 A novel series of phenithionate analogues or derivatives were designed and synthesized using phenithionate as the lead compound, and their bioactivities were studied.
A novel series of phenithionate analogues or derivatives were designed and synthesized using phenithionate as the lead compound, and their bioactivities were studied.
Abstract
A novel series of phenithionate analogues or derivatives were designed and synthesized using phenithionate as the lead compound, and their bioactivities were studied. Their structures were confirmed by 1H NMR, 13C NMR, HR-ESI-MS, and elemental analysis, respectively. The results of in vitro inhibitory activity measurement proved that compounds 5a, 5c, 5g, 5i, 5m and 5o had a better inhibitory effect on larva and imago schistosoma. Among them, the inhibitory activity of compound 5i for larva schistosoma was IC50 = 5.21 ± 0.04 μg mL–1, and for imago schistosoma it was IC50 = 6.35 ± 0.08 μg mL–1. Moreover, the experimental results of in vivo anti-schistosomiasis activity measurement showed that they had good anti-schistosomiasis activity. Therefore, these compounds had better drugability.
1. Introduction
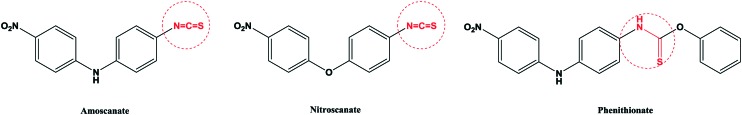
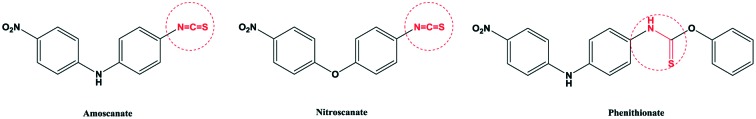
Parasitic diseases spread widely around the world. They are a common type of disease, especially in tropical and subtropical developing countries. Some parasitic diseases can develop into epidemics in a region.1–5 When parasitic diseases are prevalent, they have a serious impact on the society and economy of the region. Schistosomiasis is the most prevalent parasitic disease in the world, which is the most harmful to people's health. According to the World Health Organization (WHO), schistosomiasis is endemic in 76 countries and regions, and there are about 200 million schistosomiasis patients and 500–600 million people who are threatened by the infection. Schistosomiasis is a parasitic disease caused by the parasite Schistosoma mansoni in human veins.6–10 There are three species of the Schistosoma mansoni parasite in the human body, including Schistosoma haematobium (S. haematobium), Schistosoma mansoni (S. mansoni) and Schistosoma japonicum (S. japonicum).11–14 Among the three species, Schistosoma japonicum is the most widely distributed, mainly distributed in China, Japan, Malaysia, Indonesia and other countries.15–17 Not only can Schistosoma japonicum cause acute or chronic enteritis, cirrhosis, severe diarrhea, anemia and emaciation, but also it can cause great harm to livestock. Chemical treatment of Schistosoma japonicum was applied in 1918; antimony potassium tartrate injection was used for the treatment of Schistosoma japonicum, and a good treatment effect was achieved, but it had high toxicity. There were adverse reactions that were life-threatening according to clinical findings if people had a longer course of treatment with this medicine.18–21 The treatment of Schistosoma japonicum with this kind of antimony medicine is very toxic and must be intravenously injected.15–17 It is rarely used in the clinic, and some countries have even banned it. So looking for drugs against Schistosoma japonicum for non-antimony treatment is necessary; the first non-antimony drug nithiocyanamine and its derivative nitroscanate are shown in Fig. 1. In 1975, nithiocyanamine was designed and synthesized, and it is a broad-spectrum anthelmintic. Nithiocyanamine had a significant role in the killing of Schistosoma japonicum. The mechanism was that the body's tricarboxylic acid cycle metabolism was disturbed, causing a lack of energy supply, and this ultimately led to cell death.22–25 In the clinical treatment of various types of Schistosoma japonicum, subsequent clinical manifestations of slow metabolism can cause accumulation of poison; about 4–8% patients showed jaundice, transamination and other side effects, so it was not used clinically. The nithiocyanamine derivative nitroscanate also has an obvious anti-Schistosoma japonicum effect and its toxicity is slightly lower than that of nithiocyamine.26 The study on the structure and activity relationship shows that the isothiocyanate is not only a pharmacophore, but also a toxic group. Therefore, it is also possible to resist the effect of Schistosoma japonicum by transforming the cyano group into amino carbamate groups. The modified derivatives of the diphenyl ester led to greatly reduced toxicity. In this research, we modified the structure by replacing the linked nitrogen atom with an oxygen atom and a sulfur atom according to the principle of bioisosteres. The nitro phenithionate benzene ring was also replaced with trifluoromethyl and trichloromethyl groups. The end of the benzene ring was modified with hydroxyl and acetylamino groups, while its pharmacophore thioamide was retained (Fig. 2 and Scheme 1). The modified compounds had a good anti-Schistosoma japonicum effect, and some of them had better insecticidal activity than phenyl nitrate. According to the structure–activity relationships (SARs) of the compounds, we have retained the basic framework. A new group has been introduced into phenithionate to change the log P and pKa, which affects the activity of the drugs. When –CCl3 and –OH were introduced into the benzene ring, the activity was very high. In addition, the connecting atoms between the benzene rings also have certain effects on the activity of the drugs. When the connecting atoms are N and O, the activity is relatively high, because these atoms can form hydrogen bonds with the receptor macromolecule, which can enhance the activity of the drugs.
Fig. 1. The structures of anti-schistosomiasis drugs.
Fig. 2. Design of phenithionate analogues or derivatives.
Scheme 1. The synthesis of phenithionate analogues or derivatives.
