Abstract
Burns is a global health problem with significant morbidity and mortality. Ulinastatin, a serine protease inhibitor, has the potential to improve outcomes in burns. A retrospective comparative case note review analysis was performed to assess the impact of ulinastatin on the outcomes in acute burns patients. Acute burns patients, admitted to Masina hospital, Mumbai, from October 2012 to April 2015, who received ulinastatin, were identified from the hospital records. A similarly sized cohort of patients, admitted before the introduction of ulinastatin, was also identified. Relevant data were obtained from archived patient files. The outcomes, mortality and length of hospital stay, were compared across different groups and subgroups. Data of 97 patients, 48 of whom received ulinastatin (ulinastatin group) and 49 of whom did not (control group), were captured. Patients in ulinastatin group had received ulinastatin 100,000 IU, 8 to 12 hourly, during a mean period of 8.8 days, based on clinical judgment, in addition to standard hospital care. The in-hospital mortality was lower (60.4%) in ulinastatin group compared with control group (75.5%). The difference in mortality was statistically significant (50% vs 77.27%; P = .04) in those with 41 to 80% burnt BSA. Mean length of hospital stay, where shorter duration of hospitalization is usually associated with death, was higher in ulinastatin group compared with the control group. Ulinastatin appears to reduce mortality in acute burns patients, especially in those with intermediate extent (40 to 80%) of burnt BSA. It also appears to delay death in those who ultimately succumbed to their burn injuries.
As many as 11 million people are estimated to require medical care for burns every year. It is a global public health problem with an estimated 4.8 fire-related deaths per 100,000 population and 300,000 deaths annually. The highest prevalence rates are observed in Southeast Asia region, Western Pacific region, Eastern Mediterranean region, and the lowest in the Americas.1–3 The fire-related burns mortality rates range from around 1 to 1.3 per 100,000 population in the high-income countries of America and Europe to 5.5 in Africa and > 8 in Southeast Asia (8.3 in India).3 The low- and middle-income countries, which generally suffer from poverty, overcrowding, illiteracy, and lack of infrastructure, account for more than 90% of all mortalities and half of these occur in the Southeast Asia region.2,3 In nonfatal cases, prolonged hospitalization, disfigurement, and disability, in addition to lost wages, emotional trauma, and erosion of family resources, contribute to the adverse impact.2–4 The Southeast Asia region is, again, the most affected, with maximum fire-related burns burden, in terms of days lost.3
Current management of burns involves stabilizing the patient, controlling infection, and assisting in physiological recovery. This is achieved by the maintenance of airway and breathing, fluid resuscitation, maintenance of adequate nutrition, wound care, topical and systemic antibiotic therapy, tetanus prophylaxis, and skin grafting. Despite improvements in the early care of burns patients, systemic inflammatory response syndrome, severe sepsis, and multiple organ dysfunction syndrome remain major causes of morbidity and mortality.5 The inflammatory processes aid wound healing by recruiting leukocytes and macrophages at the site, but excess of these interfere with the healing process. The clinical challenge is the application of therapeutic intervention only when these responses become excessive.6
Multiple serine proteases act as intermediaries in the systemic inflammatory process; these include trypsin, thrombin, chymotrypsin, kallikrein, plasmin, neutrophil elastase, cathepsin, neutrophil protease-3, and coagulation factors IXa, Xa, XIa, and XlIa. These proteases have an important role in regulation of inflammation through inter- and intracellular signaling pathways. To counter the excessive effects of these enzymes, several protease inhibitors are produced by the liver in the presence of inflammation; these include acute phase reactants such as α1-antitrypsin and proteins of the inter-α-inhibitor (IaI) family.7–9
Ulinastatin is a serine protease inhibitor, belonging to the IaI family, which is available since 1985 and is currently approved in Japan, China, South Korea, and India for a variety of indications like sepsis, acute pancreatitis, and acute circulatory failure due to hemorrhagic, bacterial, or traumatic shock. Ulinastatin was approved in India for use in the management of severe sepsis in 2012 and for acute pancreatitis in 2014. Significant decrease in the production of inflammatory mediators, oxygen-free radicals, and protective effect on the functions of multiple organs with reduced fluid requirements, through inhibition of lipid peroxidation, has been reported with the use of ulinastatin in animal models of burns.10–14 Multiple clinical studies have also reported significant decrease in inflammatory mediators, edema, and wound size with protective effect on multiple organ functions as well as decrease in mortality in patients with severe burns and severe burns–induced sepsis.14–16 This retrospective comparative case note review analysis aimed to study the impact of ulinastatin on the management of hospitalized patients with burn injury with special emphasis on mortality and hospital length of stay (LOS).
