Abstract
The presence of CD28− memory CD8 T cells in the peripheral blood of renal transplant patients is a risk factor for graft rejection and resistance to CTLA-4Ig induction therapy. In vitro analyses have indicated poor alloantigen-induced CD28− memory CD8 T cell proliferation, raising questions about mechanisms mediating their clonal expansion in kidney grafts to mediate injury. Candidate proliferative cytokines were tested for synergy with alloantigen in stimulating CD28− memory CD8 T cell proliferation. Addition of IL-15, but not IL-2 or IL-7, to co-cultures of CD28− or CD28+ memory CD8 T cells and allogeneic B cells rescued proliferation of the CD28− and enhanced CD28+ memory T cell proliferation. Proliferating CD28− memory CD8 T cells produced high amounts of IFN-γ and TNFα and expressed higher levels of the cytolytic marker CD107a than CD28+ memory CD8 T cells. CTLA-4Ig inhibited alloantigen-induced proliferation of CD28+ memory CD8 T cell proliferation but had no effect on alloantigen plus IL-15-induced proliferation of either CD28− or CD28+ memory CD8 T cells. These results indicate the ability of IL-15, a cytokine produced by renal epithelial during inflammation, to provoke CD28− memory CD8 T cell proliferation and to confer memory CD8 T cell resistance to CTLA-4Ig-mediated costimulation blockade.
Introduction
High frequencies of donor-reactive memory T cells in the peripheral blood of renal transplant patients prior to transplant is associated with increased incidence of delayed graft function and poorer long-term outcome of the graft (1, 2). The underlying cause of this risk of allograft injury is the many memory T cells generated during immune responses to viral and bacterial infections and within lymphopenic environments that have high frequencies of cross-reactivity with allogeneic class I and class II MHC molecules (3–6). During immune responses in humans, many terminally differentiated memory CD4 and CD8 T cells lose expression of the costimulatory molecule CD28 (7–11). The loss of CD28 expression is more often observed on memory CD8, than CD4, T cells and the frequencies of CD28− memory CD8 T cells increase with aging (12, 13). In vitro studies have indicated that when compared to their CD28+ counterparts, CD28− memory T cells either have decreased proliferative responses to antigenic stimulation or are not able to proliferate at all (14–20).
Increased numbers of CD28− memory T cells in the peripheral blood of renal transplant recipients are associated with greater risk for poor graft outcome. In lung and renal transplant patients increased frequencies of CD4+CD28− T cells are associated with increased chronic graft dysfunction and rejection (21, 22). Higher percentages of CD8+CD28− lymphocytes are also found in long-term kidney graft recipients with chronic kidney allograft rejection when compared either to recipients with long-term grafts having stable renal function or to healthy individuals (23). We have also found that higher frequencies of pre-transplant CD28−NKG2D+ memory CD8+ T cells in the peripheral blood of kidney transplant recipients are associated with the incidence of acute cellular rejection (submitted manuscript in review). Phase III studies of a new generation CTLA-4Ig that blocks the CD28/B7 costimulation pathway reported three-year data demonstrating improvement in glomerular filtration rate in Belatacept-treated versus cyclosporine-treated renal transplant patients but that the occurrence of acute rejection was more frequent and more severe with higher Banff grades in Belatacept- -treated patients within the first 6 months of transplant (24, 25). A potential mechanism for this increase in early and more intense rejection episodes is the resistance of donor-reactive CD28− memory T cells to CTLA-4Ig.
The evidence implicating CD28− memory CD8 T cells as a risk factor for poorer graft outcome and resistance to CD28-mediated costimulatory blockade is paradoxical to observations that CD28− memory CD8 T cells are non- or poorly-proliferating cells. In order to generate sufficient numbers of effector T cells to mediate graft injury during rejection episodes, donor-reactive memory T cells mediating this injury would be expected to undergo clonal proliferation either in the recipient or within the graft. Our recent studies in mouse transplant models have documented endogenous memory CD8 T cell infiltration into cardiac allografts within 24 hours of graft reperfusion and their activation in response to graft allogeneic class I MHC to first proliferate and then produce IFN-γ in the graft (26–28). On this basis we postulated that human CD28− memory CD8 T cells might require proliferative signals that are available in vivo but missing in culture models. In this study, we tested candidate proliferative cytokines that are produced in kidneys during inflammation for the ability to synergize with alloantigen-presenting cells and provoke CD28− memory CD8 T cell proliferation. The results indicate that alloantigen plus IL-15 strongly induces these memory CD8 T cells to proliferate and express effector functions that are typically used by memory CD8 T cells to mediate graft tissue injury. Importantly, IL-15 confers CTLA-4Ig resistance of both CD28+ and CD28− memory CD8 T cells to alloantigen driven activation.
Methods
Peripheral blood mononuclear cells (PBMC) isolation and magnetic separation
Blood (40–50 ml) was collected from healthy volunteers, ages 24–65. PBMC were isolated by Ficoll-Hypaque separation (IsoPrep, Robbins Scientific Corporation, Sunnyvale, CA) and processed to isolate the CD8 memory T cells (CD8+CD45RO+CD45RA−CD56−CD57−) by negative selection using the Human CD8+ Memory T Cell Isolation Kit (MACS, Miltenyi Biotec, Auburn, CA). The unbound CD8 memory T cells were then processed using the Human CD28 Microbead Kit (MACS, Miltenyi Biotec). The unlabeled CD28− memory CD8 T cells not bound by the column were collected followed by elution of the CD28+ memory CD8 T cells from the column.
Mixed lymphocytes reaction (MLR)
The isolated CD28+ and CD28− memory CD8 T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen, Carlsbad, CA) at a concentration of 0.5 μM per 107 cells at 37°C for 10 minutes, washed and suspended in culture medium RPMI + 10% human AB serum. Allogeneic B cell lines from 3 individuals were mixed together for use as stimulator cells and were treated with 3000 Rad γ-irradiation (29). Recombinant human IL-15 (BioLegend, San Diego, CA), recombinant human IL-2 (Invitrogen, Carlsbad, CA), and recombinant human IL-7 (BioLegend, San Diego, CA) were added to the cultures. After 24–96 hours, cells were stained with antibodies and CFSE dilution was assessed by flow cytometry.
Flow cytometry
Isolated PBMC or cultured cells were analyzed for expression of surface markers using multi-color flow cytometry. Cell aliquots were treated with anti-human Fc mAb for 20 minutes and stained for 30 minutes with selected combinations of fluorochrome-conjugated antibodies: anti-human CD4-Alexa-PE 700 (Invitrogen, Carlsbad, CA), CD8-APC/Cy7 (BD Pharmingen, San Jose, CA), CD28-PE (BD Pharmingen, San Jose, CA), CD45RO-PerCP/Cy5.5 (BioLegend, San Diego, CA), CD25-PE/Cy7 (BioLegend, San Diego, CA), CD127-APC (BioLegend, San Diego, CA), ICOS-PerCP/Cy5.5 (BioLegend, San Diego, CA), IL15Rα (CD215)-PerCP (R&D systems, Minneapolis, MN), and CD107a-PE/Cy5 (BD Pharmingen, San Jose, CA). The cells were analyzed on a BD LSR II (BD Biosciences, San Jose, CA). Flow cytometry data was analyzed by FlowJo software version 9.4.5 (Tree Star, Inc. Ashland, OR).
qPCR analysis
Cells were harvested and stored at −80ºC in RNAlater. RNA was isolated using the RNeasy Micro Kit (Qiagen). The entire RNA sample was reverse transcribed in a 20 ul reaction using the High Capacity cDNA Reverse Transcription Kit (Life Technologies). cDNA aliquots were analyzed by qPCR using 1 ul of inventoried TaqMan assay for 18S rRNA (Hs99999901_s1) or IL-15ra (Hs00542604_m1) and 10 ul of Fast Universal Master Mix in a 20 ul reaction. Gene expression was quantified by the 2−ΔΔCT method (30).
