Abstract
Background
Infants born prematurely are at risk for neurodevelopmental complications. Early growth is associated with improved later cognition. The relationship of early proportionality and body composition with later cognition is not well established.
Objectives
To assess differences in fat-free mass and adiposity (fat mass, %-body fat) changes in preterm and full-term infants through preschool age and examine associations with early childhood cognition.
Methods
This is a prospective, observational study in an appropriate for gestational age cohort of 71 patients (20 preterm and 51 full-term) from infancy through preschool age. Anthropometric and body composition measurements via air displacement plethysmography were obtained during infancy at term and 3–4 months (preterm corrected ages) and at 4 years. Cognitive testing occurred at 4 years. Associations of body composition changes between visits with cognitive function were tested using linear regression.
Results
In the preterm group, higher term to 4 month corrected age %-body fat gains were associated with lower working memory performance (p=0.01), and higher 4 month corrected age to 4 year fat-free mass gains were associated with higher full-scale IQ (p=0.03) and speed of processing performance (p≤0.02). In the full-term group, higher 4 month to 4 year fat mass gains were associated with lower full-scale IQ (p=0.03).
Conclusions
Body composition gains during different time periods are associated with varying areas of cognitive function. These findings may inform interventions aimed at optimal growth.
Keywords: body composition, premature neonates, neurodevelopmental outcome, growth, fat-free mass, adiposity, processing speed, executive function, intelligence quotient
Introduction
Children born prematurely are at risk for later cognitive deficits. Growth failure after premature birth is a risk factor for poorer neurodevelopment; better infancy growth is associated with improved cognitive and motor function. Increased gains in weight, length, and head circumference both during and after NICU hospitalization are associated with improved neurodevelopment (1, 2).
Children born prematurely are also at risk for long-term metabolic health detriments. In this population the neurodevelopmental benefits of growth can be in competition with the potential harm of rapid growth given its association with later insulin-resistance, hypertension, and obesity (3, 4). Balancing enhanced neurodevelopment with minimized metabolic risk may require interventions that track (5) and target optimal proportionality and quality of weight gain (body composition). For example, improved length gains (a lean growth marker) up to 12 months corrected age (CA) are associated with improved cognition (2), while increased BMI gain (a fat growth marker) from term CA to 18 months CA is associated with later obesity (4). Notably, however, length and BMI may not accurately reflect lean and fat compartments in infancy and childhood (6, 7).
Children born prematurely have different body composition at term CA than those born full-term: lower fat-free mass and a higher percentage of fat mass (8, 9). Comparative reports of later life body composition between children born preterm versus full-term have varying results (10, 11). Emerging evidence suggests positive associations between fat-free mass (FFM) gains, but not fat mass (FM) gains, and later improvements in neurodevelopment. Specifically, greater FFM measurements at term and 4 months CA are associated with faster speed of processing at 4 months CA (12), and greater FFM gains during NICU hospitalization are associated with improved motor and cognitive scores at 12 months CA (13).
We previously reported on differences in body composition growth patterns between term and preterm infants from infancy to preschool age (11). Associations of body composition beyond infancy with early childhood cognition in preterm infants have not yet been reported. Knowledge regarding the continued influence of growth patterns on later neurodevelopment will inform optimal growth patterns and interventions promoting these patterns.
In this paper, we aimed to test the hypothesis that cognitive function, particularly memory and executive function, are associated with fat-free body mass accretion in preterm and not full-term infants.
Methods
Subjects
Subjects were recruited during infancy from the University of Minnesota Masonic Children’s Hospital (preterm, n=27; December 2008 through October 2009) and an on-going infancy body composition study (full-term, n=97; April 2009 through June 2010) (14). The initial study explored possible body composition differences between preterm and full-term infants. Preterm subjects were recruited as a convenience sample. As this was a pilot study, a power analysis was not conducted given no previous studies reporting on similar predictors and outcome variables. Included subjects were born appropriate for gestational age (AGA): birth weight between the 10th and 90th percentiles on Fenton preterm growth curves or World Health Organization full-term growth curves. Preterm infants were included if born <35 weeks GA and excluded if they were diagnosed with a condition known to affect growth (e.g. congenital viral infection, chromosomal abnormality). Further group details are in the initial report (8). The University of Minnesota Institutional Review Board approved the infancy and preschool follow-up studies. Parents gave written consent for their child’s participation in each portion of the study.
Reported birth characteristics were GA, birth weight, sex, illness, and nutritional factors from the electronic medical record (preterm) or parental report (full-term). Demographics (maternal highest level of education and race) and feeding practice (breast milk and, or formula) were collected by self-report during the infancy visits.
Protocol
Subjects participated in 3 visits at our outpatient research center. The first, Visit 1, was after NICU discharge, near term post-menstrual age for preterm infants, and about 2 weeks after hospital discharge for full-term infants (39–44 weeks post-menstrual age). The next time point, Visit 2, was at 3–4 months (preterm CA). Finally, Visit 3 or “preschool visit,” was at 4 years.
Anthropometrics and body composition
Study staff measured anthropometrics (weight, length or height, and head circumference) and body composition via air displacement plethysmography (ADP) at all visits. ADP was completed using the PEA POD at Visits 1 and 2 and the BOD POD with Pediatric Option at Visit 3 (Cosmed, Ltd.; Concord, CA). Body composition via ADP uses a participant’s weight, length (Visits 1 and 2) or height (Visit 3), and volume measurements to calculate body density. Participant FFM, FM, and percent body fat (%BF) are derived from the body density measurement using established density values of FFM and FM (15, 16), as validated in infants and children, including those born prematurely (17–19).