2. Results and discussion
2.1. Design and synthesis of phenithionate analogues or derivatives
Using phenithionate ester as the lead compound, a series of novel anti-schistosomiasis drugs were designed, and their structures were modified according to the principle of biological electron exclusion (Fig. 2). In terms of drug structure, these compounds have the same spatial structure as the phenithionate ester, so the possibility of drug formation is relatively large. During structural modification, the basic framework of the compound was retained, and the amino group with the same anti-schistosomiasis efficacy was retained. At the same time, substitution of the N atom between the benzene rings for the phenyl ester was performed and the substituent on the benzene ring was modified. In the process of structural design, N, O, and S atoms were selected as connecting atoms, the substituent R1 in the benzene ring was changed to –CF3 and –CCl3, and for R2 –OH and –NHCOCH3 were selected. Such structural design, mainly taking into account the substituents on the benzene rings and the connecting atoms, will have an impact on the log P, pKa and the interaction between drugs and receptors, which achieves the purpose of affecting the efficacy. In the synthesis process, a total of two design schemes (Fig. 3) were used. The target product was obtained via two steps, condensation and addition (Fig. 3). Since the steps of the two schemes are different in sequence, the total yield of the target and the difficulty of operation are different. If the first step in the synthesis route is condensation, followed by addition, there will be many side reactions in the condensation process, the intermediates will be difficult to handle, and the yield will be low due to the larger steric hindrance. The specific operation route scheme can be seen in Scheme 1; the addition reaction, with THF as solvent and AlCl3 as the catalyst, proceeded via refluxing for 8 hours to complete the first step reaction; the condensation reaction, with toluene as solvent and anhydrous sodium carbonate as the acid binding agent, proceeded via refluxing for 12 h, achieving the synthesis of the target product with advantages such as simple operation, mild reaction conditions, a high product yield of 80.5–93.4%.
Fig. 3. The synthetic route of the target compounds.
2.2. The biological activities
2.2.1. The inhibitory activity towards anti-schistosomiasis in vitro
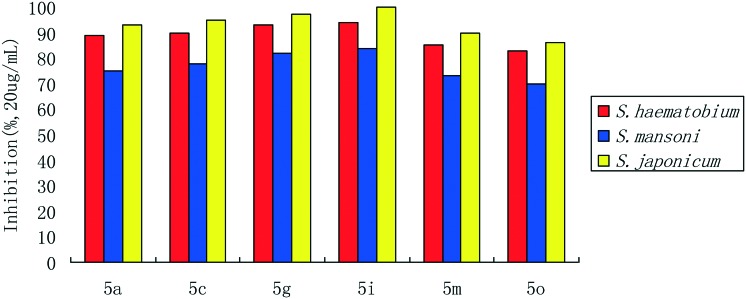
Before studying the inhibitory activity of the target compounds in vitro, we first studied the physical and chemical properties of the phenithionate analogues or derivatives, namely the lipid/water partition coefficient (log P) and dissociation constant (pKa), which might affect their activity (Table 1). As can be seen from Table 1, these compounds had good fat-solubility, and the log P is from 4.32 to 7.43. The higher the partition coefficient of lipid and water, the better the absorption of the drugs, which results in the increase of activity. In addition, from the experimental results, it could also be seen that the compounds could be digested and absorbed by the small intestine, and the pKa was from 8.99 to 9.24. The difference in the pKa of the drugs affected the effective time because the compounds needed to be digested and absorbed by the small intestine, thus the effective time would be slightly delayed. After the completion of log P and pKa studies, we focused on the anti-schistosomiasis activity in vitro. In the experiment, larva and imago schistosoma were used as the inhibitory targets, and the inhibitory activity was measured using the half maximal inhibitory concentration (IC50). Finally, the anti-imago Schistosoma haematobium (S. haematobium), Schistosoma mansoni (S. mansoni) and Schistosoma japonicum (S. japonicum) activities were determined in vitro (Fig. 4). The results showed that compounds 5a, 5c, 5g, 5i, 5m and 5o had high anti-schistosomiasis activity, especially for Schistosoma japonicum (S. japonicum).
Table 1. The inhibitory activity in vitro and some physico-chemical properties.
| Compounds | R1 | R2 | X | log P | pKa | IC50 ± SD(μg mL–1) |
|
| Larva | Imago | ||||||
| 5a | –NO2 | –OH | NH | 4.54 | 9.22 | 6.42 ± 0.12 | 8.05 ± 0.15 |
| 5b | –NO2 | –NHCOCH3 | NH | 4.32 | 9.04 | 8.22 ± 0.48 | 15.22 ± 0.37 |
| 5c | –NO2 | –OH | O | 5.01 | 9.23 | 6.38 ± 0.09 | 8.02 ± 0.12 |
| 5d | –NO2 | –NHCOCH3 | O | 4.81 | 9.04 | 8.24 ± 0.33 | 14.11 ± 0.51 |
| 5e | –NO2 | –OH | S | 5.25 | 9.21 | 15.67 ± 0.58 | 30.54 ± 0.39 |
| 5f | –NO2 | –NHCOCH3 | S | 5.06 | 8.99 | 16.66 ± 0.54 | 31.20 ± 0.38 |
| 5g | –CF3 | –OH | NH | 6.04 | 9.23 | 5.43 ± 0.11 | 6.68 ± 0.09 |
| 5h | –CF3 | –NHCOCH3 | NH | 5.32 | 9.05 | 11.20 ± 1.02 | 20.02 ± 1.28 |
| 5i | –CF3 | –OH | O | 6.11 | 9.23 | 5.21 + 0.04 | 6.35 ± 0.08 |
| 5j | –CF3 | –NHCOCH3 | O | 5.41 | 9.03 | 10.37 ± 0.89 | 17.29 ± 1.10 |
| 5k | –CF3 | –OH | S | 6.66 | 9.22 | 16.56 ± 0.83 | 31.86 ± 0.98 |
| 5l | –CF3 | –NHCOCH3 | S | 5.96 | 9.02 | 19.01 ± 0.82 | 33.69 ± 0.96 |
| 5m | –CCl3 | –OH | NH | 6.79 | 9.23 | 7.35 ± 0.26 | 12.39 ± 0.35 |
| 5n | –CCl3 | –NHCOCH3 | NH | 6.07 | 9.01 | 11.60 ± 1.20 | 20.11 ± 1.34 |
| 5o | –CCl3 | –OH | O | 6.84 | 9.24 | 7.98 ± 0.41 | 13.55 ± 0.46 |
| 5p | –CCl3 | –NHCOCH3 | O | 6.16 | 9.02 | 10.19 ± 1.01 | 18.29 ± 1.21 |
| 5q | –CCl3 | –OH | S | 7.43 | 9.22 | 21.09 ± 1.15 | 37.28 ± 1.19 |
| 5r | –CCl3 | –NHCOCH3 | S | 6.71 | 9.01 | 23.54 ± 1.22 | 40.39 ± 1.31 |
| Blank control (DMSO) | — | — | — | — | — | ||
| Phenithionate | — | — | — | 6.53 ± 0.13 | 8.21 ± 0.21 | ||
| Praziquantel | — | — | — | 6.33 ± 0.11 | 7.98 ± 0.18 | ||
Fig. 4. The inhibition rate.