Assessment of effector function
CD28+ and CD28− memory CD8 T cells were stained and analyzed for Lysosomal-associated membrane protein 1 (LAMP-1/CD107a) using anti-CD107a-PE/Cy5 mAb (BD Pharmingen, San Jose, CA). Culture supernatants were collected, stored at −80° C and subsequently analyzed for IFN-γ by Human IFN-gamma DuoSet (R&D systems, Minneapolis, MN) and TNF-α by Human TNF-alpha DuoSet (R&D systems, Minneapolis, MN) as detailed in the manufacturer’s instructions.
Statistical analysis
All analyses were performed by JMP software version 9.0 (SAS Institute Inc., Cary, NC). Values are shown as mean ± SEM. Comparison of mean values was tested using the standard t test for independent samples (two-tailed). Wilcoxon rank test was used when the continuous data was not normally distribute. Two-sided p values < 0.05 were considered to indicate statistical significance.
Results
CD28−CD8+ memory T cells proliferate poorly to allogeneic stimulators in vitro
CD28+ and CD28− memory CD8+ memory T cells were isolated by magnetic separation, labeled with CFSE and co-cultured with a mixture of 3 allogeneic B cell lines to increase the number of potential allogeneic class I HLA targets presented to each test T cell sample. The purity of the RO+CD28+CD8+ memory T cells was greater than 95% and that of RO+CD28−CD8+ memory T cells greater than 90%. Proliferation of the CFSE-labeled memory CD8+ T cell populations was compared four days after culture initiation,. The allogeneic B cells induced strong CD28+ memory CD8 T cell proliferation whereas CD28− memory CD8 T cell proliferation was low-absent (Figure 1).
Figure 1. CD28+ but not CD28− memory CD8 T cells proliferate in response to allogeneic stimulation.
(A) CD28+ and CD28− RO+ CD8 T cells were isolated from peripheral blood mononuclear cells, labeled with CFSE, and aliquots cultured with a mixture of 3 different allogeneic B cell lines. After 96 hours, the cells were collected, washed and the dilution of CFSE by the CD8 T cells was analyzed in a histogram as an indication of proliferation. Representative results from a single experiment of 6 different experiments having similar results in each are shown. (B) Cumulative data of proliferating memory CD28+ vs. CD28− memory CD8 T cells from the 6 different experiments is shown with mean percent proliferation of each T cell population ± SD. *p < 0.01
CD28nullCD8+ memory T cells proliferate with addition of IL-15 to the culture
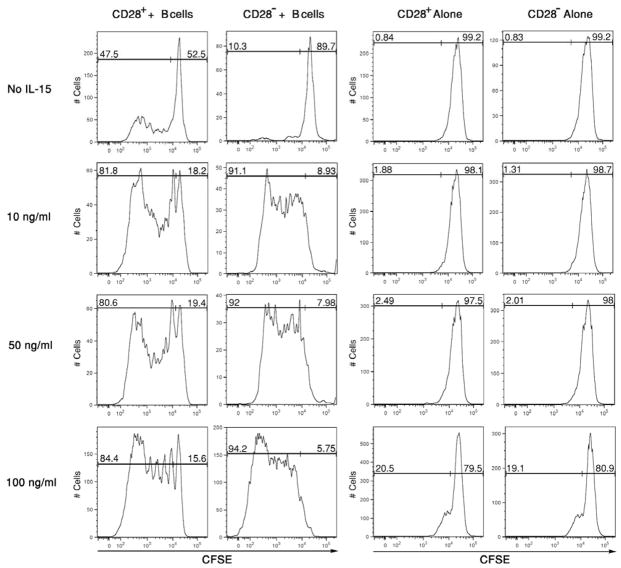
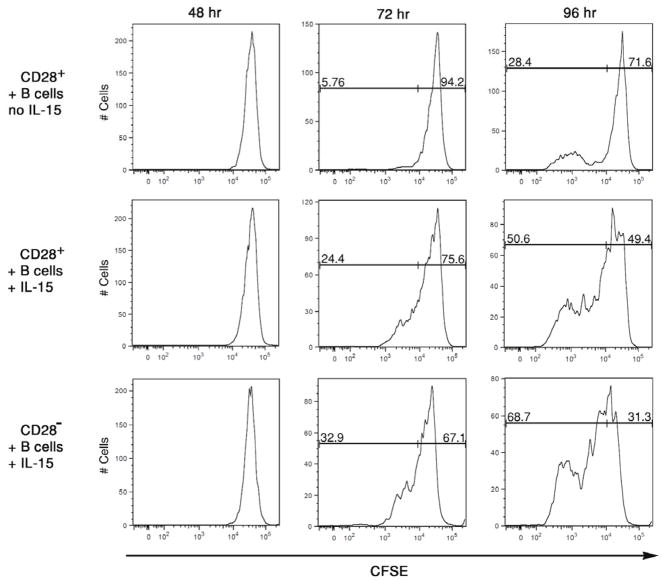
Candidate T cell proliferative cytokines were tested for the ability to induce CD28− memory CD8 T cell proliferation in the cultures. Since IL-15 is required for maintenance of memory CD8 T cell levels (31, 32), this was tested first. CD28+ and CD28− memory CD8 T cells were isolated, labeled with CFSE, and cultured for 96 hours with pooled allogeneic B cells in the presence of various doses of recombinant human IL-15. The absence of CD28− memory CD8 T cell proliferation during culture with allogeneic B cells was reversed by addition of as little as 10 ng/ml of IL-15 to the cultures and this proliferation increased with increasing amounts of the cytokine added (Figure 2). Addition of IL-15 also increased the number of proliferation cycles of CD28+ memory CD8 T cells versus that observed in co-cultures with allogeneic B cells alone. Proliferation of the CD28− memory CD8 T cells required both allogeneic stimulation plus IL-15, as no proliferation was observed in cultures without allogeneic B cells except in cultures with high amounts (100 ng/ml) of IL-15. Peaks of non-proliferating memory CD8 T cells observed with added IL-15 were similar in all cultures, consistent with low dose IL-15 enhancement of CD28+ and CD28− memory CD8 T cell proliferation restricted to T cells reactive to the allogeneic B cells. Proliferation of the CD28− and CD28+ memory CD8 T cells in cultures with allogeneic stimulator cells plus IL-15 was detectable as early as 72 hours after culture initiation (Figure 3). In contrast, proliferation of CD28+ memory CD8 T cells cultured with allogeneic B cells in the absence of IL-15 was not detected until 96 hours after culture initiation (data not shown).
