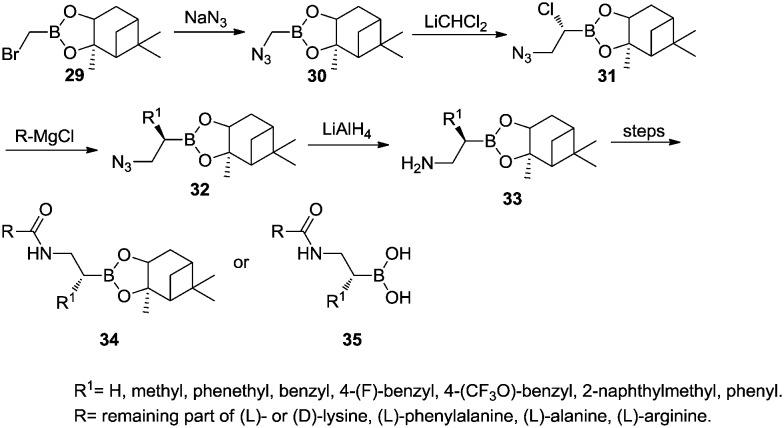
Generally, α-substituted β-aminoboronic acids were synthesized from α-bromo pinanediol boronate 29. The bromide was substituted with azide to give azidomethyl boronate 30, which was then converted to the corresponding 1-chloro-2-azidoethyl boronate 31 by Matteson homologation. Compound 31 reacted with different Grignard reagents to afford α-substituted β-azidoboronates 32 with an inversion of stereochemistry. Reduction of the azido group with lithium aluminum hydride (LiAlH4) yielded α-substituted β-aminoboronates 33 which could be coupled with various amino acids to generate peptidyl α-substituted β-azidoboronates 34.34 Free boronic acid 35 was usually obtained from its pinanediol ester 34 by transesterification with phenylboronic acid or under acidic conditions (Scheme 5).
Scheme 5. Synthesis of α-substituted β-aminoboronic acid derivatives.
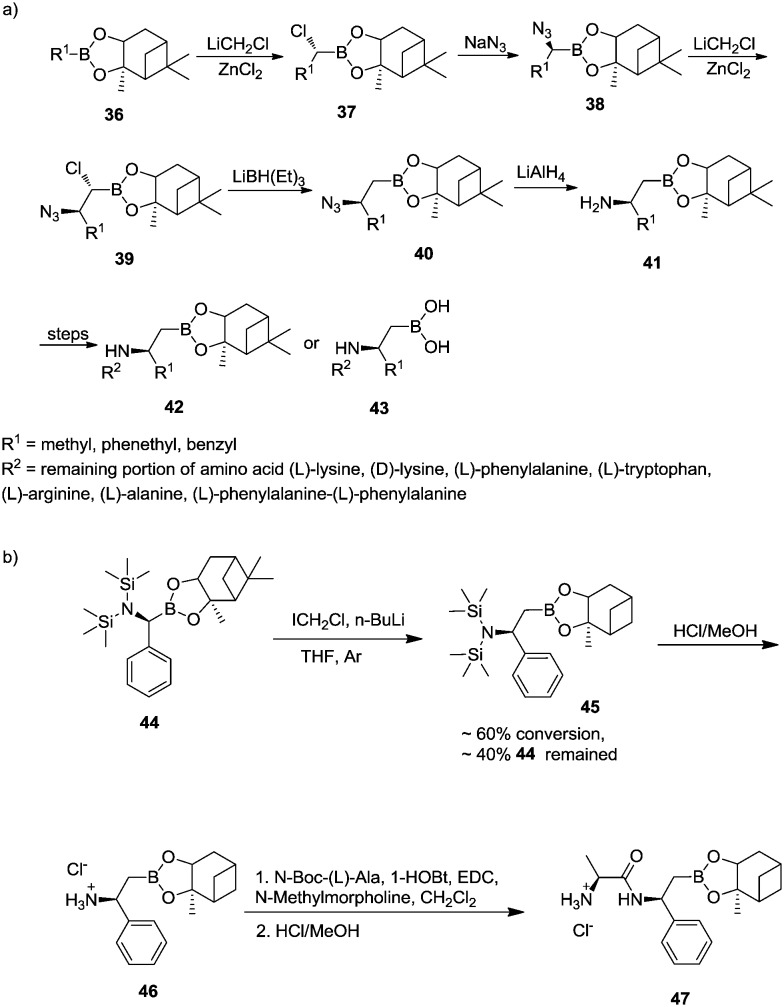
The synthesis of β-substituted β-aminoboronic acid is shown in Scheme 6. Starting from alkylboronic ester 36, α-chloroboronic ester 37 was obtained by Matteson homologation. Substitution of chloride with azide, followed by another homologation, afforded α-chloro-β-azidoboronate 39 which was reduced to β-substituted β-azido boronic ester 40 using lithium triethylborohydride (LiBHEt3). Reduction of the azido group with LiAlH4 produced β-substituted β-aminoboronic ester 41. Coupling the β-aminoboronic ester with various amino acids afforded peptidyl boronates 42 or peptidyl boronic acids 43 (Scheme 6a).35
Scheme 6. (a) Synthesis of β-substituted β-aminoboronic ester or acid derivatives. (b) Synthesis of β-phenyl β-aminoboronic esters.
However, this synthetic procedure was not suitable for introducing a phenyl group into the β-position, as the substitution of α-chloro β-phenyl β-aminoboronic esters with azide led to decomposition and formed a significant amount of benzaldehyde. Therefore, a modified procedure was employed using hexamethyldisilazane (HMDS) to introduce the amino functionality (Scheme 6b).35
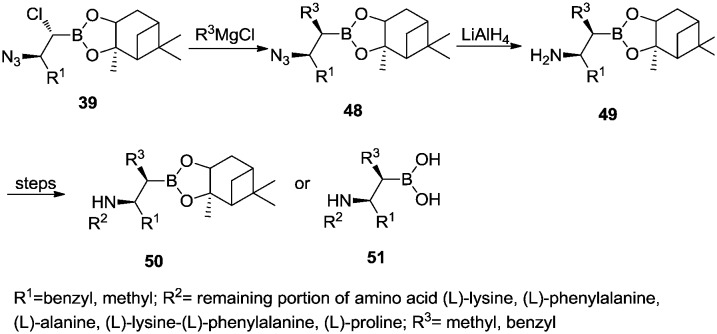
Furthermore, α-chloro-β-azidoboronate 39 could react with different Grignard reagents to afford α,β-disubstituted β-azidoboronic esters 48 (Scheme 7). The subsequent reduction of azido groups yielded α,β-disubstituted β-aminoboronic esters 49 which could be coupled with different amino acids to prepare peptidyl α,β-disubstituted β-azidoboronic esters 50. The pinanediol can also be removed in the final step to afford 51.35
Scheme 7. Synthesis of α,β-disubstituted β-aminoboronic acid derivatives.
2. Benzoxaboroles
Benzoxaboroles were first synthesized and characterized in 1957 by Torssell (Fig. 1a).36,37 They have been applied in organic synthesis, supramolecular chemistry, and glycopeptide recognition since then. This class of compounds had received little attention in medicinal chemistry until 2006, when their exceptional sugar-binding properties under physiological conditions were described.38 5-Fluorobenzoxaborole (tavaborole, Fig. 1b), used for the treatment of onychomycosis, was the first benzoxaborole drug approved by the FDA.39–41 The discovery of tavaborole has motivated the in-depth investigation of benzoxaboroles in medicinal chemistry.