2.2.2. Anti-schistosomiasis in vivo
In the course of the bioactivity study, compounds 5a, 5c, 5g, 5i, 5m and 5o with high inhibitory activity and low acute toxicity were tested in vivo (Tables 2 and 3). The oral doses were 25 mg kg–1 d–1 and 50 mg kg–1 d–1, and the anti-schistosomiasis activity was observed at different times. Phenithionate and praziquantel were used as positive references. The experimental results of inhibitory activity measurement showed that these two compounds had fast effective time and good anti-schistosomiasis activity. The experimental results of acute toxicity measurement showed that four compounds had low acute toxicity. In Table 3, as the common anti-schistosomiasis drugs, phenithionate has LD50 = 310.5 ± 4.1 mg kg–1 and praziquantel has LD50 > 500 mg kg–1. Compared with the commonly used anti-schistosomiasis drugs, compounds 5a and 5c had smaller LD50 (280.1 ± 3.2 mg kg–1 and 275.3 ± 2.6 mg kg–1), and their acute toxicity was higher. For the other compounds 5g, 5i, 5m and 5o, the LD50 was greater than 500 mg kg–1; these compounds are low toxicity or non-toxic substances.
Table 2. The inhibition activity in vivo.
| Compounds | Dosage of drugs (mg kg–1 d–1) | Time |
||||||||
| 1d | 2d | 3d | 4d | 5d | 6d | 7d | 8d | 9d | ||
| 5a | 25 | +++ | +++ | +++ | ++ | ++ | + | + | – | – |
| 50 | +++ | ++ | ++ | + | + | – | – | – | – | |
| 5c | 25 | +++ | +++ | +++ | ++ | ++ | + | + | – | – |
| 50 | +++ | ++ | ++ | + | + | – | – | – | – | |
| 5g | 25 | +++ | +++ | ++ | ++ | + | – | – | – | – |
| 50 | +++ | ++ | + | – | – | – | – | – | – | |
| 5i | 25 | +++ | +++ | ++ | ++ | + | – | – | – | – |
| 50 | +++ | ++ | + | – | – | – | – | – | – | |
| 5m | 25 | +++ | +++ | +++ | ++ | ++ | + | + | + | |
| 50 | – | |||||||||
| 5o | 25 | +++ | +++ | ++ | ++ | + | + | – | – | – |
| 50 | +++ | +++ | +++ | ++ | ++ | + | + | + | ||
| Blank control (DMSO) | 25 | – | ||||||||
| +++ | +++ | ++ | ++ | + | + | – | – | – | ||
| Phenithionate | 25 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Praziquantel | 25 | +++ | ||||||||
| +++ | +++ | +++ | ++ | ++ | + | – | – | – | ||
| +++ | +++ | +++ | ++ | ++ | + | – | – | – | ||
Table 3. The median lethal dose (LD50).
| Compounds | LD50 ± SD (mg kg–1) |
| 5a | 280.1 ± 3.2 |
| 5c | 275.3 ± 2.6 |
| 5g | >500 |
| 5i | >500 |
| 5m | >500 |
| 5o | >500 |
| Phenithionate | 310.5 ± 4.1 |
| Praziquantel | >500 |
3. Conclusion
In general, we reported the design and synthesis of a class of anti-schistosomiasis compounds, which was simple, efficient, and had high yield. All the newly synthesized compounds had good inhibitory activity and low acute toxicity. Among them, it was shown by anti-schistosomiasis experiments in vitro that compounds 5a, 5c, 5g, 5i, 5m and 5o had good anti-schistosomiasis activity, especially for Schistosoma japonicum (S. japonicum). It was also shown by the experiments in vivo that compounds 5a, 5c, 5g, 5i, 5m and 5o had good anti-schistosomiasis activity and low acute toxicity, especially compounds 5g and 5i.
4. Experimental
4.1. Synthesis of compounds 3a–3f
4-Isothiocyanatoaniline 1a (0.10 mol) and hydroquinone 2a (0.10 mol) were placed in a 250 mL round bottom flask.100 mL of tetrahydrofuran (THF) was added as solvent. Under constant pressure conditions, 0.01 mol of aluminium trichloride was added as a reaction catalyst. After addition, the reaction mixture was refluxed for 8 h. After completion of the reflux, the filtrate was filtered while it was hot. The filtrate was collected, cooled, allowed to stand for 12 h, and then filtered and dried in vacuo to give the crude product compound 3a. The crude product 3a was recrystallized in toluene, filtered and dried in vacuo to give the pure product as a white crystal. This general experimental method was used for the synthesis of compounds 3b–3f.
4.2. General method for all title analogues or derivatives 5a–5r
0.10 mol of compound 3a, 0.06 mol of anhydrous sodium carbonate, and 0.10 mol of compound 4a were placed in a 500 mL round bottom flask. 200 mL toluene was added as solvent. Under magnetic stirring, the reaction mixture was heated and refluxed for 12 h. When the reflux was completed, the filtrate was filtered while it was hot. The filtrate was collected, cooled, allowed to stand for 24 h, and then filtered and dried in vacuo to give the crude product compound 5a. The crude product 5a was recrystallized in toluene, filtered and dried in vacuo to give the pure product as a white crystal. This general experimental method was used for the synthesis of compounds 5b–5r.