Cognitive testing
Cognitive testing performed at 4 years included measurements of general intelligence plus specific domains known to be affected in the preterm population: memory, attention, and executive function. All children were able to fully participate in testing.
A trained psychometrist (SH) blinded to children’s birth status administered the standardized Wechsler Preschool and Primary Scale of Intelligence Fourth Edition (WPPSI-IV; Wechsler, 2012). Age-normed results are: Full-Scale Intelligence Quotient (FSIQ), Verbal Comprehension Index, Visual Spatial Index, Fluid Reasoning Index, Working Memory Index (WMI), and Processing Speed Index (PSI).
Four tests from the Cambridge Neuropsychological Test Automated Battery (CANTAB) were chosen and administered in a standard, scripted format by one of three trained researchers. Tasks chosen test executive functioning related to visual memory, working memory, and speed of processing (20). Performance differences in later childhood cohorts born preterm versus full-term have been reported (21).
Visual evoked potentials
Brain speed of processing was also assessed with pattern reversal visual evoked potentials (VEP) via event-related potentials. A previous report of the preterm portion of this cohort showed associations between speed of processing and infancy body composition (12). A 128-channel Sensor Net System was used. Event-related potential data were collected and recorded online using NetAmps Amplifiers and the NetStation software (Electrical Geodesics Incorporated, Eugene, OR). The child’s visual response latency (msec), referred to as “p100,” was measured at lead 76, the midline occipital active electrode (22).
Data analysis
Descriptive statistics by preterm versus full-term status were analyzed as mean (SD) or count (percentage) as appropriate for demographic, cognition, and body composition variables. Two-sample t-tests for continuous variables or Fisher’s exact tests for categorical variables were run with preterm versus full-term status for all variables to calculate p-values.
To examine associations between body composition changes and children’s performance on each cognitive test, a multivariable linear regression analysis was performed. Exposures were fat-free mass and percentage body fat change from term to 3–4 months (preterm CAs) and from 3–4 months (preterm CA) to 4 years. The outcome measures were WPPSI-IV, CANTAB, and VEP performance at 4 years. Covariates known to be associated with children’s cognitive performance-- sex, race, and maternal education-- were included in all models. Exact age at 4-year visit was additionally adjusted for in the analysis of all the CANTAB variables, as CANTAB results are not age normative. Given our hypothesis that body composition changes would be associated with cognitive testing at 4 years of age in the preterm group, but not full-term group, the two groups were assessed separately in the regression analyses.
Analyses were performed using SAS (v9.3; SAS Institute, Cary, NC). Statistical significance was defined as p<0.05.
Results
Some participants were lost to follow-up (22 of 27 preterm and 52 of 97 full-term infants participated in Visit 3). Preterm and full-term children who did versus did not complete Visit 3 had no significant group differences in birth weight, GA (13), maternal education, or race (data not shown). Three children (two preterm, one full-term) did not have body composition data collected at Visit 3 given lack of child cooperation. This analysis includes children with body composition data at all visits (preterm n=20, GA 27.0 to 34.9 weeks; full-term n=51).
Participant characteristics and preschool cognitive testing results are seen in Table 1.
Table 1.
Participant characteristics and preschool cognitive testing
| Variablea | Preterm (n=20) | Full-term (n=51) |
p-valueb |
|---|---|---|---|
| Birth data | |||
| Birth weight (g) | 1843 (586) | 3535 (459) | <0.01 |
| Birth weight z-scorec | 0.04 (0.62) | 0.47 (0.89) | 0.051 |
| Gestational age (weeks) | 31.9 (2.57) | 39.8 (1.03) | <0.01 |
| Demographics | |||
| Male sex | 13 (65) | 26 (51) | 0.31 |
| White race | 17 (85) | 38 (75) | 0.53 |
| Maternal education >High school | 14 (70) | 45 (88) | 0.084 |
| Preterm hospitalization data | |||
| Antenatal steroids | 15 (75) | - | - |
| Day 1 Score for Neonatal Acute Physiologyd | 5.5 (2–17) | - | - |
| Head ultrasound abnormale | 1 (5) | - | - |
| Postnatal steroids | 0 | - | - |
| Energy deficit, kcal/kgf | 88.4 (17.8–230) | - | - |