Fig. 1. (a) Benzoxaborole structure and ring numbering. (b) Chemical structure of tavaborole.

Benzoxaboroles with substituents on the phenyl moiety
Benzoxaboroles are internal esters of substituted ortho-boronobenzyl alcohols. These benzyl alcohols undergo intramolecular dehydration with the boronic acid group, resulting in cyclization to form benzoxaboroles.42,43 Therefore, o-(hydroxymethyl)phenylboronic acid is usually generated in situ and used directly as an intermediate in benzoxaborole synthesis.
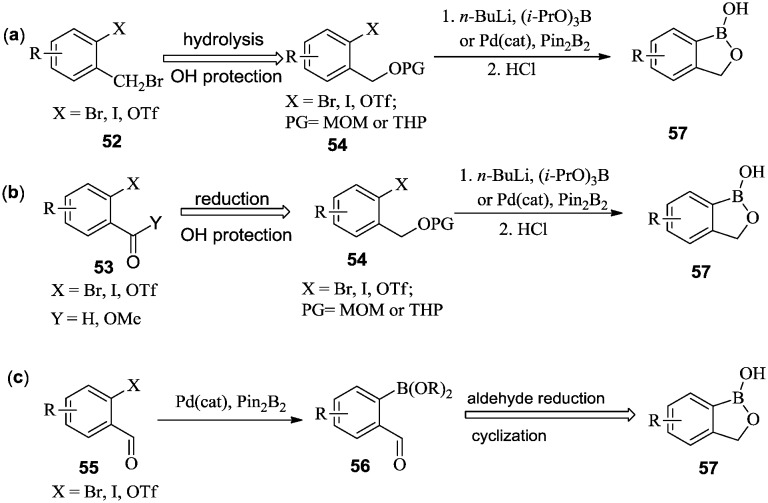
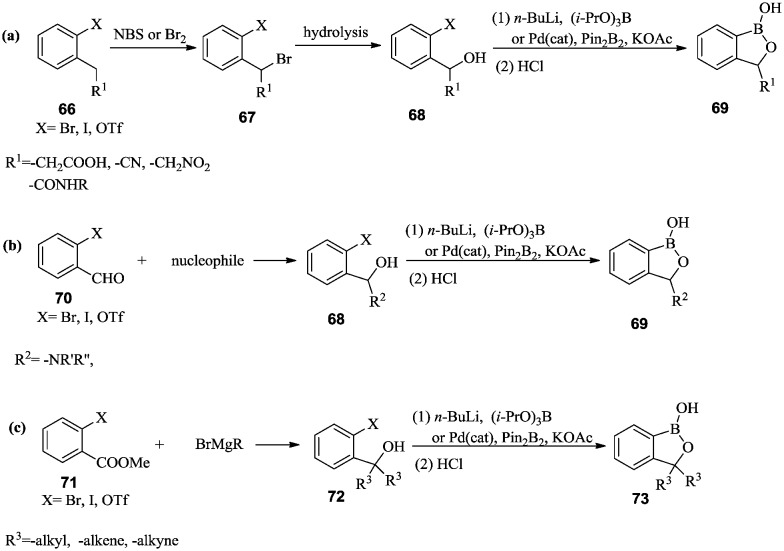
One general synthetic method for this key intermediate involves these steps: (i) the installation of a hydroxymethyl group on phenyl halides or triflates through the hydrolysis of benzyl bromide 52 (Scheme 8a), or the reduction of benzyl aldehyde or benzyl carboxymethyl ester 53 (Scheme 8b); (ii) the introduction of a boron group by reacting the aryl halide or triflate with n-butyllithium and alkyl borate, or through a Pd-catalyzed reaction with bis(pinacolato)diboron. Rational selection of a protection and deprotection strategy for the hydroxyl group that is appropriate for the reaction conditions is necessary. Finally, the boronate was hydrolyzed to a boronic acid, and the cyclization proceeded spontaneously to produce benzoxaborole 57.44–47
Scheme 8. Three general synthetic methods, (a)–(c), for benzoxaboroles.
In an alternative synthetic route, ortho-halo or trifluoromethanesulfonyl benzaldehyde 55 was converted into boronate ester 56via Pd-mediated boronylation. Subsequent reduction of aldehyde 56 and simultaneous cyclization produced benzoxaborole 57 (Scheme 8c).48–52
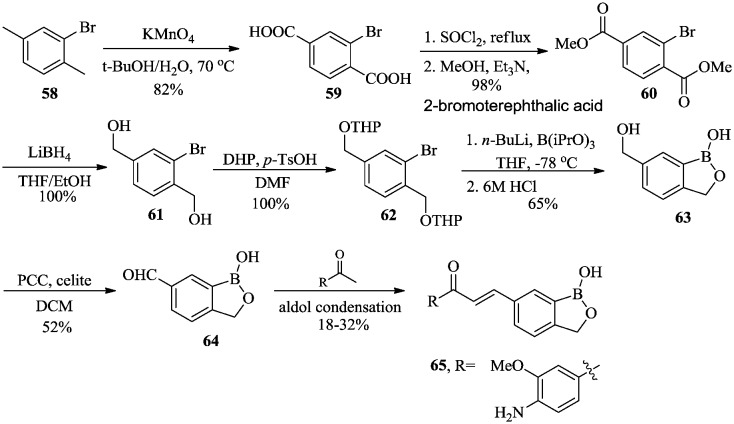
Zhou et al. designed and synthesized a series of benzoxaborole derivatives with different substituents on the phenyl moiety that showed potent antiparasitic,53–55 antibacterial,56 and anticancer57 activities. The synthesis of chalcone–benzoxaborole compound 65, which has potent antitrypanosomal activity, is outlined in Scheme 9. Dimethyl 2-bromoterephthalate 60 was obtained from the oxidation of 2-bromo-1,4-dimethylbenzene 58 using KMnO4 to form acid 59, followed by esterification. Ester 60 was reduced by lithium borohydride (LiBH4) to give 2-bromobenzenedimethanol 61, followed by protection with dihydropyran (DHP) to afford 62. Benzoxaborole 63 was obtained by adding boronic acid to 62 using n-butyllithium and triisopropyl borate, followed by deprotection and spontaneous cyclization using HCl. Aldehyde intermediate 64 was obtained by pyridinium chlorochromate (PCC) oxidation. Chalcone–benzoxaboroles 65 were prepared by aldol condensation under alkaline or acidic conditions, with E-isomers obtained as the sole isomers, as confirmed by 1H NMR. The Wittig reaction was also effective for the conversion of aldehyde intermediate 64 into substituted benzoxaboroles, which were also proved to be E-isomers by NMR. It is worth mentioning that aldehyde 64 is a versatile intermediate that can be converted to a variety of derivatives due to the chemical versatility of the formyl group. At the same time, this practical synthetic approach can be readily expanded to the synthesis of 4-, 5-, and 7-formyl benzoxaboroles.
Scheme 9. Synthesis of chalcone–benzoxaboroles as potent antitrypanosomal agents.