MATERIALS AND METHODS
Study Centre
This study was performed in the Department of Burns and Plastic Surgery, Eric Kharas Memorial Burns Centre, Masina Hospital, a 280-bedded multispecialty tertiary care hospital, located in the heart of Mumbai, the industrial and financial hub of India, with a population of 12 million people. Approval for the study was obtained from the director of the hospital.
Study Design
This is a retrospective, case note review analysis of patients from two periods—before and after introduction of ulinastatin in the management of hospitalized burns patients at Eric Kharas Memorial Burns Centre, Masina hospital. The patients from these two periods were divided into two groups and compared for outcomes.
Patients
All patients with acute burns admitted to the burns and plastic surgery department who received ulinastatin were identified. All such patients irrespective of age, gender, and extent or severity of burn injury and who either died or completed the in-patient treatment were included in the study. This comprised patients admitted from October 05, 2012, to April 27, 2015, and formed the ulinastatin group. For comparison, a cohort of similar number of patients admitted before the introduction of ulinastatin was identified and included in the control group. This group comprised patients admitted between February 01, 2011, and October 05, 2012.
Study Intervention
Patients in the ulinastatin group had received ulinastatin as intravenous infusion, for as many days as deemed necessary by the caring physician, in addition to the standard care as per hospital protocol. Ulinastatin was stopped based on clinical improvement and normalization of laboratory parameters, indicating improvement in organ functions.
Standard Care
Both groups had received all the standard care according to hospital protocol, and the aspects of patient management have not changed between the two periods. The supportive care included analgesics, intravenous fluids, packed red blood cells, fresh frozen plasma, albumin, and others, as indicated. All patients admitted for acute burns were given fluid resuscitation using Parkland formula with crystalloids, usually Ringer’s lactate solution. The wounds were cleansed with disinfectants and normal saline. Swabs from the burn wound were obtained and sent for microbial culture and antibiotic sensitivity testing. Topical antibiotics and wound dressings were applied in layers. Antitetanus immunoprophylaxis was provided as required. Prophylactic antibiotics (most commonly a third generation cephalosporin) were administered to all patients, while analgesics were given as required. This was followed by specific antibiotics based on culture and sensitivity tests performed on blood, wound, and/or other specimens. Interventions for maintaining organ system functions—assisted ventilation, vasopressor support, and/or dialysis—were instituted as required. Other intensive care and surgical interventions were provided on a case-to-case basis in coordination with the intensive care and anesthetic team.
Data
Basic patient material was identified from the medical records of the hospital. Data were obtained on a predesigned case record form from the archived patients’ files. Data on patient demographics, burn details, major interventions, medications including antibiotics, blood and blood components, and I.V. fluids and outcomes including hospital LOS, intensive care unit (ICU) stay, and in-hospital mortality were extracted from the archived patients’ files. Additionally, vital parameters and available laboratory values—serum chemistry, hematology, electrolyte values on days 0 (day of admission), 3, 6, 9, and so on, and significant laboratory, culture, or imaging findings—were recorded. In the ulinastatin group, details of ulinastatin use, including the start date, duration, and total dose, were also recorded.
Outcomes
Outcomes of interest were in-hospital mortality and LOS in hospital and ICU. These outcomes were compared in the two treatment groups. Additionally, the outcomes were compared after stratifying the treatment groups based on the burnt BSA into following strata—BSA < 40%, BSA = 40 to 80%, and BSA > 80%.