| Protein deficit, g/kgg | 2.7 (−0.14–5.9) | - | - |
| Visit 1 (Near term, preterm corrected age) | |||
| Weight z-scorec | 0.00 (0.85) | 0.10 (0.74) | 0.61 |
| Length z-scorec | −0.02 (1.2) | 0.27 (0.88) | 0.26 |
| Occiciptofrontal head circumference z-scorec | 0.92 (0.90) | 0.82 (0.90) | 0.67 |
| Breast milk, yesh | 17 (85) | 49 (96) | 0.13 |
| Visit 2 (3–4 months, preterm corrected age) | |||
| Weight z-scorec | −0.38 (1.2) | −0.055 (0.89) | 0.21 |
| Length z-scorec | −0.19 (1.2) | 0.38 (0.89) | 0.029 |
| Occiciptofrontal head circumference z-scorec | 0.77 (0.96) | 0.75 (0.76) | 0.94 |
| Breast milk, yesh | 7 (35) | 32 (63) | 0.062 |
| Visit 3 (4 years) | |||
| Weight z-scorec | −0.41 (1.2) | 0.02 (0.84) | 0.088 |
| Height z-scorei | −0.45 (0.97) | −0.08 (0.84) | 0.12 |
| WPPSI-IVi | |||
| Full scale IQ | 98.2 (13.2) | 105 (13.0) | 0.068 |
| Verbal Comprehension Index | 104 (14.3) | 108 (14.7) | 0.27 |
| Visual-Spatial Index | 97.7 (14.7) | 105 (15.3) | 0.067 |
| Fluid Reasoning Index | 97.2 (11.9) | 99.2 (12.0) | 0.53 |
| Working Memory Index | 98.9 (10.5) | 99.2 (12.8) | 0.91 |
| Processing Speed Index | 95.4 (12.2) | 103 (12.7) | 0.022 |
| CANTABj | |||
| Motor screen: average latency (msec) | 1553 (311) | 1532 (455) | 0.83 |
| Motor screen: error number | 1.32 (2.31) | 0.73 (1.67) | 0.33 |
| Spatial span longest span length | 1.68 (1.42) | 2.12 (1.47) | 0.27 |
| Spatial working memory, 4-box error | 6.61 (4.55) | 7.85 (6.19) | 0.38 |
| Spatial working memory, 6-box error | 24.0 (7.91) | 28.0 (13.72) | 0.15 |
| Spatial working memory, total errors | 30.4 (9.22) | 35.4 (16.8) | 0.14 |
| Delayed match to sample, % correct first try | 42 (12) | 41 (15) | 0.83 |
| Visual evoked potentialsk | |||
| P100 latency (msec) | 131 (19.9) | 127 (21.4) | 0.39 |
Continuous data are reported as mean (SD) and categorical data as number yes (%) unless otherwise specified in the row.
The p-value is calculated from two-sample t-test for continuous variables, and Fisher’s exact test for categorical variables.
Calculated for weight, length (Visits 1 & 2) or height (Visit 3), and head circumference on Fenton or World Health Organization growth chart as appropriate for preterm/full-term status and post-menstrual age.
Reported as mean (range).
One infant had bilateral grade 2 intraventricular hemorrhage without hydrocephalus on serial images.
Energy deficit calculated using a 120kcal/kg/day goal and subtracting or adding actual calories received each day of hospitalization and combined for entire hospitalization (kcal/kg); reported as mean (range).
Protein deficit calculated using a 3.5g/kg/day goal and subtracting or adding actual protein received each day of hospitalization and combined for entire hospitalization (g/kg); reported as mean (range).
Number of infants feeding breast milk either exclusively or partially per maternal report.
Excluding full-term participants without (n=3) or with incomplete (n=1) data.
Excluding participants without data (preterm n=1, full-term n=2) or with incomplete data (preterm n=1, full-term n=3).
Excluding participants without data (preterm n=1, full-term n=1).
Preterm infants had lower FFM and higher %BF than the full-term infants at Visit 1 but similar body composition at Visits 2 and 3 (previously reported data not shown) (8, 11). Body composition accretion from Visit 1 to Visit 2 was different for the two groups, as shown in Table 2. As compared to full-term infants, from Visit 1 to Visit 2 the premature infants gained less FM (90.7g/week versus 59.8g/week, p=0.001), less %BF (0.96%/week versus 0.39%/week, p<0.001), and similar FFM (120g/week, p=0.96). The groups had similar body composition changes from Visit 2 to Visit 3.
Table 2.
Body composition changes between visits
| Variable | Preterma (n=20) |
Full Terma (n=51) |
p-valueb | |
|---|---|---|---|---|
| Fat Free Mass (g/week) | V1–V2 rate | 120 (24.2) | 120 (32.4) | 0.96 |
| V2–V3 rate | 39.9 (6.33) | 42.6 (6.59) | 0.12 | |
| Fat Mass (g/week) | V1–V2 rate | 59.8 (29.7) | 90.7 (41.7) | 0.001 |
| V2–V3 rate | 7.56 (6.89) | 8.86 (4.84) | 0.45 | |
| Percent Body Fat (%/week) | V1–V2 rate | 0.39 (0.36) | 0.96 (0.51) | <0.001 |
| V2–V3 rate | −0.03 (0.04) | −0.02 (0.03) | 0.57 | |
V1 = Visit 1 at term or term corrected age (preterm participants)
V2 = Visit 2 at 3–4 months (preterm corrected age)
V3 = Visit 3 at 4 years
Data are reported as mean (SD).
The p-value is calculated from two-sample t-test.
Table 3 details significant results of the regression analysis for preterm children.
Table 3.