Benzoxaboroles with substituents on the oxaborole ring
3-Monosubstituted benzoxaboroles can be synthesized either by using methylene-substituted benzyl alcohol (Scheme 10a) or by nucleophilic addition to the corresponding benzaldehyde (Scheme 10b). 3-Disubstituted benzoxaboroles can be prepared by a two-step nucleophilic addition on methyl benzoate by Grignard reagents (Scheme 10c). Subsequent introduction of the boron group and spontaneous cyclization under acidic conditions yielded benzoxaboroles with substituents on the oxaborole ring.
Scheme 10. Three synthetic routes, (a)–(c), for 3-substituted benzoxaboroles.
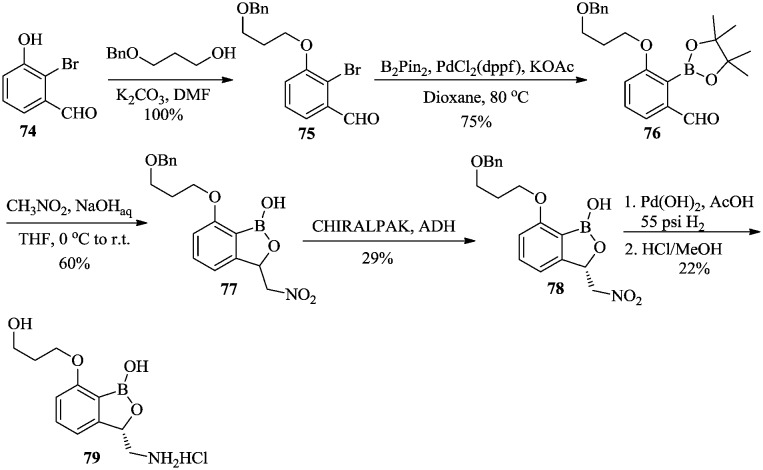
Antibacterial compound 79 (Scheme 11) is an inhibitor of bacterial leucyl-tRNA synthetase and is in development for the treatment of serious Gram-negative infections.58 The phenol 74 was converted to phenyl ether 75, followed by the introduction of pinacolatoborate to afford aldehyde 76. Racemic mixture 77 was prepared by the nucleophilic addition of compound 76 to nitromethane and resolved using a chiral column to afford 78 as an enantiomer. The nitro group was then reduced to an amino group, followed by deprotection of the benzyl group and acidification of the amine to afford target compound 79.
Scheme 11. Synthesis of compound 79 as an antibacterial agent.
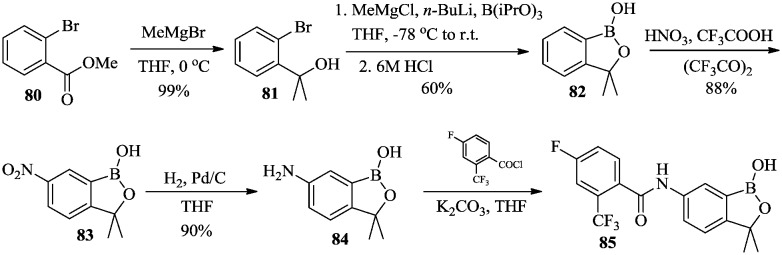
Jacobs et al.59,60 reported the synthesis of benzoxaborole 85 as a potent antitrypanosomal agent with the synthetic route shown in Scheme 12. Methyl 2-bromobenzoate 80 was converted to the corresponding dimethylbenzyl tertiary alcohol 81 using a two-step nucleophilic addition with a Grignard reagent. After extraction of proton by the Grignard reagent, boronate was introduced using n-butyllithium and triisopropyl borate, followed by spontaneous cyclization under acidic conditions to afford 3-disubstituted benzoxaborole 82. Compound 82 was then nitrated at C-6 using HNO3 to give nitro benzoxaborole 83 which was reduced to afford 6-amino benzoxaborole 84. Target compound 85 was obtained from the acylation of 84 with 4-fluoro-2-trifluoromethylbenzoyl chloride.
Scheme 12. Synthesis of compound 85 as a potent antitrypanosomal agent.
6-Amino-3,3-dimethyl benzoxaboroles were also coupled with different groups to produce 3,6-disubstituted benzoxaboroles with antiprotozoal, antibacterial, antiviral, and anti-inflammatory activities.61–66
3. Benzoxaborines
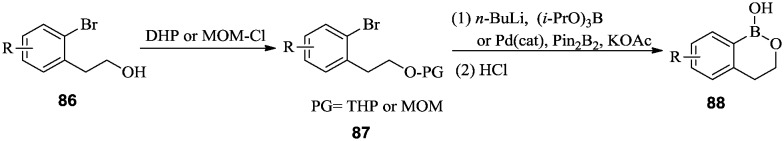
Oxaborines and benzoxaborines have shown inhibitory activities against microorganisms.67 As β-lactamase inhibitors, they have been applied in the combination therapy of multidrug resistant infections.68–70 The synthetic approach for benzoxaborines is similar to that for benzoxaboroles. ortho-Bromophenethyl alcohols 86 were protected as tetrahydropyran (THP) or methoxymethyl (MOM) ethers. Displacement of the ortho-bromide with a boron group was achieved using n-butyllithium/borate or a Pd-catalyzed reaction with bis(pinacolato)diboron, followed by ring closure under acidic conditions to afford benzoxaborines 88 (Scheme 13).
Scheme 13. General synthetic route for benzoxaborines.
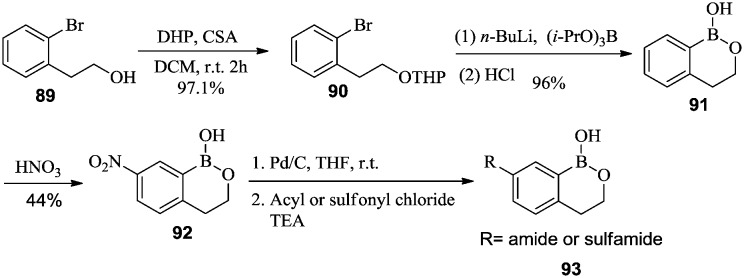
Akama et al.67 synthesized a series of benzoxaborines with amide and sulfamide phenyl substituents that showed antiprotozoal activities. First, the hydroxyl group of alcohol 89 was THP-protected to afford compound 90. The boronic acid was introduced at the ortho-bromide position of 90 using n-butyllithium/borate, followed by deprotection and cyclization to afford benzoxaborine 91 in good yield. Compound 91 was nitrated to afford 92, followed by reduction of the nitro group and acylation, which afforded target compounds 93 (Scheme 14).
Scheme 14. Synthesis of phenyl-substituted benzoxaborines as anti-protozoal agents.