Figure 2. Addition of IL-15 rescues the proliferation of CD28− memory CD8+ T cells alone in response to alloantigen stimulation.
CD28+ and CD28− RO+ CD8 T cells were isolated from peripheral blood mononuclear cells, labeled with CFSE, and aliquots cultured with or without a mixture of 3 different allogeneic B cell lines in the absence or presence of the indicated amounts of recombinant IL-15. After 96 hours, the cells were collected, washed and the dilution of CFSE by the CD8 T cells was analyzed in a histogram as an indication of proliferation. Representative results from a single experiment of 4 different experiments having similar results in each are shown.
Figure 3. IL-15 accelerates the proliferation of CD28+ memory CD8 T cells during activation by alloantigen.
CD28+ and CD28− RO+ CD8 T cells were isolated from peripheral blood mononuclear cells, labeled with CFSE, and aliquots cultured with a mixture of 3 different allogeneic B cell lines in the absence or presence of 10 ng/ml of recombinant IL-15. At the indicated time after culture initiation, the cells were collected, washed and the dilution of CFSE by the CD8 T cells was analyzed in a histogram as an indication of proliferation. Representative results from a single experiment of 4 different experiments having similar results in each are shown.
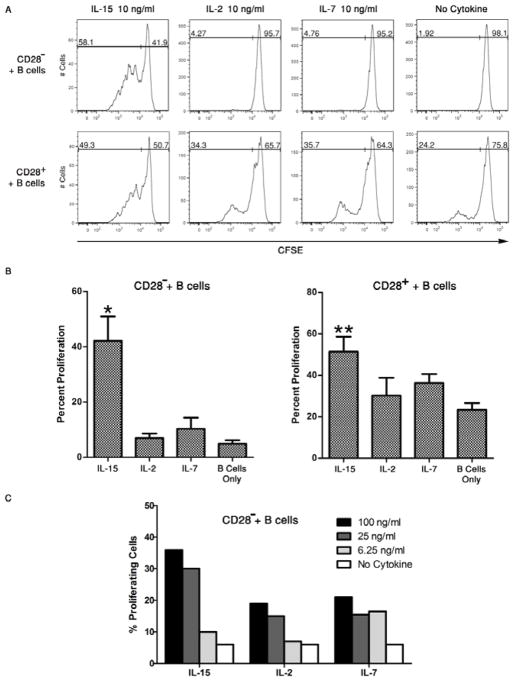
The ability of other T cell proliferative cytokines, IL-2 and IL-7, to provoke proliferation of the CD28− and CD28+ memory CD8 T cells during culture with allogeneic B cells was compared with IL-15 induced proliferation of the T cells. Equivalent amounts (10 ng/ml) of recombinant human IL-15, IL-2 or IL-7 were added at the initiation of CFSE-labeled CD28− and CD28+ CD8 memory T cell co-cultures with pooled allogeneic B cells and after 96 hours memory CD8 T cell proliferation was assessed. Of the 3 test cytokines, only IL-15 induced proliferation of the CD28− memory CD8 T cells (Figure 4A). Whereas IL-15 strongly enhanced the proliferation of the CD28+ memory CD8 T cells, proliferation of these T cells was modestly enhanced by addition of IL-2 or IL-7 to the cultures. Cumulative data from 4 individual experiments confirmed the IL-15 mediated enhancement of proliferation of both memory CD8 T cell populations and the modest effects of IL-2 and IL-7 on CD28− memory CD8 T cell proliferation and the modest effects on CD28+ memory CD8 T cell proliferation (Figure 4B). These studies were extended by titrating amounts of the IL-15, IL-2 and IL-7 added to the co-cultures of isolated CD28− memory CD8 T cells and allogeneic B cells (Figure 4C). Consistent with the previous results addition of 25 or 100 ng/ml of IL-2 or IL-7 had modest effects in stimulating proliferation of the CD28− memory CD8 T cells when compared to the proliferation induced by equivalent amounts of IL-15.
Figure 4. Proliferation of alloreactive CD28− memory CD8 T cells is rescued by IL-15 but not IL-2 or IL-7.
(A) CD28+ and CD28− RO+ CD8 T cells were isolated from peripheral blood mononuclear cells, labeled with CFSE, and aliquots cultured with or without a mixture of 3 different allogeneic B cell lines in the absence or presence of 10 ng/ml of recombinant IL-15, IL-2 or IL-7. After 96 hours, the cells were collected, washed and the dilution of CFSE by the CD8 T cells was analyzed in a histogram as an indication of proliferation. Representative results from a single experiment of 4 different experiments having similar results in each are shown. (B) Cumulative data of proliferating memory CD28+ vs. CD28− memory CD8 T cells from the 4 different experiments is shown with mean percent proliferation of each T cell population ± SD. *p < 0.001 for CD28− cells with allogeneic B cells plus IL-15 vs. all other groups and **p < 0.01 for CD28+ cells with allogeneic B cells plus IL-15 vs. T cells cultured with B cells only. (C) CD28− RO+ CD8 T cells were isolated from peripheral blood mononuclear cells, labeled with CFSE, and aliquots cultured with or without a mixture of 3 different allogeneic B cell lines in the absence or presence of the indicated concentrations of recombinant IL-15, IL-2 or IL-7. After 96 hours, the cells were collected, washed and the dilution of CFSE by the CD8 T cells was analyzed and the proliferation observed reported as in Figures 2 and 3. Representative results from a single experiment of 3 different experiments having similar results in each are shown.
Proliferating CD28−CD8+ memory T cells upregulate expression of activation markers
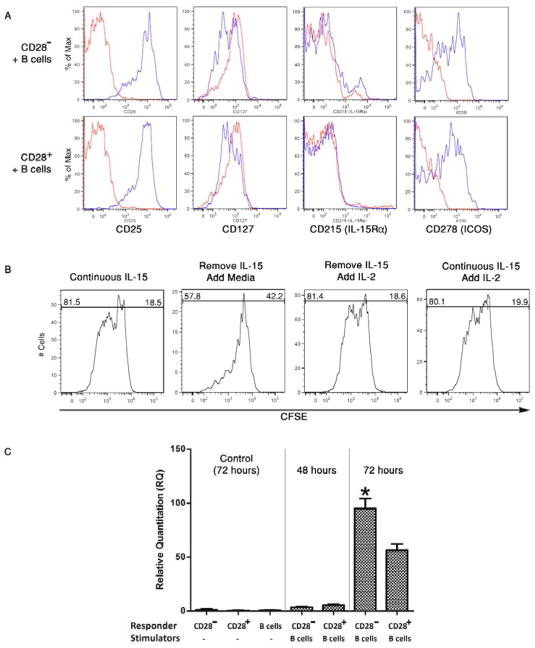
CD8 memory T cells were stained with fluorochrome-conjugated antibodies to detect selected activation markers including CD25, CD127, CD45RO, ICOS and CD215 (IL15 receptor α chain) at the start of culture and 96 hours after culture with pooled allogeneic B cells and 10 ng/ml IL-15. The fluorescent intensity of stained cells was compared between proliferating and non-proliferating cells. Upon proliferation, both CD28− and CD28+ memory CD8 T cells expressed high levels of CD25 and ICOS when compared to non-proliferating cells (Figure 5A). The expression levels of the IL-2 receptor α chain, CD25, and ICOS were comparable between the proliferating CD28− and CD28+ memory CD8 T cells and addition of IL-15 did not increase expression of these markers in the CD28+ memory CD8 T cells. In contrast, the IL-7 receptor α chain, CD127, decreased slightly in proliferating CD28− and CD28+ memory CD8 T cells when compared to non-proliferating cells. Expression of the IL-15 receptor α chain, CD215, was low-absent on both proliferating and non-proliferating CD28− and CD28+ memory CD8 T cells.
