Summary
Background
Two randomised, placebo-controlled trials—BENCHMRK-1 and BENCHMRK-2—investigated the efficacy and safety of raltegravir, an HIV-1 integrase strand-transfer inhibitor. We report final results of BENCHMRK-1 and BENCHMRK-2 combined at 3 years (the end of the double-blind phase) and 5 years (the end of the study).
Methods
Integrase-inhibitor-naive patients with HIV resistant to three classes of drug and who were failing antiretroviral therapy were enrolled. Patients were randomly assigned (2:1) to raltegravir 400 mg twice daily or placebo, both with optimised background treatment. Patients and investigators were masked to treatment allocation until week 156, after which all patients were offered open-label raltegravir until week 240. The primary endpoint was previously assessed at 16 weeks. We assessed long-term efficacy with endpoints of the proportion of patients with an HIV viral load of less than 50 copies per mL and less than 400 copies per mL, and mean change in CD4 cell count, at weeks 156 and 240.
Findings
1012 patients were screened for inclusion. 462 were treated with raltegravir and 237 with placebo. At week 156, 51% in the raltegravir group versus 22% in the placebo group (non-completer classed as failure) had viral loads of less than 50 copies per mL, and 54% versus 23% had viral loads of less than 400 copies per mL. Mean CD4 cell count increase (analysed by an observed failure approach) was 164 cells per μL versus 63 cells per μL. After week 156, 251 patients (54%) from the raltegravir group and 47 (20%) from the placebo group entered the open-label raltergravir phase; 221 (47%) versus 44 (19%) completed the entire study. At week 240, viral load was less than 50 copies per mL in 193 (42%) of all patients initially assigned to raltegravir and less than 400 copies per mL in 210 (45%); mean CD4 cell count increased by 183 cells per μL. Virological failure occurred in 166 raltegravir recipients (36%) during the double-blind phase and in 17 of all patients (6%) during the open-label phase. The most common drug-related adverse events at 5 years in both groups were nausea, headache, and diarrhoea, and occurred in similar proportions in each group. Laboratory test results were similar in both treatment groups and showed little change after year 2.
Interpretation
Raltegravir has a favourable long-term efficacy and safety profile in integrase-inhibitor-naive patients with triple-class resistant HIV in whom antiretroviral therapy is failing. Raltegravir is an alternative for treatment-experienced patients, particularly those with few treatment options.
Funding
Merck Sharp & Dohme.
Introduction
Raltegravir is an HIV-1 integrase strand-transfer inhibitor1 that is approved for use in combination treatment of HIV-1 infection in treatment-naive and previously treated patients.2 Two randomised, placebo-controlled, phase 3 trials—BENCHMRK-1 and BENCHMRK-2—investigated the efficacy and safety of raltegravir plus an optimised background regimen in previously treated patients with multidrug-resistant HIV-1 who had not previously been treated with integrase inhibitors. We have previously reported the week 483,4 and week 965 results of the combined BENCHMRK studies. These reports showed that HIV-1 RNA suppression and increase in CD4 cell count were better in patients taking raltegravir than in those taking placebo. After week 156, patients in both treatment groups could take open-label raltegravir for an additional 84 weeks. We present the final results of the BENCHMRK studies at the end of the 3 year, double-blind treatment phase, and at the end of the entire study, which is almost 5 years of treatment for patients originally assigned to the raltegravir group.
Methods
Study design and participants
BENCHMRK-1 (protocol 018; National Clinical Trial number 00293267) and BENCHMRK-2 (protocol 019; National Clinical Trial number 00293254) were multi-centre, double-blind, phase 3, randomised, placebo-controlled studies of the safety, tolerability, and efficacy of raltegravir compared with placebo, each in combination with optimised background treatment for 156 weeks. The protocols were approved by the institutional review board or ethics review committee at each site, and all participants provided written informed consent.
HIV-seropositive patients in whom previous anti-retroviral therapy had failed (ie, plasma HIV viral load >1000 copies per mL while on treatment) and who were resistant to at least one drug in each of the three classes of oral antiretroviral drugs available (nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, and protease inhibitors) were enrolled. The studies were done at 61 sites in Asia, Australia, Europe, and Peru for BENCHMRK-1 and 53 sites in North America and South America for BENCHMRK-2. Each patient’s optimised background treatment regimen was decided at study entry; the site investigator selected the best available combination of drugs on the basis of treatment history, drug-resistance testing, and laboratory data. Further details of selection of patients and optimised background treatment have been described previously.3
Using an interactive voice-response system, patients were randomly assigned (2:1) to receive raltegravir 400 mg twice a day or matching placebo twice a day in addition to their optimised background treatment. Randomisation was stratified by whether enfuvirtide was used in optimised background treatment and by viral resistance to commercially available protease inhibitors. Investigators, study site and sponsor personnel, patients, and laboratory personnel were masked to treatment allocation until all patients had completed or discontinued the study, protocol violations had been identified, and data were declared complete.
Patients who completed the double-blind phase were eligible to receive open-label raltegravir 400 mg twice a day plus optimised background treatment for an additional 84 weeks. Patients who met the definition of virological failure at week 16 or later could receive raltegravir 400 mg twice a day in an open-label phase after virological failure for a total of 240 weeks; background treatment could be optimised again at this point. The decision to enter the open-label post-virological failure phase was made by patients and their primary care physicians, who had access to all HIV viral loads and CD4 cell counts and were provided with the patients’ resistance profiles and treatment group assignment if requested. In all study phases, clinical status was assessed at regularly scheduled visits and on an ad-hoc basis if needed. Protocol-mandated laboratory tests were done in a central laboratory. HIV-1 viral loads were first measured by the standard COBAS Amplicor HIV-1 Monitor assay (version 1.5; Roche Diagnostics, Branchburg, NJ, USA; lower limit of quantification 400 copies per mL) and then by the Ultrasensitive Amplicor HIV-1 Monitor assay (version 1.5; Roche Diagnostics; lower limit of quantification 50 copies per mL) for samples with viral loads of less than 400 copies per mL according to the standard assay. Resistance testing (with PhenoSense GT; Mono gram Biosciences, San Francisco, CA, USA) was done at baseline and at the time of virological failure. All AIDS-defining conditions other than CD4 cell count of less than 200 cells per μL that occurred between randomisation and the last visit were assessed by an independent expert in HIV medicine.
Statistical analysis
The primary endpoint (proportion of patients with viral loads <400 copies per mL) and secondary endpoints (proportion of patients with viral loads <50 copies per mL, CD4 cell count) of the BENCHMRK studies were assessed at weeks 16 and 48 and have been reported previously.3 We assessed the antiretroviral activity of raltegravir compared with placebo at week 156 of the double-blind phase in exploratory analyses done in a modified intention-to-treat population. Patients were included in the treatment group to which they were assigned (provided they received at least one dose of study drug), irrespective of adherence to the entry criteria, treatment actually received, and deviations from protocol.