4. Diazaborines
2,3,1-Diazaborines
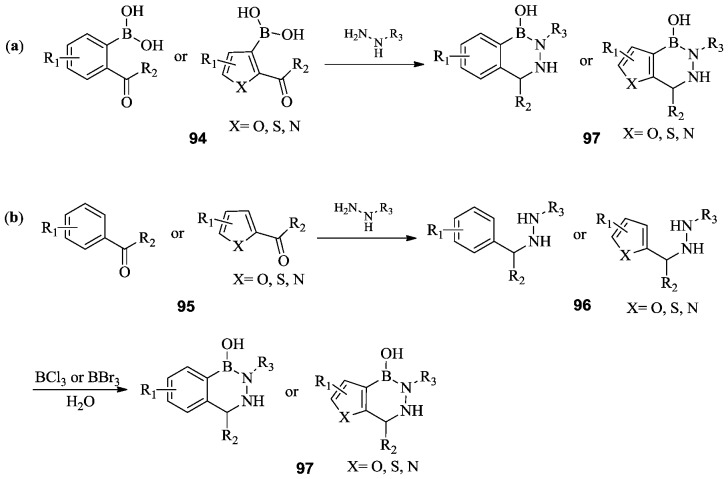
Bioactive 2,3,1-diazaborines, including 2,3,1-benzo-, furano-, thieno-, and pyrrolodiazaborines, have shown antibacterial and antifungal activities.71,72 2,3,1-Diazaborines can be prepared by the condensation of ortho-acyl/formyl aromatic or heteroaromatic boronic acids 94 with hydrazine or hydrazine derivatives, as shown in Scheme 15a.72 2,3,1-Diazaborines can also be prepared by condensation of the corresponding aromatic or heteroaromatic aldehydes or ketones 95 with hydrazine or hydrazine derivatives, followed by boronation and cyclization (Scheme 15b).73
Scheme 15. Two general synthetic routes, (a) and (b), for 2,3,1-diazaborines.
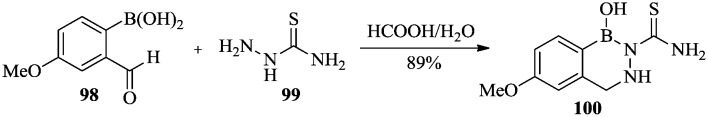
For example, Westcott et al.74 synthesized a series of 2,3,1-benzodiazaborines, of which compound 100 showed potent antifungal activity. It was obtained by condensation of the corresponding ortho-formylphenylboronic acid 98 and thiosemicarbazide in good yield (Scheme 16).
Scheme 16. Synthesis of 2,3,1-benzodiazaborine with antifungal activity.
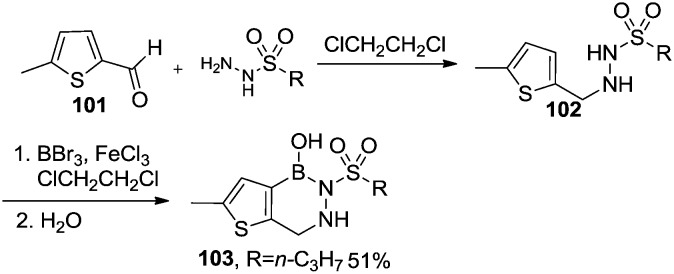
Grassberger et al.75 synthesized a series of 2,3,1-diazaborine derivatives with antibacterial activities. The 2,3,1-thienodiazaborine derivative 103 was synthesized by the condensation of 2-formyl-5-methylthiophene 101 with hydrazine derivatives, followed by installation of the boronic acid moiety with BBr3 and cyclization (Scheme 17).
Scheme 17. Synthesis of 2,3,1-thienodiazaborine with antibacterial activity.
2,4,1-Diazaborines
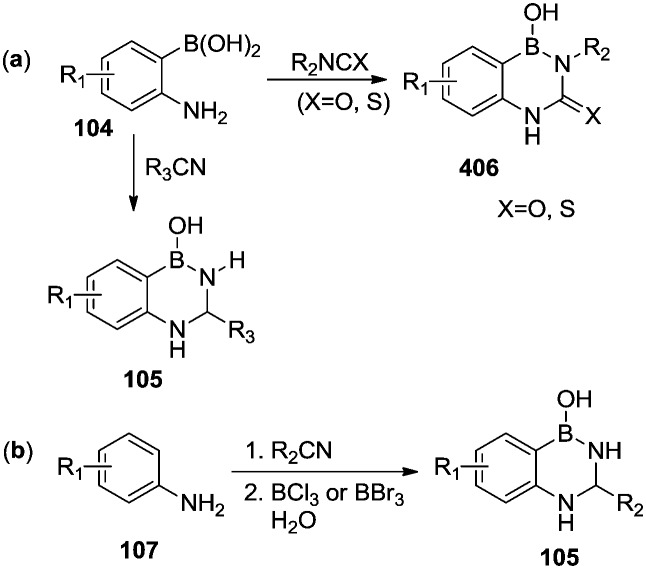
Bioactive 2,4,1-diazaborines are mainly composed of benzodiazaborines. They can be prepared by coupling ortho-aminophenylboronic acids with isocyanates, isothiocyanates, or carbonitriles (Scheme 18a). Similarly, 3-substituted 2,4,1-benzodiazaborines were obtained from the condensation of anilines with carbonitriles, followed by introducing boron and cyclization (Scheme 18b).76,77
Scheme 18. Two general synthetic routes, (a) and (b), for 2,4,1-diazaborines.
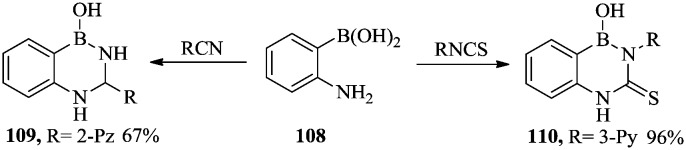
In 1998, Martin et al. reported the synthesis of 2,4,1-benzodiazaborine compounds with compounds 109 and 110 showing potent inhibitory activity against M. tuberculosis.76 The synthetic route was simple and efficient, as shown in Scheme 19. The 2-aminophenylboronic acid 108 was reacted with carbonitrile or isothiocyanate to afford 109 or 110 in good yield.
Scheme 19. Synthesis of 2,4,1-benzodiazaborine with antibacterial activity.
5. Amine carboxyborane and amine cyanoborane derivatives
The biological activities of amine carboxyboranes and amine cyanoboranes were first reported in the 1980s.78,79 These unique boron compounds are thought to possess good biological activities, such as antibacterial,80 antifungal, antiprotozoal,81 antitumor, and anti-inflammation properties.82 Recently, amine carboxyboranes were found to have carbon monoxide (CO) release properties and could be applied as therapeutics in CO delivery systems.83
Amine cyanoborane derivatives
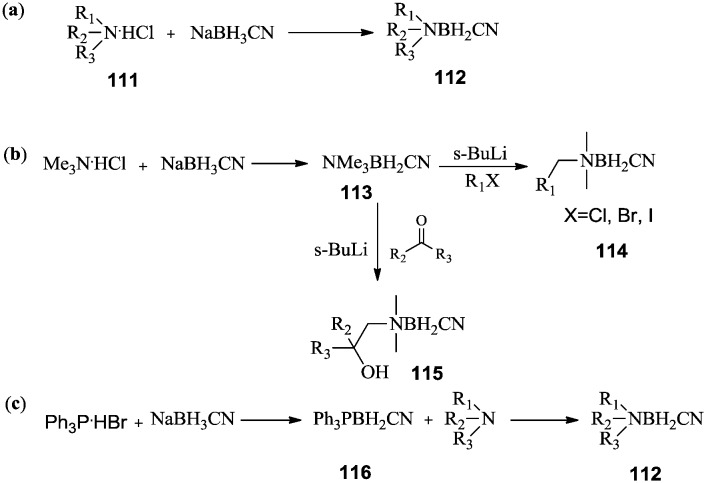
In 1978, Spielvogel et al.84 described a general synthetic approach to amine cyanoboranes (112) by reacting corresponding amines with NaBH3CN, as shown in Scheme 20a. Soon afterwards, new methods for preparing amine cyanoboranes were reported. As shown in Scheme 20b, more complex amine cyanoborane derivatives were obtained using a method based on the C-lithiation of trimethylamine cyanoborane 113 with s-BuLi, followed by alkylation with alkylhalides or ketones.82 Alternatively, amine cyanoboranes (112) have been prepared using Lewis acid exchange reactions (Scheme 20c).85 Phosphine cyanoborane 116 was prepared by reacting NaBH3CN with triphenylphosphine. Subsequently, the triphenylphosphine was exchanged with the less bulky amine to afford the amine cyanoborane. Aliphatic, aromatic, and heterocyclic amines have been used to prepare the corresponding amine cyanoboranes.86
Scheme 20. Three synthetic approaches, (a)–(c), to amine cyanoboranes.
