Abstract
The risk of infection among patients with psoriasis of varying severity in a broadly representative population remains poorly understood. Using The Health Improvement Network (THIN), an electronic medical records database representative of the general United Kingdom population, we performed a cohort study to determine the risks of serious infection, opportunistic infection, and herpes zoster among patients with versus without psoriasis and according to psoriasis severity. We identified 187,258 patients with mild, and 12,442 patients with moderate-to-severe psoriasis based on treatment patterns. Using Cox proportional hazards regression, the adjusted hazard ratios (95% confidence intervals [CI]) for serious infection were 1.18 (1.16–1.21) and 1.63 (1.52–1.75) for the mild and moderate-to-severe psoriasis groups, respectively. Among a nested cohort of 8,569 psoriasis patients with disease severity classified by body surface area involvement, similar results were obtained with the exception of an attenuated but significantly increased risk of serious infection among the moderate-to-severe psoriasis group (1.27 [1.10–1.47]). Overall, the risks of opportunistic infection and herpes zoster were significantly increased only among the moderate-to-severe psoriasis group and were associated with immunosuppressive therapy. Our analyses suggest that psoriasis is associated with an increased risk of serious infection, and psoriasis severity is a predictor of serious infection risk.
INTRODUCTION
Psoriasis is a common, chronic, immune-mediated disease primarily of the skin that affects 2–4% of the general population.(Gelfand et al., 2005; Kurd and Gelfand, 2009) Over the last decade, there have been major advances in our understanding of comorbid diseases associated with psoriasis, particularly cardiometabolic comorbidities.(Azfar et al., 2012; Gelfand et al., 2009; Gelfand et al., 2006; Langan et al., 2012; Mehta et al., 2010; Wan et al., 2013; Yeung et al., 2013) However, despite being the second leading cause of death among psoriasis patients receiving therapies for moderate-to-severe disease,(Abuabara et al., 2010) infection as a comorbidity of psoriasis remains poorly understood. With the development of several targeted biologic therapies that block key cytokines in the development of psoriasis such tumor necrosis factor (TNF), interleukin (IL)-12/23, and IL-17 in the last decade, much effort has been invested into the study of infection risk related to these newer therapies. However, little is known about the risk of infection among all patients with psoriasis and potentially attributable to the disease itself. Based on basic research that has identified increased expression of antimicrobial peptides in psoriasis skin lesions,(Hollox et al., 2008) psoriasis has historically been considered to have protective mechanisms against infection. As there are few data that actually quantify and characterize the risks of various infections among patients with psoriasis, additional studies are necessary to better understand the potential association between psoriasis and infections. Thus, the aim of our study was to determine the risk of serious infection, opportunistic infection, and herpes zoster among patients with psoriasis using a large, population-based electronic medical record database in the United Kingdom, leveraging information on important confounders and direct measures of psoriasis severity that are not typically available in other large databases.
RESULTS
Study Cohort Baseline Characteristics
For our primary study using the full The Health Improvement Network (THIN) cohort, we identified 199,700 patients with psoriasis (187,258 with mild disease and 12,442 with moderate-to-severe disease based on receipt of phototherapy or systemic therapy) and 954,315 randomly-selected patients without psoriasis. Compared with patients without psoriasis, those with psoriasis were younger and were more likely to be current or past smokers (Table 1). Patients with moderate-to-severe psoriasis had higher body mass index (BMI), were more likely to be drinkers, and were more likely to have received systemic corticosteroids than those without psoriasis. Most comorbidities were similarly prevalent among patients with and without psoriasis, reflective of psoriasis patients being generally younger. With regards to flu and pneumonia vaccinations, patients with mild psoriasis were less likely and patients with moderate-to-severe psoriasis were more likely to have received vaccinations than patients without psoriasis. Among patients with moderate-to-severe psoriasis, the majority were treated with methotrexate (69.5%) (Table S1).
Table 1.