Figure 5. IL-15 induces proliferation of alloantigen-reactive CD28− memory CD8 T cells and their expression of activation markers.
(A) CD28+ and CD28− RO+ CD8 T cells were labeled with CFSE and aliquots cultured with allogeneic B cells plus 10 ng/ml of recombinant IL-15. After 96 hours, the cultured cells were stained with fluorochrome labeled antibodies to detect expression of CD25, CD127, CD215 and ICOS on gated T cells that were (blue line) or were not (red line) proliferating as assessed by CFSE dilution. Representative results from a single experiment of 5 different experiments having similar results in each are shown. (B) CD28− memory CD8 T cells were isolated, labeled with CFSE, and aliquots cultured with pooled allogeneic B cells in culture media with 10 ng/ml IL-15. After 24 hours, the IL-15 supernatant was removed and replaced with media alone or media with 10 ng/ml IL-2. IL-2 was also added without removal of IL-15. All cells were analyzed for proliferation at 96 hours after culture initiation. Representative results from a single experiment of 4 different experiments having similar results in each are shown. (C) CD28+ and CD28− RO+ CD8 T cells were isolated, labeled with CFSE, and aliquots cultured with or without allogeneic B cell lines in the presence of 10 ng/ml IL-15. After 48 or 72 hours, the cells were collected and whole cell RNA isolated for PCR analysis of IL-15 receptor α chain. The results indicate the mean relative quantitation (RQ) of IL-15Rα in the memory CD8 T cells cultured alone or with allogeneic B cells ± SD for 4 individual samples. *p < 0.01 for expression of CD28− vs. CD28+ memory T cells.
The function of CD25 expression on proliferating CD28− memory CD8 T cells after stimulation with allogeneic B cells plus IL-15 was tested. Cultures of CFSE-labeled CD28− memory CD8 T cells and pooled allogeneic B cell stimulators were initiated in the presence of 10 ng/ml IL-15. After 24 hours, the culture supernatant was carefully removed and replaced with media alone or media containing 10 ng/ml IL-2 and the cells were analyzed for proliferation 72 hours later. IL-2 was also added in separate wells without removal of the media containing IL-15. Removal of IL-15 at 24 hours and replacement with media alone blunted proliferation of CD28− memory CD8+ T cells (Figure 5B). Addition of IL-2 at 24 hours after culture initiation with IL-15 enhanced proliferation of CD28− memory CD8 T cells whether IL-15 media was removed 24 hours after culture initiation or not, indicating that continuous stimulation with either IL-2 or IL-15 was required to provoke optimal proliferation of the IL-15 sensitized alloreactive memory T cells.
Expression of the IL-15 receptor α chain by CD28− or CD28+ memory CD8 T was further tested by qRT-PCR of RNA isolated from CD28− and CD28+ memory CD8 T cells cultured with allogeneic B cells plus IL-15 (Figure 5C). Both CD28− and CD28+ memory CD8 T cells expressed low levels of IL-15 receptor α mRNA within 48 hours after allogeneic stimulation and this expression increased markedly within another 24 hours of culture, with the highest expression observed by the CD28− versus CD28+ memory CD8 T cells.
Proliferating CD28−CD8+ memory T cells express effector functions
The cytotoxic effector function of primary effector CD8 and memory CD8 T cells is mediated in part by perforin and granzyme B stored in cytolytic granules that are released upon contact with target cells. The membrane of these cytolytic granules is lined by LAMP-1, CD107a, which is expressed on the cell surface following this degranulation and the level of CD107a expression correlates with cytotoxic activity (33, 34). The expression of CD107a was tested on proliferating CD28− and CD28+ memory CD8 T cells during culture with pooled allogeneic B cells with or without IL-15 (Figure 6A). Both proliferating and non-proliferating CD28− and CD28+ memory CD8 T cells expressed low levels of CD107a during early cycles of proliferation and expressed higher levels with increased proliferative cycles (Figure 6B). Within similar proliferative cycles, CD28− memory CD8 T cells expressed higher levels of CD107a when compared to CD28+ memory CD8 T cells (Figure 6B).
Figure 6. IL-15 induced effector function of alloantigen reactive CD28− and CD28+ memory CD8 T cells.
(A) CD28+ and CD28− RO+ CD8 T cells were labeled with CFSE and aliquots cultured with allogeneic B cell lines with 10 ng/ml of recombinant IL-15. After 96 hours, the cultured cells were stained with fluorochrome labeled antibodies to detect expression of CD107a expression. The CFSE dilution patterns were used to gate the T cells into non-proliferating (gate A), early proliferating (gate B) and late proliferating (gate C) cells and the expression levels of CD107a on T cells in each gate were determined. Representative results from a single experiment of 4 different experiments having similar results in each are shown. (B) CD107a expression was compared by overlaying histograms of late proliferating CD28− (red) and CD28+ (green) memory CD8 T cells following 96 hours of culture with allogeneic B cells plus IL-15. (C) After 96 hours, supernatants were removed from cultures of CD28+ and CD28− RO+ CD8 T cells with allogeneic B cells plus 10 ng/ml IL-15. Production of IFN-γ and TNF-α was tested by ELISA and expressed as mean concentration ± SD for 4 samples per group. *p < 0.01 for cultures with IL-15 vs. those without IL-15.
Culture supernatants were harvested after 96 hours and tested for memory CD8 T cell production of IFN-γ and TNF-α. Both CD28− and CD28+ memory CD8 T cells produced IFN-γ during culture with allogeneic B cells and IFN-γ production by CD28−, but not by CD28+, memory CD8 T cells was significantly enhanced by IL15 (Figure 6C). Similarly, CD28− memory CD8 T cells produced low levels of TNFα during culture with allogeneic B cells and this production was enhanced by IL-15. In contrast to IFN-γ production, CD28+ memory CD8 T cell production of TNFα was also significantly enhanced by IL-15. Addition of IL-15 to CD28− or CD28+ memory CD8 T cells in the absence of allogeneic stimulation did not induce IFN-γ or TNFα production.
