Abstract
Objective
Although chemotherapy-induced cognitive impairment is common among breast cancer patients, evidence for effective interventions addressing cognitive deficits is limited. This randomized controlled trial examined the feasibility and preliminary efficacy of a Tibetan Sound Meditation (TSM) program to improve cognitive function and quality of life in breast cancer patients.
Methods
Forty-seven breast cancer patients (mean age 56.3 years), who were staged I–III at diagnosis, 6–60 months post-chemotherapy, and reported cognitive impairment at study entry were recruited. Participants were randomized to either two weekly TSM sessions for 6 weeks or a wait list control group. Neuropsychological assessments were completed at baseline and 1 month post-treatment. Self-report measures of cognitive function (Functional Assessment of Cancer Therapy (FACT)-Cog), quality of life (SF-36), depressive symptoms (Center for Epidemiologic Studies Depression Scale), sleep disturbance (Pittsburgh Sleep Quality Index), fatigue (Brief Fatigue Inventory), and spirituality (FACT-Sp) were completed at baseline, the end of treatment, and 1 month later.
Results
Relative to the control group, women in the TSM group performed better on the verbal memory test (Rey Auditory Verbal Learning Test trial 1) (p = 0.06) and the short-term memory and processing speed task (Digit Symbol) (p = 0.09) and reported improved cognitive function (p = 0.06), cognitive abilities (p = 0.08), mental health (p = 0.04), and spirituality (p = 0.05) at the end of treatment but not 1 month later.
Conclusions
This randomized controlled trial revealed that TSM program appears to be a feasible and acceptable intervention and may be associated with short-term improvements in objective and subjective cognitive function as well as mental health and spirituality in breast cancer patients.
Introduction
Growing evidence suggests that standard dose chemotherapy can reduce cognitive functioning in breast cancer patients [1–12]. Estimates of cognitive declines in breast cancer patients and survivors range between 16% and 39% [3,8,9,11], and women may experience acute or delayed onset of cognitive dysfunction with longstanding problems even decades after treatment [1–3]. Neuropsychological studies suggest that chemotherapy-induced cognitive deficits are mainly observed in the domains of working memory, processing speed, executive function with visuospatial functioning, and psychomotor speed to a lesser degree [5–7,13,14]. Although these findings do not appear to be a function of clinical, demographic, or psychosocial variables (e.g., depression, fatigue, insomnia [5,15–17], neuroimaging studies have shown structural and functional brain differences in breast cancer patients who received chemotherapy in comparison with patients who did not as discussed in a recent review [18].
Surprisingly, objective assessments (i.e., performance on neuropsychological tests) of cognitive function are not consistently correlated with patients’ subjective reports [3,8,13,15,16]. Unlike objective measures, self-reported cognitive deficits in patients and survivors are more likely to be related to psychological adjustment [10–12,16,19,20]. Despite their ‘subjectivity’, subjective complaints are a concern because patients and survivors indicate that their cognitive impairment significantly undermines social and occupational functioning and thus, their overall quality of life (QOL) [21,22].
To date, evidence for effective interventions addressing both objective and subjective cognitive deficits in cancer patients is limited. Pharmacologic management (e.g., psychostimulants or erythropoietin agents) is one approach to target cognitive function; however, results are mixed at this time (cf. Gering et al. [23] for a review), and some treatments are contraindicated in patients (e.g., cardiac disease). Additionally, there are many common side effects (e.g., headaches, nausea, vomiting, insomnia, anxiety, dizziness, weight loss, irritability, hyperhidrosis, muscle and joint pain, stomatitis, depression, dysphagia, hypokalemia, and thrombosis) [24] possibly further reducing patients’ already compromised QOL.
Behavioral interventions may be another approach to improve cognitive function. Unfortunately, traditional cognitive rehabilitation programs focusing on cognitive compensatory and retraining strategies have delivered only modest, if any, improvements in cancer patients and survivors [17,25–27]. Additionally, similar to pharmacotherapy, cognitive rehabilitation tends to be single-symptom focused failing to address the broader spectrum of adverse and longstanding effects of chemotherapy (e.g., fatigue, insomnia) [23,25,28–33]. In contrast, there is some evidence that behavioral interventions using meditation and other mind–body practices may alleviate both objective and subjective cognitive impairments in addition to other treatment-related side effects in cancer patients and survivors [34–36].
Goal of the current research
We sought to further investigate the role of mind–body programs in the cognitive rehabilitation of breast cancer survivors. Specifically, we were interested in examining a meditation practice because neuroimaging and electrophysiological studies of different forms of meditation training have been associated with neurological modulation (e.g., activity, cortical thickness, and neural synchrony) pertaining to higher-order cognitive processes such as attention and working memory [37–44]. Additionally, because meditation results in relaxation and stress reduction, it has also been found to regulate aspects of the immune function (e.g., cytokine production levels) [45,46], which is believed to play a role in cognitive deficits attributable to chemotherapy [47]. Thus, there is reason to believe that a meditation practice may rehabilitate treatment-induced cognitive impairment.
We chose a Tibetan Sound Meditation (TSM), which originated from the Tibetan Bon Buddhist tradition as the foundation of this intervention. Similar to mindfulness-based stress-reduction programs [48,49], TSM training begins with focused concentration and develops mindfulness (i.e., moment-to-moment non-judgmental awareness). Importantly, in addition to breathing exercises and visualizations, as one produces the meditative sounds, one engages in cognitive tasks. In other words, rather than simply practicing a relaxed non-judgmental state of awareness, participants here engage in a series of cognitive tasks that likely activate diverse brain regions, which may rehabilitate cognitive deficits.
In summary, the goal of the current study was to establish feasibility and initial efficacy of a meditation program targeting cognitive function in breast cancer survivors reporting cognitive dysfunction. We hypothesized that women reporting cognitive dysfunction after chemotherapy who participate in a TSM program would demonstrate improved objective and subjective cognitive functions (primary outcomes) relative to women in a wait list control (WLC) group. Because meditation training may reduce other side effects of cancer treatment [50–60], we also hypothesized that women in the TSM group would report improved mood, fatigue, sleep disturbance, spiritual well-being, and overall QOL (secondary outcomes) compared with the control group.
