Abstract
Aims:
Medication nonadherence is a prevalent and costly problem among patients with type 2 diabetes. Applications of theory can inform and improve adherence promotion interventions. We used a new assessment based on the Information-Motivation-Behavioral skills (IMB) model of adherence to assess patient-reported barriers and test the theoretical model.
Methods:
Participants (N = 237) completed a card sorting task to identify barriers to adherence, a survey, and a hemoglobin A1c (HbA1c) test. We identified the most commonly reported adherence barriers and examined associations between patient characteristics and barriers mapped onto each of the IMB constructs. We used structural equation modeling to test the IMB model and determine if barriers as reported on this measure predict patients’ self-reported diabetes medication adherence and, in turn, HbA1c levels.
Results:
The most frequently reported barriers were forgetting doses, thinking brand name medicine works better than generic medicine, not seeing immediate benefit, and feeling burned out with taking diabetes medicine. Younger age and lower health literacy were associated with higher barrier scores for all IMB model constructs. Information and social motivation barriers affected adherence via behavioral skills barriers (indirect effects −0.19, CI [−0.33, −0.09] and −0.24, CI [−0.37, −0.14], respectively). The IMB barrier constructs explained 44% of the variance in diabetes medication adherence which, in turn, was significantly associated with and explained 8% of the variance in HbA1c (both p<.001).
Conclusions:
Results suggest this assessment task can identify patient-specific barriers to diabetes medication adherence. Interventions targeting patient-specific barriers using this assessment could improve adherence and HbA1c.
Keywords: medication adherence, type 2 diabetes, disparities research, behavioral change, glycemic control
1. Introduction
Adult patients with type 2 diabetes often struggle with adhering to their diabetes medication. Recent studies estimate 1 in 3 patients do not take their diabetes medication as prescribed [1, 2], with even higher rates of nonadherence among racial/ethnic minorities [2, 3] and people with low socioeconomic status (SES) [4, 5]. Medication nonadherence is a very costly issue [6], associated with suboptimal glycemic control [7], severe health complications including stroke and heart attack [8], and mortality [9, 10]. There is a critical need for effective interventions to address the costly problem of nonadherence [11]. However, interventions that do not apply theory to guide selection of targets for intervention and examine whether interventions improve those targets do little to enhance knowledge [12]. Without understanding how and why interventions do/do not improve adherence, replication and further development of effective interventions will be slow [13].
Evidence is mixed as to whether theory-based interventions are more effective than atheoretical interventions [14, 15]; in part this is due to insufficient application of a chosen theory and/or to application of theories that are inappropriate for behavior change [14, 16]. Certain criteria should be considered when choosing a theory for health behavior interventions [17, 18]. A suitable theory should demonstrate effectiveness in predicting the behavior [19], focus on modifiable targets [16, 18], provide sufficient description for how the targets explain or mediate the effects on the behavior (i.e., specified pathways) [18, 20], and include measures that appropriately operationalize the targets intended to drive behavior change [17, 18].
The Information-Motivation-Behavioral skills (IMB) model of adherence [21] meets each of these criteria with one exception – measures that operationalize the IMB constructs for diabetes medication adherence are lacking. According to the IMB model, adherence-related information, motivation (personal and social), and behavioral skills all determine adherence behavior [21]. Deficits or barriers in any of these areas reduce the likelihood of consistent performance of the behavior. The IMB model theorizes each component may have a direct effect on adherence, but that adherence-related information and motivation primarily act through adherence behavioral skills to influence behavior [21–23]. The IMB model has explained adherence to self-care behaviors in diverse samples [24, 25], including medication adherence in HIV-infected patients [21, 26, 27]. Further, interventions based on this model have effectively improved medication adherence and clinical outcomes among patients with HIV [28–30]. In diabetes, the IMB model has been shown to predict diabetes medication adherence behavior among adults with type 2 diabetes and low SES [31], but more work is needed for a robust application of this model to address IMB deficits and improve adherence.
Existing measures used to assess the IMB constructs for predicting adherence behavior are either not specific to the behavior, as necessitated by the IMB model, or fail to identify the most salient barriers or deficits. For instance, the Revised Medication Adherence Self-Efficacy Scale (MASES-R[32]), used to assess behavioral skills in a prior validation of the IMB model of diabetes medication adherence [31], asks respondents their degree of confidence in adhering in various circumstances they may not regularly encounter. As an example, the item “How confident are you that you can take your medications when they cost too much money?” may receive a mark of low confidence from a patient with financial strain even when he/she receives medications free or at a reduced cost, rendering medication costs irrelevant to his/her adherence behavior. A measure of the IMB constructs that supports patients in identifying their own adherence-related barriers or deficits could provide a more accurate assessment of the unique challenges they face, and inform more personalized and effective interventions. Also, measures used to assess social motivation often capture levels of social support or positive norms for medication adherence [31], but do not assess social barriers patients may encounter (e.g., stigma associated with needle use for insulin [33]).
Furthermore, identifying patients’ barriers to adherence using an IMB model framework allows for exploration of why patients may have worse adherence and how to intervene both at the group and individual levels. For instance, younger age [1, 4, 34], female gender [1, 4, 34], and low SES [1, 4] are associated with less adherence to diabetes medication, but it remains unclear what barriers or deficits should be targeted to improve adherence among patients with these characteristics.