IL-15 confers resistance of alloantigen-induced proliferation of CD28+ and CD28− memory CD8+ memory T cells to costimulation blockade with CTLA-4Ig
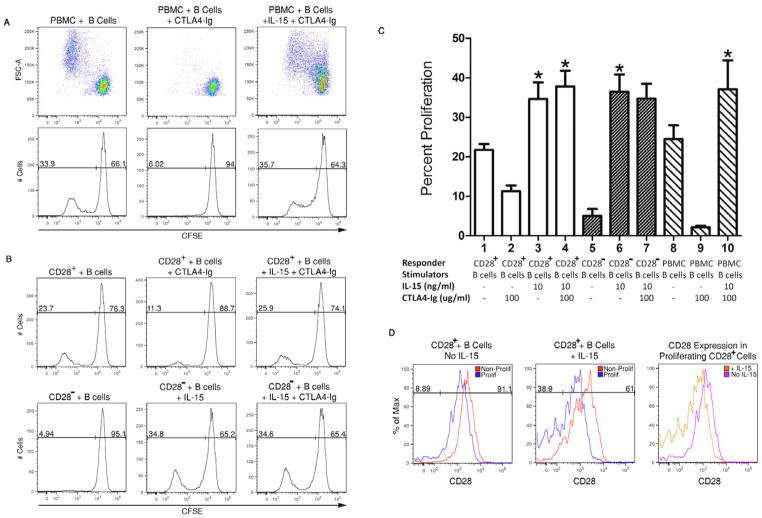
To begin to assess the proliferation of memory CD8 T cells in the presence of CD28/B7 costimulation blockade, 100 ug/ml of CTLA-4Ig was added at the initiation of PBMC co-cultures with allogeneic B cells with or without IL-15 (Figure 7A) and the proliferation of T cells in the PBMC cultures was analyzed by flow cytometry after 96 hours. CTLA-4Ig markedly inhibited proliferation of the T cells in response to allogeneic stimulation (Figure 7A), but IL-15 restored the proliferation of the alloreactive T cells in the presence of CLTA-4Ig. The impact of CTLA-4Ig on the proliferation of isolated CD28+ and CD28− memory CD8 T cells was then tested (Figure 7B). Similar to the PBMC, addition of CTLA-4Ig had a marked inhibitory effect on the alloantigen-induced proliferation of the CD28+ memory CD8 T cells but this inhibition was nullified in the presence of IL-15. Similarly, the proliferation of CD28− memory T cells induced by allogeneic B cells plus IL-15 was resistant to addition of CTLA-4Ig to the cultures. Cumulative data from 4 individual experiments confirmed that addition of CTLA-4Ig inhibited proliferation of CD28+ memory CD8 T cells during co-culture with allogeneic B cells but that this inhibition was nullified by addition of IL-15 (Figure 7C). CTLA-4Ig was also completely ineffective at inhibiting the proliferation of CD28− memory CD8 T cells cultured with allogeneic B cells in the presence of IL-15.
Figure 7. IL-15 confers resistance of alloreactive memory CD8 T cells to CD28 costimulatory blockade.
(A) Peripheral blood mononuclear cells (PBMC) were labeled with CFSE cultured with irradiated pooled allogeneic B cells +/− 10 ng/ml IL-15 in the presence of absence of 100 ug/ml CTLA-4Ig. Proliferation of the (CD3+) T cells was analyzed by flow cytometry 96 after initiation of the cultures. (B) Proliferation of CD28+ versus CD28− memory CD8 T cells alone or in response to allogeneic B cells plus 10 ng/ml IL-15 in the presence or absence of 100 ug/ml CTLA-4Ig. (C) Cumulative data from 4 separate experiments indicating the ability of IL-15 to confer resistance of alloantigen-reactive memory CD8 T cell or peripheral blood T cell proliferation to CTLA-4Ig. *p < 0.01 compared to all cultures with CTLA-4Ig but without IL-15. (D) Isolated CD28+ memory CD8 T cells were labeled with CFSE and cultured with pooled allogeneic B cells in the presence or absence of 10 ng/ml IL-15. After 96 hours, the cultured cells were washed and stained with anti-CD28 mAb and the expression of CD28 on non-proliferating and proliferating CD8 T cells was determined by flow cytometry. In the third (far right)panel, the expression of CD28 was compared on proliferating CD28+ memory CD8 T cells following 96 hours of cultures with the allogeneic B cells +/− IL-15 by overlaying the histograms from each of the other two panels.
Finally, the down-regulation of CD28 expression on proliferating CD28+ memory CD8 T cells in the presence of IL-15 was investigated as a potential mechanism underlying the resistance of the alloreactive CD28+ memory T cells to CTLA-4Ig mediated costimulation blockade (Figure 7D). Proliferating T cells from cultures of CD28+ memory CD8 T cells and allogeneic B cells in the absence of IL-15 had slight decreases in CD28 expression when compared to non-proliferating CD28+ memory CD8 T cells. In contrast, proliferating CD28+ T cells in allogeneic B cells plus IL-15 co-cultures had further decreases in CD28 expression with almost 40% of the proliferating T cells completely transitioning to CD28− cells. These decreases in CD28 expression on proliferating CD28+ memory T cells in cultures with allogeneic B cells with versus without IL-15 were more accurately depicted when the histograms of the proliferating T cells from each of the cultures were overlaid (Figure 7D).
Discussion
Accumulation of CD28− memory CD8+ T cells is a prominent feature of age-associated changes in T cell function (12, 13). The increased accumulation of CD28− memory T cells with age presents a particular problem in clinical transplantation as the incidence of end-stage organ disease also increases with age. Increased frequencies of CD28− memory T cells in renal transplant patients prior to the transplant are associated with increased incidence of acute and chronic graft injury (21–23). We have observed an association between increased frequencies of CD28− memory CD8 T cells and acute rejection within the first two years in renal transplant patients given anti-thymocyte globulin at the time of transplant (manuscript submitted). Yet a proposed role for CD28− memory T cells in graft rejection is not consistent with studies reporting suboptimal or absent proliferation of these T cells in response to culture with antigens or mitogens (14–20). Since T cell mediated graft injury and rejection requires clonal expansion to generate sufficient numbers of donor-reactive T cells to mediate the injury, it remained unclear if donor-reactive CD28− memory CD8 T cells could expand to achieve sufficient numbers in response to a graft.
We reasoned that differences in the conditions in allogeneic mixed lymphocyte cultures from the inflammatory conditions induced in an allograft are likely to underlie observations of the poor proliferation of CD28− memory CD8 T cells in vitro. Consistent with studies testing anti-T cell receptor- or mitogen-mediated activation (16, 19, 35), we report that isolated CD28− memory CD8+ T cells do not proliferate during culture with allogeneic B cells. However, robust proliferation of the CD28− memory CD8 T cells is provoked when IL-15, but not IL-2 or IL-7, is added to the co-cultures and proliferation of the CD28+ memory CD8 T cells is further enhanced in the presence of IL-15. IL-15 was considered as a primary candidate that might induce the proliferation of alloreactive CD28− memory CD8 T cells based on two sets of studies from many other laboratories. First, studies from many laboratories have demonstrated that IL-15 induces the proliferation of primary effector and memory CD8 T cells during responses to viruses, bacteria, and allografts and provides critical signals for the development and homeostasis of memory CD8 T cells, NK cells and NK T cells (36–41). Importantly in the context of acute graft injury, IL-15 also induces CD8 T cells and NK cells to express cytotoxic activity (42–45). Second, in response to inflammatory stimuli IL-15 is produced by many different cells that memory CD8 T cells encounter in allografts including endothelial cells, epithelial cells, fibroblasts, dendritic cells, and monocytes (36, 37, 39). Production of IL-15 is upregulated in many autoimmune and chronic inflammatory disorders as well as in grafts shortly after reperfusion and production by renal graft tubular epithelial cells has been implicated in the development of post-transplant renal tubulitis (46–53). Furthermore, systemic blockade of IL-15 in mouse and non-human primate models results in substantial prolongation in allograft survival (54–56), implicating IL-15 as a key cytokine in the development of CD8 T cell mediated allograft injury. As anticipated, IL-15 production by the B cell lines used as allogeneic stimulator cells in our studies is not detected (O. Traitanon, data not shown).