O-(4-Hydroxyphenyl) (4-((4-nitrophenyl)amino)phenyl)carbamothioate (5a)
Yield 90.1%; m.p. 163–165 °C; 1H NMR (300 MHz, DMSO) δ: 9.46 (s, 1H, –OH), 8.39 (s, 1H, –NH–), 8.03 (m, 2H, Ph-H), 7.44 (m, 2H, Ph-H), 7.37 (m, 2H, Ph-H), 7.25 (m, 2H, Ph-H), 6.94 (m, 2H, Ph-H), 6.70 (m, 2H, Ph-H); 13C NMR (75 MHz, DMSO) δ: 151.1, 148.5, 148.1, 145.0, 138.4, 137.4, 127.4, 126.6, 124.8, 122.0, 120.6, 117.3; HR-ESI-MS m/z: calcd for C19H15N3O4S {[M + H]+} 381.0785, found 381.4060; anal. calcd for C19H15N3O4S: C, 59.83; H, 3.96; N, 11.02; O, 16.78; S, 8.41; found: C, 59.84; H, 3.96; N, 11.01; O, 16.79; S, 8.40%.
O-(4-Acetamidophenyl) (4-((4-nitrophenyl)amino)phenyl)carbamothioate (5b)
Yield 89.3%; m.p. 171–173 °C; 1H NMR (300 MHz, DMSO) δ: 9.86 (s, 1H, –NH–); 8.39 (s, 1H, –NH–); 8.03 (m, 2H, Ph-H), 7.44 (m, 2H, Ph-H), 7.43 (m, 2H, Ph-H), 7.37 (m, 2H, Ph-H), 7.25 (m, 2H, Ph-H), 6.86 (m, 2H, Ph-H), 2.06 (s, 3H, –CH3); 13C NMR(75 MHz, DMSO) δ: 168.9, 151.1, 148.5, 145.0, 138.4, 137.4, 131.1, 127.4, 126.6, 124.8, 123.0, 122.0, 120.6, 116.1, 24.0; HR-ESI-MS m/z: calcd for C21H18N4O4S {[M + H]+} 422.1048, found 422.4591; anal. calcd for C21H18N4O4S: C, 59.71; H, 4.29; N, 13.26; O, 15.15; S, 7.59; found: C, 59.70; H, 4.30; N, 13.26; O, 15.16; S, 7.58%.
O-(4-Hydroxyphenyl) (4-(4-nitrophenoxy)phenyl)carbamothioate (5c)
Yield 92.6%; m.p. 166–168 °C; 1H NMR(300 MHz, DMSO) δ: 9.46 (s, 1H, –OH), 8.27 (m, 2H, Ph-H), 7.40 (m, 2H, Ph-H), 7.21 (m, 2H, Ph-H), 6.96 (m, 2H, Ph-H), 6.94 (m, 2H, Ph-H), 6.70 (m, 2H, Ph-H); 13C NMR (75 MHz, DMSO) δ: 163.1, 151.1, 150.2, 148.1, 145.0, 141.0, 130.2, 126.2, 124.6, 117.3, 116.2, 115.7; HR-ESI-MS m/z: calcd for C19H14N2O5S {[M + H]+} 382.0622, found 382.3902; anal. calcd for C19H14N2O5S: C, 59.68; H, 3.69; N, 7.33; O, 20.92; S, 8.38; found: C, 59.67; H, 3.69; N, 7.34; O, 20.93; S, 8.37%.
O-(4-Acetamidophenyl) (4-(4-nitrophenoxy)phenyl)carbamothioate (5d)
Yield 89.9%; m.p. 176–178 °C; 1H NMR(300 MHz, DMSO) δ: 9.86 (s, 1H, –NH–); 8.27 (m, 2H, Ph-H), 7.43 (m, 2H, Ph-H), 7.40 (m, 2H, Ph-H), 7.21 (m, 2H, Ph-H), 6.96 (m, 2H, Ph-H), 6.86 (m, 2H, Ph-H), 2.06 (s, 3H, –CH3); 13C NMR(75 MHz, DMSO) δ: 168.9, 163.1, 151.1, 150.2, 145.0, 141.0, 131.1, 130.2, 126.2, 124.6, 123.0, 116.2, 116.1, 115.7, 24.0; HR-ESI-MS m/z: calcd for C21H17N3O5S {[M + H]+} 423.0891, found 423.4431; anal. calcd for C21H17N3O5S: C, 59.57; H, 4.05; N, 9.92; O, 18.89; S, 7.57; found: C, 59.58; H, 4.05; N, 9.91; O, 18.90; S, 7.56%.
O-(4-Hydroxyphenyl) (4-((4-nitrophenyl)thio)phenyl)carbamothioate (5e)
Yield 87.3%; m.p. 170–172 °C; 1H NMR (300 MHz, DMSO) δ: 9.46 (s, 1H, –OH), 7.92 (m, 2H, Ph-H), 7.75 (m, 2H, Ph-H), 7.67 m, 2H, Ph-H), 7.29 (m, 2H, Ph-H), 6.94 (m, 2H, Ph-H), 6.70 (m, 2H, Ph-H); 13C NMR (75 MHz, DMSO) δ: 151.1, 148.1, 146.4, 145.0, 141.8, 135.6, 131.7, 131.0, 128.3, 125.7, 123.4, 117.3; HR-ESI-MS m/z: calcd for C19H14N2O4S2 {[M + H]+} 398.0397, found 398.4511; anal. calcd for C19H14N2O4S2: C, 57.27; H, 3.54; N, 7.03; O, 16.06; S, 16.09; found: C, 57.26; H, 3.54; N, 7.04; O, 16.07; S, 16.08%.