The proportions of patients with viral loads of less than 50 copies per mL and less than 400 copies per mL were assessed classifying non-completers as treatment failures. Missing data for viral loads were imputed as failures unless the flanking values were both successes, in which case the absent value was left as missing. Treatment groups were compared with a logistic regression model adjusted for baseline viral loads (log10 copies per mL), whether or not enfuvirtide was included in the optimum background treatment of patients who had not previously received enfuvirtide, whether or not darunavir was included in the optimum background treatment of patients who had not previously received darunavir, and whether or not active protease inhibitors were included in optimum background treatment as assessed by phenotypic resistance test. The change from baseline in CD4 cell count was assessed with an observed failure approach; patients who discontinued because of an absence of efficacy were assumed to have subsequently returned to their baseline value, but no other missing values were imputed and data from patients who discontinued for other reasons were censored at discontinuation. The observed failure approach was also used to assess virological and immunological outcomes in subgroups of patients on the basis of demographic and prognostic factors. Additional details of the statistical analyses have been described previously.3,4
The safety analysis was done in the all-patients-as-treated population. Because all patients received the treatment to which they were assigned, this population and the modified intention-to-treat population were the same. All patients who took at least one dose of study medication were included in the analysis of safety and tolerability. Adverse events that occurred while taking study treatment or within 14 days after discontinuation of study treatment were included. Adverse events were reported as drug-related if judged by the investigator to be definitely, probably, or possibly related to raltegravir or placebo (alone or in combination with drugs used in optimum background treatment). Severity of laboratory abnormalities was graded according to the 2004 Department of AIDS (NIH, Bethesda, MD, USA) toxic effects guidelines for adults. Adverse events and laboratory abnormalities are presented by frequency and with crude adjustment for duration of follow-up.
Role of the funding source
The sponsor had a role in study design, management, and analysis in conjunction with external investigators. The authors had access to all study data on request. The report was reviewed by the sponsor.
Results
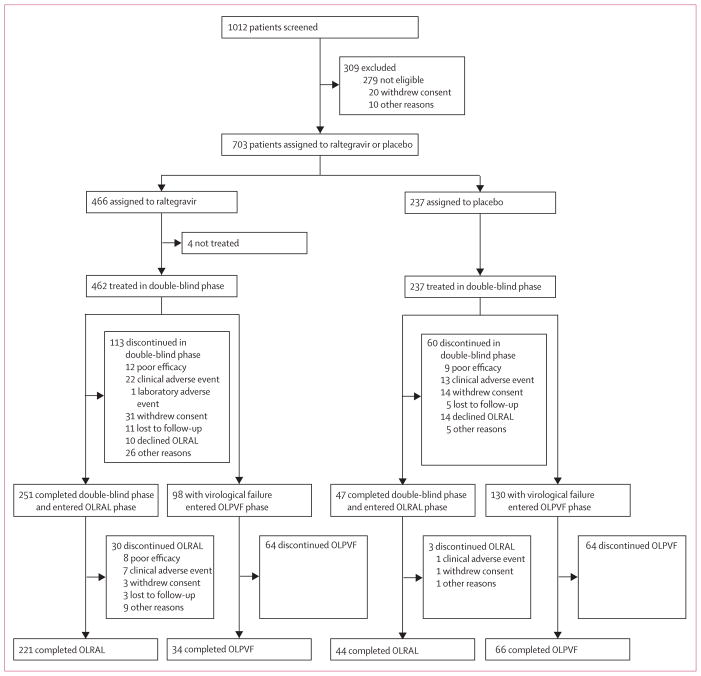
1012 patients were screened for inclusion. 699 patients were treated in the BENCHMRK studies (figure 1) between March 8, 2006, and May 16, 2011. 251 (54%) of 462 patients in the raltegravir group and 47 (20%) of 237 in the placebo group completed the double-blind phase and entered the open-label extension; 221 (48%) in the raltegravir group and 44 (19%) in the placebo group completed the entire study. More patients in the placebo group (130 of 237; 55%) than in the raltegravir group (98 of 462; 21%) discontinued because of virological failure, resulting in a longer duration of follow-up for the raltegravir group (median 208 weeks, IQR 75–242) than for the placebo group (median 38 weeks, IQR 20–158).
Figure 1. Trial profile.
OLRAL=open-label raltegravir. OLPVF=open-label post-virological failure.
Baseline characteristics were generally balanced between treatment groups (table 1). Patients had advanced HIV disease, as shown by a history of AIDS in 92% of patients, a median CD4 count of 120 cells per μL, and 35% with baseline viral loads of more than 100 000 copies per mL (table 2). The most commonly used anti-infective drugs were co-trimoxazole (337 of 699 patients, 48%), azithromycin (220 of 699, 31%), and fluconazole (197 of 699, 28%). The most commonly used antiretroviral drugs in optimum background treatment were darunavir (349 of 699; 50%), emtricitabine with tenofovir (334 of 699; 48%), enfuvirtide (272 of 699; 39%), tenofovir (245 of 699; 35%), and tipranavir (146 of 699; 21%). Many patients had few, if any, remaining treatment options at study entry. Fully active drugs were unavailable to 26% of patients according to genotypic sensitivity scores and to 16% according to phenotypic sensitivity scores.
Table 1.