Materials and methods
Study eligibility
Women were eligible to participate if they were (i) diagnosed with stages I–III breast cancer; (ii) at least 18 years old; (iii) proficient in English; (iv) had received chemotherapy 6–60 months prior to recruitment; (v) reporting subjectively assessed cognitive impairment since the initiation of chemotherapy (four items of the FACT-Cog [61]; see measures); (vi) living within 2 hours driving distance of the institution in order to participate in the meditation sessions; and (vii) receiving hormone therapy at the time of recruitment. Women were excluded from the study if they had (i) a documented diagnosis of a formal thought disorder (e.g., schizophrenia); (ii) any neurological injury; (iii) a Mini-Mental State Examination [62] score of ≤23; (iv) metastatic or recurrent cancer; and/or (v) those who reported prior regular meditation practice (self-defined) within the past year.
Procedure
This randomized controlled trial (RCT) was conducted after approval from MD Anderson Institutional Review Board and is registered as NCT00556218 at www.clinicaltrials.gov. Research staff identified potentially eligible patients through the institution’s electronic medical records system and mailed recruitment letters to potential participants inviting them to contact the research team if interested in study participation. Participants provided informed consent prior to any data collection and were screened during a phone-based standardized structured interview including four questions from the FACT-Cog [61] (see Measures) and the Mini-Mental State Examination to confirm study eligibility. If participants were deemed eligible, they completed baseline assessments of self-report instruments and objective cognitive function tests (i.e., computerized neuropsychological assessment). Then, participants were randomly assigned to either the TSM or WLC group by a form of adaptive randomization called minimization [63] so that the groups were evenly balanced according to age, time since diagnosis, menopausal status, receptor status, stage of disease, surgical procedure, type of hormone treatment (e.g., tamoxifen vs. AIs), other medications with possible cognitive effects (e.g., Effexor), and baseline subjective reports of cognitive function (FACT-Cog total score). Participants in the treatment group followed the TSM program as described. Women in the WLC group received usual care and were asked to refrain from participating in any meditation class/practice for the duration of the study. Participants in both groups completed follow-up self-report assessments during the last week of the meditation program (T2) (on a day separate from the meditation class) and 1 month after the end of the meditation program (T3). Objective cognitive tests were administered at T3 but not T2 to avoid practice effects through repeated exposure within a relatively short period. Patients received small gift ($25 value) for completing each assessment. Women who were assigned to the WLC group were given the option of attending the TSM program after the completion of the last assessment, but data were not collected.
Tibetan Sound Meditation program
Participants in the treatment group participated in two weekly meditation classes (60 min. each) over the course of 6 weeks. Three meditation instructors were available for teaching the classes, but there was continuity of the same instructor for each patient. Session attendance ranged from one to three participants. The TSM program consists of two main components as follows: (i) breathing, awareness, and concentration techniques and (ii) visualization and sound exercises. There are three separate, yet interrelated, stages for the visualization and sound exercises. Each stage consists of a different cognitive activity and corresponds to a ‘healing sound’ (i.e., Ah, Om, and Hoong), location in the body (i.e., forehead, throat, and heart), and a specific color (i.e., white, red, and blue) in the form of light to help focus their attention. Cognitive activities focused on Stage 1: acknowledging, cleansing, and releasing negative thoughts and obstacles; Stage 2: identifying and retrieving a positive supportive quality (e.g., love, peace, and joy) that is needed; and Stage 3: integrating that quality into everyday life. Participants were given a CD with a recording of the TSM program and some printed materials with similar instructions and encouraged to practice on their own on the days when they did not meet with the instructor and daily thereafter.
Measures
Screening questions for cognitive dysfunction
To screen for cognitive impairment that patients attributed to their chemotherapy, participants were asked four questions from the FACT-Cog [61] (‘I have had trouble concentrating’; ‘I have trouble remembering whether I did things I was supposed to do, such as taking medicine or buying something I needed’; ‘I am able to pay attention and keep track of what I am doing without extra effort’; and ‘My thinking has been slower than usual’; rated from 0 to 4). If they scored greater than or equal to 2 on any item or greater than or equal to 1 on two or more items, they were deemed eligible.
Primary outcome measures
To measure objective cognitive performance, we administered four common neuropsychological tests to assess domains relevant to cognitive deficits observed in patients with a history of chemotherapy[64–66]. The digit span test [67] was used to assess attention and working memory. Digit symbol test [67] was used to measure visuomotor coordination, attention, and processing speed. Verbal fluency was assessed with the controlled oral word association test [68,69] and verbal memory with the Rey Auditory Verbal Learning Test [70]. Higher scores on all tests indicate better functioning.
Perceived cognitive function was assessed with the FACT-Cog-Version 3[61]. The measure contains questions regarding the patient’s perceived change of cognitive function prior to diagnosis and treatment, how their present cognitive function affects their daily life, and how others perceive the patient’s cognitive function yielding four subscales (perceived cognitive impairment, perceived cognitive abilities, comments from others, and impact on QOL). Participants rated the frequency with which each statement had occurred in the past 7 days on a five-point Likert scale (from 0 = never to 4 = several times a day) with higher scores indicating less impairment.
Secondary outcome measures
Symptoms of depression were assessed with the Center for Epidemiologic Studies Depression Scale (CES-D) [71], a 20-item self-report measure of depression that focuses on depressive feelings and behaviors over the past week, with higher scores representing worse symptoms. A cutoff score of ≥16 indicates ‘caseness’ warranting further psychological evaluation for clinical depression.
Sleep disturbances were assessed using the Pittsburgh Sleep Quality Index (PSQI), an 18-item self-rated questionnaire that assesses sleep disturbances over the past month with higher scores indicating worse sleep disturbances [72].
Fatigue was assessed using the Brief Fatigue Inventory (BFI) [73], a nine-item questionnaire asking participants to rate the severity of their fatigue at that moment and how much it interfered with their lives during the previous 24 h.