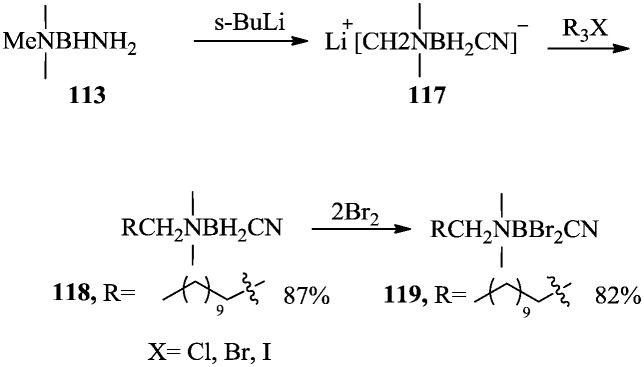
A series of new amine cyanoborane derivatives that exhibit antifungal activity were developed by Srebnik et al.87 Amine cyanoborane 118 and amine halocyanoborane 119 with long aliphatic chains showed antifungal activities against the most important human pathogenic fungi, C. albicans and A. fumigatus. The synthetic approach is shown in Scheme 21. The N-methyl group of 113 was exchanged using s-BuLi to give lithium salt 117, followed by coupling with alkyl halides to afford amine cyanoboranes 118. Amine cyanodibromoboranes 119 were prepared by treating the amine cyanoboranes with excess bromine.
Scheme 21. Synthesis of amine cyanoboranes with antifungal activity.
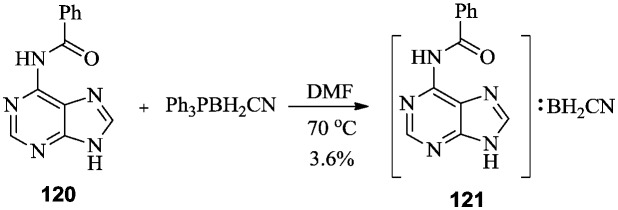
Burnham et al. synthesized purine cyanoborane derivative 121 as an antitumor agent. The Lewis exchange reaction of triphenylphosphine cyanoborane with 6-benzoylpurine 120 afforded the target purine cyanoborane 121 (Scheme 22). Notably, these boron exchange reactions must be carried out under anhydrous conditions to avoid the hydrolysis of cyanoborane to boric acid.88
Scheme 22. Synthesis of purine cyanoborane with antitumor activity.
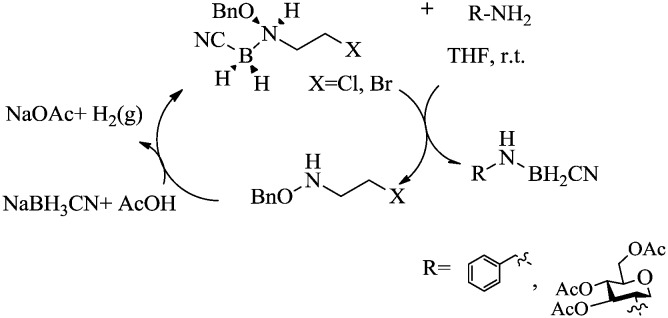
Fernández-Bolaños et al. reported alkoxyamine–cyanoborane adducts as efficient cyanoborane transfer agents to various types of amines, and in glucoside formation with a reducing sugar.89 The mechanism of cyanoborane transfer is shown in Scheme 23.
Scheme 23. Cyanoborane transfer agents for different amines.
Amine carboxyborane derivatives
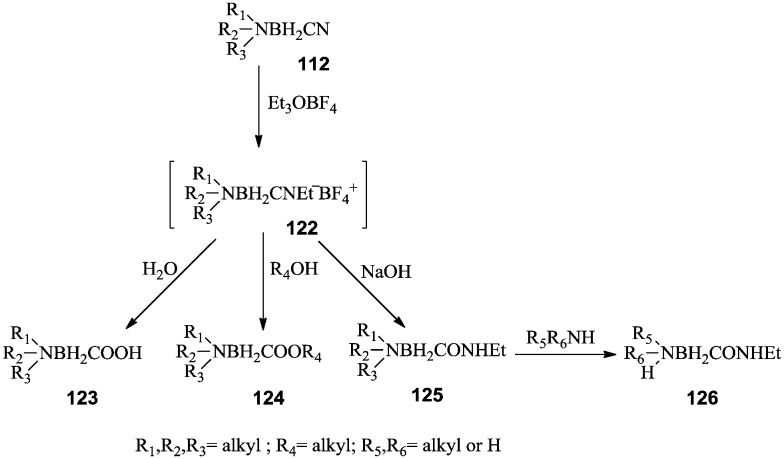
Generally, amine carboxyborane derivatives 124–126 have been prepared from their corresponding amine cyanoborane 112. The key intermediate, N-ethylnitrilium salt 122, was prepared by reaction of the amine cyanoboranes with triethyloxonium tetrafluoroborate. Subsequent addition of water converted nitrilium salt 122 to amine carboxyboranes 123. Alternatively, the nitrilium salt could be treated with alcohol to yield amine carboxyborane esters 124, or with a hydroxide base to yield amine N-ethylcarbamoylboranes 125 (Scheme 24).90–92
Scheme 24. Synthesis of amine carboxyborane derivatives.
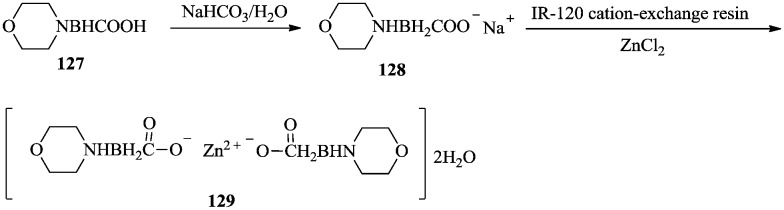
Hall et al.93,94 synthesized metal complexes of amine carboxyborane adduct 129 and discovered its antitumor activity. Morpholine carboxyborane 127 was converted to sodium salt 128 using aqueous NaHCO3. Metal complex 129 was successfully synthesized from amine carboxyborane sodium salt 128 using IR-120 cation-exchange resin charged with the desired cation (Scheme 25).
Scheme 25. Synthesis of a carboxyborane metal complex with antitumor activity.
Conclusion
The number of boron-containing compounds in clinical trials and pre-clinical studies, under development in laboratories, and used as FDA-approved drugs, has rapidly increased with recent advances in synthetic methods for this class of compounds. Syntheses of peptidyl boronates or boronic acids are mainly based on Matteson asymmetric homologation, while benzoxaboroles and diazaborines are mainly obtained through intramolecular cyclization. The boron-containing compounds discussed in this review have been proven to be stable compounds under ambient conditions.77,79,95,96 They showed degradation under oxidative conditions via cleavage of boronic acid as exemplified by the oxidative degradation of peptidyl boronates.97,98 We believe that novel structures and synthetic methods will continue to emerge and contribute to the discovery of useful pharmaceutical applications.