Baseline characteristics of patients
| Raltegravir group (n=462) | Placebo group (n=237) | |
|---|---|---|
| Sex | ||
|
| ||
| Male | 405 (88%) | 210 (89%) |
| Female | 57 (12%) | 27 (11%) |
|
| ||
| Ethnic origin | ||
|
| ||
| White | 301 (65%) | 173 (73%) |
| Black | 65 (14%) | 26 (11%) |
| Asian | 16 (3%) | 6 (3%) |
| Hispanic | 53 (11%) | 19 (8%) |
| Other | 27 (6%) | 13 (5%) |
|
| ||
| Region | ||
|
| ||
| North America | 192 (42%) | 99 (42%) |
| South America | 61 (13%) | 31 (13%) |
| Asia and Australia | 38 (8%) | 20 (8%) |
| Europe | 171 (37%) | 87 (37%) |
|
| ||
| Age (years) | ||
|
| ||
| Mean (SD) | 45·7 (8·6) | 45·1 (8·1) |
| Median (IQR) | 45·0 (41·0–51·0) | 45·0 (40·0–50·0) |
|
| ||
| CD4 cell count (cells per μL) | ||
|
| ||
| Mean (SD) | 151·3 (141·1) | 158·0 (150·4) |
| Median (IQR) | 119 (31–233) | 123 (32–238) |
|
| ||
| Plasma HIV viral load (log10 copies per mL) | ||
|
| ||
| Mean (SD) | 4·7 (0·8) | 4·6 (0·8) |
| Median (IQR) | 4·8 (4·1–5·2) | 4·7 (4·1–5·1) |
|
| ||
| History of AIDS | ||
|
| ||
| Yes | 426 (92%) | 214 (90%) |
|
| ||
| Previous antiretroviral drug use | ||
|
| ||
| Median years of use (IQR) | 10·1 (7·4–12·1) | 10·2 (7·9–12·4) |
| Median number of drugs (IQR) | 12·5 (9·0–15·0) | 12·0 (9·0–15·0) |
|
| ||
| Viral RNA subtype | ||
|
| ||
| Clade B | 416 (90%) | 219 (92%) |
| Other* | 40 (9%) | 15 (6%) |
| Missing data | 6 (1%) | 3 (1%) |
|
| ||
| Hepatitis co-infection† | ||
|
| ||
| No hepatitis B or C | 385 (83%) | 200 (84%) |
| Hepatitis B only | 36 (8%) | 7 (3%) |
| Hepatitis C only | 37 (8%) | 28 (12%) |
| Hepatitis B and C | 4 (1%) | 2 (1%) |
|
| ||
| Randomisation strata | ||
|
| ||
| Enfuvirtide in optimum background treatment | 175 (38%) | 89 (38%) |
| Resistant to two or more protease inhibitors | 447 (97%) | 226 (95%) |
Data are n (%) unless otherwise stated.
Other viral RNA subtypes were A, A/D, A1, AE, AG, B/G, BF, C, D, D/F, F, F1, and G.
Hepatitis B surface antigen positive or hepatitis C antibody positive.
Table 2.
Baseline prognostic factors
| Raltegravir group (n=462) | Placebo group (n=237) | |
|---|---|---|
| Plasma HIV viral load (copies per mL) | ||
|
| ||
| ≤50 000 | 216 (47%) | 125 (53%) |
| >50 000 | 246 (53%) | 112 (47%) |
| ≤100 000 | 297 (64%) | 159 (67%) |
| >100 000 | 165 (36%) | 78 (33%) |
|
| ||
| CD4 cell count (cells per μL) | ||
|
| ||
| ≤50 | 146 (32%) | 78 (33%) |
| 51–200 | 173 (37%) | 85 (36%) |
| >200 | 142 (31%) | 74 (31%) |
|
| ||
| Number of antiretroviral drugs in optimum background treatment | ||
|
| ||
| Median (IQR) | 4·0 (3–4) | 4·0 (3–4) |
|
| ||
| Enfuvirtide used in optimum background treatment | ||
|
| ||
| No | 287 (62%) | 148 (62%) |
| Yes (enfuvirtide-experienced patients) | 83 (18%) | 41 (17%) |
| Yes (enfuvirtide-naive patients) | 92 (20%) | 48 (20%) |
|
| ||
| Darunavir used in optimum background treatment | ||
|
| ||
| No | 278 (60%) | 138 (58%) |
| Yes (darunavir-experienced patients) | 18 (4%) | 9 (4%) |
| Yes (darunavir-naive patients) | 166 (36%) | 90 (38%) |
|
| ||
| Active protease inhibitors in optimum background treatment* | ||
|
| ||
| No | 165 (36%) | 96 (41%) |
| Yes | 278 (60%) | 137 (58%) |
| Missing data | 19 (4%) | 4 (2%) |
|
| ||
| Phenotypic sensitivity score† | ||
|
| ||
| 0 | 67 (15%) | 43 (18%) |
| 1 | 144 (31%) | 71 (30%) |
| 2 | 142 (31%) | 66 (28%) |
| 3 or more | 85 (18%) | 48 (20%) |
| Missing data | 24 (5%) | 9 (4%) |
|
| ||
| Genotypic sensitivity score† | ||
|
| ||
| 0 | 116 (25%) | 65 (27%) |
| 1 | 177 (38%) | 95 (40%) |
| 2 | 111 (24%) | 49 (21%) |
| 3 or more | 51 (11%) | 23 (10%) |
| Missing data | 7 (2%) | 5 (2%) |
Darunavir use in optimum background treatment in darunavir-naive patients was counted as one active protease inhibitor.
Defined as the total number of antiretroviral drugs in optimal background treatment to which HIV was fully susceptible according to phenotypic or genotypic resistance testing. For the 33 patients missing baseline data, the number of active protease inhibitors in optimum background treatment was assigned a value ≥1 when darunavir was used and the patient was darunavir-naive.
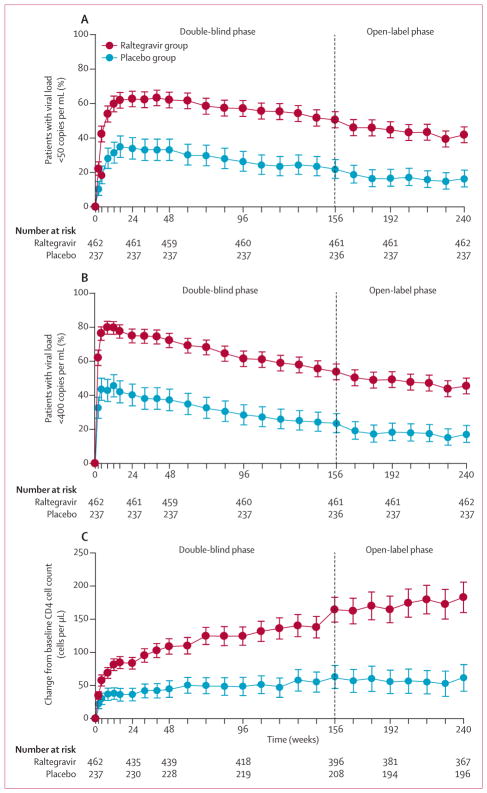
At the end of the double-blind phase, virological and immunological outcomes were consistent between the individual studies (appendix). In the raltegravir groups from both studies combined, 57% of patients at week 96 and 51% at week 156 had viral loads of less than 50 copies per mL (figure 2A). Likewise, 62% of the raltegravir group at week 96 and 54% at week 156 had viral loads of less than 400 copies per mL (figure 2B). Differences between raltegravir and placebo groups were significant at week 156 (relative difference of 29% for viral loads <50 copies per mL, and 31% for viral loads <400 copies per mL; nominal p<0·0001 for both comparisons) and were similar to those at week 96 (31% and 34%, respectively). The mean change in CD4 cell count increased from 125 cells per μL at week 96 to 164 cells per μL at week 156 in the raltegravir group, and from 49 cells per μL to 63 cells per μL in the placebo group (figure 2C).
Figure 2. Virological and immunological outcomes.