O-(4-Acetamidophenyl) (4-((4-nitrophenyl)thio)phenyl)carbamothioate (5f)
Yield 85.6%; m.p. 184–186 °C; 1H NMR(300 MHz, DMSO) δ: 9.86 (s, 1H, –NH–); 7.92 (m, 2H, Ph-H), 7.75 (m, 2H, Ph-H), 7.67 (m, 2H, Ph-H), 7.43 (m, 2H, Ph-H), 7.29 (m, 2H, Ph-H), 6.86 (m, 2H, Ph-H), 2.06 (s, 3H, –CH3); 13C NMR (75 MHz, DMSO) δ: 168.9, 151.1, 146.4, 145.0, 141.8, 135.6, 131.7, 131.1, 131.0, 128.3, 125.7, 123.4, 123.0, 116.1, 24.0; HR-ESI-MS m/z: calcd for C21H17N3O4S2 {[M + H]+} 439.0660, found 439.5042; anal. calcd for C21H17N3O4S2: C, 57.39; H, 3.90; N, 9.56; O, 14.56; S, 14.59; found: C, 57.37; H, 3.91; N, 9.57; O, 14.57; S, 14.58%.
O-(4-Hydroxyphenyl) (4-((4-(trifluoromethyl)phenyl)amino)phenyl)carbamothioate (5g)
Yield 92.1%; m.p. 152–154 °C; 1H NMR (300 MHz, DMSO) δ: 9.46 (s, 1H, –OH), 7.79 (s, 1H, –NH–) 7.43 (m, 2H, Ph-H), 7.37 (m, 2H, Ph-H), 31 (m, 2H, Ph-H), 7.25 (m, 2H, Ph-H), 6.94 (m, 2H, Ph-H), 6.70 (m, 2H, Ph-H); 13C NMR (75 MHz, DMSO) δ: 151.1, 148.1, 145.7, 145.0, 138.4, 127.4, 126.6, 126.0, 124.1, 123.5, 120.6, 117.3; HR-ESI-MS m/z: calcd for C20H15F3N2O2S {[M + H]+} 404.0802, found 404.4071; anal. calcd for C20H15F3N2O2S: C, 59.40; H, 3.74; F, 14.09; N, 6.93; O, 7.91; S, 7.93; found: C, 59.41; H, 3.74; F, 14.08; N, 6.93; O, 7.92; S, 7.92%.
O-(4-Acetamidophenyl) (4-((4-(trifluoromethyl)phenyl)amino)phenyl)carbamothioate (5h)
Yield 90.4%; m.p. 157–159 °C; 1H NMR(300 MHz, DMSO) δ: 9.86 (s, 1H, –NH–); 7.79 (s, 1H, –NH–); 7.43 (m, 4H, Ph-H), 7.37 (m, 2H, Ph-H), 7.31 (m, 2H, Ph-H), 7.25 (m, 2H, Ph-H), 6.86 (m, 2H, Ph-H), 2.06 (s, 3H, –CH3); 13C NMR(75 MHz, DMSO) δ: 168.9, 151.1, 145.7, 145.0, 138.4, 131.1, 127.4, 126.6, 126.0, 124.1, 123.5, 123.0, 120.6, 116.1, 24.0; HR-ESI-MS m/z: calcd for C22H18F3N3O2S {[M + H]+} 445.1073, found 445.4601; anal. calcd for C22H18F3N3O2S: C, 59.32; H, 4.07; F, 12.79; N, 9.43; O, 7.18; S, 7.20; found: C, 59.33; H, 4.06; F, 12.77; N, 9.43; O, 7.19; S, 7.21%.
O-(4-Hydroxyphenyl) (4-(4-(trifluoromethyl)phenoxy)phenyl)carbamothioate (5i)
Yield 93.4%; m.p. 170–172 °C; 1H NMR (300 MHz, DMSO) δ: 9.46 (s, 1H, –OH), 7.43 (m, 2H, Ph-H), 7.40 (m, 2H, Ph-H), 7.26 (m, 2H, Ph-H), 96 (m, 2H, Ph-H), 6.94 (m, 2H, Ph-H), 6.70 (m, 2H, Ph-H); 13C NMR (75 MHz, DMSO) δ: 160.3, 151.1, 150.2, 148.1, 145.0, 130.2, 126.9, 126.2, 124, 122.0, 117.3, 115.7; HR-ESI-MS m/z: calcd for C20H14F3NO3S {[M + H]+} 405.0645, found 405.3913; anal. calcd for C20H14F3NO3S: C, 59.26; H, 3.48; F, 14.06; N, 3.46; O, 11.84; S, 7.91; found: C, 59.27; H, 3.49; F, 14.05; N, 3.46; O, 11.83; S, 7.90%.
O-(4-Acetamidophenyl) (4-(4-(trifluoromethyl)phenoxy)phenyl)carbamothioate (5j)
Yield 90.2%; m.p. 172–174 °C; 1H NMR (300 MHz, DMSO) δ: 9.86 (s, 1H, –NH–); 7.43 (m, 4H, Ph-H), 7.40 (m, 2H, Ph-H), 7.26 (m, 2H, Ph-H), 96 (m, 2H, Ph-H), 6.86 (m, 2H, Ph-H), 2.06 (s, 3H, –CH3); 13C NMR (75 MHz, DMSO) δ: 168.9, 160.3, 151.1, 150.2, 145.0, 131.1, 130.2, 126.9, 126.2, 124.1, 123.0, 122.0, 116.1, 115.7, 24.0; HR-ESI-MS m/z: calcd for C22H17F3N2O3S {[M + H]+} 446.0913, found 446.4443; anal. calcd for C22H17F3N2O3S: C, 59.19; H, 3.84; F, 12.77; N, 6.27; O, 10.75; S, 7.18; found: C, 59.18; H, 3.85; F, 12.77; N, 6.26; O, 10.75; S, 7.19%.