Cognitive factors associated with body composition changes in preterm infants at 4 years of age
| % Body fat Rate (%/week) | Fat-free mass Rate (gram/week) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Visit 1–Visit 2 | Visit 2–Visit3 | Visit 1–Visit 2 | Visit 2–Visit3 | ||||||
| Variable | Test | Estimate (SE) |
p-value | Estimate (SE) |
p-value | Estimate (SE) |
p-value | Estimate (SE) |
p-value |
|
| |||||||||
| Working Memory | WMI | −20.9 (6.29) | 0.005 | −18.6 (72.1) | 0.80 | −0.14 (0.18) | 0.47 | 0.28 (0.43) | 0.52 |
|
| |||||||||
| SWM Total errorsa | 21.6 (6.08) | 0.005 | −58.8 (83.7) | 0.50 | 0.22 (0.17) | 0.21 | 0.45 (0.51) | 0.40 | |
|
| |||||||||
| IQ | Full Scale IQ | −5.63 (7.90) | 0.49 | −62.0 (68.2) | 0.38 | 0.03 (0.18) | 0.86 | 0.85 (0.36) | 0.033 |
|
| |||||||||
| Processing Speed | PSI | 1.47 (8.15) | 0.86 | −41.0 (70.3) | 0.57 | 0.07 (0.18) | 0.69 | 0.95 (0.35) | 0.015 |
|
| |||||||||
| DMS Mean Correct Latencya | 431 (1530) | 0.78 | 7110 (1270) | 0.59 | −13.1 (29.7) | 0.67 | −244 (43.6) | <0.001 | |
|
| |||||||||
| VEP p100 Latency | −12.5 (15.8) | 0.44 | 331 (94.8) | 0.004 | 0.27 (0.33) | 0.43 | 0.01 (0.78) | 0.99 | |
Multivariable linear regression analysis with adjustment for sex, race (white versus non-white), and maternal education (≤high school versus >high school).
CANTAB analysis additionally adjusted for age at V3
Visit 1 at term (preterm corrected age)
Visit 2 at 3–4 months (preterm corrected age)
Visit 3 at 4 years
WMI= WPPSI-IV Working Memory Index
SWM= CANTAB Spatial Working Memory
Full Scale IQ = WPPSI-IV Full Scale Intelligence Quotient
PSI= WPPSI-IV Processing Speed Index
DMS= CANTAB Delayed Match to Sample
VEP= Visual evoked potential
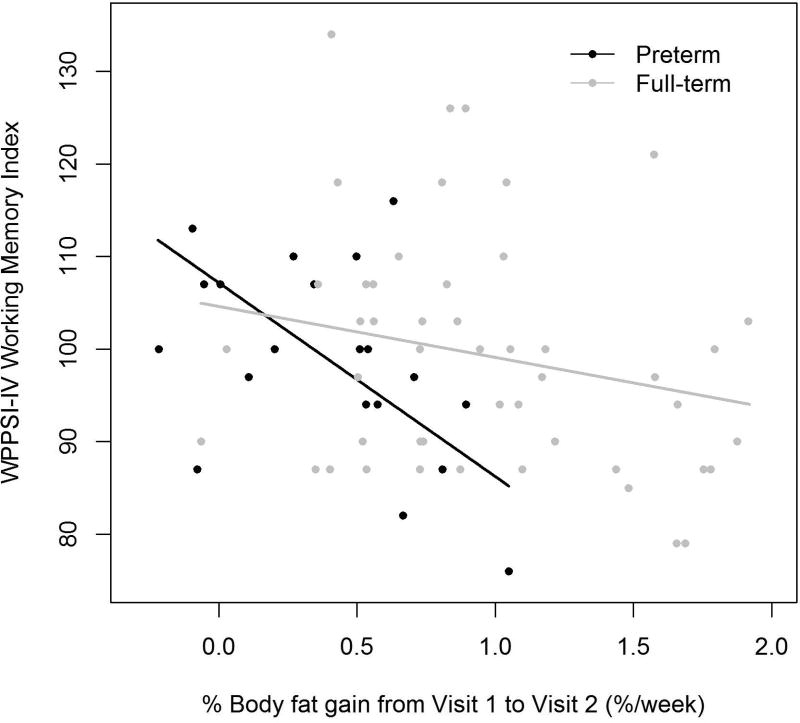
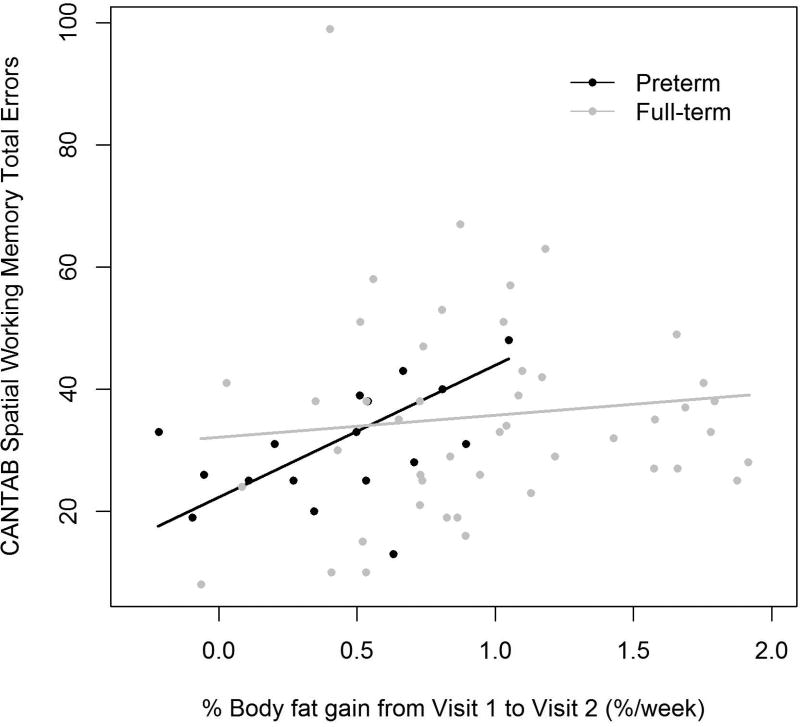
Early changes in proportionality (%BF) were associated with the preterm children’s performance on working memory tests at preschool age: greater gains in %BF from Visit 1 to Visit 2 were associated with a lower WPPSI-IV WMI score and a higher number of errors on CANTAB Spatial Working Memory (SWM) test. Figure 1 illustrates these significant associations found in the preterm but not full-term group. Each percentage point higher %BF per week from Visit 1 to Visit 2, was associated with a 21-point lower WPPSI-IV WMI at Visit 3 (SE 6.3, p=0.005) and 22 more total errors on CANTAB SWM (SE 6.1, p=0.005) in preterm participants.