Proportion of patients with plasma viral loads less than 50 copies per mL (A) and less than 400 copies per mL (B). The proportion of patients with viral loads below the limit of quantification for the ultrasensitive assay and the standard assay are shown for the non-completer classed as failure analysis. Error bars are 95% CIs. Change from baseline in CD4 cell counts over time by treatment group (C). The mean change in CD4 cell counts per μL from baseline used an observed failure approach, carrying baseline values forward (thereby assigning a value of 0 to change from baseline) for all failures. Error bars are 95% CIs.
After 5 years of treatment (week 240), 42% of patients initially assigned to raltegravir had viral loads of less than 50 copies per mL, 45% had viral loads of less than 400 copies per mL, and the mean rise in CD4 cell count from baseline was 183 cells per μL. Of the patients who entered the open-label raltegravir phase at week 156, viral loads stayed at less than 50 copies per mL at week 240 in 193 (77%) of 251 participants from the original raltegravir group versus 38 (81%) of 47 from the original placebo group; mean CD4 cell count increased by 293 cells per μL from baseline to week 240 in patients from the raltegravir group compared with 267 cells per μL in those from the placebo group. Discontinuation because of no efficacy after week 156 was low, occurring in eight (3%) of 251 patients assigned to raltegravir who entered the open-label phase.
During the double-blind phase, 22 raltegravir recipients (4·8%; 2·1/100 person-years) and 11 placebo recipients (4·6%; 3·4/100 person-years) had new or recurrent AIDS-defining conditions (relative risk [RR] 0·625, 95% CI 0·290–1·426). At the end of the study, the rate of confirmed AIDS-defining conditions was 1·8/100 person-years for patients who received raltegravir in both phases and 2·9/100 person-years for patients who received placebo followed by open-label raltegravir (RR 0·611, 0·335–1·132).
At week 156, efficacy by prognostic factors was generally consistent with the overall analysis (appendix). Raltegravir was more efficacious than placebo over several baseline prognostic factors, including those that predict poor response to antiretroviral therapy, for example high viral load, low CD4 cell count, and low phenotypic and genotypic sensitivity score. In both treatment groups, virological response rates at week 156 were higher among patients with a phenotypic or genotypic sensitivity score of 1 or higher compared with those with a score of 0. However, treatment with raltegravir was beneficial compared with placebo in each subcategory of phenotypic and genotypic sensitivity score, including scores of 0. The smallest difference between groups was for patients with virus susceptible to several drugs in their optimum back ground treatment regimen (phenotypic or genotypic sensitivity score ≥3). We recorded high response rates for patients who received raltegravir with enfuvirtide (79%) or darunavir (72%) for the first time as part of their optimum background treatment. Analyses by viral subtype and patients’ demographic factors—including by geographic region—showed that those in the raltegravir group had better virological and immunological outcomes than those in the placebo group (appendix).
Of the 302 patients continuing in the raltegravir group after week 96, 16 (5%) had virological failure between week 96 and week 156. Overall, 166 (36%) of 462 patients in the raltegravir group had virological failure during the double-blind phase. Raltegravir signature resistance mutations were detected in 86 (60%) of 144 patients for whom integrase genotyping was available (table 3). From year 3 to year 5 (when all patients received open-label raltegravir), virological failure occurred in 14 (6%) of 251 patients from the original raltegravir group and in three (6%) of 47 patients from the original placebo group. Because few additional virological failures occurred in the raltegravir group in the open-label raltegravir phase, raltegravir resistance mutations in the entire study were generally similar to those in the double-blind phase (table 3). None of the patients assigned to placebo who had virological failure in the open-label raltegravir phase developed raltegravir resistance mutations.
Table 3.
HIV integrase mutations in viral isolates from patients with virological failure
| Week 156 (double-blind phase; n=144) | Week 240 (entire study; n=148) | |
|---|---|---|
| Mutations at position 143, 148, or 155 | 86 (60%) | 89 (60%) |
| Position 143 | 18 (13%) | 18 (12%) |
| Tyr143Cys | 12 (8%) | 12 (8%) |
| Tyr143His | 5 (3%) | 5 (3%) |
| Tyr143Arg | 8 (6%) | 8 (5%) |
| Position 148 | 35 (24%) | 36 (24%) |
| Gln148His | 20 (14%) | 21 (14%) |
| Gln148Lys | 5 (3%) | 5 (3%) |
| Gln148Arg | 20 (14%) | 21 (14%) |
| Position 155 (Asn155His) | 56 (39%) | 58 (39%) |
| No mutations at position 143, 148, or 155 | 58 (40%) | 59 (40%) |
| Other raltegravir resistance mutations* | 5 (3%) | 6 (4%) |
Data are n (%). Includes patients assigned to raltegravir who had virological failure and resistance testing done. Virological failure was defined as non-response (did not have >1·0 log10 reduction in HIV RNA or <400 copies per mL by week 16) or viral rebound (virological relapse starting at week 16 or later, which could be either HIV viral load >400 copies per mL [in two consecutive measurements at least 1 week apart] after initial response with HIV viral load <400 copies per mL, or >1·0 log10 increase in HIV RNA above nadir [two consecutive measurements at least 1 week apart]). After week 48, virological failure was defined as confirmed HIV viral load greater than 50 copies per mL (two consecutive measurements at least 1 week apart).
Includes Leu74Met, Glu92Gln, Thr97Ala, Glu138Ala, Glu138Lys, Gly140Ala, Gly140Ser, Gly163Arg, and Ser230Arg.
At the end of the double-blind phase (year 3), the proportions of patients with drug-related adverse events, serious adverse events, and discontinuations because of adverse events were similar in the raltegravir and placebo groups (table 4). Exposure-adjusted rates for these events were lower in the raltegravir group (table 4). The most common drug-related clinical adverse events in both treatment groups were diarrhoea, nausea, and headache. Two patients in the placebo group had fatal adverse events that were thought to be possibly drug-related (AIDS dementia complex and lymphoma); all other deaths were deemed not related to treatment. Most clinical adverse events were mild to moderate in intensity and did not limit treatment. After adjusting for the duration of exposure, rates of almost all grade 3 and grade 4 laboratory test abnormalities were similar in the raltegravir and placebo groups (table 5). In both treatment groups, the frequencies of adverse events changed little from year 2 to year 3 and year 3 to year 5. Of the 251 patients from the original raltegravir group who entered the open-label raltegravir phase, seven (3%) discontinued because of an adverse event after year 3; one of these events (breast cancer) was deemed possibly related to treatment by the site investigator.
Table 4.