Health-related QOL was assessed with the Medical Outcomes Study 36-item short-form survey (SF-36) measuring several distinct domains [74]. We report on the standardized mental health (mental component summary (MCS)) and physical health component summary scales (physical component summary (PCS)).
Spiritual well-being was measured with the Functional Assessment of Cancer Therapy Spiritual Well-Being Scale (version 4) consisting of 12 items pertaining to meaning, peace, and faith [75].
Demographic and medical factors and tracking data, demographic items (e.g., age, marital status) were included in the baseline questionnaires. Medical data were extracted from patients’ charts. Tracking data were kept regarding class attendance, completion of questionnaires, and attrition. Participants in the TSM group completed weekly brief evaluations of the meditation classes to document their satisfaction with and acceptance of the TSM program.
Data analyses
The main objective of this study was to establish feasibility and preliminary efficacy of a TSM program to improve cognitive function and indices of QOL in breast cancer patients. We calculated descriptive statistics to characterize the sample. Because our inclusion criteria were based on subject reports, we also established an objective index of women’s cognitive dysfunction by operationalizing clinically significant cognitive impairment based on data from the neuropsychological tests using the criteria of Wefel et al. [65]. To establish feasibility, we calculated frequencies of class attendance, assessment completion, and program satisfaction. We used an intent-to-treat analysis approach and conducted all analyses in sas (version 9.2, Cary, NC, USA). We compared differences in outcomes between the TSM and WLC groups using analysis of variance (ANOVA) at the end of the meditation program (T2) for subjective measures and 1 month after the end of the program (T3) for objective and subjective measures controlling for baseline levels of the given outcome by including them as covariates in the ANOVA.
Neuropsychological tests were standardized on the basis of population means so that raw scores were transformed into z-scores prior to conducting the main analyses. Because of the relatively small sample size, power for inferential tests was limited. For example, given our sample size of 18 and 24 evaluable patients per group, differences between groups would need to be greater than 0.9 standard deviations (SD) (effect size 0.9; large effect) to be declared as statistically significant, with 80% power and a two-sided significance level of 0.05. Consequently, we calculated the effect size (Cohen’s d [76]) associated with each between group comparison controlling for baseline scores using pooled standard deviations to complement the p-value and interpreted the effect size in terms of Cohen’s taxonomy (‘small’ effect, d = 0.2; ‘medium’ effect, d = 0.5; and ‘large’ effect, d = 0.8) to determine whether future larger trials would be warranted.
Results
Recruitment and study sample
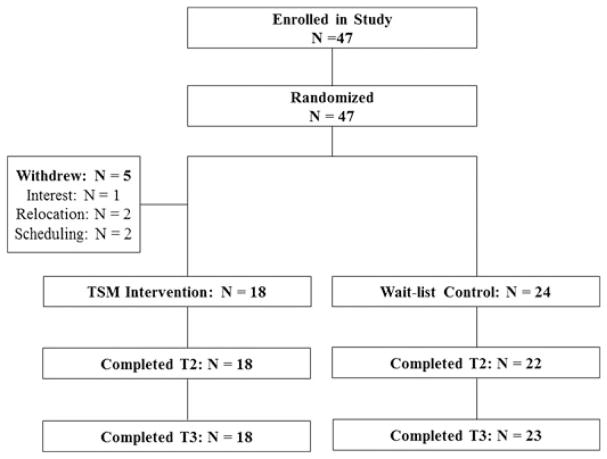
We mailed 1378 letters to breast cancer survivors from the hospital database and the first 47 consecutive women expressing interest were all eligible, consented, and then randomized, with 23 women assigned to the TSM group and 24 women to the WLC group. After baseline completion and randomization, five participants withdrew (one expressed a lack of interest, two relocated, and two had scheduling difficulties) from the TSM group prior to starting the intervention leaving 18 women in the TSM group (Figure 1). Women who withdrew from the study did not significantly differ regarding demographic and medical characteristics and baseline study variables. There were also no significant group differences in patient characteristics (except for ethnicity) or any of the objective and subjective study outcome variables (see Table 1).
Figure 1.
Consort chart
Table 1.
Participant demographics and clinical characteristics by group at baseline
| Variable | TSM (n = 23) | WLC (n = 24) | p-value |
|---|---|---|---|
| Mean age, years ± SD, (range) | 53.0 ± 6.6 (39–66) | 54.1 ± 8.6 (39–69) | 0.63 |
| Marital status, n (%) | |||
| Married | 16 (69.6) | 16 (66.7) | 0.90 |
| Ethnicity, n (%) | |||
| White | 17 (73.9) | 15 (62.5) | |
| African-American | 2 (8.7) | 0 | |
| Asian-American | 3 (13.0) | 1 (4.2) | |
| Latino/Hispanic | 1 (4.3) | 8 (33.3) | 0.05 |
| Highest level of education, n (%) | |||
| Some college or higher | 22 (95.6) | 18 (74.9) | 0.38 |
| Income, n (%) | |||
| 50,000 or more | 15 (65.2) | 16 (66.7) | 0.37 |
| Employment status, n (%) | |||
| Full-time | 13 (56.5) | 9 (37.5) | |
| Retired | 5 (21.7) | 7 (29.2) | 0.41 |
| Stage at diagnosis, n (%) | |||
| I | 6 (26.1) | 6 (25.0) | |
| II | 13 (56.5) | 13 (54.2) | |
| III | 4 (17.4) | 5 (20.8) | 0.96 |
| Time since diagnoses, months ± SD, (range) | 35.0 ± 14.6 (9–60) | 34.33 ± 14.6 (8–60) | 0.87 |
| Radiation, n (%) | |||
| Yes | 17 (73.9) | 19 (79.2) | 0.74 |
| Surgery, n (%) | |||
| Yes | 20 (87.0) | 24 (100) | 0.56 |
| Type of surgery, n (%) | |||
| Lumpectomy | 1 (4.3) | 2 (8.3) | |
| Segmented mastectomy | 8 (34.8) | 9 (37.5) | |
| Skin-sparing mastectomy | -- | 2 (8.3) | |
| Radical mastectomy | 7 (30.4) | 7 (29.2) | |
| Modified radical mastectomy | 4 (17.4) | 4 (16.7) | 0.50 |
| Menopausal status, n (%) | |||
| Post-menopausal | 19 (82.6) | 19 (79.2) | 0.53 |
TSM, Tibetan Sound Meditation; WLC, wait list control; SD, standard deviation.