Figure 1. Rate of % Body Fat Gain from Visit 1 to Visit 2 Associations with Visit 3 Working Memory Testing.
a.) WPPSI-IV Working Memory Index
Preterm β (SE)=−20.9 (6.29), p=0.005, R=0.51
Full-term β (SE)=−5.50 (3.69), p=0.14, R=0.15
b.) CANTAB Spatial Working Memory Total Errors
Preterm β (SE)=21.6 (6.08), p=0.005, R=0.63
Full-term β (SE)=3.61 (5.00), p=0.47, R=0.15
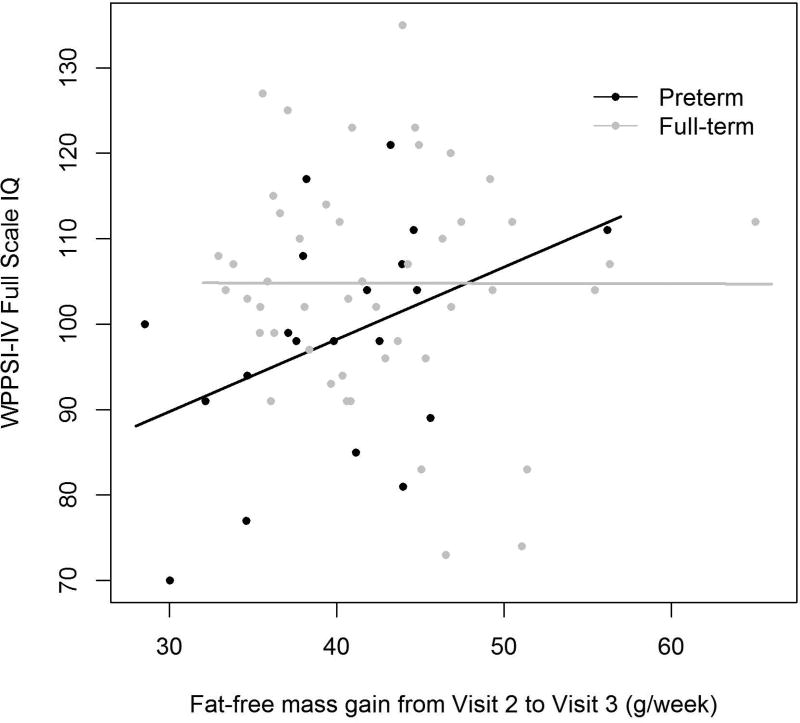
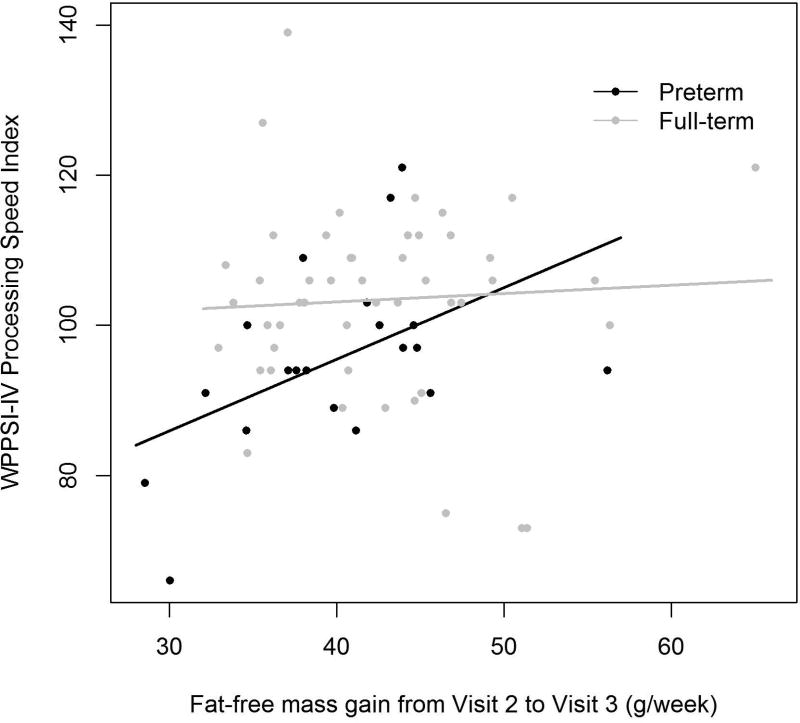
Later body composition and proportionality changes were associated with preterm children’s general cognition (WPPSI-IV FSIQ) and tests of processing speed. Higher FFM gain from infancy to early childhood (Visit 2 to Visit 3) was positively associated with preschool full-scale IQ and PSI in the preterm group, as seen in Figure 2. For a preterm participant each gram of FFM gained per week from Visit 2 to Visit 3 was associated with a 0.85-point higher WPPSI-IV FSIQ (SE 0.36, p=0.033) and 0.95-point higher WPPSI-IV PSI (SE 0.35, p=0.015). Higher FFM gain during this time period was also associated with a 244 msec lower CANTAB Delayed Match to Sample (DMS) latency for correct answer (SE 43.6, p<0.001), i.e. a faster time. Additionally, for each %BF gain per week from Visit 2 to Visit 3, VEP p100 latency increased by 331 msec (SE 94.8, p=0.004), i.e. a slower time.