Clinical adverse events
| Up to week 96 (double-blind phase) | Up to week 156 (double-blind phase) | Up to week 240 (entire study) | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Raltegravir (n=462; 824 person-years) | Placebo (n=237; 269 person-years) | Raltegravir (n=462; 1061 person-years) | Placebo (n=237; 326 person-years) | Raltegravir and open- label raltegravir (n=462; 1433 person-years) | Placebo and open-label raltegravir (n=237; 397 person-years) | |
| Any adverse event | 429 (93%; 52·1) | 210 (89%; 78·0) | 435 (94%; 41·0) | 214 (90%; 65·6) | 438 (95%; 30·6) | 214 (90%; 53·9) |
|
| ||||||
| Drug-related adverse event* | 270 (58%; 32·8) | 139 (59%; 51·6) | 277 (60%; 26·1) | 145 (61%; 44·5) | 281 (61%; 19·6) | 146 (62%; 36·8) |
|
| ||||||
| Serious adverse event | 117 (25%; 14·2) | 53 (22%; 19·7) | 135 (29%; 12·7) | 54 (23%; 16·6) | 155 (34%; 10·8) | 61 (26%; 15·4) |
|
| ||||||
| Serious drug-related adverse event | 13 (3%; 1·6) | 9 (4%; 3·3) | 16 (4%; 1·5) | 10 (4%; 3·1) | 17 (4%; 1·2) | 10 (4%; 2·5) |
|
| ||||||
| Deaths | 13 (3%; 1·6) | 7 (3%; 2·6) | 17 (4%; 1·6) | 8 (3%; 2·5) | 23 (5%; 1·6) | 9 (4%; 2·3) |
|
| ||||||
| Discontinued because of adverse event | 17 (4%; 2·1) | 12 (5%; 4·5) | 23 (5%; 2·2) | 13 (5%; 4·0) | 29 (6%; 2·0) | 14 (6%; 3·5) |
|
| ||||||
| Most common† drug-related adverse events | ||||||
| Abdominal distension | 10 (2%; 1·2) | 4 (2%; 1·5) | 11 (2%; 1·0) | 4 (2%; 1·2) | 11 (2%; 0·8) | 4 (2%; 1·0) |
| Diarrhoea | 15 (3%; 1·8) | 12 (5%; 4·5) | 17 (4%; 1·6) | 12 (5%; 3·7) | 18 (4%; 1·3) | 12 (5%; 3·0) |
| Nausea | 19 (4%; 2·3) | 11 (5%; 4·1) | 19 (4%; 1·8) | 12 (5%; 3·7) | 19 (4%; 1·3) | 12 (5%; 3·0) |
| Vomiting | 7 (2%; 0·8) | 5 (2%; 1·9) | 6 (1%; 0·6) | 5 (2%; 1·5) | 6 (1%; 0·4) | 5 (2%; 1·3) |
| Fatigue | 15 (3%; 1·8) | 2 (1%; 0·7) | 15 (3%; 1·4) | 3 (1%; 0·9) | 16 (3%; 1·1) | 4 (2%; 1·0) |
| Pyrexia | 4 (1%; 0·5) | 6 (3%; 2·2) | 4 (1%; 0·4) | 6 (3%; 1·8) | 4 (1%; 0·3) | 6 (3%; 1·5) |
| Headache | 22 (5%; 2·7) | 12 (5%; 4·5) | 22 (5%; 2·1) | 12 (5%; 3·7) | 22 (5%; 1·5) | 12 (5%; 3·0) |
Data are n (%; events per 100 person-years at risk).
Decided by the investigator to be possibly, probably, or definitely related to raltegravir or placebo (alone or in combination with optimum background treatment).
Incidence ≥2% in any group, of any intensity.
Table 5.
Grade 3 and 4 laboratory test abnormalities
| Criteria | Week 96 (double-blind phase) | Week 156 (double-blind phase) | Week 240 (entire study) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Raltegravir (n=462; 824 person-years) | Placebo (n=237; 269 person-years) | Raltegravir (n=462; 1061 person-years) | Placebo (n=237; 326 person-years) | Raltegravir and open-label raltegravir (n=462; 1433 person-years) | Placebo and open-label raltegravir (n=237; 397 person-years) | ||
| Absolute neutrophil count (10³ cells per μL) | |||||||
|
| |||||||
| Grade 3 | 0·50–0·749 | 14/460 (3%; 1·7) | 8/237 (3%; 3·0) | 14/460 (3%; 1·3) | 8/237 (3%; 2·5) | 17/460 (4%; 1·2) | 8/237 (3%; 2·0) |
| Grade 4 | <0·50 | 6/460 (1%; 0·7) | 3/237 (1%; 1·1) | 7/460 (2%; 0·7) | 2/237 (1%; 0·6) | 7/460 (2%; 0·5) | 2/237 (1%; 0·5) |
|
| |||||||
| Haemoglobin (g/L) | |||||||
|
| |||||||
| Grade 3 | 65–74 | 4/461 (1%; 0·5) | 2/237 (1%; 0·7) | 4/461 (1%; 0·4) | 2/237 (1%; 0·6) | 4/461 (1%; 0·3) | 2/237 (1%; 0·5) |
| Grade 4 | <65 | 1/461 (<1%; 0·1) | 0/237 (0%; 0·0) | 1/461 (<1%; 0·1) | 0/237 (0%; 0·0) | 1/461 (<1%; 0·1) | 1/237 (<1%; 0·3) |
|
| |||||||
| Platelet count (10³ per μL) | |||||||
|
| |||||||
| Grade 3 | 25<50 | 3/455 (1%; 0·4) | 1/234 (<1%; 0·4) | 3/455 (1%; 0·3) | 2/234 (1%; 0·6) | 5/455 (1%; 0·3) | 2/234 (1%; 0·5) |
| Grade 4 | <25 | 4/455 (1%; 0·5) | 1/234 (<1%; 0·4) | 3/455 (1%; 0·3) | 1/234 (<1%; 0·3) | 3/455 (1%; 0·2) | 1/234 (<1%; 0·3) |
|
| |||||||
| Fasting LDL cholesterol (mmol/L) | |||||||
|
| |||||||
| Grade 3 | ≥4·91 | 26/349 (7%; 3·2) | 11/181 (6%; 4·1) | 23/349 (7%; 2·2) | 11/170 (6%; 3·4) | 27/352 (8%; 1·9) | 11/170 (6%; 2·8) |