Statistical Analysis
Data were collated in a Microsoft® (Microsoft Corporation, U.S.) Excel® spreadsheet and statistical analysis was performed using SAS® software version 9.4 (‘SAS Institute, U.S.). The demographic and baseline characteristics have been summarized by treatment groups using descriptive statistics (number of patients [n], mean, SD, median) and were compared using either Pearson’s χ2 test or Mann–Whitney U test, as appropriate. Mortality data have been summarized using frequency counts (n) and percentages (%) and compared using Pearson’s χ2 test. Statistical significance was considered at a probability of P value < .05.
RESULTS AND ANALYSIS
Patients
A total of 48 patients with acute burns were identified to have received ulinastatin during the period starting from October 05, 2012; these comprised the ulinastatin group. The control group included 49 subjects admitted to the hospital before October 05, 2012, starting from February 05, 2011. Thus, a total of 97 patients were enrolled in the study. All patients belonged to Asian race. The mean age of patients in the ulinastatin and control groups were 37.8 ± 14.1 and 35.3 ± 17.8 years, respectively. The male:female distribution in the ulinastatin group was 1:1 and 1:1.72 in the control group. The mean body temperature, systolic and diastolic blood pressures, hematocrit, leukocyte count, platelet count, serum creatinine, and serum bilirubin values at baseline in both groups have been provided in Table 1. Patients in the ulinastatin group had a mean burnt BSA of 60.1%, while in the control group, it was 58.6%. The distribution of patients across different strata (<40%, 41–60%, 61–80%, and >80%) of burnt BSA was similar between the groups (Table 2). A similar proportion of patients in the ulinastatin group and control group (25% and 22.4%, respectively), were diagnosed to have inhalation injury identified by clinical manifestations and radio-imaging findings (pulmonary edema, congestion, effusion, etc).
Table 1.
Demographic and baseline characteristics
| Particular | Group | P Value | |
|---|---|---|---|
| Ulinastatin (N = 48) | Control (N = 49) | ||
| Age (y) mean (SD) | 37.8 (14.1) | 35.3 (17.8) | .1 |
| Gender (male:female) | 24:24 | 18:31 | .19 |
| Burnt surface area (%) mean (SD) | 60.1 (18.4) | 58.6 (25.7) | .7 |
| Inhalation injury (yes:no) | 12: 36 | 11: 38 | .8 |
| Temperature (°C) mean (SD) | 101.4 (1.6) | 98.8 (1.0) | <.0001 |
| Hematocrit (%) mean (SD) | 36.5 (7.4) | 42.6 (10.0) | .001 |
| Leukocyte count (/mm3) mean (SD) | 11,054 (8065) | 21,887 (14,040) | <.0001 |
| Platelet count (/mm3) mean (SD) | 195,000 (119,981) | 316,200 (185,251) | .0002 |
| Serum creatinine (mg/dL) mean (SD) | 0.9 (0.54) | 1.2 (1.39) | .166 |
| Serum bilirubin (mg/dL) mean (SD) | 1.46 (1.27) | 1.04 (1.1) | .085 |
| Systolic BP (mm Hg) mean (SD) | 130.4 (16.0) | 128.2 (19.6) | .55 |
| Diastolic BP (mm Hg) mean (SD) | 72.4 (10.7) | 76 (14.0) | .16 |
Table 2.
Distribution of cases based on burnt BSA in the two groups
| Burnt BSA (%) | Group | Ulinastatin (n) | Control (n) | |
|---|---|---|---|---|
| <40 | 5 | 13 | ||
| 40–80 | 32 | 22 | ||
| ≥80 | 11 | 14 | ||
P value = .11.
Study Drug Use
The patients in the ulinastatin group received ulinastatin in addition to standard care; the commonly used regimen was 100,000 IU given as intravenous infusion every 8 to 12 hours, dissolved in normal saline and given over 30 to 60 minutes. The mean duration of ulinastatin use was 8.8 days (range 1–26 days), and the mean cumulative dose was 2,400,000 IU (Table 3).