Conflicts of interest
The authors confirm that there are no conflicts of interest.
Acknowledgments
This work was funded by the National Key Research and Development Program of China (2017YFA0505200), the National Science Foundation of China (81222042; 81573264; 21402118), and the E-Institutes of Shanghai Universities (EISU) Chemical Biology Division.
Footnotes
†The authors declare no competing interests.
References
- Koehler K. A., Lienhard G. E. Biochemistry. 1971;10:2477–2483. doi: 10.1021/bi00789a008. [DOI] [PubMed] [Google Scholar]
- Philipp M., Bender M. L. Proc. Natl. Acad. Sci. U. S. A. 1971;68:478–480. doi: 10.1073/pnas.68.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D. A., Alden R. A., Birktoft J. J., Freer S. T., Kraut J. J. Biol. Chem. 1975;250:7120–7126. [PubMed] [Google Scholar]
- Ban H. S., Nakamura H. Chem. Rec. 2015;15:616–635. doi: 10.1002/tcr.201402100. [DOI] [PubMed] [Google Scholar]
- Smoum R., Rubinstein A., Dembitsky V. M., Srebnik M. Chem. Rev. 2012;112:4156–4220. doi: 10.1021/cr608202m. [DOI] [PubMed] [Google Scholar]
- Sorianoursúa M. A., Das B. C., Trujilloferrara J. G. Expert Opin. Ther. Pat. 2014;24:485–500. doi: 10.1517/13543776.2014.881472. [DOI] [PubMed] [Google Scholar]
- Das B. C., Thapa P., Karki R., Schinke C., Das S., Kambhampati S., Banerjee S. K., Van V. P., Verma A., Weiss L. M. Future Med. Chem. 2013;5:653–676. doi: 10.4155/fmc.13.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani L., Ristori S. Expert Opin. Drug Discovery. 2012;7:1017–1027. doi: 10.1517/17460441.2012.717530. [DOI] [PubMed] [Google Scholar]
- Hiratake J., Oda J. Biochemistry. 1997;61:211–218. [Google Scholar]
- Han L., Wen Y., Li R., Xu B., Ge Z., Wang X., Cheng T., Cui J., Li R. Bioorg. Med. Chem. 2017;25:4031–4044. doi: 10.1016/j.bmc.2017.05.049. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Wu G., Zhu X., Ma Y., Zhao X., Li Y., Yuan Y., Yang J., Yu S., Shao F. J. Med. Chem. 2010;53:8619–8626. doi: 10.1021/jm1009742. [DOI] [PubMed] [Google Scholar]
- Venugopal D. V. R., Rao A. K., Devi P. U., Sastry Y. N., Lakshmi K. A., Ramji M. T., Shiralgi Y. Res. Chem. Intermed. 2017;43:5775–5778. [Google Scholar]
- Kostova M. B., Rosen D. M., Chen Y., Mease R. C., Denmeade S. R. J. Med. Chem. 2013;56:4224–4235. doi: 10.1021/jm301718c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Gao X., Wang B. Med. Res. Rev. 2003;23:346–368. doi: 10.1002/med.10043. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Tomsho J. W., Benkovic S. J. Chem. Soc. Rev. 2011;40:4279–4285. doi: 10.1039/c0cs00131g. [DOI] [PubMed] [Google Scholar]
- Ruggeri B., Miknyoczki S., Dorsey B., Hui A. M. Adv. Pharmacol. 2009;57:91–135. doi: 10.1016/S1054-3589(08)57003-7. [DOI] [PubMed] [Google Scholar]
- Matteson D. S., Majumdar D. Organometallics. 1983;2:1529–1535. [Google Scholar]
- Matteson D. S., Sadhu K. M. Organometallics. 1984;3:614–618. [Google Scholar]
- Matteson D. S., Jesthi P. K., Sadhu K. M. Organometallics. 1984;3:1284–1288. [Google Scholar]
- Matteson D. S., Ray R., Rocks R. R., Tsai D. J. Organometallics. 1983;2:1536–1543. [Google Scholar]
- Matteson D. S. Acc. Chem. Res. 1988;21:294–300. [Google Scholar]
- Adams J., Behnke M., Chen S., Cruickshank A. A., Dick L. R., Grenier L., Klunder J. M., Ma Y. T., Plamondon L., Stein R. L. Bioorg. Med. Chem. Lett. 1998;8:333–338. doi: 10.1016/s0960-894x(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Paramore A., Frantz S. Nat. Rev. Drug Discovery. 2003;2:611–612. doi: 10.1038/nrd1159. [DOI] [PubMed] [Google Scholar]
- Scorei R. I., Popa R. Anticancer Agents Med. Chem. 2010;10:346–351. doi: 10.2174/187152010791162289. [DOI] [PubMed] [Google Scholar]
- Rentsch A., Landsberg D., Brodmann T., Bülow L., Girbig A. K., Kalesse M. Angew. Chem., Int. Ed. 2013;52:5450–5488. doi: 10.1002/anie.201207900. [DOI] [PubMed] [Google Scholar]
- Mulakayala N., Reddy CH U., Iqbal J., Pal M. Tetrahedron. 2010;66:4919–4938. [Google Scholar]
- Coutts S. J., Kelly T. A., Snow R. J., Kennedy C. A., Barton R. W., Adams J., Krolikowski D. A., Freeman D. M., Campbell S. J., Ksiazek J. F. J. Med. Chem. 1996;39:2087–2094. doi: 10.1021/jm950732f. [DOI] [PubMed] [Google Scholar]
- Wityak J., Earl R. A., Abelman M. M., Bethel Y. B., Fisher B. N., Kauffman G. S., Kettner C. A., Ma P., McMillan J. L. J. Org. Chem. 1995;60:3717–3722. [Google Scholar]
- Boloor A., Hanway D., Joshi M., Winn D. T., Mendez G., Walls M., Wei P., Qian F., Zhang X., Zhang Y. Bioorg. Med. Chem. Lett. 2009;19:5708–5711. doi: 10.1016/j.bmcl.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang Y.-K., Liu Y., Ding C. Z., Zhou Y., Li Q., Plattner J. J., Baker S. J., Zhang S., Kazmierski W. M. Bioorg. Med. Chem. Lett. 2010;20:5695–5700. doi: 10.1016/j.bmcl.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang Y.-K., Liu Y., Ding C. Z., Li Q., Zhou Y., Plattner J. J., Baker S. J., Qian X., Fan D. Bioorg. Med. Chem. Lett. 2010;20:3550–3556. doi: 10.1016/j.bmcl.2010.04.129. [DOI] [PubMed] [Google Scholar]