O-(4-Hydroxyphenyl) (4-((4-(trifluoromethyl)phenyl)thio)phenyl)carbamothioate (5k)
Yield 90.2%; m.p. 173–175 °C; 1H NMR (300 MHz, DMSO) δ: 9.46 (s, 1H, –OH), 7.75 (m, 2H, Ph-H), 7.45 (m, 2H, Ph-H), 7.29 (m, 2H, Ph-H), 7.19 (m, 2H, Ph-H), 6.94 (m, 2H, Ph-H), 6.70 (m, 2H, Ph-H); 13C NMR (75 MHz, DMSO) δ: 151.1, 148.1, 145.0, 139.0, 135.6, 131.7, 131.5, 129.5, 128.3, 125.7, 124.1, 117.3; HR-ESI-MS m/z: calcd for C20H14F3NO2S2 {[M + H]+} 421.0417, found 421.4523; anal. calcd for C20H14F3NO2S2: C, 57.00; H, 3.35; F, 13.52; N, 3.32; O, 7.59; S, 15.21; found: C, 57.01; H, 3.36; F, 13.50; N, 3.32; O, 7.58; S, 15.22%.
O-(4-Acetamidophenyl) (4-((4-(trifluoromethyl)phenyl)thio)phenyl)carbamothioate (5l)
Yield 90.2%; m.p. 173–175 °C; 1H NMR (300 MHz, DMSO) δ: 9.86 (s, 1H, –NH–); 7.75 (m, 2H, Ph-H), 7.45 (m, 2H, Ph-H), 7.43 (m, 2H, Ph-H), 7.29 (m, 2H, Ph-H), 7.19 (m, 2H, Ph-H), 6.86 (m, 2H, Ph-H), 2.06 (s, 3H, –CH3); 13C NMR (75 MHz, DMSO) δ: 168.9, 151.1, 145.0, 139.0, 135.6, 131.7, 131.5, 131.1, 129.5, 128.3, 125.7, 124.1, 123.0, 116.124.0; HR-ESI-MS m/z: calcd for C22H17F3N2O2S2 {[M + H]+} 462.0685, found 462.5053; anal. calcd for C22H17F3N2O2S2: C, 57.13; H, 3.71; F, 12.32; N, 6.06; O, 6.92; S, 13.86; found: C, 57.12; H, 3.72; F, 12.32; N, 6.06; O, 6.91; S, 13.87%.
O-(4-Hydroxyphenyl) (4-((4-(trichloromethyl)phenyl)amino)phenyl)carbamothioate (5m)
Yield 91.3%; m.p. 165–167 °C; 1H NMR (300 MHz, DMSO) δ: 9.46 (s, 1H, –OH); 7.79 (s, 1H, –NH–); 7.78 (m, 2H, Ph-H), 7.37 (m, 2H, Ph-H), 7.31 (m, 2H, Ph-H), 7.25 (m, 2H, Ph-H), 6.94 (m, 2H, Ph-H), 6.70 (m, 2H, Ph-H); 13C NMR (75 MHz, DMSO) δ: 151.1, 148.1, 145.0, 144.2, 138.4, 133.5, 127.5, 127.4, 126.6, 123.1, 120.6, 117.3, 97.8; HR-ESI-MS m/z: calcd for C20H15Cl3N2O2S {[M + H]+} 451.9921, found 453.7623; anal. calcd for C20H15Cl3N2O2S: C, 52.94; H, 3.33; Cl, 23.44; N, 6.17; O, 7.05; S, 7.07; found: C, 52.93; H, 3.32; Cl, 23.43; N, 6.17; O, 7.05; S, 7.08%.
O-(4-Acetamidophenyl) (4-((4-(trichloromethyl)phenyl)amino)phenyl)carbamothioate (5n)
Yield 91.3%; m.p. 165–167 °C; 1H NMR (300 MHz, DMSO) δ: 9.86 (s, 1H, –NH–), 7.79 (s, 1H, –NH–), 7.78 (m, 2H, Ph-H), 7.43 (m, 2H, Ph-H), 7.37 (m, 2H, Ph-H), 7.31 (m, 2H, Ph-H), 7.25 (m, 2H, Ph-H), 6.86 (m, 2H, Ph-H), 2.06 (s, 3H, –CH3); 13C NMR (75 MHz, DMSO) δ: 168.9, 151.1, 145.0, 144.2, 138.4, 133.5, 131.1, 127.5, 127.4, 126.6, 123.1, 123.0, 120.6, 116.1, 97.8, 24.0; HR-ESI-MS m/z: calcd for C22H18Cl3N3O2S {[M + H]+} 493.0184, found 494.8152; anal. calcd for C22H18Cl3N3O2S: C, 53.40; H, 3.67; Cl, 21.49; N, 8.49; O, 6.47; S, 6.48; found: C, 53.41; H, 3.66; Cl, 21.49; N, 8.49; O, 6.48; S, 6.47%.
O-(4-Hydroxyphenyl) (4-((4-(trichloromethyl)phenoxy)phenyl)carbamothioate (5o)
Yield 92.3%; m.p. 177–179 °C; 1H NMR (300 MHz, DMSO) δ: 9.46 (s, 1H, –OH); 7.78 (m, 2H, Ph-H), 7.40 (m, 2H, Ph-H), 7.26 (m, 2H, Ph-H), 96 (m, 2H, Ph-H), 6.94 (m, 2H, Ph-H), 6.70 m, 2H, Ph-H); 13C NMR (75 MHz, DMSO) δ: 158.8, 151.1, 150.2, 148.1, 145.0, 137.1, 130.2, 126.3, 126.2, 121.6, 117.3, 115.7, 97.8; HR-ESI-MS m/z: calcd for C20H14Cl3NO3S {[M + H]+} 452.9762, found 454.7463; anal. calcd for C20H14Cl3NO3S: C, 52.83; H, 3.10; Cl, 23.39; N, 3.08; O, 10.55; S, 7.05; found: C, 52.82; H, 3.10; Cl, 23.38; N, 3.09; O, 10.55; S, 7.06%.