1.1. Objective
To advance applications of the IMB model to diabetes medication adherence, we sought to evaluate an operationalization of patients’ IMB barriers. We used a new IMB model-based measure to assess barriers to diabetes medication adherence among adults with type 2 diabetes. We examined associations between patient characteristics and barriers reported on each of the IMB constructs, and used structural equation modeling (SEM) to test whether our operationalization of IMB barriers performs as stipulated by the model to predict patients’ diabetes medication adherence behavior and, in turn, their hemoglobin A1c (HbA1c) levels.
2. Materials and methods
We previously conducted a thorough review of published studies describing patient-reported barriers to adherence for oral hypoglycemic medications and/or insulin among adults with type 2 diabetes [35]. Across 30 studies, we identified 68 barriers to taking medications and 7 barriers to taking insulin. We collapsed similar barriers, resulting in 36 adherence barriers (5 of which were insulin-specific) and mapped those barriers to the IMB model’s information, motivation, and behavioral skills constructs. Using these patient-reported barriers identified in the literature, we developed a barrier assessment [35] in which each barrier is written as a first-person statement (e.g., “Seeing no immediate benefit from taking medication” was modified to read “I’m disappointed when my medicine doesn’t improve my diabetes right away”). We enrolled adults with type 2 diabetes in a randomized controlled trial (RCT) [36] to evaluate a text messaging intervention which uses participants’ barriers identified with this assessment to tailor text message content. For the present analysis, we examine baseline data from the larger trial to understand if this assessment performs as stipulated by the IMB model.
2.1. Participants
We recruited patients from clinic sites across Davidson County in Nashville, Tennessee, USA including 13 Federally Qualified Health Centers (FQHCs) and 3 Vanderbilt University Medical Center primary care clinics. Recruitment strategies included flyers, interest cards, referrals from clinic staff, and in-person contact with patients in the clinic waiting room or at clinic and community events. Additionally, we made phone calls to potentially eligible patients identified through the electronic health record (EHR) who did not respond to opt-out letters. We prioritized patients with public health insurance and/or racial and ethnic minorities from the Vanderbilt clinics to obtain a sample similar to those from the FQHCs. Eligible participants had a type 2 diabetes diagnosis, were prescribed at least one daily diabetes medication (oral, insulin, and/or non-insulin injectables), were responsible for taking their diabetes medication (i.e., a caregiver did not administer medication), had a cell phone with text messaging, were at least 18 years of age, and could speak and read in English. Exclusion criteria included a HbA1c <6.8% (51 mmol/mol) as the most recent value within 12 months, auditory limitations, an inability to communicate orally, failing a brief cognitive screening instrument [37], or being unable to receive/read/send text messages after demonstration by a research assistant (RA).
2.2. Procedures
The Vanderbilt University IRB approved all study procedures. RAs met with interested patients to describe the study and verify eligibility in a private room at the patient’s clinic. RAs obtained informed consent before verbally administering survey measures. Unless participants had a HbA1c test within the past three weeks or had one scheduled for the day of study enrollment, we either requested that their provider order a blood-drawn HbA1c test or asked participants to complete a mail-in HbA1c test kit [38, 39] depending on clinic preference.
RAs accessed participants’ EHRs to confirm a type 2 diabetes diagnosis and the type and quantity of prescribed diabetes medications and to collect the results of clinic HbA1c tests. Participants received $10 for completing the survey and another $10 for the HbA1c test.
2.3. Measures
Demographic and clinical characteristics.
We collected self-reported age, gender, race, ethnicity, education (i.e., years in school), income, insurance status, diabetes duration (i.e., years since a diabetes diagnosis), the number of diabetes medications prescribed and insulin status. We later confirmed prescribed diabetes medications and insulin status by EHR review.
Barriers to medication adherence
We used a two-step, interactive procedure to boost reporting of barriers to diabetes medication adherence for purposes of tailoring the intervention to participants’ barriers [35]. First, participants were asked to complete a card-sorting task. Depending on participant preference, RAs either gave participants cards with individual barriers printed on them or read the barriers on each card aloud to participants (Table 1). RAs then asked participants to place each card in one of two piles, labeled “Sometimes” or “Never,” based on whether or not the barrier applied to them (see Supplemental Figure 1). There were 31 barriers plus an additional 5 insulin-specific barriers for participants prescribed insulin. Barriers participants placed in the “Never” pile were scored as 1. Next, RAs presented participants with a board that included ten card pockets with the numbers 1 through 10 printed on them. For the barriers the participant placed in the “Sometimes” pile, participants were asked to rate the degree to which each applies to them from “a little” (pocket #1) to “a lot” (pocket #10) by placing each card in the respective pocket (see Supplemental Figure 2). Participants were told they could place more than one card in each pocket. Barriers placed in the “Sometimes” pile were scored based on the pocket they were placed in – however, if participants placed one of these cards in pocket #1 (“a little”), we gave the barrier a score of 2 to signify that the participant had originally chosen “Sometimes” rather than “Never.” Barrier cards placed in pockets 2–10 were scored with the pocket’s respective number, so every barrer received a score ranging from “never” (1) to “a lot” (10).
Table 1.