Table 3.
Ulinastatin use summary
| N | Ulinastatin | Mean Duration | Mean Dose | Median Dose | ||
|---|---|---|---|---|---|---|
| 48 | 8.8 days | 2,400,000 IU | 2,550,000 IU | |||
Length of Hospital Stay
The median hospital LOS was higher in the ulinastatin group compared with the control group (20.5 days vs 9.5 days). Similarly the median ICU LOS was also higher in the ulinastatin group (15.5 days vs 6 days). The median duration of hospital LOS was higher in the ulinastatin group, both in survivors (61 days vs 36.5 days) and nonsurvivors (13 days vs 6 days; Table 4).
Table 4.
Hospital and ICU length of stay among survivors and nonsurvivors
| Population | Hospitalization (Median) Days | ICU Stay (Median) Days | ||
|---|---|---|---|---|
| Ulinastatin Group | Control Group | Ulinastatin Group | Control Group | |
| All patients | 20.5 | 9.5 | 15.5 | 6 |
| Survivors | 61 | 36.5 | 24 | 12 |
| Nonsurvivors | 13 | 6 | 13 | 6 |
ICU, intensive care unit.
Mortality Rates
The overall in-hospital mortality among the studied patients was 68% (66 of 97). The in-hospital mortality (Table 5) was higher (75.5%) in the control group than in the ulinastatin group (60.4%; Figure 1). All patients (11 in ulinastatin group and 14 in control group) with more than 80% burnt BSA had died. In patients with burnt BSA < 40%, a similar proportion had died in both the groups (40% in ulinastatin and 46.2% in control). In patients with 41 to 80% burnt BSA, 16 of 32 had died in the ulinastatin group, while 17 of 22 had died in the control group ( Figure 2). This difference was statistically significant (77.27% vs 50%; P = .04; Table 6). Seventy-five percent (9 of 12) of patients with inhalation injury in the ulinastatin group and 91% (10 of 11) of similar patients in the control group had died.
Table 5.
Mortality in the two groups
| Outcome | Group | χ2 Test | |
|---|---|---|---|
| Ulinastatin (n) | Control (n) | P Value | |
| Died | 29 (60.4%) | 37 (75.5%) | 0.19 |
| Survived | 19 (39.6%) | 12 (24.5%) | |
Figure 1.
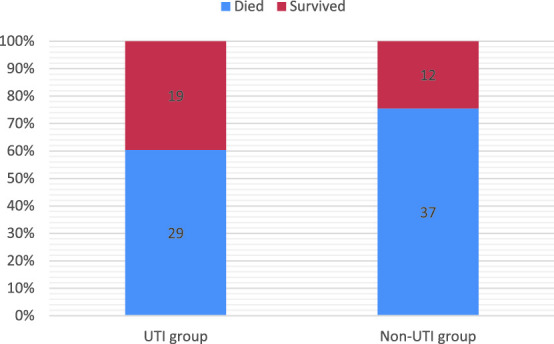
Survival outcomes in ulinastatin and control groups.
Figure 2.
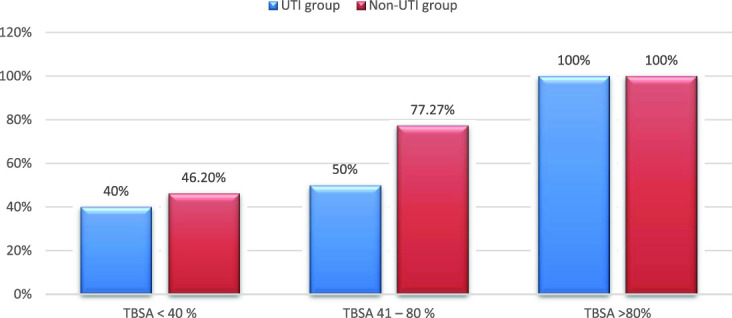
Mortality rates in ulinastatin and control groups based on burnt BSA stratification.
Table 6.