Baseline Characteristics: THIN Cohort
| Characteristic N (%) |
No Psoriasis N=954,315 |
All Psoriasis N=199,700 |
Standardized Differencea | Mild Psoriasis N=187,258 |
Standardized Differencea | Moderate-to-Severe Psoriasis N=12,442 |
Standardized Differencea |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Age | |||||||
| mean (SD) | 49.9 (17.6) | 46.6 (17.4) | 0.19 | 46.4 (17.5) | 0.20 | 49.4 (15.1) | 0.07 |
| median (IQR) | 49 (36, 63) | 45 (32, 60) | 45 (32, 60) | 49 (38, 60) | |||
|
| |||||||
| Male sex | 422,290 (44.3) | 96,701 (48.4) | 0.08 | 90,643 (48.4) | 0.08 | 6,058 (48.7) | 0.10 |
|
| |||||||
| Asthma/COPD | 135,925 (14.2) | 28,012 (14.0) | 0.006 | 26,079 (13.9) | 0.009 | 1,933 (15.5) | 0.04 |
|
| |||||||
| Cardiovascular Disease | 92,571 (9.7) | 16,628 (8.3) | 0.05 | 15,486 (8.3) | 0.05 | 1,142 (9.2) | 0.04 |
|
| |||||||
| Chronic Kidney Disease | 21,534 (2.3) | 3,487 (1.8) | 0.04 | 3,131 (1.7) | 0.04 | 356 (2.9) | 0.05 |
|
| |||||||
| Congestive Heart Failure | 15,776 (1.7) | 2,577 (1.3) | 0.03 | 2,400 (1.3) | 0.03 | 177 (1.4) | 0.02 |
|
| |||||||
| Dementia | 5,017 (0.53) | 1,177 (0.59) | 0.009 | 1,137 (0.61) | 0.01 | 40 (0.32) | 0.03 |
|
| |||||||
| Depression | 159,551 (16.7) | 34,472 (17.3) | 0.01 | 31,828 (17.0) | 0.007 | 2,644 (21.3) | 0.12 |
|
| |||||||
| Diabetes Types 1 and 2 | 61,194 (6.4) | 11,817 (5.9) | 0.02 | 10,678 (5.7) | 0.03 | 1,139 (9.2) | 0.11 |
|
| |||||||
| Inflammatory Bowel Disease | 11,005 (1.2) | 2,663 (1.3) | 0.02 | 2,400 (1.3) | 0.01 | 263 (2.1) | 0.07 |
|
| |||||||
| Liver Disease | 4,900 (0.51) | 1,464 (0.73) | 0.03 | 1,320 (0.70) | 0.02 | 144 (1.2) | 0.08 |
|
| |||||||
| Osteoarthritis | 110,495 (11.6) | 20,450 (10.2) | 0.04 | 18,616 (9.9) | 0.05 | 1,834 (14.7) | 0.10 |
|
| |||||||
| Psoriatic Arthritis | 0 (0) | 10,078 (5.0) | NA | 5,365 (2.9) | NA | 4,715 (37.9) | NA |
|
| |||||||
| Rheumatoid Arthritis | 263 (0.03) | 3,063 (1.5) | 0.17 | 1,495 (0.8) | 0.12 | 1,568 (12.6) | 0.53 |
|
| |||||||
| Systemic Lupus Erythematosus | 1,457 (0.15) | 369 (0.18) | 0.008 | 312 (0.17) | 0.004 | 57 (0.46) | 0.05 |
|
| |||||||
| Inhaled Corticosteroids within 90 days prior to start date | 40,612 (4.3) | 7,677 (3.8) | 0.02 | 7,120 (3.8) | 0.02 | 557 (4.5) | 0.009 |
|
| |||||||
| Systemic Corticosteroids within90 days prior to start date | 20,654 (2.2) | 5,199 (2.6) | 0.03 | 3,931 (2.1) | 0.005 | 1,268 (10.2) | 0.34 |
|
| |||||||
| Flu Vaccine | 290,414 (30.4) | 51,485 (25.8) | 0.10 | 46,630 (24.9) | 0.12 | 4,855 (39.0) | 0.18 |
|
| |||||||
| Pneumonia Vaccine | 141,187 (14.8) | 24,000 (12.0) | 0.08 | 21,566 (11.5) | 0.10 | 2,434 (19.6) | 0.13 |
|
| |||||||
| Herpes Zoster Vaccine | 121 (0.01) | 15 (0.01) | 0.005 | 13 (0.01) | 0.006 | 2 (0.02) | 0.004 |
|
| |||||||
| Any hospitalization within 30 days prior to start date | 15,110 (1.6) | 1,760 (0.88) | 0.06 | 1,444 (0.77) | 0.08 | 316 (2.5) | 0.08 |
|
| |||||||
| Infection within 30 days prior to start date | 45,826 (4.8) | 5,870 (2.9) | 0.10 | 5,567 (3.0) | 0.10 | 303 (2.4) | 0.10 |
|
| |||||||
| History of opportunistic infection | 9,696 (1.0) | 1,658 (0.83) | 0.02 | 1,539 (0.82) | 0.02 | 119 (0.96) | 0.009 |
|
| |||||||
| History of herpes zoster | 40,333 (4.2) | 7,451 (3.7) | 0.03 | 6,924 (3.7) | 0.03 | 527 (4.2) | 0.01 |
|
| |||||||
| Smoking History | |||||||
| None | 457,358 (47.9) | 77,739 (38.9) | 72,903 (38.9) | 4,836 (38.9) | |||
| Current | 208,690 (21.9) | 57,717 (28.9) | 0.20 | 54,417 (29.1) | 0.20 | 3,300 (26.5) | 0.33 |
| Past | 191,630 (20.1) | 43,722 (21.9) | 40,154 (21.4) | 3,568 (28.7) | |||
| Missing | 96,637 (10.1) | 20,522 (10.3) | 19,784 (10.6) | 738 (5.9) | |||
|
| |||||||
| Drinking History | |||||||