Items assessing barriers to medication adherence (N=237)
| Barrier Item | Participants reporting the barrier (%) |
Average score among participants reporting the barrier from a2 = “a little” to 10 = “a lot” (M ± SD) |
|
|---|---|---|---|
| Information | I think brand name medicine works better than generic medicine | 40% | 6.2 ± 3.1 |
| I’m disappointed when my medicine doesn’t improve my diabetes right away | 37% | 6.4 ± 2.8 | |
| I’m not sure what my diabetes medicine is supposed to do | 16% | 5.6 ± 2.5 | |
| I think taking medicine won’t help control my blood sugars or prevent me from having complications | 16% | 5.5 ± 2.6 | |
| I’m not sure why my doctor sometimes changes my dose or type of medicine | 15% | 4.7 ± 2.5 | |
| I think it is OK to skip or stop taking my medicine on my own | 14% | 5.2 ± 2.6 | |
| I think diabetes medicine is not important when I feel well | 9% | 4.9 ± 2.3 | |
| I think medicine isn’t important for managing diabetes | 7% | 6.2 ± 3.0 | |
| Personal Motivation | I feel burned out with having to take diabetes medicines | 36% | 6.3 ± 2.6 |
| I worry that taking diabetes medicines for a long time will be bad for me | 34% | 6.6 ± 2.8 | |
| I’m afraid of experiencing a side effect from my diabetes medicine | 31% |
6.1 ± 2.9 | |
| I believe diabetes medication can be harmful | 27% | 5.2 ± 2.8 | |
| I believe my health will get worse no matter how often I take my medicine | 25% | 6.2 ± 2.6 | |
| bI am afraid of the side effects of taking insulin | 26% | 5.8 ± 2.6 | |
| My diabetes medicine is unpleasant to take | 25% | 5.4 ± 2.6 | |
| I worry that taking diabetes medicines will cause me to gain weight | 21% | 6.3 ± 2.4 | |
| Social Motivation | Juggling other responsibilities makes medicine difficult | 30% | 4.9 ± 2.3 |
| Friends and family nag and annoy me about remembering to take my medicine | 28% | 5.5 ± 3.0 | |
| bI am embarrassed to take my insulin in front of other people | 24% | 6.3 ±3.0 | |
| When my family or friends remind me to take my medicine, it makes me feel like a child | 18% | 5.5 ± 3.0 | |
| I feel embarrassed when taking medicine in front of others | 13% | 5.9 ± 2.8 | |
| bI worry that people judge me because I take insulin | 13% | 5.3 ± 2.3 | |
| I feel others judge me for taking diabetes medicine | 10% | 5.4 ± 2.9 | |
| The people I care about don’t support my efforts to take my diabetes medicines | 9% | 5.0 ± 2.5 | |
| People close to me say taking my medicine isn’t important | 3% | 6.7 ± 3.1 | |
| Family or friends say I shouldn’t take diabetes medicine | 3% | 4.1 ± 2.7 | |
| Behavioral Skills | I forget to take my medicine | 49% | 4.0 ± 2.3 |
| bI have problems with pain when injecting insulin | 32% | 4.9 ±2.7 | |
| I have trouble paying for medicine | 30% | 6.1 ±2.7 | |
| I forget to order refills | 25% | 4.2 ± 2.1 | |
| I have trouble picking up refills | 20% | 4.3 ± 2.7 | |
| I have trouble reading medicine labels | 19% | 6.4 ± 2.7 | |
| bTaking insulin disrupts my daily activities | 20% | 4.4 ± 2.5 | |
| Taking diabetes medicine disrupts my daily activities | 16% | 4.9 ± 2.5 | |
| My daily medicine routine is too complicated to keep track of | 12% | 5.0 ± 2.7 | |
| It is hard for me to ask my doctor about problems with my diabetes medicine | 6% | 4.0 ± 2.7 | |
Barriers not reported (i.e., barrier cards placed in the “Never” pile for the card sorting task) were scored as 1
Only assessed among participants prescribed insulin
We calculated barrier scores in two ways. First, we looked at individual barriers by identifying how many participants reported each barrier (i.e., scored >1) and the average barrier score among participants reporting the barrier (Table 1). Next, we averaged scores across barriers mapped onto each IMB construct among all participants to generate a scale score for each of the IMB model’s constructs. Eight barriers mapped onto information, 8 onto personal motivation, 10 onto social motivation, and 10 onto behavioral skills (Table 1).
Health literacy.
We assessed health literacy with the Brief Health Literacy Screen (BHLS) [40] which is comprised of three items. Two items ask, “How often do you have someone help you read hospital or clinic materials?” and “How often do you have problems learning about your medical condition because of difficulty understanding written information?” Response options range from “never” (1) to “always” (5). The third item asks, “How confident are you filling out medical forms by yourself?” Response options for that item range from “not at all” (1) to “extremely” (5). Responses to the first two items are reverse coded. We summed all three items to create a score ranging from 3 to 15, with higher scores indicating higher health literacy.
Medication adherence.
We assessed diabetes medication adherence using two valid and reliable self-report measures: The Adherence to Refills and Medications Scale for Diabetes (ARMS-D) [41] and the Summary of Diabetes Self-Care Activities medications subscale (SDSCA-MS) [42]. We used these measures as indicators of a latent variable of diabetes mediation adherence, which was used in the prior validation of the IMB model of diabetes medication adherence [31]. The SDSCA-MS is a commonly used and widely accepted measure of diabetes medication adherence [43] which asks about number of days adherent, whereas the ARMS-D is a more sensitive measure that asks about perceived frequency of nonadherence, and is a stronger predictor of HbA1c [41]. We included both measures to reduce measurement error.
The ARMS-D consists of 11 items asking about participants “daily experiences, on average” with taking or refilling their diabetes medication. Response options range from “none of the time” (1) to “all of the time” (4). Items are summed to create a score ranging from 11 to 44. We reverse-scored the ARMS-D items so higher scores indicate greater adherence.
The SDSCA-MS consists of two items asking the participant, “On how many of the last 7 days did you…take this medication?” and “…take the correct number of pills/injections for this medication.” Response options range from 0–7. We asked the two items for each of the participant’s prescribed diabetes medications and then averaged scores across medications so scores could range from 0–7 [41] with higher scores indicating more days adherent.