- Morandi F., Caselli E., Morandi S., Focia P. J., Blázquez J., Shoichet B. K., Prati F. J. Am. Chem. Soc. 2003;125:685–695. doi: 10.1021/ja0288338. [DOI] [PubMed] [Google Scholar]
- Eidam O., Romagnoli C., Caselli E., Babaoglu K., Pohlhaus D. T., Karpiak J., Bonnet R., Shoichet B. K., Prati F. J. Med. Chem. 2010;53:7852–7863. doi: 10.1021/jm101015z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovoy A. S., Gozhina O. V., Svendsen J. S., Domorad A. A., Tetz G. V., Tetz V. V., Lejon T. Chem. Biol. Drug Des. 2013;81:408–413. doi: 10.1111/cbdd.12091. [DOI] [PubMed] [Google Scholar]
- Gorovoy A. S., Gozhina O., Svendsen J. S., Tetz G. V., Domorad A., Tetz V. V., Lejon T. J. Pept. Sci. 2013;19:613–618. doi: 10.1002/psc.2537. [DOI] [PubMed] [Google Scholar]
- Torssell K. Ark. Kemi. 1957;10:507–511. [Google Scholar]
- Haynes R. R., Snyder H. R. J. Org. Chem. 1964;29:3229–3233. [Google Scholar]
- Dowlut M., Hall D. G. J. Am. Chem. Soc. 2006;128:4226–4227. doi: 10.1021/ja057798c. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Zhang Y.-K., Akama T., Lau A., Zhou H., Hernandez V., Mao W., Alley M., Sanders V., Plattner J. J. J. Med. Chem. 2006;49:4447–4450. doi: 10.1021/jm0603724. [DOI] [PubMed] [Google Scholar]
- Rock F. L., Mao W., Yaremchuk A., Tukalo M., Crépin T., Zhou H., Zhang Y.-K., Hernandez V., Akama T., Baker S. J. Science. 2007;316:1759–1761. doi: 10.1126/science.1142189. [DOI] [PubMed] [Google Scholar]
- Markham A. Drugs. 2014;74:1555–1558. doi: 10.1007/s40265-014-0276-7. [DOI] [PubMed] [Google Scholar]
- Adamczyk-Woźniak A., Borys K. M., Sporzynski A. Chem. Rev. 2015;115:5224–5247. doi: 10.1021/cr500642d. [DOI] [PubMed] [Google Scholar]
- Liu C. T., Tomsho J. W., Benkovic S. J. Bioorg. Med. Chem. 2014;22:4462–4473. doi: 10.1016/j.bmc.2014.04.065. [DOI] [PubMed] [Google Scholar]
- Zhang J., Zhu M., Lin Y., Zhou H. Sci. China: Chem. 2013;56:1372–1381. [Google Scholar]
- Adamczyk-Woźniak A., Cyrański M. K., Żubrowska A., Sporzyński A. J. Organomet. Chem. 2009;694:3533–3541. [Google Scholar]
- Adamczyk-Woźniak A., Madura I., Velders A. H., Sporzyński A. Tetrahedron Lett. 2010;51:6181–6185. [Google Scholar]
- Zhdankin V. V., Persichini P., Zhang L., Fix S., Kiprof P. Tetrahedron Lett. 1999;40:6705–6708. [Google Scholar]
- Zhang Y.-K., Plattner J. J., Easom E. E., Jacobs R. T., Guo D., Freund Y. R., Berry P., Ciaravino V., Erve J. C., Rosenthal P. J. J. Med. Chem. 2017;60:5889–5908. doi: 10.1021/acs.jmedchem.7b00621. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-K., Plattner J. J., Easom E. E., Jacobs R. T., Guo D., Sanders V., Freund Y. R., Campo B., Rosenthal P. J., Bu W. J. Med. Chem. 2015;58:5344–5354. doi: 10.1021/acs.jmedchem.5b00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-K., Plattner J. J., Freund Y. R., Easom E. E., Zhou Y., Ye L., Zhou H., Waterson D., Gamo F.-J., Sanz L. M. Bioorg. Med. Chem. Lett. 2012;22:1299–1307. doi: 10.1016/j.bmcl.2011.12.096. [DOI] [PubMed] [Google Scholar]
- Zhang Y. K., Plattner J. J., Easom E. E., Liu L., Retz D. M., Ge M., Zhou H. H. J. Labelled Compd. Radiopharm. 2012;55:201–205. [Google Scholar]
- Zhang Y.-K., Plattner J. J., Freund Y. R., Easom E. E., Zhou Y., Gut J., Rosenthal P. J., Waterson D., Gamo F.-J., Angulo-Barturen I. Bioorg. Med. Chem. Lett. 2011;21:644–651. doi: 10.1016/j.bmcl.2010.12.034. [DOI] [PubMed] [Google Scholar]
- Ye L., Ding D., Feng Y., Xie D., Wu P., Guo H., Meng Q., Zhou H. Tetrahedron. 2009;65:8738–8744. [Google Scholar]
- Qiao Z., Wang Q., Zhang F., Wang Z., Bowling T., Nare B., Jacobs R. T., Zhang J., Ding D., Liu Y. J. Med. Chem. 2012;55:3553–3557. doi: 10.1021/jm2012408. [DOI] [PubMed] [Google Scholar]
- Ding D., Zhao Y., Meng Q., Xie D., Nare B., Chen D., Bacchi C. J., Yarlett N., Zhang Y.-K., Hernandez V. ACS Med. Chem. Lett. 2010;1:165–169. doi: 10.1021/ml100013s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q.-H., Liu R.-J., Fang Z.-P., Zhang J., Ding Y.-Y., Tan M., Wang M., Pan W., Zhou H.-C., Wang E.-D. Sci. Rep. 2013;3:2475. doi: 10.1038/srep02475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Yang F., Qiao Z., Zhu M., Zhou H. Bioorg. Med. Chem. Lett. 2016;26:5797–5801. doi: 10.1016/j.bmcl.2016.10.024. [DOI] [PubMed] [Google Scholar]
- Hernandez V., Akama T., Alley M., Baker S., Mao W., Rock F., Zhang Y., Zhang Y., Zhou Y. and Crepin T., Discovery and mechanism of action of AN3365: a novel boron containing antibacterial agent in clinical development for Gram-negative infections, 50th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Boston, 2010, p. 12. [Google Scholar]
- Jacobs R. T., Nare B., Wring S. A., Orr M. D., Chen D., Sligar J. M., Jenks M. X., Noe R. A., Bowling T. S., Mercer L. T. PLoS Neglected Trop. Dis. 2011;5:e1151. doi: 10.1371/journal.pntd.0001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Orr M., Sligar J., Jacobs R. and Plattner J. J., Preparation of benzoxaborole molecules as antiprotozoal agents and their use in the treatment of protozoal infection, PCT Pat., WO 2011019618, 2011.