| None | 108,057 (11.3) | 20,901 (10.5) | 19,524 (10.4) | 1,377 (11.1) | |||
| Some | 609,093 (63.8) | 129,754 (65.0) | 0.03 | 121,476 (64.9) | 0.04 | 8,278 (66.5) | 0.19 |
| A lot | 39,820 (4.2) | 7,883 (4.0) | 7,064 (3.8) | 819 (6.6) | |||
| Missing | 197,345 (20.7) | 41,162 (20.6) | 39,194 (20.9) | 1,968 (15.8) | |||
|
| |||||||
| Body Mass Index | |||||||
| Underweight/Normal | 343,611 (36.0) | 67,717 (33.9) | 64,177 (34.3) | 3,540 (28.5) | |||
| Overweight | 261,401 (27.4) | 54,246 (27.2) | 0.06 | 50,593 (27.0) | 0.05 | 3,653 (29.4) | 0.31 |
| Obese | 158,487 (16.6) | 36,798 (18.4) | 33,424 (17.9) | 3,374 (27.1) | |||
| Missing | 190,816 (20.0) | 40,939 (20.5) | 39,064 (20.9) | 1,875 (15.1) | |||
|
| |||||||
| Townsend Score | |||||||
| 1st Quintile | 237,254 (24.9) | 46,107 (23.1) | 43,106 (23.0) | 3,001 (24.1) | |||
| 2nd Quintile | 200,546 (21.0) | 40,474 (20.3) | 37,855 (20.2) | 2,619 (21.1) | |||
| 3rd Quintile | 190,980 (20.0) | 40,528 (20.3) | 0.06 | 38,030 (20.3) | 0.06 | 2,498 (20.1) | 0.05 |
| 4th Quintile | 168,753 (17.7) | 36,405 (18.2) | 34,225 (18.3) | 2,180 (17.5) | |||
| 5th Quintile | 117,224 (12.3) | 26,442 (13.2) | 24,844 (13.3) | 1,598 (12.8) | |||
| Missing | 39,558 (4.2) | 9,744 (4.9) | 9,198 (4.9) | 546 (4.4) | |||
IQR, interquartile range; SD, standard deviation; NA, not applicable
Standardized difference ≥ 0.1 is considered to indicate meaningful imbalance between groups.(Austin, 2009)
In the Incident Health Outcomes and Psoriasis Events (iHOPE) cohort, we identified 8,569 patients with psoriasis (4,437 had mild disease defined by < 3% body surface area [BSA] involvement; 4,132 had moderate-to-severe disease defined by ≥ 3% BSA involvement, of whom 25.7% had > 10% BSA involved) and 83,540 matched patients without psoriasis (Table S2). Patients with psoriasis had higher BMI and were more likely to be current or past smokers. Comorbidities were similarly prevalent among patients with and without psoriasis, and patients with moderate-to-severe psoriasis were more likely to have received pneumonia vaccination than those without psoriasis.
Serious Infection
The incidence rates of serious infection derived from the full THIN cohort are summarized in Table 2. Patients with psoriasis had a higher incidence of serious infection than patients without psoriasis; the incidence rate was highest among patients with moderate-to-severe disease. The serious infections with the highest incidence rates among patients with psoriasis in descending order were lower respiratory tract, skin and soft tissue, and upper respiratory tract infections (Table 3). In multivariable analyses, psoriasis was associated with an increased risk of serious infection with adjusted hazard ratios (HRs) of 1.21 (95% confidence interval [CI], 1.18–1.23), 1.18 (1.16–1.21), and 1.63 (1.52–1.75) for the overall, mild, and moderate-to-severe psoriasis groups, respectively (Table 2). While effect modification by age and sex were each found to be statistically significant, differences among hazard ratios stratified by age and sex, respectively, were numerically small and, thus, not reported. The results were robust to multiple sensitivity analyses including an analysis that excluded patients who had received immunosuppressive psoriasis treatments (Table 4). The attributable risks of serious infection among all patients with psoriasis and those with mild and moderate-to-severe disease were 16.2, 14.4, and 49.5 per 10,000 person-years, respectively; the excess risks were one serious infection per 616, 693, and 201 patients with any, mild, and moderate-to-severe psoriasis, respectively, per year (data not shown).