Glycemic control (HbA1c).
We collected participants’ HbA1c using either their HbA1c test result obtained from their clinic EHR or, if completing a mail-in HbA1c test kit, the result returned from CoreMedica Laboratories (Lees Summit, MO) via a secure web portal.
2.4. Statistical Analyses
We used Stata 14 for descriptive statistics and tests of difference. We used Spearman’s, Mann-Whitney U, and Kruskal-Wallis tests to examine associations with participant characteristics (i.e., age, gender, race/ethnicity, education, income, insurance status, diabetes duration, number of diabetes medications, insulin status, and health literacy) and each of the IMB construct scores.
Next, we used AMOS 24, a SEM program, to test statistical assumptions and estimate models. We estimated a model in which personal and social motivation were independent, based on prior research on the IMB model of diabetes medication adherence [31]. We also created a latent variable for adherence behavior using both observed self-report medication adherence measures (i.e., the ARMS-D and SDSCA-MS). One participant was missing SDSCA-MS values and was excluded from SEM analyses; no other data were missing. We first tested whether our data met the assumption of multivariate non-normality, considering variables with skew<2.0 and kurtosis<7.0 as acceptable [44] and examining the critical ratio (c.r.) of Madia’s coefficient (c.r.<1.96 acceptable). All variables had acceptable skew and kurtosis except for Social Motivation (skew>2.0), and Madia’s coefficient was 37.8 with a c.r.>1.96. Therefore, we tested for multivariate outliers using Mahalanobis distance [45]. We identified and removed five cases with a Mahalnobis distance p<.001, which resulted in acceptable skew and kurtosis and reduced but did not correct multivariate non-normality (Madia’s coefficient=22.4, c.r.=15.2). Violations of multivariate non-normality may bias results [45]. Therefore, we used 5,000 bootstrapped samples to calculate Bollen-Stine x2 and to estimate robust standard errors and bias-corrected p values for parameter estimates and 95% confidence intervals (CI) for indirect effects [46].
Stable parameter estimates are indicated by low bias (i.e., bootstrapped and maximum likelihood estimation [MLE] are similar) and an unbiased and good fitting model is indicated by a Bollin-Stine x2 with p>0.05 calculated using the bootstrapped distribution [46]. Model fit was assessed with multiple additional indices: comparative fit index (CFI) and Tucker Lewis Index (TLI) ≥0.95 indicates good fit, standardized root mean square residual (SRMR) <0.08 indicates acceptable fit while SRMR = 0 indicates perfect fit, and root mean square error of approximation (RMSEA) ≤0.06 with confidence interval 0.00–0.08 indicates good fit [45].
3. Results
As shown in Table 2, the sample (n=237) had a mean age of 54.7±9.8 years, was 53% female and 55% racial/ethnic minority (49% African American). Nearly half (47%) had less than a high school degree or equivalent, 53% had incomes less than $25,000 (USD), and 60% were either uninsured or had public insurance. The mean HbA1c was 8.6±1.9 (70±20.8 mmol/mol) and almost half (46%) were prescribed insulin.
Table 2.
Participant characteristics (N=237).
| Characteristic | M ± SD or n (%) | Range |
|---|---|---|
| Age, years | 54.7 ± 9.8 | 25.4 – 75.9 |
| Gender, Male | 111 (47) | |
| Race/Ethnicity a | ||
| Non-Hispanic White | 105 (45) | |
| African American (non-Hispanic) | 115 (49) | |
| Other race (non-Hispanic) | 6 (3) | |
| Hispanic | 9 (4) | |
| Education, years | 13.7 ± 3.3 | 2.0 – 30.0 |
| Annual Household Income, US$ b | ||
| <10,000 | 49 (23) | |
| 10,000-24,999 | 64 (30) | |
| 25,000-44,999 | 47 (22) | |
| >45,000 | 52 (25) | |
| Insurance status c | ||
| Private | 93 (40) | |
| Public | 71 (30) | |
| None | 70 (30) | |
| Diabetes duration, years | 10.0 ± 7.4 | 0.1 – 42.0 |
| Prescribed diabetes medications, # | 1.9 ± 0.8 | 1.0 – 4.0 |
| Insulin status, taking insulin | 109 (46) | |
| Health Literacy (BHLS) | 12.8 ± 2.6 | 4.0 – 15.0 |
| Medication Adherence (ARMS-D) | 39.5 ± 3.9 | 27.0 – 44.0 |
| Medication Adherence (SDSCA-MS) | 6.1 ± 1.4 | 0.0 – 7.0 |
| HbA1c % (mmol/mol) d | 8.6 ± 1.9 (70 ± 20.8) | 5.5 – 16.1 (37 – 152) |
2 (1%) refused
25 participants did not report income
3 participants did not report insurance
Although we excluded patients with a most recent HbA1c value <6.8%, we did not withdraw any participant if the HbA1c test taken at study enrollment returned a result <6.8%
Only 7% (n=16) of participants reported no adherence barriers. The maximum number of barriers reported was 24 with participants reporting an average of 7.0±5.5 barriers. The most frequently reported barriers (Table 1) were forgetting to take doses (49% of participants said this barrier applied to them), thinking brand name medicine works better than generic medicine (40%), being disappointed when medicine doesn’t improve diabetes right away (37%), and feeling burned out with having to take diabetes medicines (36%). The most commonly reported insulin-specific barrier was having problems with pain when injecting insulin (32% of participants prescribed insulin). Average rating among participants who reported (i.e., scored >1) each barrier are shown in Table 1.