IL-15 binds to the induced IL-15 receptor α chain in complex with the IL-2R/IL-15Rβ (CD122) and the common gamma (γc) chain (CD132) to deliver proliferative signaling to CD8 T cells and NK cells. Consistent with the IL-15 driven proliferation, memory CD28−CD8 T cells stimulated with allogeneic B cells induced IL-15 receptor α chain expression within two days of culture initiation. PCR analysis of alloantigen stimulated cells indicated higher expression of the IL-15 receptor α chain in the CD28− versus CD28+ memory CD8 T cells suggesting either the presence of mechanisms regulating this expression following T cell receptor engagement with allogeneic class I MHC between the two memory CD8 T cell populations or differences in the frequencies of alloreactive cells in the two populations. Preliminary studies using ELISPOT assays indicate that the CD28− memory CD8 T cells do not have higher alloreactive frequencies when compared to the CD28+ memory CD8 T cells (J. Bechtel, data not shown). Importantly, IL-15 did induce the CD28− memory CD8 T cells to express CD25 and conferred responsiveness of the T cells to IL-2-mediated proliferative signaling. Since memory CD4 T cells also infiltrate grafts and are activated to produce IL-2, the presence of IL-15 is likely to synergize with the IL-2 to enhance proliferation of the CD28− memory CD8 T cells within the graft.
IL-15 also induced CD28− and CD28+ memory CD8 T cells to express the costimulatory molecule ICOS and CD107a, an indicator of cytolytic function. Within similar proliferative cycles, CD28− memory CD8 T cells had higher CD107a expression when compared to CD28+ memory CD8 T cells (Figure 7B), suggesting greater sensitivity to IL-15 through the increased expression of the IL-15 receptor α chain. These results are consistent with observations of increased cytolytic activity by human CD28− versus CD28+ memory CD8 T cells (15, 18, 57). In a mouse model, allograft-infiltrating memory CD8 T cell proliferation induces expression of ICOS and interaction of ICOS with its ligand B7RP-1 within the graft is required for memory CD8 T cell expression of effector function to produce IFN-γ (26). The possible ICOS-induced production of IFN-γ and/or expression of CD107a by CD28− or CD28+ memory CD8 T cells are currently under investigation.
The source of IL-15 that would induce the proliferation and activation of the CD28− memory CD8 T cells in response to and within grafts is unknown at this time. One potential source is that IL-15 is produced in peripheral lymphoid tissues during CD28− memory CD28 T cells interaction with alloantigen. Lymphoid endothelial cells, monocyte/macrophages and/or dendritic cells may produce and/or present IL-15 to provoke activation of the memory CD8 T cells interacting with donor-derived antigen-presenting cells in the spleen. However, our previous and current studies in mouse models do not indicate memory CD8 T cell proliferation in allograft recipient spleens and that it is in within the allograft that memory CD8 T cells are stimulated to proliferate (26, 58). In order to become activated to infiltrate the allograft, the CD28− memory CD8 T cells would have to come to arrest on the allograft endothelium and IL-15 would have to be trans-presented by the graft endothelium. This presented IL-15 could be potentially produced directly by the graft endothelial cells, by tubular epithelial cells in the case of kidney grafts, or by donor- or recipient-derived monocytes/macrophages that are interacting with the endothelial as well as within the inflammatory environment in the graft. These possible mechanisms require the endothelium to acquire the IL-15 produced within the graft and present it to the CD28− memory CD8 T cells. In support of such a potential mechanism in allografts, recent studies have documented the trans-presentation of IL-15 by endothelial cells to activate memory T cells and NK cells (59, 60).
Alloantigen-induced proliferation of CD28+ memory CD8 T cells and peripheral blood T cells was completely inhibited in the presence of CTLA-4Ig in the co-cultures. However, sensitization of CD28− and CD28+ memory CD8 T cells with IL-15 conferred resistance to CTLA-4 Ig, suggesting a potential role for both CD28− and CD28+ memory CD8 T cells in the higher early rejection rate observed in the Belatacept study (61). It is important to note that approximately 60% of the proliferating alloantigen-reactive CD8 memory T cells retained low expression of CD28 and yet remained resistant to the presence of CTLA-4Ig. This suggests that down-regulation of CD28 expression may not be the sole mechanism by which IL-15 signaling confers resistance of CD28+ memory CD8 T cells to CTLA-4Ig mediated costimulation blockade. The ability of TNFα to down regulate CD28 expression on human T cells stimulated with anti-T cell receptor antibody has been previously reported (62, 63), but whether this occurs during alloantigen-induced activation of CD28− and/or CD28+ memory CD8 T cells is unknown and warrants further investigation, particularly in light of the availability of TNFα neutralizing reagents approved for clinical use.
In summary, we have demonstrated that CD28− memory CD8 T cells are activated to proliferate and express effector functions in response to allogeneic cells when IL-15 is present, supporting this as an underlying mechanism of CD28− memory CD8 T cell mediated allograft injury. Furthermore, the production of IL-15 within grafts has the added detrimental effect of conferring resistance of donor-reactive CD28+ memory CD8 T cells to CTLA-4Ig. These results suggest that the risk of memory CD8 T cell mediated graft injury is dependent not only on the frequency of the donor-reactive memory T cells but on the constituents of the inflammatory environment that dictate both the functional activities of the infiltrating memory T cells and their ability to resist immunosuppression.
Acknowledgments
This study was supported by NIH grants RO1-AI40459 (RLF) and K23-AI68824 (EDP) and a research grant from the Roche Organ Transplant Research Fund (EDP).
Footnotes
Disclosure
The authors have no financial conflict of interest to declare.