Mortality rates based on burnt BSA strata
| Burnt BSA | Mortality | χ2 Test | |
|---|---|---|---|
| Ulinastatin Group | Control Group | P Value | |
| <40 % | 2/5 (40%) | 6/13 (46.2) | .81 |
| 41–80% | 16/32 (50%) | 17/22 (77.27%) | .04 |
| >80% | 11/11 (100%) | 14/14 (100%) | 1 |
Bold indicates statistical significance of P value.
DISCUSSION
In India, the incidence of burn injuries continues to be high, with more than 1,000,000 people moderately or severely burnt every year, making it an endemic health hazard.4 The mortality rates reported in studies from tertiary centers in India range from 40 to 68%.17–22 This retrospective single-centre case note review analysis showed lower mortality in patients hospitalized for acute burns when ulinastatin was added to the standard care of therapy.
The pathophysiology of the burn wound involves an inflammatory reaction leading to rapid edema formation as a result of increased microvascular permeability accompanied by vasodilation and increased extravascular osmotic activity.23 Lipid peroxidation, leading to generation of reactive oxygen species, plays a critical role in burn-induced plasma leakage by contributing to the increased microvascular permeability, edema formation, and tissue damage after burn injury.12,23 Septicemia/sepsis, shock, and multiorgan failure are recognized as the most common causes of death in burn injury patients.6,19,22,24–26 Burn shock can develop rapidly after major burn injury, and current treatment mainly includes early adequate fluid resuscitation with the aim of maintaining sufficient tissue perfusion. However, shock can develop and progress despite fluid resuscitation. Patients with larger burns often develop prolonged hypermetabolism, chronic inflammation, and lean body mass wasting, all of which may impair wound healing. The associated alteration in the immune status increases the susceptibility to infection via the burn wound, further exacerbating systemic inflammation.6,23
Increased serum levels of proinflammatory cytokines characterize the systemic response to burns. Cytokines, including interleukin (IL) 1β and tumor necrosis factor α, contribute to the production of fever, acute-phase proteins, and an overall state of catabolism. They also upregulate the production of prostaglandin E2, IL-6, and platelet-activating factor by endothelial cells and macrophages. High levels of IL-6 have been associated with increased rates of morbidity and mortality.5 There is a paucity of therapies specifically aimed at controlling these events. Even in the developed world, despite the practice of advanced surgical techniques and availability of tissue-engineered biomaterials, management of burns and their sequelae remains a challenge.3 There has been no major breakthrough in the treatment of burns since many years now, and with the increasing resistance of bacteria to antimicrobials, the management of burns patients has become more complicated. Therapies aimed at controlling the inflammation and improving the immunological integrity have a role in treatment and can improve outcomes in such patients.5 These therapies can act as adjuvants to antimicrobials and fluid management.
Ulinastatin (also known as urinary trypsin inhibitor, HI-30, or bikunin) is a multifunctional Kunitz-type serine protease inhibitor belonging to the IaI family, produced by hepatocytes and found in human urine and blood. During inflammation, ulinastatin is cleaved from IaI family proteins through proteolytic cleavage by neutrophil elastase in the peripheral circulation or at sites of inflammation.7–9 It has inhibitory action on a wide variety of serine proteases, including trypsin, thrombin, chymotrypsin, kallikrein, plasmin, elastase, cathepsin, and factors IXa, Xa, XIa, and XIIa. It also inhibits inflammation by suppressing the infiltration of neutrophils and release of elastase and inflammatory mediators from neutrophils. Ulinastatin also inhibits the production of tumor necrosis factor α, IL-1, and IL-6 possibly through the suppression of mitogen-activated protein kinase (MAPK) signaling pathway.7–9 It also inhibits coagulation and fibrinolysis, which promotes microperfusion.7,8 Thus, ulinastatin acts as an agent for control of inflammation and immune modulation to prevent organ dysfunction and promote homeostasis. Based on these mechanisms and clinical evidence, ulinastatin is approved for management of conditions like sepsis, acute pancreatitis, and acute circulatory failure due to hemorrhagic, bacterial, or traumatic shock in countries like Japan, China, South Korea, and India.