Table 2.
Incidence and Risk of Serious Infection
| Full THIN Cohort | No Psoriasis N=954,315 |
All Psoriasis N=199,700 |
Mild Psoriasis N=187,258 |
Moderate-to-Severe Psoriasis N=12,442 |
|---|---|---|---|---|
|
| ||||
| Follow-up time (yrs) | ||||
| Mean (SD) | 6.4 (4.7) | 6.0 (4.7) | 6.1 (4.7) | 5.1 (4.0) |
| Median (IQR) | 5.5 (2.3, 9.8) | 5.0 (2.1, 9.4) | 5.1 (2.1, 9.5) | 4.2 (1.8, 7.5) |
|
| ||||
| Number of person-years | 6,096,846 | 1,207,499 | 1,143,873 | 63,625 |
|
| ||||
| Number (%) of serious infections | 47,875 (5.0) | 10,734 (5.4) | 9,807 (5.2) | 927 (7.5) |
|
| ||||
| Incidence per 10,000 person years (95% CI) | 78.5 (77.8, 79.2) | 88.9 (87.2, 90.6) | 85.7 (84.1, 87.4) | 145.7 (136.6, 155.4) |
|
| ||||
| Unadjusted hazard ratio (95% CI) | Reference | 1.14 (1.12, 1.16) | 1.10 (1.07, 1.12) | 1.97 (1.84, 2.10) |
|
| ||||
| Adjusted hazard ratio (95% CI)a | Reference | 1.21 (1.18, 1.23) | 1.18 (1.16, 1.21) | 1.63 (1.52, 1.75) |
|
| ||||
| iHOPE Cohort |
No Psoriasis N=83,540 |
All Psoriasis N=8,569 |
Mild Psoriasis N=4,437 |
Moderate-to-Severe Psoriasis N=4,132 |
|
| ||||
| Follow-up time (yrs) | ||||
| Mean (SD) | 4.1 (1.7) | 4.2 (1.6) | 4.2 (1.6) | 4.2 (1.6) |
| Median (IQR) | 4.4 (3.1, 5.6) | 4.4 (3.3, 5.6) | 4.4 (3.3, 5.6) | 4.4 (3.3, 5.5) |
|
| ||||
| Number of person-years | 342,169 | 35,664 | 18,435 | 17,229 |
|
| ||||
| Number (%) of serious infections | 2,588 (3.1) | 371 (4.3) | 180 (4.1) | 191 (4.6) |
|
| ||||
| Incidence per 10,000 person years (95% CI) | 75.6 (72.8, 78.6) | 104.0 (94.0, 115.2) | 97.6 (84.4, 113.0) | 110.9 (96.2, 127.8) |
|
| ||||
| Unadjusted hazard ratio (95% CI) | Reference | 1.38 (1.23, 1.53) | 1.29 (1.11, 1.50) | 1.47 (1.27, 1.70) |
|
| ||||
| Adjusted hazard ratio (95% CI)b | Reference | 1.21 (1.09, 1.35) | 1.16 (0.99, 1.35) | 1.27 (1.10, 1.47) |
CI, confidence interval; IQR, interquartile range; SD, standard deviation; yrs, years
Adjusted for age, sex, BMI, smoking and drinking status, asthma/COPD, CVD, CKD, CHF, dementia, depression, diabetes, IBD, liver disease, OA, RA, systemic corticosteroid use within 90 days prior to start date, flu and pneumonia vaccination status, hospitalization or infection within 30 days prior to start date, and Townsend score.
Adjusted for age, sex, BMI, smoking status, asthma/COPD, CVD, CKD, CHF, dementia, depression, diabetes, IBD, liver disease, OA, systemic corticosteroid use within 90 days prior to start date, flu and pneumonia vaccination status, hospitalization or infection within 30 days prior to start date.
Table 3.