|
| |||||||
| Fasting total cholesterol (mmol/L) | |||||||
|
| |||||||
| Grade 3 | >7·77 | 49/446 (11%; 6·0) | 15/228 (7%; 5·6) | 49/446 (11%; 4·6) | 14/225 (6%; 4·3) | 55/446 (12%; 3·8) | 15/225 (7%; 3·8) |
|
| |||||||
| Fasting triglyceride (mmol/L) | |||||||
|
| |||||||
| Grade 3 | 8·49–13·56 | 30/466 (7%; 3·6) | 10/228 (4%; 3·7) | 28/446 (6%; 2·6) | 13/224 (6%; 4·0) | 30/446 (7%; 2·1) | 13/224 (6%; 3·3) |
| Grade 4 | >13·56 | 19/466 (4%; 2·3) | 5/228 (2%; 1·9) | 20/446 (4%; 1·9) | 5/224 (2%; 1·5) | 22/446 (5%; 1·5) | 7/224 (3%; 1·8) |
|
| |||||||
| Fasting glucose (mmol/L) | |||||||
|
| |||||||
| Grade 3 | 13·89–27·75 | 12/451 (3%; 1·5) | 5/229 (2%; 1·9) | 13/451 (3%; 1·2) | 3/227 (1%; 0·9) | 13/451 (3%; 0·9) | 4/227 (2%; 1·0) |
| Grade 4 | >27·75 | 0/451 (0%; 0·0) | 0/229 (0%; 0·0) | 0/451 (0%; 0·0) | 0/237 (0%; 0·0) | 0/451 (0%; 0·0) | 0/227 (0%; 0·0) |
|
| |||||||
| Creatinine | |||||||
|
| |||||||
| Grade 3 | 1·9–3·4×ULN | 7/461 (2%; 0·8) | 2/237 (1%; 0·7) | 8/461 (2%; 0·8) | 3/237 (1%; 0·9) | 7/461 (2%; 0·5) | 3/237 (1%; 0·8) |
| Grade 4 | ≥3·5×ULN | 1/461 (<1%; 0·1) | 1/237 (<1%; 0·4) | 1/461 (<1%; 0·1) | 1/237 (<1%; 0·3) | 3/461 (1%; 0·2) | 1/237 (<1%; 0·3) |
|
| |||||||
| Total bilirubin | |||||||
|
| |||||||
| Grade 3 | 2·6–5·0×ULN | 14/461 (3%; 1·7) | 7/237 (3%; 2·6) | 14/461 (3%; 1·3) | 6/237 (3%; 1·8) | 16/461 (3%; 1·1) | 6/237 (3%; 1·5) |
| Grade 4 | >5·0×ULN | 4/461 (1%; 0·5) | 0/237 (0%; 0·0) | 4/461 (1%; 0·4) | 0/237 (0%; 0·0) | 7/461 (2%; 0·5) | 0/237 (0%; 0·0) |
|
| |||||||
| Aspartate aminotransferase | |||||||
|
| |||||||
| Grade 3 | 5·1–10·0×ULN | 20/461 (4%; 2·4) | 7/236 (3%; 2·6) | 20/461 (4%; 1·9) | 7/236 (3%; 2·1) | 20/461 (4%; 1·4) | 7/236 (3%; 1·8) |
| Grade 4 | >10·0×ULN | 3/461 (1%; 0·4) | 3/236 (1%; 1·1) | 3/461 (1%; 0·3) | 3/236 (1%; 0·9) | 7/461 (2%; 0·5) | 3/236 (1%; 0·8) |
|
| |||||||
| Alanine aminotransferase | |||||||
|
| |||||||
| Grade 3 | 5·1–10·0×ULN | 19/461 (4%; 2·3) | 6/237 (3%; 2·2) | 22/461 (5%; 2·1) | 6/237 (3%; 1·8) | 23/461 (5%; 1·6) | 6/237 (3%; 1·5) |
| Grade 4 | >10·0×ULN | 6/461 (1%; 0·7) | 4/237 (2%; 1·5) | 6/461 (1%; 0·6) | 4/237 (2%; 1·2) | 10/461 (2%; 0·7) | 4/237 (2%; 1·0) |
|
| |||||||
| Alkaline phosphatase | |||||||
|
| |||||||
| Grade 3 | 5·1–10·0×ULN | 2/461 (<1%; 0·2) | 3/237 (1%; 1·1) | 2/461 (<1%; 0·2) | 3/237 (1%; 0·9) | 2/461 (<1%; 0·1) | 3/237 (1%; 0·8) |
| Grade 4 | >10·0×ULN | 3/461 (1%; 0·4) | 1/237 (<1%; 0·4) | 3/461 (1%; 0·3) | 1/237 (<1%; 0·3) | 3/461 (1%; 0·2) | 1/237 (<1%; 0·3) |
|
| |||||||
| Pancreatic amylase* | |||||||
|
| |||||||
| Grade 3 | 2·1–5·0×ULN | 23/461 (5%; 2·8) | 7/237 (3%; 2·6) | 20/461 (4%; 1·9) | 7/237 (3%; 2·1) | 21/461 (5%; 1·5) | 7/237 (3%; 1·8) |
| Grade 4 | >5·0×ULN | 1/461 (<1%; 0·1) | 1/237 (<1%; 0·4) | 1/461 (<1%; 0·1) | 1/237 (<1%; 0·3) | 2/461 (<1%; 0·1) | 1/237 (<1%; 0·3) |
|
| |||||||
| Lipase | |||||||
|
| |||||||
| Grade 3 | 3·1–5·0×ULN | 9/461 (2%; 1·1) | 2/237 (1%; 0·7) | 11/461 (2%; 1·0) | 3/237 (1%; 0·9) | 9/461 (2%; 0·6) | 3/237 (1%; 0·8) |
| Grade 4 | >5·0×ULN | 0/461 (0%; 0·0) | 0/237 (0%; 0·0) | 1/461 (<1%; 0·1) | 0/237 (0%; 0·0) | 3/461 (1%; 0·2) | 0/237 (0%; 0·0) |
|
| |||||||
| Creatine kinase | |||||||
|
| |||||||
| Grade 3 | 10·0–19·9×ULN | 18/461 (4%; 2·2) | 6/237 (3%; 2·2) | 19/461 (4%; 1·8) | 6/237 (3%; 1·8) | 21/461 (5%; 1·5) | 7/237 (3%; 1·8) |
| Grade 4 | ≥20·0×ULN | 14/461 (3%; 1·7) | 2/237 (1%; 0·7) | 14/461 (3%; 1·3) | 3/237 (1%; 0·9) | 14/461 (3%; 1·0) | 4/237 (2%; 1·0) |
Data are n/N (%; events per 100 person-years). A baseline measurement and at least one on-treatment measurement were required. An event was graded as the highest grade reached during treatment. Events were only included if the laboratory measurement was worse than baseline. ULN=upper limit of normal range.
Defined as (number of patients meeting pancreatic amylase criteria)/(number of patients with an amylase test result).