For the following analyses, barrier scores that mapped onto each IMB construct, including barriers scored 1, were averaged for all participants. Personal motivation barriers were rated the highest (2.4 ± 1.7), followed by information (1.9 ± 1.1), behavioral skills (1.9 ± 1.0) and social motivation (1.6 ± 0.9). There were no significant associations between gender, insurance status, or diabetes duration and any of the IMB barrier construct scores. All significant associations between participant characteristics and IMB barrier construct scores along with relevant statistics are reported in Table 3. Younger age and lower health literacy were associated with higher barrier scores for information, personal motivation, social motivation, and behavioral skills. Non-white participants and participants with less education reported higher barrier scores for information. Non-white participants also reported higher scores for personal and social motivation. Participants with lower income and participants prescribed insulin reported higher barrier scores for behavioral skills.
Table 3.
Associations between patient characteristics and IMB construct scale scores a
| Type of Test |
Patient Characteristic | IMB construct |
|||
|---|---|---|---|---|---|
| Information | Personal Motivation | Social Motivation | Behavioral Skills | ||
| Spearman’s rho | Age, years | −0.21** | −0.33*** | −0.28*** | −0.25*** |
| Education, years | −0.16* | ||||
| Prescribed diabetes medications, # |
0.17** | ||||
| Health literacy | −0.28*** | 0.19** | −0.22*** | −0.30*** | |
| Mann-Whitney U or Kruskal-Wallis |
Race/Ethnicity b |
U=5344.0, z=3.07** |
U=5627.0, z=2.51* |
U=5344.0, z=2.00* |
|
| Income c | bχ2(4)=12.75* | ||||
| Insulin status |
U=5935.5, z=−2.00* |
U=5488.5, z=−2.67** |
|||
We examined associations between participant characteristics (i.e., age, gender, race/ethnicity, education, income, insurance status, diabetes duration, number of diabetes medications, and insulin status) and IMB construct scale scores; only significant associations are reported in table.
Analyzed as non-Hispanic White vs. Minority
Post-hoc tests of difference indicated patients with incomes <$45,000 reported higher barrier scores for behavioral skills [median = 1.7, IQR: 1.1, 2.4] than those with incomes ≥$45,000 [median = 1.25, IQR: 1.0, 1.7].
p<.001
p<.01
p<.05
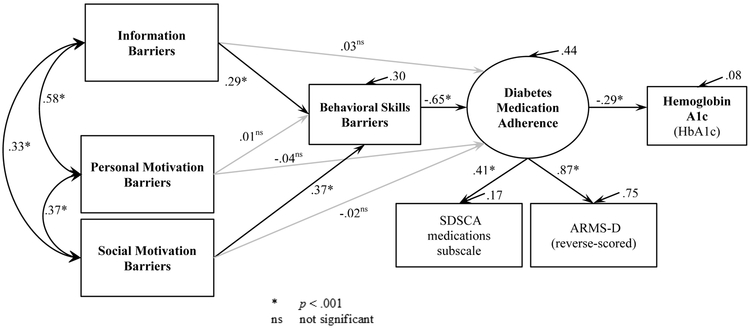
The estimated model of diabetes medication adherence in Figure 1 (n=231 after removing n=1 for missing SDSCA-MS and n=5 multivariate outliers) had good data fit: CFI=0.98; TLI=0.96; RMSEA=0.6; and SRMR=0.04. The RMSEA CI (0.0 – 0.10) had an upper value outside the preferred range, but model fit is best assessed by agreement across indices [45] and all others indicated good model fit. Bias on parameter estimates ranged from −0.006 to 0.006 indicating they were stable and unbiased by multivariate non-normality, and the Bollen-Stine x2 p=0.62 indicated an unbiased model. Figure 1 shows the parameter estimates and the proportion of variance explained in each endogenous variable (R2 or squared multiple correlations, all p<.01).
Figure 1.
Estimated model of diabetes medication adherence using patient-reported barriers from an assessment based on the Information-Motivation-Behavioral skills model.
Paths from information barriers to behavioral skills barriers and from social motivation to behavioral skills barriers were significant and in the predicted direction. Behavioral skills barriers were significantly related to worse diabetes medication adherence, and also mediated the effects of information barriers (indirect effect −0.19, CI [−0.33, −0.09], p=.001) and social motivation barriers (indirect effect −0.24, CI [−0.37, −0.14], p<.001) on adherence. Personal motivation barriers were not related to behavioral skills barriers or medication adherence, directly or indirectly. The IMB barrier constructs explained 44% of the variance in the latent construct of diabetes medication adherence which, in turn, was significantly associated with and explained 8% of the variance in HbA1c levels (both p<.001).
4. Discussion
We used the IMB model as a guiding framework to assess barriers to diabetes medication adherence among a sample of patients with type 2 diabetes, diverse with respect to race, gender, and SES. The most frequently reported barriers to diabetes medication adherence in our sample were forgetting doses (behavioral skills barrier), concern that generic medications are less effective than brand name medications (information barrier), disappointment when medicine doesn’t improve diabetes right away (information barrier), and feeling burned out with taking diabetes medications (personal motivation barrier). Overall, barriers explained 44% of the variance in self-reported diabetes medication adherence which in turn, explained 8% of the variation in HbA1c.