Descriptive findings
Thirteen women (30.2%) met the criteria for clinically significant cognitive impairment [65] at baseline. The sample mean of CES-D scores at baseline was 13.0 (SD ± 8.0) with 28.3% of the sample meeting the CES-D criterion for caseness. None of the objective cognitive function tests were significantly correlated at p <0.05 with self-reported cognitive function or any of the secondary outcomes. The FACT-Cog total score was significantly negatively correlated with depressive symptoms (r = −00.43, p <0.01; higher functioning, lower depressive symptoms) and positively correlated with SF-36 PCS scores (r = 0.35, p <0.05; higher functioning, higher PCS levels).
Feasibility of Tibetan Sound Meditation program
Out of 12 total TSM sessions, 23.5% of women attended all sessions, 72.2% attended at least 75% of the session, and 0% attended less than 50% of the classes (mean = 9.71 sessions; SD = 1.90; range = 7–12). Over the course of the program, on average, 33.4% practiced every day outside of class, 46.5% practiced more than twice per week but not every day, 10.1% practiced once per week, and 9.5% practiced not at all. Regarding assessment completion, 100% of participants completed the baseline measures, 95% completed the T2 assessments, and 98% completed the T3 assessments. The TSM program was highly rated by the participants, with 74.4% of the sample indicating that the program was ‘very useful’ and 83.7% indicating that the program was ‘definitely beneficial’. Open-ended questions regarding participants’ experiences with the program revealed almost exclusively positive feedback. Sample comments included after meditation, my brain feels uncluttered; I feel more calm and focused and can concentrate better…; and I love it—It is calming yet invigorating—peaceful—energy potent. Helps you release negative thoughts deeds and actions—very, very helpful—I love, love it.
Preliminary evaluation of intervention efficacy
Primary outcomes
Objective cognitive function
Compared with the WLC group, the TSM group revealed marginally significantly better verbal memory (RAVLT test 1: p = 0.06; least square means: TSM = 0.44 vs. WLC = −0.22; medium effect) and improved short-term memory and processing speed (Digit Symbol Test: p = 0.09; least square means: TSM = 0.75 vs. WLC = 0.49; small effect) when controlling for baseline measures of cognitive function. Results for the other tests were not significant and effect sizes were mainly small. Raw means and effect sizes for all objective outcomes are presented in Table 2.
Table 2.
Population standardized means of neuropsychological tests by group at each assessment point and Cohen’s d for between group comparison at T3 controlling for baseline
| Measure | Baseline
|
1-Month post-program (T3)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| TSM
|
WLC
|
TSM
|
WLC
|
ES
|
|||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | d | |
| Digit span | 0.31 | 0.73 | −0.09 | 0.95 | 0.49 | 0.96 | 0.06 | 0.99 | 0.06 |
| Digit symbol | 0.32 | 0.78 | 0.09 | 0.77 | 0.87 | 0.81 | 0.41 | 1.16 | 0.25 |
| COWA | 0.64 | 0.94 | 0.22 | 1.01 | 0.65 | 1.13 | 0.65 | 0.99 | 0.30 |
| RALVT1 | 0.24 | 1.18 | 0.27 | 1.54 | 0.43 | 0.95 | −0.21 | 1.35 | 0.56 |
| RALVT2 | 0.75 | 1.13 | 0.45 | 1.19 | 0.68 | 0.97 | 0.23 | 1.15 | 0.27 |
| RALVT3 | 0.99 | 1.07 | 0.40 | 1.20 | 0.57 | 1.03 | 0.25 | 1.09 | 0.04 |
| RALVT4 | 0.69 | 0.89 | 0.13 | 1.23 | 0.51 | 0.79 | 0.33 | 1.09 | 0.16 |
| RALVT5 | 0.65 | 0.89 | 0.29 | 1.06 | 0.63 | 0.76 | 0.29 | 1.02 | 0.17 |
| RALVT list B | 0.33 | 0.95 | 0.17 | 1.38 | 0.50 | 0.95 | 0.09 | 1.2 | 0.31 |
| RALVT recall | 0.44 | 1.17 | 0.31 | 0.93 | 0.42 | 0.96 | 0.24 | 0.98 | 0.19 |
d, Cohen’s d using least square means for group comparisons adjusted for baseline scores. Small effect, d = 0.2; medium effect, d = 0.5; and large effect, d = 0.8 [76].
TSM, Tibetan Sound Meditation; WLC, wait list control; ES, effect size; SD, standard deviation; Digit span, digit span test, COWA, controlled oral word association test; RALVT, Rey Auditory Verbal Learning Test.
Perceived cognitive function
Controlling for baseline, ANOVA revealed marginally significantly lower levels of Perceived Cognitive Impairment at T2 for the TSM relative to the WLC group (p = 0.06; least square means for baseline scores: TSM = 75.7 vs. WLC = 67.6; small effect) but not at T3 (p = 0.13). Similarly, women in the TSM group had marginally significantly better perceived cognitive abilities at T2 compared with women in the WLC group (p = 0.08; least square means: TSM = 22.4 vs. WLC = 18.5; medium effect) but not T3 (p = 0.71). There were no group differences on the other two subscales (see Table 3 for raw means and effect sizes).
Table 3.