Incidence Rates of Serious Infection Subtypes: THIN Cohort
| Serious Infection Type | No Psoriasis N=954,315 |
All Psoriasis N=199,700 |
Mild Psoriasis N=187,258 |
Moderate-to-Severe Psoriasis N=12,442 |
||||
|---|---|---|---|---|---|---|---|---|
| N (%) | Ratea (95% CI) | N (%) | Ratea (95% CI) | N (%) | Ratea (95% CI) | N (%) | Ratea (95% CI) | |
| Lower respiratory tract | 16,156 (1.7) | 26.1 (25.7, 26.5) | 3,571 (1.8) | 29.0 (28.1, 30.0) | 3,244 (1.7) | 27.8 (26.9, 28.8) | 327 (2.6) | 49.8 (44.7, 55.5) |
| Skin and soft tissue | 7,426 (0.78) | 11.9 (11.7, 12.2) | 2,022 (1.0) | 16.4 (15.7, 17.1) | 1,817 (0.97) | 15.5 (14.9, 16.3) | 205 (1.6) | 31.1 (27.1, 35.6) |
| Upper respiratory tract | 8,631 (0.90) | 13.9 (13.6, 14.2) | 2,006 (1.0) | 16.3 (15.6, 17.0) | 1,817 (0.97) | 15.6 (14.9, 16.3) | 189 (1.5) | 28.7 (24.9, 33.1) |
| Urinary tract | 7,956 (0.83) | 12.8 (12.5, 13.1) | 1,581 (0.79) | 12.8 (12.2, 13.4) | 1,447 (0.77) | 12.4 (11.7, 13.0) | 134 (1.1) | 20.2 (17.1, 24.0) |
| Conjunctivitis | 1,491 (0.16) | 2.4 (2.3, 2.5) | 348 (0.17) | 2.8 (2.5, 3.1) | 325 (0.17) | 2.8 (2.5, 3.1) | 23 (0.18) | 3.5 (2.3, 5.2) |
| Gastrointestinal | 1,334 (0.14) | 2.1 (2.0, 2.3) | 312 (0.16) | 2.5 (2.3, 2.8) | 279 (0.15) | 2.4 (2.1, 2.7) | 33 (0.27) | 5.0 (3.5, 7.0) |
| Sepsis | 1,257 (0.13) | 2.0 (1.9, 2.1) | 305 (0.15) | 2.5 (2.2, 2.8) | 269 (0.14) | 2.3 (2.0, 2.6) | 36 (0.29) | 5.4 (3.9, 7.5) |
| Cholecystitis/cholangitis | 1,271 (0.13) | 2.0 (1.9, 2.2) | 253 (0.13) | 2.0 (1.8, 2.3) | 234 (0.12) | 2.0 (1.8, 2.3) | 19 (0.15) | 2.9 (1.8, 4.5) |
| Abdominal abscess | 177 (0.019) | 0.28 (0.25, 0.33) | 43 (0.022) | 0.35 (0.26, 0.47) | 42 (0.022) | 0.36 (0.26, 0.48) | 1 (0.008) | 0.15 (0.02, 1.1) |
| Septic arthritis | 128 (0.013) | 0.20 (0.17, 0.24) | 39 (0.020) | 0.31 (0.23, 0.43) | 31 (0.017) | 0.26 (0.19, 0.38) | 8 (0.064) | 1.2 (0.60, 2.4) |
| Osteomyelitis | 127 (0.013) | 0.20 (0.17, 0.24) | 35 (0.018) | 0.28 (0.20, 0.39) | 32 (0.017) | 0.27 (0.19, 0.39) | 3 (0.024) | 0.45 (0.15, 1.4) |
| Breast abscess | 108 (0.011) | 0.17 (0.14, 0.21) | 34 (0.017) | 0.27 (0.20, 0.38) | 31 (0.017) | 0.26 (0.19, 0.38) | 3 (0.024) | 0.45 (0.15, 1.4) |
| Endocarditis | 58 (0.006) | 0.093 (0.072, 0.12) | 12 (0.006) | 0.097 (0.055, 0.17) | 11 (0.006) | 0.094 (0.052, 0.17) | 1 (0.008) | 0.15 (0.02, 1.07) |
| Prostate | 38 (0.004) | 0.061 (0.044, 0.084) | 8 (0.004) | 0.064 (0.032, 0.13) | 7 (0.004) | 0.060 (0.028, 0.13) | 1 (0.008) | 0.15 (0.021, 1.1) |
| Central nervous system | 37 (0.004) | 0.059 (0.043, 0.082) | 8 (0.004) | 0.064 (0.032, 0.13) | 8 (0.004) | 0.068 (0.034, 0.14) | 0 | 0 |
| Device-associated | 6 (0.0006) | 0.0096 (0.004, 0.021) | 3 (0.0015) | 0.024 (0.008, 0.075) | 3 (0.0016) | 0.026 (0.008, 0.079) | 0 | 0 |
| Central nervous system abscess | 6 (0.0006) | 0.0096 (0.004, 0.021) | 2 (0.001) | 0.016 (0.004, 0.064) | 2 (0.001) | 0.017 (0.004, 0.068) | 0 | 0 |
CI, confidence interval
Per 10,000 person-years
Table 4.