Luo et al, in 2013,12 reported attenuation of increase in vascular permeability and net fluid accumulation along with reduced fluid requirements in a swine model with 40% TBSA burn injury, with the use of ulinastatin. Ulinastatin has also been studied in other animal models of burn injury and has demonstrated significant decrease in the levels of inflammatory mediators, oxygen-free radicals, and protective effect on multiple organs.10–14 Many of these findings have been substantiated in clinical studies in limited number of patients with severe burn and severe burn–induced sepsis, which have reported decrease in inflammatory mediators, edema, and wound size, accompanied by reduced morbidity and mortality.14–16
Multiple studies in India have reported overall mortality rates ranging from 40.3 to 68.5% among burn injury patients.17–22 The observed overall mortality of 68% in this study is in line with these reports. These values are, however, much higher than those reported from the developed world. The American Burn Association-National Burn Repository 2015 (ABA-NBR 2015) reported an overall mortality of 3.2% among more than 172,000 burn injury patients from different centers across the United States.27 Similarly, a systematic review of more than 186,500 patients from 76 studies by Brusselaers et al24 reported that mortality rates usually ranged between 1.4 and 18% (maximum, 34%) in hospitalized burn injury patients in Europe. Burnt BSA appears to be the major determinant here. O’Mara et al, in 2000,28 reported a mortality of 72% among 39 patients with >60% BSA burns admitted to a burn trauma unit in Pennsylvania, PA. The ABA-NBR 2015 data had only 3.1 % cases with a total burn size of 40% BSA or more. Similarly, in the European data, the mean BSA burns was 11 to 24%. These values are in stark contrast to the high mean BSA burns reported in Indian studies, which range from 47.5 to 67%, with 40 to 50% patients having burn size of 60% BSA or more.17,19–21 In our study, the mean BSA burns was 60.1% and 58.6% in the ulinastatin and control groups, respectively, and half of the patients in each group had >60% BSA burns.
Age is another important determinant of burn injury outcome, mortality increasing with age.29,30 Studies conducted in India have also shown increasing mortality with increase in mean age of the patients.17–20 The mean age (37.8 years in ulinastatin group and 35.3 years in control group) and mortality in this study correlates well with that reported by Subrahmanyam and Joshi, in 2003,17 in a retrospective study of 254 patients, with a mean age of 38 years and an overall mortality rate of 68.5%.20
The mortality in the ulinastatin group (60.4%) was, however, lower than in the control group (75.5%). The difference in mortality between the groups was more evident in those belonging to the intermediate stratum of burnt BSA (41–80 %). There was a significantly lower incidence of mortality in the ulinastatin group, than in the control group, in this stratum (50% vs 77.27%; P = .04).
In many low- and middle-income countries, mortality increases and reaches a plateau of 100% at 60% BSA burned.3 Even in developed countries where resources are abundant, treatment failure is a common occurrence in patients with BSA burns of more than 60%.28 In this study, all patients with >80% BSA burns died; however, notably, although in the control group all patients with > 60% BSA burns died, 41.7% (5 of 12 patients) of those with 61 to 80% BSA burns survived in the ulinastatin group. This finding, in addition to earlier Indian studies that have reported a 100% mortality in patients with >55 to 60% BSA burns,17,20 indicates a favorable impact of ulinastatin in patients with large area burns.
As per ABA-NBR 2015 data, nonsurvivors generally tend to have shorter LOS compared with survivors; and among the survivors, the hospital stay was approximately 1 day for each percent BSA burns.27 A prospective study in 278 patients by Macedo and Santos, in 2007,30 indicated that shorter hospital LOS, among other factors, predicted increased mortality. The mean LOS reported in the study was 17.2 ± 14.9 days among nonsurvivors and 11.9 ± 9.3 days among survivors. The review by Brusselaers et al24 reported a mean LOS in the general population with burn injuries of 7 to 33 days (median, 3–18 days). Bain et al, in 2014,19 in a retrospective analysis of 2499 patients from central India also reported lower median LOS (4 days) of stay in nonsurvivors compared with overall LOS of 8 days. Akther et al, in 2010,22 in a retrospective and prospective study of 714 burns patients admitted in the surgery department of a tertiary hospital in India reported a mean LOS of 11 to 30 days in those who died and >30 days in those who survived. Shorter duration of hospitalization is associated with death and, thus, is not a predictor of mortality but rather a consequence of the outcome. The median LOS was higher in the ulinastatin group (20.5 days) compared with control group (15.5 days). This was also reflected in LOS for both survivors and nonsurvivors, which were higher in the ulinastatin group compared with the control group.