References
- 1.Augustine JJ, Siu DS, Clemente MJ, Schulak JA, Heeger PS, Hricik DE. Pre-transplant IFN-gamma ELISPOTs are associated with post-transplant renal function in African American renal transplant recipients. Am J Transplant. 2005;5:1971–1975. doi: 10.1111/j.1600-6143.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 2.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphcytes is a manifestation of immunologic memory and correlates with the risk of post-transplant rejection episodes. J Immunol. 1999;163:2267–2275. [PubMed] [Google Scholar]
- 3.Adams AB, Williams MR, Jones TR, Shirasugi N, Durham MM, Kaech SM, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford ML, Kirk AD, Larsen CP. Donor-reactive T-cell stimulation history and precursor frequency: barriers to tolerance induction. Transplantation. 2009;87:S69–S74. doi: 10.1097/TP.0b013e3181a2a701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford ML, Larsen CP. Overcoming the memory barrier in tolerance induction: molecular mimicry and functional heterogeneity among pathogen-specific T-cell populations. Curr Opin Organ Transplant. 2010;15:405–410. doi: 10.1097/MOT.0b013e32833b7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welsh RM, markees TG, Woda BA, Daniels KA, Brehm MA, Mordes JP, et al. Virus-induced abrogation of transplantation tolerance induced by donor-specific transfusion and anti-CD154 antibody. J Virol. 2000;74:2210–2218. doi: 10.1128/jvi.74.5.2210-2218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamann D, Kostense S, Wolthers KC, Otto SA, Baars PA, Miedema F, et al. Evidence that human CD8+CD45RA+CD27− cells are induced by antigen and evolve through extensive rounds fo division. Int Immunol. 1999;11:1027–1033. doi: 10.1093/intimm/11.7.1027. [DOI] [PubMed] [Google Scholar]
- 8.Rufer N, Zippelius A, Batard P, Pittet MJ, Kurth I, Corthesy P, et al. Ex vivo characterization of human CD8+ T subsets with distinctive replicative history and partial effector functions. Blood. 2003;102:1779–1787. doi: 10.1182/blood-2003-02-0420. [DOI] [PubMed] [Google Scholar]
- 9.Sze DM, Giesajtis G, Brown RD, Raitakari M, Gibson J, Ho J, et al. Clonal cytotoxic T cells are expanded in myeloma and reside int he CD8+CD57+CD28− compartment. Blood. 2001;98:2817–2827. doi: 10.1182/blood.v98.9.2817. [DOI] [PubMed] [Google Scholar]
- 10.Weekes MP, Carmichael AJ, Wills MR, Maynard K, Sissons JG. Human CD28−CD8+ T cells contain greatly expanded functional virus-specific memory CTL clones. J Immunol. 1999;162:7569–7577. [PubMed] [Google Scholar]
- 11.Wills MR, Okecha G, Weekes MP, Gandhi MK, Sissons PJG, Carmichael AJ. Identification of naive or antigen-experienced human CD8+ T cells by expression of co-stimulation and chemokine receptors: analysis of the human cytomegalovirus-specific CD8+ T cell response. J Immunol. 2002;168:5455–5464. doi: 10.4049/jimmunol.168.11.5455. [DOI] [PubMed] [Google Scholar]
- 12.Boucher N, Dufeu-Duchesne T, Vicaut E, Farge D, Effros RB, Schachter F. CD28 expression in T cell aging and human longevity. Exp Gerontol. 1998;33:267–282. doi: 10.1016/s0531-5565(97)00132-0. [DOI] [PubMed] [Google Scholar]
- 13.Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, et al. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almanzar G, Schwaiger S, Jenewein B, Keller M, Herndler-Brandstetter D, Wurzner R, et al. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005;79:3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azuma M, Phillips JH, Lanier LL. CD28− T lymphocytes: antigenic and functional properties. J Immunol. 1993;150:1147–1159. [PubMed] [Google Scholar]
- 16.Brzezinska A, Magalska A, Szybinska A, Sikora E. Proliferation and apoptosis of human CD8+CD28+ and CD8+CD28− lymphocytes during aging. Exp Gerontol. 2004;39:539–544. doi: 10.1016/j.exger.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 17.Nociari MM, Telford W, Russo C. Posthymic development of CD28−CD8+ T cell subset: age-associated expansion and shift from memory to naive phenotype. J Immunol. 1999;162:3327–3335. [PubMed] [Google Scholar]
- 18.Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy”. J Exp Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheuring UJ, Sabzevari H, Theofilopoulos AN. Proliferative arrest and cell cycle regulation in CD8+CD28− versus CD8+CD28+ T cells. Hum Immunol. 2002;63:1000–1009. doi: 10.1016/s0198-8859(02)00683-3. [DOI] [PubMed] [Google Scholar]
- 20.Tortorella C, Pisconti A, Piazzolla GAS. APC-dependent impairment of T cell proliferation in aging: role of CD28− and IL-12/IL-15-mediated signaling. Mech Ageing Dev. 2002;123:1389–1402. doi: 10.1016/s0047-6374(02)00079-9. [DOI] [PubMed] [Google Scholar]
- 21.Pawlik A, Florczak M, Masiuk M, Dutkiewicz G, Machalinski B, Rozanski J, et al. The expansion of CD4+CD28− T cells in patients with chronic kidney graft rejection. Transplant Proc. 2003;35:2902–2904. doi: 10.1016/j.transproceed.2003.10.061. [DOI] [PubMed] [Google Scholar]
- 22.Studer SM, George MP, Zhu X, Song Y, Velentine VG, Stoner MW, et al. CD28 down-regulation on CD4 T cells is a marker for graft dysfunction in lung transplant recipients. Am J Respir Crit Care Med. 2008;178:765–773. doi: 10.1164/rccm.200701-013OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baeten D, Louis S, Braud C, Braudeau C, Ballet C, Moizant F, et al. Phenotypically and functionally distinct CD8+ lymphocyte populations in long-term drug-free tolerance and chronic rejection in human kidney graft recipients. J Am Soc Nephrol. 2006;17:294–304. doi: 10.1681/ASN.2005020178. [DOI] [PubMed] [Google Scholar]
- 24.Durrbach A, Pestana JM, Pearson T, Vincenti F, Garcia VD, Campistol J, et al. A phase III study of Belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT Study) Am J Transplant. 2010;10:547–557. doi: 10.1111/j.1600-6143.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 25.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Breshahan B, Darji P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transpalnt recipients (BENEFIT Study) Am J Transplant. 2010;10:535–546. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 26.Schenk AD, Gorbacheva V, Rabant M, Fairchild RL, Valujskikh A. Effector functions of donor-reactive CD8 memory T cells are dependent on ICOS induced during division in cardiac grafts. Am J Transplant. 2009;9:64–73. doi: 10.1111/j.1600-6143.2008.02460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schenk AD, Nozaki T, Rabant M, Valujskikh A, Fairchild RL. Donor-reactive CD8 memory T cells infiltrate cardiac allografts within 24-h posttransplant in naive recipients. Am J Transplant. 2008;8:1652–1661. doi: 10.1111/j.1600-6143.2008.02302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Setoguchi K, Hattori Y, Iida S, Baldwin WMr, Fairchild RL. Endogenous memory CD8 T cells are activated within cardiac allografts without mediating rejection. Am J Transplant. 2013;13:2293–2307. doi: 10.1111/ajt.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poggio ED, Clemente M, Hricik DE, Heeger PS. Panel of reactive T cells as a measurement of primed cellular alloimmunity in kidney transplant candidates. J Am Soc Nephrol. 