Sensitivity Analyses for Serious Infection, Opportunistic Infection, and Herpes Zoster Outcomes: THIN Cohort
| Sensitivity Analysis Model by Outcome | Number of Patients | All Psoriasis HR (95% CI) |
Mild Psoriasis HR (95% CI) |
Moderate-to-Severe Psoriasis HR (95% CI) |
|
|---|---|---|---|---|---|
| No Psoriasis | Psoriasis | ||||
| Serious Infection | |||||
| Primary Model | 954,315 | 199,700 | 1.21 (1.18, 1.23) | 1.18 (1.16, 1.21) | 1.63 (1.52, 1.75) |
| Multiple Imputation | 954,315 | 199,700 | 1.20 (1.18, 1.23) | 1.18 (1.15, 1.21) | 1.63 (1.52, 1.75) |
| Exclude patients seen by GP less than once per year | 909,728 | 192,303 | 1.20 (1.18, 1.23) | 1.18 (1.15, 1.21) | 1.61 (1.50, 1.73) |
| Exclude IBD and RA | 943,052 | 194,047 | 1.21 (1.18, 1.23) | 1.19 (1.16, 1.21) | 1.64 (1.52, 1.76) |
| Exclude PsA | 954,315 | 189,622 | 1.20 (1.17, 1.23) | 1.18 (1.16, 1.21) | 1.71 (1.57, 1.86) |
| Exclude patients with history of infection | 366,203 | 89,312 | 1.25 (1.21, 1.30) | 1.23 (1.18, 1.28) | 1.73 (1.52, 1.98) |
| Exclude patients who had received immunosuppressive psoriasis treatments | 951,676 | 190,055 | 1.19 (1.16, 1.22) | 1.19 (1.16, 1.21) | 1.56 (1.35, 1.82) |
| Opportunistic Infection | |||||
| Primary Model | 954,315 | 199,700 | 0.91 (0.79, 1.04) | 0.87 (0.75, 1.00) | 1.57 (1.06, 2.34) |
| Multiple imputation | 954,315 | 199,700 | 0.91 (0.79, 1.05) | 0.87 (0.75, 1.00) | 1.61 (1.08, 2.39) |
| Exclude patients seen by GP less than once per year | 909,728 | 192,303 | 0.90 (0.79, 1.04) | 0.86 (0.75, 1.00) | 1.54 (1.03, 2.29) |
| Exclude IBD and RA | 943,052 | 194,047 | 0.92 (0.80, 1.06) | 0.89 (0.76, 1.03) | 1.64 (1.06, 2.53) |
| Exclude PsA | 954,315 | 189,622 | 0.91 (0.79, 1.04) | 0.87 (0.75, 1.01) | 1.66 (1.03, 2.68) |
| Exclude patients with history of opportunistic infection | 944,619 | 198,042 | 0.92 (0.79, 1.06) | 0.86 (0.74, 1.01) | 1.76 (1.18, 2.65) |
| Exclude patients who had received immunosuppressive psoriasis treatments | 951,676 | 190,055 | 0.88 (0.76, 1.02) | 0.88 (0.76, 1.02) | 1.17 (0.44, 3.12) |
| Herpes Zoster | |||||
| Primary Model | 954,315 | 199,700 | 1.08 (1.05, 1.11) | 1.07 (1.05, 1.10) | 1.17 (1.06, 1.30) |
| Multiple imputation | 954,315 | 199,700 | 1.08 (1.05, 1.11) | 1.09 (1.06, 1.13) | 1.20 (1.08, 1.33) |
| Exclude patients seen by GP less than once per year | 909,728 | 192,303 | 1.08 (1.05, 1.11) | 1.07 (1.04, 1.10) | 1.16 (1.05, 1.28) |
| Exclude IBD and RA | 943,052 | 194,047 | 1.08 (1.05, 1.11) | 1.08 (1.05, 1.11) | 1.12 (1.00, 1.26) |
| Exclude PsA | 954,315 | 189,622 | 1.08 (1.05, 1.11) | 1.08 (1.05, 1.11) | 1.16 (1.02, 1.32) |
| Exclude patients with history of herpes zoster | 913,982 | 192,249 | 1.07 (1.04, 1.10) | 1.07 (1.04, 1.10) | 1.19 (1.07, 1.32) |
| Exclude patients who had received immunosuppressive psoriasis treatments | 951,676 | 190,055 | 1.08 (1.05, 1.11) | 1.08 (1.05, 1.11) | 0.97 (0.76, 1.23) |