228 patients entered the open-label post-virological failure phase; 98 (21%) from the raltegravir group and 130 (55%) from the placebo group (figure 1). Median duration of treatment in the open-label post-virological failure phase was 161·4 weeks (IQR 64·9–220·7) for patients from the placebo group and 100·6 weeks (IQR 43·1–170·0) for patients from the raltegravir group. The percentage of patients with viral loads of less than 50 copies per mL in this phase was higher in patients initially assigned to placebo (47% at week 48, 49% at week 96, 43% at week 156, and 22% at week 228) compared with patients initially assigned to raltegravir (22%, 23%, 18%, and 7%, respectively). This pattern is not unexpected, because patients from the placebo group received raltegravir as a new active drug during the open-label post-virological failure phase, whereas those who had received raltegravir had already failed a raltegravir-containing regimen. Clinical adverse events were reported by 200 patients (88%) in the open-label post-virological failure phase and were considered drug-related in 85 (37%), serious in 79 (35%), and led to discontinuation in 16 (7%). Laboratory adverse events were reported in 75 patients (33%) in the open-label post-virological failure phase and were thought to be drug-related in 36 (16%), serious in one (<1%), and led to treatment discontinuation in two (1%). The profile of adverse events reported in the open-label post-virological failure phase was consistent with that of patients receiving raltegravir in the double-blind phase (data not shown).
Discussion
Raltegravir had greater efficacy than placebo after 3 years. 51% of the raltegravir group versus 22% of the placebo group had viral loads of less than 50 copies per mL at year 3; the difference between groups (29%) is similar to that at year 2 (31%), which shows that the greater efficacy of raltegravir compared with placebo in previously treated patients is durable. An immunological benefit—measured by increases in CD4 cell count—was also maintained after year 2 in patients receiving raltegravir.
The studies have some limitations. In the subgroup analyses, the virological and immunological effects of raltegravir were consistent across various demographic and prognostic factors, including those that predict poor response to antiretroviral therapy. However, the studies were not designed to make formal inferences in these subgroups, therefore these results should be interpreted with caution. Another limitation is the change from masked treatment allocation in both groups to open-label raltegravir after week 156, which precludes any further masked comparisons between raltegravir and placebo. However, data collected during the open-label phase does provide important information, particularly for patients initially assigned raltegravir, who were continuously treated with raltegravir for the entire study. Patients in this group had sustained responses up to week 240. Of the patients who entered the extension phase, maintenance of virological sup pression below 50 copies per mL was similar to those who continued on raltegravir and those who switched from placebo to raltegravir at week 156, and CD4 cell counts continued to increase in both groups.
In patients originally assigned raltegravir, most virological failures occurred during the first 2 years.4,5 After year 2, virological failure occurred in 5% of patients by year 3, and in 6% of patients between year 3 and year 5. 64% of patients for whom genotyping was available after virological failure had treatment-emergent mutations in HIV integrase. In 60% of patients, viruses isolated at virological failure had a primary mutation at position 155, 148, or 143, the main pathways associated with resistance to raltegravir.6–8 Furthermore, most viruses with a primary integrase mutation also had one or more secondary mutations at other positions in the integrase. These secondary mutations can augment raltegravir resistance and, in some cases, improve viral fitness.6,9,10 The pattern and number of raltegravir resistance mutations affects dolutegravir activity in vivo.11 Treatment with dolutegravir for 10 days decreases plasma HIV viral load in patients with viral variants containing primary integrase resistance mutations at codons 155 or 143 (with or without additional mutations), and to a lesser extent, in those with mutations at codon 148, particularly when additional integrase resistance mutations are present.
Considering data from both approaches, raltegravir had an overall safety profile similar to that of placebo after 3 years of treatment. Because more patients in the placebo group than in the raltegravir group discontinued because of virological failure, the treatment groups were not balanced in terms of time at risk for adverse events. To address this limitation, safety data were reported in two ways: the percentage of patients with events that have not been adjusted for time at risk, and crude exposure-adjusted rates (the number of events per 100 person-years of exposure), which accounted for the different time at risk between the raltegravir and placebo groups.
Randomised clinical trials are often criticised for lack of external validity, which is related to factors including selection of patients, appropriate treatment, clinically relevant outcome measures, and length of follow-up.12,13 Several aspects of the BENCHMRK studies suggest that the results are relevant to the broader population of patients seen in clinical practice. The entry criteria were chosen to minimise exclusions because of concomitant medications and comorbidities—eg, patients with chronic hepatitis or stable cancer were not excluded, very few drugs were prohibited, and exceptions could be made if medically necessary and the benefits outweighed the risks. Background regimens were optimised for each patient and could include any approved antiretroviral therapy, as well as investigational drugs under review for licensure (if certain conditions were met); changes were allowed during the study for toxic effects, intolerance, or administrative reasons (eg, availability of a new formulation), as well as for virological failure. Efficacy measures included HIV-1 viral load, CD4 cell counts, and genotypic resistance testing in cases of confirmed virological failure, in line with current guidelines for monitoring the response to antiretroviral therapy in clinical practice.14 The analysis population was as broad as possible, including all patients who received at least one dose of study drug irrespective of protocol deviations. Finally, the treatment duration of almost 5 years provides long-term efficacy and safety data that are essential for making decisions about treatment of a chronic condition.
Our results support the long-term efficacy and safety of raltegravir (panel) when added to an optimised background antiretroviral therapy regimen in previously treated patients, including those with few or no remaining treatment options.
Supplementary Material
Panel: Research in context.
Systematic review
Two compilations of clinical studies of raltegravir have been published since 2012: a systematic review and meta-analysis of HIV integrase inhibitors, published in January 2013,15 and a review of the published work on raltegravir for management of HIV-1, published in April 2012.16 Both reviews included available clinical studies of raltegravir in previously treated patients with virological failure. Treatment duration in these studies ranged from 24 to 96 weeks.
Interpretation
Our report extends the evidence for raltegravir in previously treated patients to 3 years of masked treatment compared with placebo, and to 5 years of treatment overall. Raltegravir with optimum background treatment has a favourable long-term efficacy and safety profile in integrase-inhibitor-naive patients with triple-class resistant HIV and virological failure on their previous antiretroviral therapy regimen. Our results provide additional support for the conclusion that HIV integrase inhibitors are a beneficial addition for previously treated patients with virological failure,15 including those with limited treatment options.16
Acknowledgments
The opinions expressed in the report are the collective views of the authors and do not necessarily reflect the official position of Merck or the institutions affiliated with the authors. The BENCHMRK studies were sponsored and funded by Merck, which markets raltegravir under the brand name ISENTRESS. We thank all of the patients and their caregivers who participated in the BENCHMRK studies. We also thank the investigators for their contributions. We thank Anthony Rodgers for statistical review and support, and Tu Ly, Deborah Hepler, and Desmond Ryan for clinical trial support.