LIMITATIONS
This was a small, retrospective case note study, and comprehensive details of important variables like serial values of laboratory parameters at specific time points, development of infection, surgical intervention, need for blood transfusion, presence of multiresistant bacteria in wound, presence of fungi in wound, etc were not available for analysis and hence not studied. Our study was limited to in-hospital events only and hence longer term outcomes have not been considered. Finally, detailed study of associated morbidity was also not possible. Thus, it can be used only to generate a preliminary hypothesis and additional testing.
CONCLUSION
Burn injury continues to be a challenge to treat and is associated with significant mortality in those with larger area of burns. In the absence of any major medical breakthrough in the treatment of burns in recent years, therapies that control inflammation and improve the immunological integrity can prove useful adjuvants to fluid management and antimicrobials. Ulinastatin, a serine protease inhibitor, by achieving this, might be a useful addition to burns treatment regimens. In this study, ulinastatin when added to current standard therapy of burns appeared to reduce mortality. This benefit was more prominently observed in those with intermediate extent (40–80%) of burnt BSA. It also increased the time to death in those who died, as evident from the increased hospital LOS in them.
REFERENCES
- 1. The global burden of disease: 2004 update. World Health Organization; 2008. [online]. Available at: http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf. Accessed October 20, 2015.
- 2. WHO | Burns. [online]. Available at: http://www.who.int/violence_injury_prevention/other_injury/burns/en/. Accessed October 20, 2015.
- 3. Atiyeh B, Masellis A, Conte C. Optimizing burn treatment in developing low- and middle-income countries with limited health care resources (part 1). Ann Burns Fire Disasters 2009;22:121–5. [PMC free article] [PubMed] [Google Scholar]
- 4. WHO | Burns. [online]. Available at: http://www.who.int/mediacentre/factsheets/fs365/en/. Accessed October 20, 2015.
- 5. Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev 2006;19:403–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rowan MP, Cancio LC, Elster EA, et al. Burn wound healing and treatment: review and advancements. Critical Care 2015;19:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Han JI. Urinary trypsin inhibitor: miraculous medicine in many surgical situations? Korean J Anesthesiol 2010;58:325–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nishiyama T, Yokoyama T, Yamashita K. Effects of a protease inhibitor, ulinastatin, on coagulation and fibrinolysis in abdominal surgery. J Anesth 2006;20:179–82. [DOI] [PubMed] [Google Scholar]
- 9. Linder A, Russell JA. An exciting candidate therapy for sepsis: ulinastatin, a urinary protease inhibitor. Intensive Care Med 2014;40:1164–7. [DOI] [PubMed] [Google Scholar]
- 10. Koga Y, Fujita M, Tsuruta R, et al. Urinary trypsin inhibitor suppresses excessive superoxide anion radical generation in blood, oxidative stress, early inflammation, and endothelial injury in forebrain ischemia/reperfusion rats. Neurol Res 2010;32:925–32. [DOI] [PubMed] [Google Scholar]
- 11. Tanaka R, Fujita M, Tsuruta R, et al. Urinary trypsin inhibitor suppresses excessive generation of superoxide anion radical, systemic inflammation, oxidative stress, and endothelial injury in endotoxemic rats. Inflamm Res 2010;59:597–606. [DOI] [PubMed] [Google Scholar]