Raw means of self-report measures by group at each assessment point and effect sizes for between group comparisons controlling for baseline
| Measure | Baseline
|
Post-program (T2)
|
1-month post-program (T3)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TSM
|
WLC
|
TSM
|
WLC
|
ES
|
TSM
|
WLC
|
ES
|
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | d | Mean | SD | Mean | SD | d | |
| Impairment | 61.6 | 22.4 | 64.8 | 19.8 | 73.6 | 20.8 | 69.3 | 21.8 | 0.40 | 79.9 | 18.2 | 76.2 | 20.6 | 0.35 |
| Abilities | 18.8 | 7.0 | 16.8 | 5.5 | 22.9 | 5.2 | 18.1 | 8.7 | 0.64 | 22.0 | 7.4 | 20.6 | 7.0 | 0.12 |
| Comments | 10.7 | 3.8 | 10.0 | 3.0 | 12.3 | 3.1 | 10.7 | 3.0 | 0.25 | 12.4 | 3.2 | 12.0 | 3.2 | 0.05 |
| Impact | 17.5 | 6.4 | 18.9 | 8.2 | 19.4 | 7.0 | 19.8 | 8.2 | 0.08 | 21.9 | 7.1 | 21.8 | 7.1 | 0.02 |
| CES-D | 13.6 | 9.9 | 12.5 | 5.9 | 8.0 | 4.9 | 12.4 | 9.0 | 0.59 | 9.8 | 8.8 | 10.7 | 7.6 | 0.22 |
| BFI | 3.3 | 1.7 | 3.2 | 2.02 | 2.8 | 2.4 | 3.2 | 2.0 | 0.25 | 2.1 | 2.0 | 2.5 | 2.0 | 0.30 |
| PSQI | 8.6 | 4.1 | 6.4 | 3.8 | 6.8 | 3.4 | 6.4 | 3.0 | 0.23 | 7.4 | 4.9 | 6.1 | 3.1 | 0.32 |
| Mental health | 44.7 | 10.6 | 46.6 | 11.3 | 53.2 | 6.5 | 47.4 | 10.9 | 0.57 | 53.3 | 9.4 | 49.1 | 10.4 | 0.46 |
| Physical health | 45.8 | 12.7 | 46.6 | 8.3 | 42.5 | 13.3 | 44.7 | 11.7 | 0.19 | 45.5 | 11.2 | 46.7 | 10.7 | 0.09 |
| Spirituality | 37.5 | 7.4 | 37.1 | 8.0 | 42.2 | 6.1 | 36.7 | 8.7 | 0.52 | 41.4 | 5.2 | 39.2 | 7.4 | 0.25 |
d, Cohen’s d using least square means adjusted for baseline scores. Small effect, d = 0.2; medium effect, d = 0.5; and large effect, d = 0.8 [76].
TSM, Tibetan Sound Meditation; WLC, wait list control; ES, effect size; SD, standard deviation; Impairment, FACT-Cog Perceived Cognitive Impairment Subscale; Abilities, FACT-Cog Perceived Cognitive Abilities Subscale; Comments, FACT-Cog Comments from Others Subscale; Impact, FACT-Cog Impact on Quality of Life Subscale; CES-D, Center for Epidemiologic Studies Depression Scale; IES, Impact of Events Scale; BFI, Brief Fatigue Inventory; PSQI, Pittsburgh Sleep Quality Index; Mental Health, SF-36 Mental Health Component Subscale; Physical Health, SF-36 Physical Health Component Subscale; Spirituality, FACIT-Sp.
Secondary outcomes
At T2, women in the TSM group reported significantly less depressive symptoms (CES-D; p = 0.05; least square means: TSM = 8.2 vs. WLC = 12.6; medium effect); better mental health (SF-36 MCS: p <0.04; least square means: TSM = 53.3 vs. WLC = 47.2; medium effect); and spiritual well-being (FACT-SP: p = 0.05; least square means: TSM = 41.3 vs. WLC = 37.4; medium effect); compared with women in the WLC group when controlling for baseline levels. Results for the other secondary outcomes were not significant, and effect sizes were mainly small. There were no significant group differences by the 1-month post-intervention (T3) time point (see Table 3 for raw means and effect sizes).
Practice effects
Class attendance and home-practice rates were not significantly associated with the objective and subjective (primary and secondary) outcomes.
Discussion
As cancer treatment becomes more successful, treatment side effects undermining patients’ ability to perform usual activities of daily living must be addressed. The current study revealed that meditation training may be a feasible approach to improve cognitive declines in breast cancer survivors after chemotherapy. The TSM program was highly rated by the participants as indicated by the high rates of class attendance and home practice as well as positive program evaluations. In comparison with the previously reported drug and cognitive rehabilitation trials reporting high rates of study withdrawals [23], all study withdrawals of the RCT happened prior to intervention initiation, and no adverse events were reported. There is also suggestive preliminary evidence that the TSM intervention improves objective and subjective measures of cognitive function. Even though improvements were modest with small to medium effect sizes, this is, to the best of our knowledge, the first meditation study and one of the few non-pharmacological RCTs in general aiming to improve cognitive deficits in cancer patients. Additionally, in the context of the cognitive rehabilitation literature, these post-treatment effect sizes are rather promising [23,77] considering the small sample size and that some previous interventions did not report any significant results [17]. Findings were also promising regarding short-term improvement in mental health outcomes and spiritual well-being.
Consistent with previous studies, objective and subjective measures of cognitive function were not correlated and only subjective reports were correlated with depressive symptoms [3,8,13,15,16,20]. In fact, even though all participants met our inclusion criteria of subjective cognitive impairment, only 30% were objectively impaired, which may possibly explain a lack of greater improvement on the neurological assessments. This research also supports previous investigations pointing to the benefits of meditation programs on improving aspects of QOL [50–60].