Our results provide support for the IMB model, which posits that information and motivation barriers operate through behavioral skills barriers to influence adherence and disease control, and support this operationalization of the IMB constructs to guide interventions. However, personal motivation did not perform as expected in this study, in that it was not significantly associated with behavioral skills or with medication adherence. The reasons are uncertain but may be related to study participation (i.e., participants demonstrated a certain level of motivation by going to clinic and deciding to join the study).
Reasons for medication nonadherence can vary considerably across patients [1, 2, 48]; therefore, it is optimal to assess a comprehensive list of barriers to ensure any and all barriers are captured for an individual patient. Our approach resulted in nearly all participants (93%) reporting at least one barrier to diabetes medication adherence and provided a second step to rate the importance of that barrier. Our approach allowed for consideration of a large number of potential barriers, without posing an excessive cognitive burden on respondents. However, with 36 items, the measure may be too lengthy for some purposes, particularly in clinical practice. When a briefer measure is needed, we recommend assessing the behavioral skills barriers (8 or 10 items, depending on insulin status) because this scale independently explained the variance in medication adherence. Our findings can also guide education and intervention when no barrier assessment is possible. Several patient characteristics we found to be associated with higher IMB barrier scores have been reported in other studies as predictors of lower diabetes medication adherence (e.g., younger age [1, 4, 34], lower SES [1, 4], lower health literacy [47]). Our results indicate types of barriers that are common overall can be targeted in subgroups of interest when individual-level barrier assessment is not feasible. For example, specifically targeting behavioral skills and self-efficacy among patients with less income and those prescribed insulin may be effective.
There are several limitations to this study. First, medication adherence was assessed using self-report measures only which may have led to more recall and social desirability bias than if we had used objective measures. However, using two self-report measures to create a latent variable in SEM helped account for measurement error associated with either measure. Further, cross-sectional data preclude conclusions about true causal direct effects and mediation. Lastly, although our sample was diverse with respect to gender, race, and SES, recruiting from clinics in a single county in Tennessee may limit the generalizability of the findings to other patient populations. There may be regional differences in barriers to adherence, as many of the barriers assessed are related to social context and the availability of information and resources. The contribution of each of the IMB constructs may be different in different samples as well, which may be another reason why personal motivation was not associated with behavior skills or adherence behavior in the current study.
5. Conclusions
Our findings support application of the IMB model to identify and address patients’ barriers to medication adherence using theory-driven methods. In a recent cohort study using a large EHR-administrative claims database, poor medication adherence was the primary factor explaining smaller HbA1c reductions among patients receiving usual care in the real-word compared to participants in RCTs [49]. In order to realize HbA1c improvements achieved in RCTs, approaches for improving adherence must be easily implemented in real-world clinical practice and account for multiple adherence barriers [50]. In future research, we plan to improve the efficiency of our barriers assessment, both by reducing the number of items and exploring alternative methods of administration (e.g., via tablet, phone, or kiosks in clinic waiting rooms).
Our barriers to diabetes medication adherence assessment can be used by both diabetes researchers and clinicians to identify patients’ unique adherence barriers to support intervention efforts. As it relates to research, intervention content can be tailored to address individuals’ unique barriers. For example, if a patient indicated, per the assessment, that pain with injecting insulin applied to him/her more than other barriers, content could focus on strategies for overcoming this specific barrier (e.g., tips to warm the vial or pen in one’s hands for a few minutes before injecting, and to try exhaling or coughing upon injection to relieve pain). In clinical care, use of this assessment could lead to more personalized care by initiating conversations specific to patients’ barriers; this may include education about medications and/or changes to the medication regimen in response to adherence barriers (e.g., switching from generic to brand name medication or prescribing a medication that does not have a specific feared side effect). Our results also inform interventions at the group level by detailing which domains should be targeted based on participant characteristics. Finally, including the barriers assessment in intervention trials will support determining if reductions in barriers drive improvements in medication adherence.
Supplementary Material
Acknowledgments
The authors thank the REACH team, our partnering clinics (i.e., Faith Family Medical Center, The Clinic at Mercury Courts, ConnectUsHealth, Shade Tree Clinic, United Neighborhood Health Services, Vanderbilt Adult Primary Care), and the participants for their contributions to this research. Dr. Chandra Y. Osborn designed the parent study.
Funding
This research is funded by the National Institutes of Health NIH/NIDDK R01-DK100694. L.S.M. is also supported by a career development award NIH/NIDDK K01-DK106306.
Footnotes
Duality of Interest
K.A.W. is on the Advisory Board of EdLogics, Inc. No other potential conflicts of interest relevant to this article were reported.