Figure 2. Rate of Fat-free Mass Gain from Visit 2 to Visit 3 Associations with Visit 3 Cognitive Testing.
a.) WPPSI-IV Full Scale IQ
Preterm β (SE)=0.85 (0.36), p=0.033, R=0.63
Full-term β (SE)=−0.004 (0.33), p=0.99, R=0.03
b.) WPPSI-IV Processing Speed Index
Preterm β (SE)=0.95 (0.35), p=0.015, R=0.59
Full-term β (SE)=0.11 (0.32), p=0.73, R=0.039
Body composition changes in the preterm children were not associated with other WPPSI-IV sub-scores nor CANTAB Spatial Span or other DMS measures (data not shown).
In the full-term group later adiposity gains were negatively associated with preschool cognition. Specifically, FM gains from Visit 2 to Visit 3 were associated only in the full-term group with lower FSIQ (β= −0.92, SE 0.41, p=0.031), lower PSI (β= −0.77, SE 0.38, p=0.049), and lower maximum span length on CANTAB Spatial Span (β=−0.1, SE 0.04, p=0.029).
Discussion
Early growth is important for preterm children’s later cognition. Concern exists regarding potential adverse metabolic consequences of excess growth (i.e. later obesity, hypertension, and insulin resistance). Evidence increasingly supports augmenting lean tissue growth to optimize the competing cognitive and metabolic outcomes (2, 4). Recent literature specifically suggests that gains in FFM, but not FM, are associated with improved infancy cognition (12, 13). To our knowledge, this is the first report relating body composition changes beyond infancy with preschool age cognition.
As compared to full-term infants, this heterogeneous group of AGA preterm infants had less FFM and higher % body fat at term CA (8). Then the preterm group gained significantly less FM and less %BF but similar FFM from term CA to 3–4 months CA (see Table 2), and the groups had similar body composition (8). The groups were also similar at 4 years (11). Except for slightly lower WPPSI-IV Processing Speed Index in the preterm group, cognitive testing results including general cognition were normal and similar for both groups, demonstrating a preterm group relatively unaffected by major neurodevelopmental disability.
In the preterm cohort, greater %BF gain (greater FM gain relative to less FFM gain) from term CA to 4 months CA (Visit 1 to Visit 2) was negatively associated with preschool working memory. Poorer working memory performance has been described in very low birth weight and late preterm infants (21, 23). These tasks are a function of the dorsolateral prefrontal cortex and hippocampus (21). Structural changes in these areas are associated with working memory performance in preterm children (24). These brain areas undergo rapid structural and functional changes during infancy and are vulnerable to stress-related injury including nutritional deprivation (25). Infancy growth patterns in association with working memory performance have not been previously reported.
Our findings infer that higher adiposity and/or lack of appropriate FFM growth from term to 4 months CA may be detrimental to developmental trajectories in these brain areas and preterm children’s on-going working memory performance. Weight and length growth during the early infancy/post-NICU discharge time period are recognized as important for later neurodevelopment (4). Therefore, we suggest that targeting overall growth while minimizing FM and %BF gains may promote improved working memory function development. Working memory and other executive functioning continue to develop beyond preschool age. Longer-term studies are needed to determine persistence of these findings.
Greater infancy to preschool age (Visit 2 to Visit 3) FFM gains, but not adiposity gains, were associated with improved overall cognition (FSIQ) and processing speed task performance (WPPSI-IV PSI, CANTAB DMS latency, VEP p100 latency) among the preterm cohort. Differences in processing speed between preterm and full-term children are well known, and our study confirms these findings. Processing speed and general cognition are associated with brain imaging changes. FFM is a reflection of overall protein accretion, which along with growth factors such as IGF-1, is necessary for overall brain growth and myelination (25). Soria-Pastor et al. studied a group of adolescents born prematurely and found both overall intelligence and processing speed were correlated with decreased brain white matter on MRI (26). Murray et al. also reported in a group of 7-year-olds born prematurely that decreased processing speed was associated with global brain and white matter abnormalities on MRI (27).
Our findings indicate that specific body composition changes continue to incur neurodevelopmental benefit beyond infancy. Literature best supports growth in the first 4 months after discharge from the NICU is associated with improved neurodevelopment. However, Belfort found that higher BMI gains from 4 to 12 months CA were also associated with lower odds of IQ below 85 (4), and Ramel reported higher linear growth to 12 months CA was associated with improved 24 months CA cognitive performance (2). The relatively long time between Visits 2 and 3 limits more specific definition of the most beneficial time periods for FFM gain. While one period of growth may be influenced by growth in the prior period (28), we found that growth during each time period was associated with different cognitive changes, pointing to sensitive growth periods for each affected domain. Future studies with more frequent follow-up visits will be important to define these critical periods, particularly as excess growth during this timeframe may incur increased metabolic risk (4).