Although the primary focus of this trial was establishing feasibility and initial efficacy and not to determine definitive evidence for the TSM program, it has laid an important foundation to systematically examine the role of meditation training in cognitive rehabilitation in cancer patients and survivors. Further research is warranted because meditation has no contraindications or side effects, it has demonstrated feasibility, and on the basis of participants’ ratings, is perceived as helpful and enjoyable. Additionally, meditation may be helpful to a wide range of patients and survivors as it appears to improve objective and subjective cognitive function as well as other aspects of QOL. Meditation training may be more cost-effective compared with traditional psychotherapy and accessible to patients with various physical performance status compared with an exercise intervention [35,36,78]. Of great interest would be to examine the benefits of meditation in combination with other treatments. Meditation research also has potential in uncovering mechanisms underlying chemotherapy-induced cognitive changes. Imaging and electrophysiological studies examining neuromodulation as a function of meditation training may be particularly helpful. Additionally, because meditation appears to reduce cytokine dysregulation in cancer patients, inflammatory processes may also be worthwhile exploring as they have been postulated to play a role in cognitive side effects [52,79].
Even though this research points to potentially new and exciting treatment approaches, prudence is warranted in interpreting these preliminary results. For one, the RCT involved a small sample and lacked an attention control group. Given the small sample, inferential tests were underpowered. Consequently, we did not control for multiple comparisons but rather reported effect sizes to convey a meaningful picture of the data. Also, participants were self-selected on the basis of rather leniently defined subjective as opposed to objective impairment. In fact, the relatively modest improvement in objective outcomes may be explained that only 30% of the sample experienced objectively measured cognitive dysfunction at baseline. We did not collect data on refusers so that it is impossible to distinguish the women who participated in the trial from the ones who did not. Importantly, even though completion rates were relatively high, accrual rates were very low, which seemingly is a common problem in the literature reporting on interventions to improve cognitive deficits in cancer survivors [23]. Further, we did not measure home practice during the follow-up period (between T2 and T3) so that we can only speculate that a reduction in effect sizes is a function of reduced treatment exposure. Moreover, we did not assess if women in the WLC group did in fact refrain from meditation training during the assessment period as instructed; however, any form of noncompliance would actually strengthen our study results. We did not assess participants’ involvement in co-interventions (e.g., herbal use, support groups); however, considering that our design involved random assignment, a potential effect on outcomes should be minimal at most. Lastly, we did not measure outcome expectations in women in the TSM group, which may have had positive effects on cognitive function.
In conclusion, this RCT found preliminary evidence that a TSM program may improve verbal memory, short-term memory, and processing speed as well as survivors’ perception of cognitive function in addition to mental health and spiritual well-being. Yet, future adequately powered larger trials involving appropriate control groups and including participants who have both objective and subjective cognitive impairments are needed to compellingly establish treatment efficacy. Future research involving imaging and biomarkers of inflammatory processes are needed to explore underlying mechanisms.
Acknowledgments
We would like to thank the Wolff Family Foundation and the Hines Family Foundation.
Footnotes
Conflict of interest
The authors have declared no conflicts of interest.
References
- 1.Koppelmans V, Breteler MM, Boogerd W, et al. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012;30:1080–1086. doi: 10.1200/JCO.2011.37.0189. [DOI] [PubMed] [Google Scholar]
- 2.de Ruiter MB, Reneman L, Boogerd W, et al. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp. 2011;32:1206–1219. doi: 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahles TA, Saykin AJ, Furstenberg CT, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20:485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- 4.Yamada TH, Denburg NL, Beglinger LJ, Schultz SK. Neuropsychological outcomes of older breast cancer survivors: cognitive features ten or more years after chemotherapy. J Neuropsychiatry Clin Neurosci. 2010;22:48–54. doi: 10.1176/appi.neuropsych.22.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wefel JS, Vardy J, Ahles T, Schagen SB. International cognition and cancer task force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12:703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 6.Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- 7.Vardy J, Wefel JS, Ahles T, Tannock IF, Schagen SB. Cancer and cancer-therapy related cognitive dysfunction: an international perspective from the Venice cognitive workshop. Ann Oncol. 2008;19:623–629. doi: 10.1093/annonc/mdm500. [DOI] [PubMed] [Google Scholar]
- 8.Schagen SB, van Dam FS, Muller MJ, et al. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85:640–650. doi: 10.1002/(sici)1097-0142(19990201)85:3<640::aid-cncr14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 9.van Dam FS, Schagen SB, Muller MJ, et al. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst. 1998;90:210–218. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- 10.Downie FP, Mar Fan HG, Houede-Tchen N, Yi Q, Tannock IF. Cognitive function, fatigue, and menopausal symptoms in breast cancer patients receiving adjuvant chemotherapy: evaluation with patient interview after formal assessment. Psychooncology. 2006;15:921–930. doi: 10.1002/pon.1035. [DOI] [PubMed] [Google Scholar]
- 11.Tchen N, Juffs HG, Downie FP, et al. Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. J Clin Oncol. 2003;21:4175–4183. doi: 10.1200/JCO.2003.01.119. [DOI] [PubMed] [Google Scholar]
- 12.Fan HG, Houede-Tchen N, Yi QL, et al. Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective controlled study. J Clin Oncol. 2005;23:8025–8032. doi: 10.1200/JCO.2005.01.6550. [DOI] [PubMed] [Google Scholar]
- 13.Castellon SA, Ganz PA, Bower JE, et al. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004;26:955–969. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- 14.Tager FA, McKinley PS, Schnabel FR, et al. The cognitive effects of chemotherapy in post-menopausal breast cancer patients: a controlled longitudinal study. Breast Cancer Res Treat. 2010;123:25–34. doi: 10.1007/s10549-009-0606-8. [DOI] [PubMed] [Google Scholar]
- 15.Weis J, Poppelreuter M, Bartsch HH. Cognitive deficits as long-term side-effects of adjuvant therapy in breast cancer patients: ’subjective’ complaints and ’objective’ neuropsychological test results. Psychooncology. 2009;18:775–782. doi: 10.1002/pon.1472. [DOI] [PubMed] [Google Scholar]
- 16.Poppelreuter M, Weis J, Kulz AK, et al. Cognitive dysfunction and subjective complaints of cancer patients. A cross-sectional study in a cancer rehabilitation centre. Eur J Cancer. 2004;40:43–49. doi: 10.1016/j.ejca.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Poppelreuter M, Weis J, Mumm A, Orth HB, Bartsch HH. Rehabilitation of therapy-related cognitive deficits in patients after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:79–90. doi: 10.1038/sj.bmt.1705884. [DOI] [PubMed] [Google Scholar]
- 18.Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012;12:267–275. doi: 10.1007/s11910-012-0264-9. [DOI] [PubMed] [Google Scholar]
- 19.Vardy J, Dhillon H. The fog hasn’t lifted on “chemobrain” yet: ongoing uncertainty regarding the effects of chemotherapy and breast cancer on cognition. Breast Cancer Res Treat. 2010;123:35–37. doi: 10.1007/s10549-009-0719-0. [DOI] [PubMed] [Google Scholar]
- 20.Kayl AE, Wefel JS, Meyers CA. Chemotherapy and cognition: effects, potential mechanisms, and management. Am J Ther. 2006;13:362–369. doi: 10.1097/00045391-200607000-00013. [DOI] [PubMed] [Google Scholar]
- 21.NCI. Cancer care issues in the United States quality of care, quality of life, January 1, 1997 to December 31, 1998. National Cancer Institute; President’s cancer panel meeting. [Google Scholar]
- 22.NCCS, editor. Palliative care and symptom management. [accessed February 2007];Cognitive Issues [cited; Available from. http://www.canceradvocacy.org/resources/essential/effects/cognitive_test.aspx.