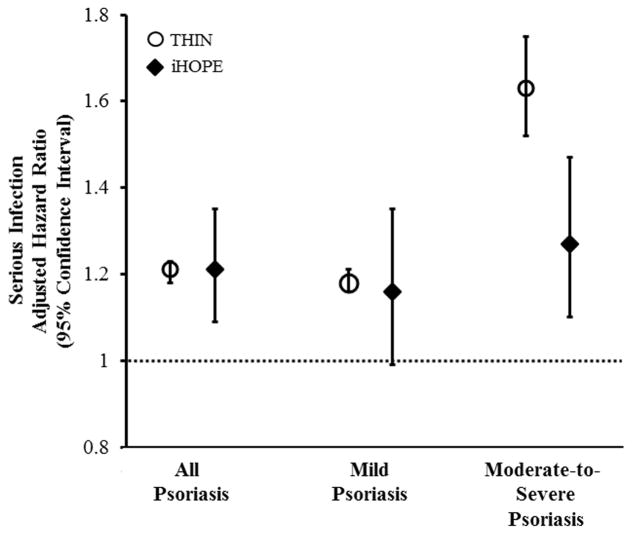
In the iHOPE cohort, the adjusted HRs (95% CI) for serious infection were 1.21 (1.09–1.35), 1.16 (0.99–1.35), and 1.27 (1.10–1.47) among all patients with psoriasis and patients with mild and moderate-to-severe disease as defined by BSA involved by psoriasis, respectively (Table 2). A significant dose-dependent relationship between BSA involvement and serious infection was observed (P for trend = 0.005). When patients who had received immunosuppressive psoriasis treatments were excluded from the iHOPE analyses (N=557), the HRs for serious infection remained similar among all patients with psoriasis and those with mild and moderate-to-severe disease: 1.18 (1.05–1.32), 1.15 (0.99–1.34) and 1.21 (1.03–1.42), respectively (data not shown). Importantly, the risk of serious infection was observed to be similar in both the full THIN and iHOPE cohorts with the exception of the moderate-to-severe psoriasis subgroup among whom the risk of serious infection was attenuated but still significantly elevated in the iHOPE versus full THIN cohort (Figure 1). Using the iHOPE cohort-derived HRs for serious infection, the attributable and excess risks of serious infection among all patients with psoriasis and those with mild and moderate-to-severe disease were 16.2, 12.2, and 20.4 per 10,000 person-years, respectively, and one serious infection per 617, 817, and 489 patients with any, mild, and moderate-to-severe psoriasis, respectively, per year (data not shown).
Figure 1. Adjusted Risk of Serious Infection by Psoriasis Severity: THIN Versus iHOPE Cohort.
Moderate-to-severe psoriasis defined by receipt of phototherapy or systemic therapy in THIN cohort and ≥3% BSA in iHOPE cohort.
Opportunistic Infection
The incidence rates of opportunistic infection derived from the entire THIN cohort are summarized in Table 5. Opportunistic infection incidence rates were similar between patients with mild psoriasis and those without psoriasis. Patients receiving therapies for moderate-to-severe psoriasis had a higher incidence of opportunistic infection than all other groups with or without psoriasis. By far, the most common opportunistic infection among all groups was tuberculosis with incidence rates of 1.05, 0.94, and 3.00 per 10,000 person-years among all patients with psoriasis and those with mild and moderate-to-severe disease, respectively, versus 1.15 per 10,000 person-years among those without psoriasis (data not shown). In multivariable analyses, only patients with moderate-to-severe psoriasis had an increased risk of opportunistic infection (HR 1.57; 95% CI 1.06–2.34). Neither age nor sex was a significant effect modifier. In sensitivity analyses, the risk of opportunistic infection was substantially attenuated when patients who had received immunosuppressive psoriasis treatments were excluded (HR 1.17; 95% CI 0.44–3.12) (Table 4).
Table 5.