O-(4-Acetamidophenyl) (4-(4-(trichloromethyl)phenoxy)phenyl)carbamothioate (5p)
Yield 92.3%; m.p. 181–183 °C; 1HNMR (300 MHz, DMSO) δ: 9.86 (s, 1H, –NH–), 7.78 (m, 2H, Ph-H), 7.43 (m, 2H, Ph-H), 7.40 (m, 2H, Ph-H), 26 (m, 2H, Ph-H), 6.96 (m, 2H, Ph-H), 6.86 (m, 2H, Ph-H), 2.06 (s, 3H, –CH3); 13C NMR (75 MHz, DMSO) δ: 168.9, 158.8, 151.1, 150.2, 145.0, 137.1, 131.1, 130.2, 126.3, 126.2, 123.0, 121.6, 116.1, 115.7, 97.8, 24.0; HR-ESI-MS m/z: calcd for C22H17Cl3N2O3S {[M + H]+} 494.0026, found 495.7992; anal. calcd for C22H17Cl3N2O3S: C, 53.30; H, 3.46; Cl, 21.45; N, 5.65; O, 9.68; S, 6.47; found: C, 53.31; H, 3.45; Cl, 21.45; N, 5.64; O, 9.68; S, 6.48%.
O-(4-Hydroxyphenyl) (4-((4-(trichloromethyl)phenyl)thio)phenyl)carbamothioate (5q)
Yield 84.1%; m.p. 175–177 °C; 1H NMR (300 MHz, DMSO) δ: 9.46 (s, 1H, –OH); 7.75 (m, 2H, Ph-H), 7.63 (m, 2H, Ph-H), 7.45 (m, 2H, Ph-H), 7.29 (m, 2H, Ph-H), 6.94 (m, 2H, Ph-H), 6.70 m, 2H, Ph-H); 13C NMR (75 MHz, DMSO) δ: 151.1, 148.1, 145.0, 142.5, 137.5, 135.6, 131.7, 131.1, 128.3, 127.2, 125.6, 117.3, 97.8; HR-ESI-MS m/z: calcd for C20H14Cl3NO2S2 {[M + H]+} 468.9533, found 470.8072; anal. calcd for C20H14Cl3NO2S2: C, 51.02; H, 3.00; Cl, 22.59; N, 2.98; O, 6.80; S, 13.62; found: C, 51.03; H, 3.00; Cl, 22.59; N, 2.97; O, 6.81; S, 13.61%.
O-(4-Acetamidophenyl) (4-((4-(trichloromethyl)phenyl)thio)phenyl)carbamothioate (5r)
Yield 80.1%; m.p. 189–191 °C; 1H NMR (300 MHz, DMSO) δ: 9.86 (s, 1H, –NH–), 7.75 (m, 2H, Ph-H), 7.63 (m, 2H, Ph-H), 7.45 (m, 2H, Ph-H), 7.43 (m, 2H, Ph-H), 7.25 (m, 2H, Ph-H), 6.86 (m, 2H, Ph-H), 2.06 (s, 3H, –CH3); 13C NMR (75 MHz, DMSO) δ: 168.9, 151.1, 145.0, 142.5, 137.5, 135.6, 131.7, 131.1, 128.3, 127.2, 125.7, 123.0, 116.1, 97.8, 24.0; HR-ESI-MS m/z: calcd for C22H17Cl3N2O2S2 {[M + H]+} 509.9798, found 511.8605; anal. calcd for C22H17Cl3N2O2S2: C, 51.62; H, 3.35; Cl, 20.78; N, 5.47; O, 6.25; S, 12.53; found: C, 51.63; H, 3.36; Cl, 20.78; N, 5.47; O, 6.24; S, 12.52%.
4.3. Biological activities
4.3.1. Screening of biological activity of compounds in vitro
A total of 100 healthy male Mus musculus mice weighing 20–22 g were selected. Each Mus musculus was divided into two parts and 50 mice underwent abdominal shaving. Each of them was infected via their abdominal skin with 260–280 cercariae of Schistosoma japonicum. The Mus musculus mice were killed after 15 days of infection. Low temperature Hank's balanced salt solution containing heparin (HBSS solution) was injected through the thoracic aorta or mesenteric vein of Schistosoma japonicum, which was washed with HBSS solution for 3–4 times after in vitro culture experiments for larva. In addition, a Mus musculus mouse was infected with 120–140 cercariaes via abdominal skin infection; after 35 days, the Mus musculus was killed with the same HBSS solution, and the worms were collected for imago culture experiments in vitro. 10% calf serum-RPMI 1640 culture medium containing penicillin sodium salt (150 IU mL–1), streptomycin (150 IU mL–1) and amphotericin B (1 μg mL–1) was used to culture Schistosoma japonicum larva and imago worms in vitro. The larva and imago worms were cultured in a 6 well culture plate; 5 male and female worms were placed in each well and incubated in a constant temperature incubator at 37 °C with 5% CO2 for 4 h, then different concentrations of the compounds were added. The culture was continued for 72 h. The viability of the worms for 72 h was observed and recorded. When the survival rate was 50%, it was considered as the half maximal inhibitory concentration (IC50) of the compound. DMSO was used as blank, and phenithionate and praziquantel were used as positive controls.
4.3.2. Biological activity of anti-schistosomiasis compounds in vivo
A total of 50 healthy male Mus musculus mice weighing 20–22 g were injected with 120–140 cercariae of Schistosoma japonicum to infect their abdominal skin. After 35 days of infection, oral doses of 25 mg kg–1 d–1 and 50 mg kg–1 d–1 of the compounds were administered for 5 days. After 5 days of oral administration, the venous blood of Mus musculus was collected and coagulated; the serum was centrifuged for 15 min at 3500 rpm and then collected. The obtained serum was detected by triple dot ELISA, and a round spot color was observed. When the round spot is brown, it indicates strong positive (+ + +); yellow, positive (+ +); slight yellow, weak positive (+); colorless, negative (–). DMSO was used as blank and phenithionate and praziquantel were used as positive controls.