- 23.Gehring K, Roukema JA, Sitskoorn MM. Review of recent studies on interventions for cognitive deficits in patients with cancer. Expert Rev Anticancer Ther. 2012;12:255–269. doi: 10.1586/era.11.202. [DOI] [PubMed] [Google Scholar]
- 24.FDA. Drugs@FDA, FDA approved drug products. www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
- 25.Ferguson RJ, McDonald BC, Rocque MA, et al. Development of CBT for chemotherapy-related cognitive change: results of a waitlist control trial. Psychooncology. 2012;21:176–186. doi: 10.1002/pon.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson RJ, Ahles TA, Saykin AJ, et al. Cognitive-behavioral management of chemotherapy-related cognitive change. Psychooncology. 2007;16:772–777. doi: 10.1002/pon.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDougall GJ, Becker H, Acee TW, Vaughan PW, Delville CL. Symptom management of affective and cognitive disturbance with a group of cancer survivors. Arch Psychiatr Nurs. 2011;25:24–35. doi: 10.1016/j.apnu.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cimprich B. Development of an intervention to restore attention in cancer patients. Cancer Nurs. 1993;16:83–92. [PubMed] [Google Scholar]
- 29.Crandall C, Petersen L, Ganz PA, Greendale GA. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519–530. doi: 10.1097/01.gme.0000117061.40493.ab. [DOI] [PubMed] [Google Scholar]
- 30.Schag CA, Ganz PA, Polinsky ML, et al. Characteristics of women at risk for psychosocial distress in the year after breast cancer. J Clin Oncol. 1993;11:783–793. doi: 10.1200/JCO.1993.11.4.783. [DOI] [PubMed] [Google Scholar]
- 31.Bower JE, Ganz PA, Irwin MR, et al. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29:3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Society AC. Breast Cancer Facts & Figures 2011–2012. Atlanta: 2011. [Google Scholar]
- 33.Henson HK. Breast cancer and sexuality. Sex Disabil. 2000;20:261–275. [Google Scholar]
- 34.Biegler KA, Chaoul MA, Cohen L. Cancer, cognitive impairment, and meditation. Acta Oncol. 2009;48:18–26. doi: 10.1080/02841860802415535. [DOI] [PubMed] [Google Scholar]
- 35.Oh B, Butow PN, Mullan BA, et al. Effect of medical Qigong on cognitive function, quality of life, and a biomarker of inflammation in cancer patients: a randomized controlled trial. Support Care Cancer. 2011;20:1235–1242. doi: 10.1007/s00520-011-1209-6. [DOI] [PubMed] [Google Scholar]
- 36.Reid-Arndt SA, Matsuda S, Cox CR. Tai Chi effects on neuropsychological, emotional, and physical functioning following cancer treatment: a pilot study. Complement Ther Clin Pract. 2012;18:26–30. doi: 10.1016/j.ctcp.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Kang DH, Jo HJ, Jung WH, et al. The effect of meditation on brain structure: cortical thickness mapping and diffusion tensor imaging. Soc Cogn Affect Neurosci. 2012;8:27–33. doi: 10.1093/scan/nss056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cogn Affect Behav Neurosci. 2007;7:109–119. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- 39.Kozasa EH, Sato JR, Lacerda SS, et al. Meditation training increases brain efficiency in an attention task. Neuroimage. 2012;59:745–749. doi: 10.1016/j.neuroimage.2011.06.088. [DOI] [PubMed] [Google Scholar]
- 40.Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci U S A. 2007;104:11483–11488. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slagter HA, Lutz A, Greischar LL, et al. Mental training affects distribution of limited brain resources. PLoS Biol. 2007;5:e138. doi: 10.1371/journal.pbio.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lutz A, Greischar LL, Rawlings NB, Ricard M, Davidson RJ. Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proc Natl Acad Sci U S A. 2004;101:16369–16373. doi: 10.1073/pnas.0407401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lutz A, Slagter HA, Dunne JD, Davidson RJ. Cognitive-emotional interactions - Attention regulation and monitoring in meditation. Trends Cogn Sci. 2008;12:163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Leeuwen S, Singer W, Melloni L. Meditation increases the depth of information processing and improves the allocation of attention in space. Front Hum Neurosci. 2012;6:133. doi: 10.3389/fnhum.2012.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davidson RJ, Kabat-Zinn J, Schumacher J, et al. Alterations in brain and immune function produced by mindfulness meditation. Psychosom Med. 2003;65:564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- 46.Witek-Janusek L, Albuquerque K, Chroniak KR, et al. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain Behav Immun. 2008;22:969–981. doi: 10.1016/j.bbi.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiatry. 1982;4:33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- 49.Ludwig DS, Kabat-Zinn J. Mindfulness in medicine. JAMA. 2008;300:1350–1352. doi: 10.1001/jama.300.11.1350. [DOI] [PubMed] [Google Scholar]
- 50.Kwekkeboom KL, Cherwin CH, Lee JW, Wanta B. Mind-body treatments for the pain-fatigue-sleep disturbance symptom cluster in persons with cancer. J Pain Symptom Manage. 2010;39:126–138. doi: 10.1016/j.jpainsymman.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lengacher CA, Reich RR, Post-White J, et al. Mindfulness based stress reduction in post-treatment breast cancer patients: an examination of symptoms and symptom clusters. J Behav Med. 2012;35:86–94. doi: 10.1007/s10865-011-9346-4. [DOI] [PubMed] [Google Scholar]