2006;17:564–572. doi: 10.1681/ASN.2005030293. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expreession data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 33.Aktas E, Kucuksezer UC, Bilgic S, Erten G, Deniz G. Relationship between CD107a expression and cytotoxic activity. Cell Immunol. 2009;254:149–154. doi: 10.1016/j.cellimm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 35.Chiu WK, Fann M, Weng N-P. Generation and growth of CD28nullCD8+ memory T cells mediated by IL-15 and its induced cytokines. J Immunol. 2006;177:7802–7810. doi: 10.4049/jimmunol.177.11.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–280. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Di Sabatino, Calarota SA, Vidali F, Macdonald TT, Corazza GR. Role of IL-15 in immune-mediiated and infectious diseases. Cytokine Growth Factor Rev. 2011;22:19–33. doi: 10.1016/j.cytogfr.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Li XC, Demirci G, Ferrari-Lacraz S, Groves C, Coyle A, Malek TR, et al. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat Med. 2001;7:114–118. doi: 10.1038/83253. [DOI] [PubMed] [Google Scholar]
- 39.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 40.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagay Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory pool. J Clin Invest. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fehniger TA, Caligiuri MA. Interleukin-15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 42.Bamford RN, Grant AJ, Burton JD, Peters C, Kurys G, Goldman CK, et al. The interleukin (IL) 2 receptor beta chin is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci USA. 1994;91:4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burton JD, Bamford RN, Peters C, Grant AJ, Kurys G, Goldman CK, et al. A lymphokine, provisionally designated interleukin T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci USA. 1994;91:4935–4939. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawamura T, Koka R, Ma A, Kumar V. Differential roles for IL-15 alpha-chain in NK cell development and Ly-49 induction. J Immunol. 2003;171:5085–5090. doi: 10.4049/jimmunol.171.10.5085. [DOI] [PubMed] [Google Scholar]
- 45.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 46.Ajjan RA, Watson PF, Weetman AP. Detection of IL-12, IL-13, and IL-15 messenger ribonucleic acid in the thyroid of patients with autoimmune thyroid disease. J Clin Endocrinol Metab. 1997;82:666–669. doi: 10.1210/jcem.82.2.3760. [DOI] [PubMed] [Google Scholar]
- 47.Bhorade SM, Yu A, Vigneswaran WT, Alex CG, Garrity ER. Elevation of interleukin-15 protein expression in bronchoalveolar fluid in acute lung allograft rejection. Chest. 2007;131:533–538. doi: 10.1378/chest.06-1257. [DOI] [PubMed] [Google Scholar]
- 48.Manfro RC, Roy-Chaudhury P, Zheng XX, Steiger J, Nickerson PW, Li Y, et al. Interleukin-15 gene transcripts are present in rejecting islet allografts. Transplant Proc. 1997;29:1077–1078. doi: 10.1016/s0041-1345(96)00410-1. [DOI] [PubMed] [Google Scholar]
- 49.Pavlakis M, Strehlau J, Lipman M, Shapiro M, Maslinksi W, Strom TB. Intragraft IL-15 transcripts are increased in human renal allograft rejection. Transplantation. 1996;62:543–545. doi: 10.1097/00007890-199608270-00020. [DOI] [PubMed] [Google Scholar]
- 50.Reksten TR, Jonsson MV, Szyszko EA, Brun JG, Jonsson R, Brokstad KA. Cytokine and autoantibody profiling related to histopathological features in primary Sjogren’s syndrome. Rheumatology. 2009;48:1102–1106. doi: 10.1093/rheumatology/kep149. [DOI] [PubMed] [Google Scholar]
- 51.Robertson H, Kirby JA. Post-transplant renal tubulitis: the recruitment, differentiation, and persistence of intra-epithelial T cells. Am J Transplant. 2003;3:3–10. doi: 10.1034/j.1600-6143.2003.30102.x. [DOI] [PubMed] [Google Scholar]
- 52.Wang WL, Yao MY, Jin J, Jia CK, Gao LH, Xie HY, et al. Increased expression of non-interleukin-2 T cell growth factors and thier implications during liver allograft rejection in rats. J Gastroenterol Hepatol. 2007;22:1141–1147. doi: 10.1111/j.1440-1746.2007.04925.x. [DOI] [PubMed] [Google Scholar]
- 53.Weiler M, rogashev B, Einbinder T, Hausmann MJ, Kaneti J, Cahimovitz C, et al. Interleukin-15, a leukocyte activator and growth factor, is produced by cortical tubular epithelial cells. J Am Soc Nephro. 1998;9:1194–1201. doi: 10.1681/ASN.V971194. [DOI] [PubMed] [Google Scholar]
- 54.Ferrari-Lacraz S, Zheng XX, Kim YS, Li Y, Maslinski W, Li XC, et al. An antagonist IL-15/Fc protein prevents costimulation blockade-resistant rejection. J Immunol. 2001;167:3478–3485. doi: 10.4049/jimmunol.167.6.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrari-Lacraz S, Zheng XX, Sanchez-Fueyo S, Maslinski W, Moll T, Strom TB. CD8+ T cells resistant to costimulatory blockade are controlled by an antagonist interleukin-15/Fc protein. Transplantation. 2006;82:1510–1517. doi: 10.1097/01.tp.0000243168.53126.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koulmanda M, Qipo A, Fan Z, Smith N, Auchincloss H, Zheng XX, et al. Prolonged survival of allogeneic islets in cynomolgus monkeys after short-term triple therapy. Am J Transplant. 2012;12:1296–1302. doi: 10.1111/j.1600-6143.2012.03973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fagnoni FF, Vescovini R, Mazzola M, Bologna G, Nigro E, Lavagetto G, et al. Expansion of cytotoxic CD8+C28− T cells in healthy ageing people, including centenarians. Immunology. 1966;88:501–507. doi: 10.1046/j.1365-2567.1996.d01-689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Su CA, Iida S, Abe T, Fairchild RL. Endogenous memory CD8 T cells directly mediate cardiac allograft rejection. Am J Transplant. 2014:14. doi: 10.1111/ajt.12605. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Menthon M, Lambert M, Guiard E, Tognarelli S, Bienvenu B, Karras A, et al. Excessive interleukin-15 transpresentation endows NKG2D+CD4+ T cells with inante-like capacity to lyse vascular endothelium in granulomatosis with polyangiitis (Wegener’s) Arth Rheum. 2011;63:2116–2126. doi: 10.1002/art.30355. [DOI] [PubMed] [Google Scholar]
- 60.Kitaya K, Yasua T. Regulatory role of membrane-bound form interleukin-15 on human uterine microvascular endothelial cells in circulating CD16(−) natural killer cell extravasation into human endometrium. Biol Reprod. 2013;89:1–7. doi: 10.1095/biolreprod.113.111138. [DOI] [PubMed] [Google Scholar]
- 61.Vincenti F, Larsen CP, Alberu J, Bresnahan B, Garcia VD, Kothan J, et al. Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. Am J Transplant. 2012;12:210–217. doi: 10.1111/j.1600-6143.2011.03785.x. [DOI] [PubMed] [Google Scholar]
- 62.Bryl E, Vallejo AN, Weyand CM, Goronzy JJ. Down-regulation of CD28 expression by TNF-α. J Immunol. 2001;167:3231–3238. doi: 10.4049/jimmunol.167.6.3231. [DOI] [PubMed] [Google Scholar]
- 63.Lewis D, Merched-Sauvage EM, Goronzy JJ, Weyand CM, Vallejo AN. Tumor necrosis factor-α and CD80 modulate CD28 expression through a similar mechanim of T-cell receptor-independent inhibition of transcription. J Biol Chem. 2004;279:29130–29138. doi: 10.1074/jbc.M402194200. [DOI] [PubMed] [Google Scholar]