References
- [1].Kirkman MS, Rowan-Martin MT, Levin R, Fonseca VA, Schmittdiel JA, Herman WH, et al. Determinants of adherence to diabetes medications: Findings from a large pharmacy claims database. Diabetes Care. 2015;38:604–9. PMID: 10.2337/dc14-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Capoccia K, Odegard PS, Letassy N. Medication adherence with diabetes medication: A systematic review of the literature. Diabetes Educ 2016;42:34–71. PMID: 10.1177/0145721715619038. [DOI] [PubMed] [Google Scholar]
- [3].Mayberry LS, Bergner EM, Chakkalakal RJ, Elasy TA, Osborn CY. Self-care disparities among adults with type 2 diabetes in the USA. Curr Diab Rep 2016;16:113 PMID: 10.1007/s11892-016-0796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rolnick SJ, Pawloski PA, Hedblom BD, Asche SE, Bruzek RJ. Patient characteristics associated with medication adherence. Clin Med Res 2013;11:54–65. PMID: 10.3121/cmr.2013.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Berkowitz SA, Seligman HK, Choudhry NK. Treat or eat: Food insecurity, cost-related medication underuse, and unmet needs. Am J Med 2014;127:303–10 e3. PMID: 10.1016/j.amjmed.2014.01.002. [DOI] [PubMed] [Google Scholar]
- [6].Egede LE, Gebregziabher M, Dismuke CE, Lynch CP, Axon RN, Zhao Y, et al. Medication nonadherence in diabetes: Longitudinal effects on costs and potential cost savings from improvement. Diabetes Care. 2012;35:2533–9. PMID: 10.2337/dc12-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Aikens JE, Piette JD. Longitudinal association between medication adherence and glycaemic control in type 2 diabetes. Diabetic Medicine. 2013;30:338–44. PMID: 10.1111/dme.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Centers for Disease Control and Prevention. Reports to congress: Diabetes report card 2014. 2014. [Google Scholar]
- [9].Currie CJ, Peyrot M, Morgan CL, Poole CD, Jenkins-Jones S, Rubin RR, et al. The impact of treatment noncompliance on mortality in people with type 2 diabetes. Diabetes Care. 2012;35:1279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ho P, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Archives of Internal Medicine. 2006;166:1836–41. 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- [11].Adherence to long term therapies, evidence for action. In: Sabaté E, editor. Geneva: World Health Organization; 2003. [PubMed] [Google Scholar]
- [12].Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: The new medical research council guidance. International journal of nursing studies. 2013;50:587–92. [DOI] [PubMed] [Google Scholar]
- [13].Glasgow RE, Fisher L, Strycker LA, Hessler D, Toobert DJ, King DK, et al. Minimal intervention needed for change: Definition, use, and value for improving health and health research. Translational behavioral medicine. 2014;4:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Prestwich A, Webb TL, Conner M. Using theory to develop and test interventions to promote changes in health behaviour: Evidence, issues, and recommendations. Current Opinion in Psychology. 2015;5:1–5. [Google Scholar]
- [15].Glanz K, Bishop DB. The role of behavioral science theory in development and implementation of public health interventions. Annu Rev Public Health. 2010;31:399–418. PMID: 10.1146/annurev.publhealth.012809.103604. [DOI] [PubMed] [Google Scholar]
- [16].Munro S, Lewin S, Swart T, Volmink J. A review of health behaviour theories: How useful are these for developing interventions to promote long-term medication adherence for tb and hiv/aids? BMC Public Health. 2007;7:104 PMID: 10.1186/1471-2458-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Brawley LR, Culos-Reed SN. Studying adherence to therapeutic regimens: Overview, theories, recommendations. Control Clin Trials. 2000;21:156S–63S. PMID: . [DOI] [PubMed] [Google Scholar]
- [18].Brawley LR. The practicality of using social psychological theories for exercise and health research and intervention. Journal of Applied Sport Psychology. 1993;5:99–115. 10.1080/10413209308411309. [DOI] [Google Scholar]
- [19].Baranowski T, Lin LS, Wetter DW, Resnicow K, Hearn MD. Theory as mediating variables: Why aren’t community interventions working as desired? Annals of Epidemiology. 1997;7:S89–S95. [Google Scholar]
- [20].Rejeski WJ, Brawley LR, McAuley E, Rapp S. An examination of theory and behavior change in randomized clinical trials. Control Clin Trials. 2000;21:164S–70S. PMID: . doi:S019724560000074X [pii]. [DOI] [PubMed] [Google Scholar]
- [21].Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2006;25:462–73. PMID: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- [22].Amico KR, Toro-Alfonso J, Fisher JD. An empirical test of the information, motivation and behavioral skills model of antiretroviral therapy adherence. AIDS care. 2005;17:661–73. PMID: 10.1080/09540120500038058. [DOI] [PubMed] [Google Scholar]
- [23].Starace F, Massa A, Amico KR, Fisher JD. Adherence to antiretroviral therapy: An empirical test of the information-motivation-behavioral skills model. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2006;25:153–62. PMID: . [DOI] [PubMed] [Google Scholar]
- [24].Zarani F, Besharat MA, Sadeghian S, Sarami G. The effectiveness of the information-motivation-behavioral skills model in promoting adherence in cabg patients. J Health Psychol 2010;15:828–37. PMID: . doi:1359105309357092 [pii] 10.1177/1359105309357092. [DOI] [PubMed] [Google Scholar]