- 12. Luo HM, Du MH, Lin ZL, et al. Ulinastatin suppresses burn-induced lipid peroxidation and reduces fluid requirements in a Swine model. Oxid Med Cell Longev 2013;2013:904370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luo HM, Hu S, Zhou GY, et al. The effects of ulinastatin on systemic inflammation, visceral vasopermeability and tissue water content in rats with scald injury. Burns 2013;39:916–22. [DOI] [PubMed] [Google Scholar]
- 14. Hu XH, Zhang HY, Ge YL, et al. Protective effects of ulinastatin against multiple organic damage after severe burn injury: experimental and clinic studies. Zhonghua Yi Xue Za Zhi 2005;85:2889–94. [PubMed] [Google Scholar]
- 15. Chun-hua W, Hu DL, Yu YX, Fang LS, Xu QL, Chang-Rong W. Clinical effects of ulinastatin in patients with severe burn sepsis. Anhui Medical and Pharmaceutical Journal. 2011. [Google Scholar]
- 16. Xu YB, Qi SH, Xie JL, Yuan JS, Zhang T, Chen XD, Shu B, Liu P, Li TZ. Protective effects of ulinastatin on organ function in patients with severe burn. Zhongguo wei zhong bing ji jiu yi xue 2006;18:39–41. [PubMed] [Google Scholar]
- 17. Subrahmanyam M, Joshi AV. Analysis of burn injuries treated during a one-year period at a district hospital in India. Ann Burns Fire Disasters 2003;16:74–6. [Google Scholar]
- 18. Shankar G, Naik VA, Powar R. Epidemiolgical study of burn injuries admitted in two hospitals of north Karnataka. Indian J Community Med 2010;35:509–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bain J, Lal S, Baghel VS, Yedalwar V, Gupta R, Singh AK. Decadorial of a burn center in central India. J Nat Sci Biol Med 2014;5:116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahuja RB, Bhattacharya S. An analysis of 11,196 burn admissions and evaluation of conservative management techniques. Burns 2002;28:555–61. [DOI] [PubMed] [Google Scholar]
- 21. Shanmugakrishnan RR, Narayanan V, Thirumalaikolundusubramanian P. Epidemiology of burns in a teaching hospital in south India. Indian J Plast Surg 2008;41:34–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Akther JM, Nerker NE, Reddy PS, Khan MI, Chauhan MK, Shahapurkar VV. Epidemiology of burned patients admitted in burn unit of a rural tertiary teaching hospital. Pravara Med Rev 2010;5:11–7. [Google Scholar]
- 23. Arturson G. Pathophysiology of the burn wound. Ann Chir Gynaecol 1980;69:178–90. [PubMed] [Google Scholar]
- 24. Brusselaers N, Monstrey S, Vogelaers D, Hoste E, Blot S. Severe burn injury in Europe: a systematic review of the incidence, etiology, morbidity, and mortality. Crit Care 2010;14:R188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lal S, Yadav GK, Gupta R, Shrivastava GP, Singh S, Bain J. Mortality pattern of burn patients admitted in SGM Hospital Rewa: a teaching institute of central India. J Sci Soc 2012;39:130. [Google Scholar]
- 26. Mazumder A, Patowary A. A study of pattern of burn injury cases. J Indian Acad Forensic Med 2013;35:44–6. [Google Scholar]
- 27. American Burn Association, National Burn Repository® 2015. Version 11.0. [online]. Available at: http://www.ameriburn.org/2015NBRAnnualReport.pdf. Accessed October 20 2015.
- 28. O’Mara MS, Caushaj P, Goldfarb IW, Slater H. Treatment and mortality trends among massively burned patients. Ann Burns Fire Disasters 2000;13:73–6. [Google Scholar]
- 29. Taylor SL, Lawless M, Curri T, Sen S, Greenhalgh DG, Palmieri TL. Predicting mortality from burns: the need for age-group specific models. Burns 2014;40:1106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Macedo JL, Santos JB. Predictive factors of mortality in burn patients. Rev Inst Med Trop Sao Paulo 2007;49:365–70. [DOI] [PubMed] [Google Scholar]