- Ding C. Z., Zhang Y.-K., Li X., Liu Y., Zhang S., Zhou Y., Plattner J. J., Baker S. J., Liu L., Duan M. Bioorg.Bioorg. Med. Chem. Lett.Med. Chem. Lett. 2010;20:7317–7322. doi: 10.1016/j.bmcl.2010.10.071. [DOI] [PubMed] [Google Scholar]
- Xia Y., Cao K., Zhou Y., Alley M., Rock F., Mohan M., Meewan M., Baker S. J., Lux S., Ding C. Z. Bioorg. Med. Chem. Lett. 2011;21:2533–2536. doi: 10.1016/j.bmcl.2011.02.024. [DOI] [PubMed] [Google Scholar]
- Akama T., Virtucio C., Dong C., Kimura R., Zhang Y.-K., Nieman J. A., Sharma R., Lu X., Sales M., Singh R. Bioorg. Med. Chem. Lett. 2013;23:1680–1683. doi: 10.1016/j.bmcl.2013.01.072. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang S., Zhang Y.-K., Liu Y., Ding C. Z., Zhou Y., Plattner J. J., Baker S. J., Bu W., Liu L. Bioorg. Med. Chem. Lett. 2011;21:2048–2054. doi: 10.1016/j.bmcl.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang Y.-K., Liu Y., Zhang S., Ding C. Z., Zhou Y., Plattner J. J., Baker S. J., Liu L., Bu W. Bioorg. Med. Chem. Lett. 2010;20:7493–7497. doi: 10.1016/j.bmcl.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Printsevskaya S. S., Reznikova M. I., Korolev A. M., Lapa G. B., Olsufyeva E. N., Preobrazhenskaya M. N., Plattner J. J., Zhang Y. K. Future Med. Chem. 2013;5:641–652. doi: 10.4155/fmc.13.16. [DOI] [PubMed] [Google Scholar]
- Akama T., Easom E., Freund Y., Plattner J. J., Sligar J., Chen D., Freeman J. and Perales J., Boron-containing small molecules as antiprotozoal agents, PCT Pat., WO2014121124, 2014.
- Burns C. J., Pevear D. C., Trout R. E. L., Jackson R. W., Hamrick J., Zulli A. L., Mesaros E. F. and Boyd S. A., Preparation of oxaborinine compounds useful as Beta-lactamase inhibitors, PCT Pat., WO 2017100537, 2017.
- Hecker S. and Reddy R. K., Boronic acid derivatives and therapeutic uses thereof, PCT Pat., WO2016149393, 2016.
- Bush K. ACS Infect. Dis. 2015;1:509–511. doi: 10.1021/acsinfecdis.5b00100. [DOI] [PubMed] [Google Scholar]
- Hicks J. W., Kyle C. B., Vogels C. M., Wheaton S. L., Baerlocher F. J., Decken A., Westcott S. A. Chem. Biodiversity. 2008;5:2415–2422. doi: 10.1002/cbdv.200890206. [DOI] [PubMed] [Google Scholar]
- Kanichar D., Roppiyakuda L., Kosmowska E., Faust M. A., Tran K. P., Chow F., Buglo E., Groziak M. P., Sarina E. A., Olmstead M. M. Chem. Biodiversity. 2014;11:1381–1397. doi: 10.1002/cbdv.201400007. [DOI] [PubMed] [Google Scholar]
- Rajan R., Budihal V., Renold P. and Stierli D., Preparation of boron containing fungicides and their use in compns. and methods for the control and/or prevention of microbial infection in plants, PCT Pat., WO2014167133, 2014.
- Yang J., Johnson B. J., Letourneau A. A., Vogels C. M., Decken A., Baerlocher F. J., Westcott S. A. Aust. J. Chem. 2015;68:366–372. [Google Scholar]
- Grassberger M. A., Turnowsky F., Hildebrandt J. J. Med. Chem. 1984;27:947–953. doi: 10.1021/jm00374a003. [DOI] [PubMed] [Google Scholar]
- Davis M. C., Franzblau S. G., Martin A. R. Bioorg. Med. Chem. Lett. 1998;8:843–846. doi: 10.1016/s0960-894x(98)00126-7. [DOI] [PubMed] [Google Scholar]
- Lee G. T., Prasad K., Repič O. Tetrahedron Lett. 2002;43:3255–3257. [Google Scholar]
- Hall I. H., Starnes C. O., McPhail A. T., Wisian-neilson P., Das M. K., Harchelroad F., Spielvogel B. F. J. Pharm. Sci. 1980;69:1025–1029. doi: 10.1002/jps.2600690912. [DOI] [PubMed] [Google Scholar]
- Hall I. H., Chen S. Y., Rajendran K. G., Sood A., Spielvogel B. F., Shih J. Environ. Health Perspect. 1994;102:21–30. doi: 10.1289/ehp.94102s721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour A., Smoum R., Takrouri K., Shalom E., Zaks B., Steinberg D., Rubinstein A., Goldberg I., Katzhendler J., Srebnik M. Pure Appl. Chem. 2006;78:1425–1453. [Google Scholar]
- Takrouri K., Srebnik M., Katzhendler Y. and Polacheck I., Use of amine-borane compounds as antimicrobial agents, PCT Pat., WO 2007032004, 2007.
- Spielvogel B. F., Wojnowich L., Das M. K., McPhail A. T., Hargrave K. D. J. Am. Chem. Soc. 1976;98:5702–5703. doi: 10.1021/ja00434a053. [DOI] [PubMed] [Google Scholar]
- Ayudhya T. I., Raymond C. C., Dingra N. N. Dalton Trans. 2017;46:882–889. doi: 10.1039/c6dt03856e. [DOI] [PubMed] [Google Scholar]
- Wisian-Neilson P., Das M. K., Spielvogel B. F. Inorg. Chem. 1978;17:2327–2329. [Google Scholar]
- Sood C. K., Sood A., Spielvogel B. F., Yousef J. A., Burnham B., Hall I. J. Pharm. Sci. 1991;80:1133–1140. doi: 10.1002/jps.2600801209. [DOI] [PubMed] [Google Scholar]
- Burnham B. S. Curr. Med. Chem. 2005;12:1995–2010. doi: 10.2174/0929867054546573. [DOI] [PubMed] [Google Scholar]
- Takrouri K., Oren G., Polacheck I., Sionov E. J. Med. Chem. 2006;49:4879–4885. doi: 10.1021/jm060476e. [DOI] [PubMed] [Google Scholar]
- Scarlett T. C., Durham R. W., Hall I. H., Crosswicks R. J., Berkowitz J. D., Burnham B. S. Met.-Based Drugs. 2002;9:19–32. doi: 10.1155/MBD.2002.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márquez J. M., Martínez-Castro E., Gabrielli S., López Ó., Maya I., Angulo M., Álvarez E., Fernández-Bolaños J. G. Chem. Commun. 2011;47:5617–5619. doi: 10.1039/c1cc11345c. [DOI] [PubMed] [Google Scholar]
- Spielvogel B. F., Ahmed F. U., Silvey G. L., Wisian-Neilson P., McPhail A. T. Inorg. Chem. 1984;23:4322–4324. [Google Scholar]
- Dembitsky V. M., Srebnik M. Tetrahedron. 2003;59:579–593. [Google Scholar]
- Spielvogel B. F., Ahmed F. U., Morse K. W., McPhail A. T. Inorg. Chem. 1984;23:1776–1777. [Google Scholar]
- Miller 3rd M. C., Sood A., Spielvogel B. F., Hall I. H. Anticancer Res. 1997;17:3299–3306. [PubMed] [Google Scholar]
- Miller 3rd M. C., Sood A., Spievogel B. F., Hall I. H. Appl. Organomet. Chem. 1998;12:87–97. [Google Scholar]
- Snyder H., Reedy A. J., Lennarz W. J. J. Am. Chem. Soc. 1958;80:835–838. [Google Scholar]
- Dewar M., Dietz R. Tetrahedron. 1961;15:26–34. [Google Scholar]
- Wu S., Waugh W., Stella V. J. J. Pharm. Sci. 2000;89:758–765. doi: 10.1002/(SICI)1520-6017(200006)89:6<758::AID-JPS7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Takrouri K., Dembistky V. M., Srebnik M. Stud. Inorg. Chem. 2005;22:495. [Google Scholar]