Our primary aim of the study was to explore the relationship of body composition changes with cognition in the preterm children, yet we also found that increased adiposity during early childhood was associated with poorer cognitive performance in the full-term children. Previous research suggests adequate early growth is important for preschool cognition in full-term AGA children (29), while rapid early growth does not incur additional neurodevelopmental benefit (30) and is, in fact, associated with lower cognitive scores (29). We suggest increasing adiposity during early childhood may be detrimental to long-term cognitive outcomes and should be monitored more closely.
Our study has some limitations. The preterm group was small and heterogeneous in regards to GA. Also, follow-up at Visit 3 was suboptimal for the full-term group, though those seen were similar to those who did not follow-up (11). We were unable, given the sample size, to adjust for additional covariates that may affect body composition or cognition (e.g. nutrition, physical versus sedentary activity, neonatal illness). Larger studies with additional follow-up visits are important to confirm these preliminary findings.
We report preliminary evidence in a heterogeneous preterm population that body composition changes following NICU discharge-- decreased early adiposity and increased later FFM-- are associated with enhanced preschool cognitive performance. Lower infancy to preschool adiposity gains may also incur neurodevelopmental benefit in the full-term population. Further research aimed to improve neurodevelopment and minimize metabolic risk will define optimal body composition growth patterns. Ultimately, intervention trials targeting these patterns will inform best practices from the NICU through childhood.
Acknowledgments
The authors wish to acknowledge the significant contributions of Katherine Hoversten, MD and Neely Miller, BS. We sincerely thank the participating children and families who enabled successful study completion.
Footnotes
Declarations
The authors declare no conflicts of interest.
References
- 1.Franz AR, Pohlandt F, Bode H, Mihatsch WA, Sander S, Kron M, et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics. 2009 Jan;123(1):e101–9. doi: 10.1542/peds.2008-1352. [DOI] [PubMed] [Google Scholar]
- 2.Ramel SE, Demerath EW, Gray HL, Younge N, Boys C, Georgieff MK. The relationship of poor linear growth velocity with neonatal illness and two-year neurodevelopment in preterm infants. Neonatology. 2012;102(1):19–24. doi: 10.1159/000336127. [DOI] [PubMed] [Google Scholar]
- 3.Lapillonne A, Griffin IJ. Feeding preterm infants today for later metabolic and cardiovascular outcomes. J Pediatr. 2013 Mar;162(3 Suppl):S7–16. doi: 10.1016/j.jpeds.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 4.Belfort MB, Gillman MW, Buka SL, Casey PH, McCormick MC. Preterm infant linear growth and adiposity gain: trade-offs for later weight status and intelligence quotient. J Pediatr. 2013 Dec;163(6):1564–1569.e2. doi: 10.1016/j.jpeds.2013.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demerath EW, Johnson W, Davern BA, Anderson CG, Shenberger JS, Misra S, et al. New body composition reference charts for preterm infants. Am J Clin Nutr. 2017 Jan;105(1):70–7. doi: 10.3945/ajcn.116.138248. [DOI] [PubMed] [Google Scholar]
- 6.Ramel SE, Zhang L, Misra S, Anderson CG, Demerath EW. Do anthropometric measures accurately reflect body composition in preterm infants? Pediatr Obes. 2016 Sep 16; doi: 10.1111/ijpo.12181. [DOI] [PubMed] [Google Scholar]
- 7.Perng W, Ringham BM, Glueck DH, Sauder KA, Starling AP, Belfort MB, et al. An observational cohort study of weight- and length-derived anthropometric indicators with body composition at birth and 5 mo: the Healthy Start study. Am J Clin Nutr. 2017 Aug;106(2):559–67. doi: 10.3945/ajcn.116.149617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramel SE, Gray HL, Ode KL, Younge N, Georgieff MK, Demerath EW. Body composition changes in preterm infants following hospital discharge: comparison with term infants. J Pediatr Gastroenterol Nutr. 2011 Sep;53(3):333–8. doi: 10.1097/MPG.0b013e3182243aa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson MJ, Wootton SA, Leaf AA, Jackson AA. Preterm birth and body composition at term equivalent age: a systematic review and meta-analysis. Pediatrics. 2012 Sep;130(3):e640–9. doi: 10.1542/peds.2011-3379. [DOI] [PubMed] [Google Scholar]
- 10.Gianni ML, Roggero P, Piemontese P, Morlacchi L, Bracco B, Taroni F, et al. Boys who are born preterm show a relative lack of fat-free mass at 5 years of age compared to their peers. Acta Paediatr. 2015 Mar;104(3):e119–23. doi: 10.1111/apa.12856. [DOI] [PubMed] [Google Scholar]
- 11.Scheurer JM, Zhang L, Gray HL, Weir K, Demerath EW, Ramel SE. Body Composition Trajectories From Infancy to Preschool in Children Born Premature Versus Full-term. J Pediatr Gastroenterol Nutr. 2017 Jun;64(6):e147–53. doi: 10.1097/MPG.0000000000001494. [DOI] [PubMed] [Google Scholar]