Live subject statement
The reported experiments were in accordance with the standards set forth in the 8th edition of Guide for the Care and Use of Laboratory Animals published by the National Academy of Sciences, The National Academies Press, Washington DC, United States of America. The academic committee of Chongqing Normal University has approved the experiment.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
This project was sponsored by the Innovation Team Project of Universities in Chongqing (No. CXTDX201601018). This project was also sponsored by the Chongqing Scientific and Technological Innovation Special Project of Social Undertakings and People's Livelihood Guarantee (No. cstc2015shmszx80060), the Open Foundation Project of Engineering Research Center of Ministry of Education for Biotechnology of Active Substances (No. 20150408; AS201605; AS201605; AS201610; AS201608; AS201607), Chongqing University Students' Training Project of Innovation and Undertaking (201510637085), Doctoral Program of Chongqing Normal University (No. 12XLB006), and the Outstanding Achievements Transformation Project in Chongqing Normal University (No.15XZH08), China. At the same time, this project was sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry. This work was also supported by the Chongqing Key Research Project of Basic Science & Frontier Technology (No. cstc2017jcyjBX0012), the Scientific and Technological Research Program of Chongqing Municipal Education Commission (Grant No. KJ1400523), the Foundation Project of Chongqing Normal University (No. 14XYY020), the Chongqing General Research Program of Basic Research and Frontier Technology (No. cstc2015jcyjA10054), Chongqing Normal University Postgraduate's Research and Innovation Project (No. YKC17004), the Open Foundation Project of Engineering Research Center for Bioactive Substances (No. AS201614), and Chongqing University Students' Training Project of Innovation & Undertaking (No. 201610637076), China.
References
- Tappel A. L. J. Biol. Chem. 1955;217(2):721–733. [PubMed] [Google Scholar]
- Chou A. C., Chevli R., Fitch C. D. J. Clin. Invest. 1980;66(4):856–858. doi: 10.1172/JCI109925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orjih A. U., Banyal H. S., Chevli R. Science. 1981;214(4521):667–669. doi: 10.1126/science.7027441. [DOI] [PubMed] [Google Scholar]
- Orjih A. U., Chevli R., Fitch C. D. Am. J. Trop. Med. Hyg. 1985;34(2):223–227. doi: 10.4269/ajtmh.1985.34.223. [DOI] [PubMed] [Google Scholar]
- Pagola S., Stephens P. W., Bohle D. S. Nature. 2000;404(6775):307–310. doi: 10.1038/35005132. [DOI] [PubMed] [Google Scholar]
- Oliveira M. F., d'Avila J. C., Torres C. R. Mol. Biochem. Parasitol. 2000;111(1):217–221. doi: 10.1016/s0166-6851(00)00299-1. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Smith A. Biochemistry. 1988;256(3):861–865. doi: 10.1042/bj2560861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira M. F., d'Avila J. C., Tempone A. J. J. Infect. Dis. 2004;190(4):843–852. doi: 10.1086/422759. [DOI] [PubMed] [Google Scholar]
- Egan T. J. Inorg. Biochem. 2002;91(1):19–26. doi: 10.1016/s0162-0134(02)00372-0. [DOI] [PubMed] [Google Scholar]
- Kaschula C. H., Egan T. J., Hunter R. J. Med. Chem. 2002;45(16):3531–3539. doi: 10.1021/jm020858u. [DOI] [PubMed] [Google Scholar]
- Egan T. J. J. Inorg. Biochem. 2006;100(5–6):916–926. doi: 10.1016/j.jinorgbio.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Corrêa Soares J. B., Menezes D., Vannier-Santos M. A. PLoS Neglected Trop. Dis. 2009;3(7):e477. doi: 10.1371/journal.pntd.0000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loup C., Lelie'vre J., Benoit-Vical F. Antimicrob. Agents Chemother. 2007;51(10):3768–3770. doi: 10.1128/AAC.00239-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S. H., Catto B. A. Antimicrob. Agents Chemother. 1989;33(9):1557–1562. doi: 10.1128/aac.33.9.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S. H., Chollet J., Utzinger J. Med. Hyg. 2001;95(1):67–71. doi: 10.1016/s0035-9203(01)90336-0. [DOI] [PubMed] [Google Scholar]
- Xiao S. H., Wu Y. L., Tanner M. Parasitol. Res. 2003;89(6):459–466. doi: 10.1007/s00436-002-0786-1. [DOI] [PubMed] [Google Scholar]
- Xiao S. H., May J. Y., Jiao P. Y. Parasitol. Res. 2009;106(1):237–246. doi: 10.1007/s00436-009-1656-x. [DOI] [PubMed] [Google Scholar]
- Keiser J., Manneck T., Vargas M. J. Antimicrob. Chemother. 2011;66(8):1791–1797. doi: 10.1093/jac/dkr178. [DOI] [PubMed] [Google Scholar]
- Xiao S. H., Xue J., Zhang H. B. Parasitol. Res. 2012;110(3):1239–1248. doi: 10.1007/s00436-011-2621-z. [DOI] [PubMed] [Google Scholar]
- Keiser J., Chollet J., Xiao S. H. PLoS Neglected Trop. Dis. 2009;3(1):e350. doi: 10.1371/journal.pntd.0000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S. H., Mei J. Y., Jiao P. Y. Parasitol. Res. 2009;106(1):131–138. doi: 10.1007/s00436-009-1640-5. [DOI] [PubMed] [Google Scholar]
- Egan T. J., Mavuso W. W., Ncokazi K. K. Biochemistry. 2001;40(1):204–213. doi: 10.1021/bi0013501. [DOI] [PubMed] [Google Scholar]
- Orjih A. U. Exp. Biol. Med. 2001;226(8):746–752. doi: 10.1177/153537020222600806. [DOI] [PubMed] [Google Scholar]
- Ncokazi K. K., Egan T. J. A. Anal. Biochem. 2005;338(2):306–319. doi: 10.1016/j.ab.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Auparakkitanon S., Chapoomram S., Kuaha K. Antimicrob. Agents Chemother. 2006;50(6):2197–2200. doi: 10.1128/AAC.00119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. N., Wang L. Y., Chen M. G. Acta Trop. 2005;96(2–3):97–105. doi: 10.1016/j.actatropica.2005.07.005. [DOI] [PubMed] [Google Scholar]