- [25].Osborn CY, Amico KR, Cruz N, O’Connell AA, Perez-Escamilla R, Kalichman SC, et al. A brief culturally tailored intervention for puerto ricans with type 2 diabetes. Health Educ Behav 2010;37:849–62. PMID: . doi:1090198110366004 [pii] 10.1177/1090198110366004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Amico KR, Barta W, Konkle-Parker DJ, Fisher JD, Cornman DH, Shuper PA, et al. The information-motivation-behavioral skills model of art adherence in a deep south hiv+ clinic sample. AIDS Behav 2009;13:66–75. PMID: 10.1007/s10461-007-9311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kalichman SC, Rompa D, DiFonzo K, Simpson D, Austin J, Luke W, et al. Hiv treatment adherence in women living with hiv/aids: Research based on the information-motivation-behavioral skills model of health behavior. J Assoc Nurses AIDS Care. 2001;12:58–67. PMID: 10.1016/S1055-3290(06)60217-3. [DOI] [PubMed] [Google Scholar]
- [28].Fisher JD, Amico KR, Fisher WA, Cornman DH, Shuper PA, Trayling C, et al. Computer-based intervention in hiv clinical care setting improves antiretroviral adherence: The lifewindows project. AIDS Behav 2011;15:1635–46. PMID: 10.1007/s10461-011-9926-x. [DOI] [PubMed] [Google Scholar]
- [29].Sabin LL, DeSilva MB, Hamer DH, Xu K, Zhang J, Li T, et al. Using electronic drug monitor feedback to improve adherence to antiretroviral therapy among hiv-positive patients in china. AIDS Behav 2010;14:580–9. PMID: 10.1007/s10461-009-9615-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve hiv medication adherence among hazardous drinkers: A randomized controlled trial. J Acquir Immune Defic Syndr 2007;46:443–50. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mayberry LS, Osborn CY. Empirical validation of the information-motivation-behavioral skills model of diabetes medication adherence: A framework for intervention. Diabetes Care. 2014;37:1246–53. PMID: 10.2337/dc13-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fernandez S, Chaplin W, Schoenthaler AM, Ogedegbe G. Revision and validation of the medication adherence self-efficacy scale (mases) in hypertensive african americans. J Behav Med 2008;31:453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Davies MJ, Gagliardino JJ, Gray LJ, Khunti K, Mohan V, Hughes R. Real-world factors affecting adherence to insulin therapy in patients with type 1 or type 2 diabetes mellitus: A systematic review. Diabetic medicine : a journal of the British Diabetic Association. 2013;30:512–24. PMID: 10.1111/dme.12128. [DOI] [PubMed] [Google Scholar]
- [34].Curkendall SM, Thomas N, Bell KF, Juneau PL, Weiss AJ. Predictors of medication adherence in patients with type 2 diabetes mellitus. Curr Med Res Opin 2013;29:1275–86. PMID: 10.1185/03007995.2013.821056. [DOI] [PubMed] [Google Scholar]
- [35].Nelson LA, Mayberry LS, Wallston K, Kripalani S, Bergner EM, Osborn CY. Development and usability of reach: A tailored theory-based text messaging intervention for disadvantaged adults with type 2 diabetes. JMIR Hum Factors. 2016;3:e23 PMID: 10.2196/humanfactors.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nelson L, Wallston K, Kripalani S, Greevy RJ, Elasy T, Bergner E, et al. Mobile phone support for diabetes self-care among diverse adults: Protocol for a 3-arm randomized controlled trial evaluating long-term outcomes. JMIR Research Protocols 2018;(forthcoming/in press). 10.2196/resprot.9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Medical care. 2002;40:771–81. [DOI] [PubMed] [Google Scholar]
- [38].Lorig K, Ritter PL, Moreland C, Laurent DD. Can a box of mailed materials achieve the triple aims of health care? The mailed chronic disease self-management tool kit study. Health Promot Pract 2015;16:765–74. PMID: 10.1177/1524839915571633. [DOI] [PubMed] [Google Scholar]
- [39].Jones TG, Warber KD, Roberts BD. Analysis of hemoglobin a1c from dried blood spot samples with the tina-quantr ii immunoturbidimetric method. J Diabetes Sci Technol 2010;4:244–9. PMID: 10.1177/193229681000400203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wallston KA, Cawthon C, McNaughton CD, Rothman RL, Osborn CY, Kripalani S. Psychometric properties of the brief health literacy screen in clinical practice. J Gen Intern Med 2013. PMID: 10.1007/s11606-013-2568-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mayberry LS, Gonzalez JS, Wallston KA, Kripalani S, Osborn CY. The arms-d out performs the sdsca, but both are reliable, valid, and predict glycemic control. Diabetes Res Clin Pract 2013;102:96–104. PMID: 10.1016/j.diabres.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: Results from 7 studies and a revised scale. Diabetes Care. 2000;23:943–50. PMID: . [DOI] [PubMed] [Google Scholar]
- [43].Gonzalez JS, Schneider HE, Wexler DJ, Psaros C, Delahanty LM, Cagliero E, et al. The validity of medication adherence self-reports in adults with type 2 diabetes. Diabetes Care. 2012. PMID: . doi:dc12-0410 [pii] 10.2337/dc12-0410.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd ed. Mahwah, NJ: Lawrence Erlbaum; 2013. [Google Scholar]
- [45].Kline RB. Principles and practices of structural equation modeling. 2nd ed. New York, NY: The Guilford Press; 2005. [Google Scholar]
- [46].Chernick MR. Bootstrap methods: A guide for practitioners and researchers, 2nd edition. 2nd ed. New York, NY: Wiley; 2008. [Google Scholar]
- [47].Osborn CY, Cavanaugh K, Wallston KA, Kripalani S, Elasy TA, Rothman RL, et al. Health literacy explains racial disparities in diabetes medication adherence. J HEALTH COMMUN 2011;16 Suppl 3:268–78. PMID: 10.1080/10810730.2011.604388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Odegard PS, Capoccia K. Medication taking and diabetes: A systematic review of the literature. Diabetes Educ 2007;33:1014–29; discussion 30–1. PMID: . doi:33/6/1014 [pii] 10.1177/0145721707308407. [DOI] [PubMed] [Google Scholar]
- [49].Carls GS, Tuttle E, Tan RD, Huynh J, Yee J, Edelman SV, et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of glp-1 ra and dpp-4 therapies in patients with type 2 diabetes. Diabetes Care. 2017;40:1469–78. PMID: 10.2337/dc16-2725. [DOI] [PubMed] [Google Scholar]
- [50].Edelman SV, Polonsky WH. Type 2 diabetes in the real world: The elusive nature of glycemic control. Diabetes Care. 2017;40:1425–32. PMID: 10.2337/dc16-1974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.