- 12.Pfister KM, Gray HL, Miller NC, Demerath EW, Georgieff MK, Ramel SE. Exploratory study of the relationship of fat-free mass to speed of brain processing in preterm infants. Pediatr Res. 2013 Nov;74(5):576–83. doi: 10.1038/pr.2013.138. [DOI] [PubMed] [Google Scholar]
- 13.Ramel SE, Gray HL, Christiansen E, Boys C, Georgieff MK, Demerath EW. Greater Early Gains in Fat-Free Mass, but Not Fat Mass, Are Associated with Improved Neurodevelopment at 1 Year Corrected Age for Prematurity in Very Low Birth Weight Preterm Infants. J Pediatr. 2016 Jun;173:108–15. doi: 10.1016/j.jpeds.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Ode KL, Gray HL, Ramel SE, Georgieff MK, Demerath EW. Decelerated early growth in infants of overweight and obese mothers. J Pediatr. 2012 Dec;161(6):1028–34. doi: 10.1016/j.jpeds.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fomon SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 years. Am J Clin Nutr. 1982 May;35(5 Suppl):1169–75. doi: 10.1093/ajcn/35.5.1169. [DOI] [PubMed] [Google Scholar]
- 16.Lohman TG. Applicability of body composition techniques and constants for children and youths. Exerc Sport Sci Rev. 1986;14:325–57. [PubMed] [Google Scholar]
- 17.Ellis KJ, Yao M, Shypailo RJ, Urlando A, Wong WW, Heird WC. Body-composition assessment in infancy: air-displacement plethysmography compared with a reference 4-compartment model. Am J Clin Nutr. 2007 Jan;85(1):90–5. doi: 10.1093/ajcn/85.1.90. [DOI] [PubMed] [Google Scholar]
- 18.Fields DA, Allison DB. Air-displacement plethysmography pediatric option in 2–6 years old using the four-compartment model as a criterion method. Obesity (Silver Spring) 2012 Aug;20(8):1732–7. doi: 10.1038/oby.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roggero P, Gianni ML, Amato O, Piemontese P, Morniroli D, Wong WW, et al. Evaluation of air-displacement plethysmography for body composition assessment in preterm infants. Pediatr Res. 2012 Sep;72(3):316–20. doi: 10.1038/pr.2012.75. [DOI] [PubMed] [Google Scholar]
- 20.Luciana M, Nelson CA. The functional emergence of prefrontally-guided working memory systems in four- to eight-year-old children. Neuropsychologia. 1998 Mar;36(3):273–93. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- 21.Luciana M, Lindeke L, Georgieff M, Mills M, Nelson CA. Neurobehavioral evidence for working-memory deficits in school-aged children with histories of prematurity. Dev Med Child Neurol. 1999 Aug;41(8):521–33. doi: 10.1017/s0012162299001140. [DOI] [PubMed] [Google Scholar]
- 22.Odom JV, Bach M, Barber C, Brigell M, Marmor MF, Tormene AP, et al. Visual evoked potentials standard (2004) Doc Ophthalmol. 2004 Mar;108(2):115–23. doi: 10.1023/b:doop.0000036790.67234.22. [DOI] [PubMed] [Google Scholar]
- 23.Fitzpatrick A, Carter J, Quigley MA. Association of Gestational Age With Verbal Ability and Spatial Working Memory at Age 11. Pediatrics. 2016 Dec;138(6):e20160578. doi: 10.1542/peds.2016-0578. Epub 2016 Nov 3. [DOI] [PubMed] [Google Scholar]
- 24.Nosarti C, Froudist-Walsh S. Alterations in development of hippocampal and cortical memory mechanisms following very preterm birth. Dev Med Child Neurol. 2016 Mar;58(Suppl 4):35–45. doi: 10.1111/dmcn.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuglestad A, Rao R, Georgieff M. The role of nutrition in cognitive development. In: Nelson C, Luciana L, editors. Handbook of Developmental Cognitive Neuroscience. 2. Cambridge, MA: MIT Press; 2008. pp. 623–42. [Google Scholar]
- 26.Soria-Pastor S, Gimenez M, Narberhaus A, Falcon C, Botet F, Bargallo N, et al. Patterns of cerebral white matter damage and cognitive impairment in adolescents born very preterm. Int J Dev Neurosci. 2008 Nov;26(7):647–54. doi: 10.1016/j.ijdevneu.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Murray AL, Scratch SE, Thompson DK, Inder TE, Doyle LW, Anderson JF, et al. Neonatal brain pathology predicts adverse attention and processing speed outcomes in very preterm and/or very low birth weight children. Neuropsychology. 2014 Jul;28(4):552–62. doi: 10.1037/neu0000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kramer MS, Martin RM, Bogdanovich N, Vilchuk K, Dahhou M, Oken E. Is restricted fetal growth associated with later adiposity? Observational analysis of a randomized trial. Am J Clin Nutr. 2014 Jul;100(1):176–81. doi: 10.3945/ajcn.113.079590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinonen K, Raikkonen K, Pesonen AK, Kajantie E, Andersson S, Eriksson JG, et al. Prenatal and postnatal growth and cognitive abilities at 56 months of age: a longitudinal study of infants born at term. Pediatrics. 2008 May;121(5):e1325–33. doi: 10.1542/peds.2007-1172. [DOI] [PubMed] [Google Scholar]
- 30.Beyerlein A, Ness AR, Streuling I, Hadders-Algra M, von Kries R. Early rapid growth: no association with later cognitive functions in children born not small for gestational age. Am J Clin Nutr. 2010 Sep;92(3):585–93. doi: 10.3945/ajcn.2009.29116. [DOI] [PubMed] [Google Scholar]