Footnotes
Contributors
JJE, DAC, RTS, BC, JMG, PNK, JKR, MS, MM, PY, MRL, AL, and JLL enrolled patients, collected and interpreted the data, and reviewed and revised the report. KMS interpreted the data and wrote and revised the report. HW did the statistical analysis and reviewed and revised the report. RJOB did the resistance analyses and reviewed and revised the report. B-YTN and HT designed the study, interpreted the data, and reviewed and revised the report. All authors approved the final version of the report for submission.
Conflicts of interest
JJE has received research support from Merck, GlaxoSmithKline/ViiV Healthcare, and Bristol-Myers Squibb, and consulting fees from Merck, Bristol-Myers Squibb, GlaxoSmithKline/ViiV Healthcare, Gilead Sciences, Janssen Pharmaceuticals. DAC has received research support, speaker fees, and consulting fees from Merck. RTS has received research support, speaker fees, and consulting fees from Merck. BC has been a consultant on advisory boards, participated in speakers’ bureaus, or done clinical trials with Roche, Boehringer-Ingelheim, Abbott, Bristol-Myers Squibb, GlaxoSmithKline, Gilead Sciences, Tibotec, Janssen Pharmaceuticals, Merck, Pfizer, Siemens, Monogram Biosciences, and Panacos. JMG has received research support, speaker fees, and consulting fees from Merck. PNK has been an investigator for Merck, GlaxoSmithKline, Janssen Pharmaceuticals, and Bristol-Myers Squibb; a paid consultant for Bristol-Myers Squibb, ViiV Healthcare, and Janssen; has received speaker fees from Janssen Pharmaceuticals and ViiV Healthcare; and owns stock in Pfizer, GlaxoSmithKline, Gilead Sciences, and Janssen Pharmaceuticals. JKR has received honoraria for lectures or participation in advisory boards from Merck, Roche, GlaxoSmithKline, Bristol-Myers Squibb, Tibotec, Pfizer, Gilead Sciences, Abbott, ViiV Healthcare, and Boehringer Ingelheim. MS has received research support from Merck, Bristol-Myers Squibb, GlaxoSmithKline, Gilead Sciences; and has acted as a speaker and consulted for Abbott, Bristol-Myers Squibb, GlaxoSmithKline, Gilead Sciences, and Merck. MM has received research support from Merck, Gilead Sciences, GlaxoSmithKline, and Tobira; speaker fees from Gilead Sciences and Tibotec; and consulting fees from Merck, Gilead Sciences, Tibotec, and ViiV Healthcare. MRL has received research support from Merck. AL has been an adviser for Merck Sharp & Dohme, GlaxoSmithKline, Bristol-Myers Squibb, Gilead Sciences, Monogram, Abbott, and Tibotec; has received lecture fees from Merck Sharp & Dohme, Bristol-Myers Squibb, Abbott, Pfizer, Roche, and Boehringer-Ingelheim; and has received research support from Merck Sharp & Dohme, GlaxoSmithKline, Bristol-Myers Squibb, Gilead Sciences, Pfizer, Roche, and Schering-Plough. JLL has received research support and speaker’s fees from Merck, and has served on Merck’s antiretroviral scientific advisory board. He has also received research support from Gilead, GlaxoSmith Kline, and Pfizer. KMS, HW, RJOB, BTN, and HT are current or former employees of Merck Sharp & Dohme, a subsidiary of Merck, and own stock or stock options in the company. PY declares that he has no conflicts of interest.
References
- 1.Hazuda DJ, Felock P, Witmer M, et al. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science. 2000;287:646–50. doi: 10.1126/science.287.5453.646. [DOI] [PubMed] [Google Scholar]
- 2.Merck Prescribing information for ISENTRESS (raltegravir) tablets [Google Scholar]
- 3.Steigbigel RT, Cooper DA, Kumar PN, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359:339–54. doi: 10.1056/NEJMoa0708975. [DOI] [PubMed] [Google Scholar]
- 4.Cooper DA, Steigbigel RT, Gatell JM, et al. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N Engl J Med. 2008;359:355–65. doi: 10.1056/NEJMoa0708978. [DOI] [PubMed] [Google Scholar]
- 5.Steigbigel RT, Cooper DA, Teppler H, et al. Long-term efficacy and safety of raltegravir combined with optimized background therapy in treatment-experienced patients with resistant HIV infection: week 96 results of the BENCHMRK 1 and 2 phase III trials. Clin Infect Dis. 2010;50:605–12. doi: 10.1086/650002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fransen S, Gupta S, Danovich R, et al. Loss of raltegravir susceptibility by human immunodeficiency virus type 1 is conferred via multiple nonoverlapping genetic pathways. J Virol. 2009;83:11440–46. doi: 10.1128/JVI.01168-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malet I, Delelis O, Soulie C, et al. Quasispecies variant dynamics during emergence of resistance to raltegravir in HIV-1-infected patients. J Antimicrob Chemother. 2009;63:795–804. doi: 10.1093/jac/dkp014. [DOI] [PubMed] [Google Scholar]
- 8.Delelis O, Thierry S, Subra F, et al. Impact of Y143 HIV-1 integrase mutations on resistance to raltegravir in vitro and in vivo. Antimicrob Agents Chemother. 2010;54:491–501. doi: 10.1128/AAC.01075-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delelis O, Malet I, Na L, et al. The G140S mutation in HIV integrases from raltegravir-resistant patients rescues catalytic defect due to the resistance Q148H mutation. Nucleic Acids Res. 2009;37:1193–201. doi: 10.1093/nar/gkn1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quercia R, Dam E, Perez-Bercoff D, Clavel F. Selective-advantage profile of human immunodeficiency virus type 1 integrase mutants explains in vivo evolution of raltegravir resistance genotypes. J Virol. 2009;83:10245–49. doi: 10.1128/JVI.00894-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eron JJ, Clotet B, Durant J, et al. Safety and efficacy of dolutegravir in treatment-experienced subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING study. J Infect Dis. 2013;207:740–48. doi: 10.1093/infdis/jis750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothwell PM. Factors that can affect the external validity of randomised controlled trials. PLoS Clin Trials. 2006;1:e9. doi: 10.1371/journal.pctr.0010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gartlehnera G, Hansen RA, Nissman D, Lohr KN, Carey TS. A simple and valid tool distinguished efficacy from effectiveness studies. J Clin Epidemiol. 2006;59:1040–48. doi: 10.1016/j.jclinepi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 15.Messiaen P, Wensing AMJ, Fun A, Nijhuis M, Brusselaers N, Vandekerckhove L. Clinical use of HIV integrase inhibitors: a systematic review and meta-analysis. PLoS One. 2013;8:e52562. doi: 10.1371/journal.pone.0052562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rokas KEE, Bookstaver PB, Shamroe CL, et al. Role of raltegravir in HIV-1 management. Ann Pharmacother. 2012;46:578–89. doi: 10.1345/aph.1Q616. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.