- 52.Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress, and immune parameters in breast and prostate cancer outpatients. Psychosom Med. 2003;65:571–581. doi: 10.1097/01.psy.0000074003.35911.41. [DOI] [PubMed] [Google Scholar]
- 53.Carlson LE, Ursuliak Z, Goodey E, Angen M, Speca M. The effects of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients: 6-month follow-up. Support Care Cancer. 2001;9:112–123. doi: 10.1007/s005200000206. [DOI] [PubMed] [Google Scholar]
- 54.Cohen L, Warneke C, Fouladi RT, Rodriguez MA, Chaoul-Reich A. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100:2253–2260. doi: 10.1002/cncr.20236. [DOI] [PubMed] [Google Scholar]
- 55.Ospina MB, Bond K, Karkhaneh M, et al. Clinical trials of meditation practices in health care: characteristics and quality. J Altern Complement Med. 2008;14:1199–1213. doi: 10.1089/acm.2008.0307. [DOI] [PubMed] [Google Scholar]
- 56.Matchim Y, Armer JM, Stewart BR. Effects of mindfulness-based stress reduction (MBSR) on health among breast cancer survivors. West J Nurs Res. 2011;33:996–1016. doi: 10.1177/0193945910385363. [DOI] [PubMed] [Google Scholar]
- 57.Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology. 2009;18:1261–1272. doi: 10.1002/pon.1529. [DOI] [PubMed] [Google Scholar]
- 58.Ando M, Morita T, Akechi T, et al. The efficacy of mindfulness-based meditation therapy on anxiety, depression, and spirituality in Japanese patients with cancer. J Palliat Med. 2009;12:1091–1094. doi: 10.1089/jpm.2009.0143. [DOI] [PubMed] [Google Scholar]
- 59.Speca M, Carlson LE, Goodey E, Angen M. A randomized, wait-list controlled clinical trial: the effect of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients. Psychosom Med. 2000;62:613–622. doi: 10.1097/00006842-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 60.Hoffman CJ, Ersser SJ, Hopkinson JB, et al. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III Breast Cancer: a randomized, controlled trial. J Clin Oncol. 2012;30:1335–1342. doi: 10.1200/JCO.2010.34.0331. [DOI] [PubMed] [Google Scholar]
- 61.Jacobs SR, Jacobsen PB, Booth-Jones M, et al. Evaluation of the functional assessment of cancer therapy cognitive scale with hematopoietic stem cell transplant patients. J Pain Symptom Manage. 2007;33:13–23. doi: 10.1016/j.jpainsymman.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 62.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and education level. J Am Med Assoc. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 63.Pocock SJ. Clinical Trials: A Practical Approach. New York: John Wiley & Sons; 1983. [Google Scholar]
- 64.Jacobs SR, Jacobsen PB, Booth-Jones M, Wagner LI, Anasetti C. Evaluation of the functional assessment of cancer therapy cognitive scale with hematopoietic stem cell transplant patients. J Pain Symptom Manage. 2007;33:13–23. doi: 10.1016/j.jpainsymman.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 65.Wefel JS, Lenzi R, Theriault R, et al. ’Chemobrain’ in breast carcinoma? A prologue. Cancer. 2004;101:466–475. doi: 10.1002/cncr.20393. [DOI] [PubMed] [Google Scholar]
- 66.Taillibert S, Voillery D, Bernard-Marty C. Chemobrain: is systemic chemotherapy neurotoxic? Curr Opin Oncol. 2007;19:623–627. doi: 10.1097/CCO.0b013e3282f0e224. [DOI] [PubMed] [Google Scholar]
- 67.Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 68.Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. Iowa City: AJA Associates; 1989. [Google Scholar]
- 69.Fletcher JM. Memory for verbal and nonverbal stimuli in learning disabled subgroups: analysis by selective reminding. J Exp Child Psychol. 1985;40:244–259. doi: 10.1016/0022-0965(85)90088-8. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt M. Rey Auditory and Verbal Learning Test. A handbook. Los Angeles, CA: Western Psychological Services; 1996. [Google Scholar]
- 71.Radloff LS. The CES-D scale: a new self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 72.Buysse DJ, Reynolds CF, Monk TH, Berman SR. Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 73.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 74.Ware JE, Johnston SA, Davies-Avery A, et al. Conceptualization and measurement of health for adults in the Health Insurance Study (Mental Health R-1987/3-HEW: 3) Santa Monica, CA: RAND Corporation; 1994. [Google Scholar]
- 75.Peterson AH, Fitchett GD, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: the Functional Assessment of Chronic Illness Therapy-Spiritual Well-Being Scale (FACIT-Sp) Ann Behav Med. 2002;24:49–58. doi: 10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- 76.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 77.Rohling ML, Demakis GJ. A meta-analysis of the neuropsychological effects of occupational exposure to mercury. Clin Neuropsychol. 2006;20:108–132. doi: 10.1080/13854040500203324. [DOI] [PubMed] [Google Scholar]
- 78.Cramp F, Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2008;11:1–38. CD006145. doi: 10.1002/14651858.CD006145.pub2. [DOI] [PubMed] [Google Scholar]
- 79.Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008;13:1285–1295. doi: 10.1634/theoncologist.2008-0130. [DOI] [PubMed] [Google Scholar]