Incidence and Risk of Opportunistic Infection and Herpes Zoster: THIN Cohort
| Full THIN Cohort | No Psoriasis N=954,315 |
All Psoriasis N=199,700 |
Mild Psoriasis N=187,258 |
Moderate-to-Severe Psoriasis N=12,442 |
|---|---|---|---|---|
|
| ||||
| Opportunistic Infection | ||||
|
| ||||
| Follow-up time (yrs) | ||||
| Mean (SD) | 6.5 (4.8) | 6.2 (4.7) | 6.3 (4.8) | 5.3 (4.1) |
| Median (IQR) | 5.6 (2.4, 10.0) | 5.3 (2.2, 9.7) | 5.3 (2.2, 9.7) | 4.4 (1.9, 7.9) |
|
| ||||
| Number of person-years | 6,240,165 | 1,239,794 | 1,173,285 | 66,509 |
|
| ||||
| Number (%) of opportunistic infections | 1,329 (0.14) | 236 (0.12) | 211 (0.11) | 25 (0.20) |
|
| ||||
| Incidence per 10,000 person-years (95% CI) | 2.13 (2.02, 2.25) | 1.90 (1.68, 2.16) | 1.80 (1.57, 2.06) | 3.76 (2.54, 5.56) |
|
| ||||
| Unadjusted hazard ratio (95% CI) | Reference | 0.89 (0.78, 1.02) | 0.84 (0.73, 0.97) | 1.73 (1.16, 2.57) |
|
| ||||
| Adjusted hazard ratio (95% CI)a | Reference | 0.91 (0.79, 1.04) | 0.87 (0.75, 1.00) | 1.57 (1.06, 2.34) |
|
| ||||
| Herpes Zoster | ||||
|
| ||||
| Follow-up time (yrs) | ||||
| Mean (SD) | 6.4 (4.7) | 6.1 (4.7) | 6.1 (4.7) | 5.2 (4.1) |
| Median (IQR) | 5.5 (2.3, 9.8) | 5.0 (2.1, 9.4) | 5.1 (2.1, 9.5) | 4.2 (1.9, 7.7) |
|
| ||||
| Number of person-years | 6,084,648 | 1,209,265 | 1,144,308 | 64,957 |
|
| ||||
| Number (%) of herpes zoster cases | 33,115 (3.5) | 6,537 (3.3) | 6,117 (3.3) | 420 (3.4) |
|
| ||||
| Incidence per 10,000 person years (95% CI) | 54.4 (53.8, 55.0) | 54.1 (52.8, 55.4) | 53.5 (52.1, 54.8) | 64.7 (58.8, 71.1) |
|
| ||||
| Unadjusted hazard ratio (95% CI) | Reference | 1.00 (0.97, 1.02) | 0.98 (0.96, 1.01) | 1.21 (1.10, 1.34) |
|
| ||||
| Adjusted hazard ratio (95% CI)b | Reference | 1.08 (1.05, 1.11) | 1.07 (1.05, 1.10) | 1.17 (1.06, 1.30) |
CI, confidence interval; IQR, interquartile range; SD, standard deviation; yrs, years
Adjusted for age, sex, BMI, smoking and drinking status, asthma/COPD, CVD, diabetes, IBD, inhaled and systemic corticosteroid use within 90 days prior to start date, hospitalization or infection within 30 days prior to start date, prior opportunistic infection, and Townsend score.
Adjusted for age, sex, smoking status, asthma/COPD, CVD, dementia, depression, IBD, OA, RA, SLE, systemic corticosteroid use within 90 days prior to start date, infection within 30 days prior to start date, prior herpes zoster.
Herpes Zoster
The incidence rates of herpes zoster derived from the entire THIN cohort are summarized in Table 5. Incidence rates were the highest among patients receiving therapies for moderate-to-severe psoriasis. In multivariable analyses, patients with moderate-to-severe psoriasis had the greatest risk of herpes zoster (HR 1.17; 95% CI 1.06–1.30); mild psoriasis was also associated with a significant but small increased risk of herpes zoster (HR 1.07; 95% CI 1.05–1.10). Neither age nor sex was a significant effect modifier. In sensitivity analyses, exclusion of patients who had received immunosuppressive psoriasis treatments resulted in complete attenuation of the association between moderate-to-severe psoriasis and herpes zoster (HR 0.97; 95% CI 0.76–1.23) (Table 4).
DISCUSSION
Our population-based study in the UK demonstrates that psoriasis is associated with an increased risk of serious infection, independent of traditional risk factors for infection that are captured in routine medical practice. The risk of serious infection was greatest among patients with moderate-to-severe disease whether defined indirectly by treatment pattern in the full THIN cohort or directly by BSA involvement in the nested iHOPE cohort. While the serious infection risk among patients with moderate-to-severe psoriasis was attenuated in the iHOPE versus full THIN cohort, it remained significantly higher by 20–30% when psoriasis severity was defined by BSA involvement, even when those who had received immunosuppressive therapies were excluded. Collectively, our findings support the idea that psoriasis severity, defined by either treatment pattern or affected BSA, is a predictor of serious infection risk beyond traditional risk factors for infection that are identifiable and collected in routine medical practice. In contrast, higher risks of opportunistic infections and herpes zoster were essentially limited to those patients receiving therapies for moderate-to-severe psoriasis and were entirely (or nearly entirely) associated with immunosuppressive therapies used to treat more severe psoriasis.
The pathophysiologic mechanisms for increased risk of serious infection among patients with psoriasis remain poorly understood. Underlying immune dysfunction characterized by TNF and IL-17 induced inflammation in patients with psoriasis may be hypothesized to alter psoriasis patients’ infection risk profile. For example, several proinflammatory cytokines including TNF, IL-6, and IL-17 have been found to be elevated in the serum of patients with psoriasis compared to those without the disease.(Arican et al., 2005; Suarez-Farinas et al., 2012) Notably, in a single study of elderly individuals in the U.S., elevated levels of TNF and IL-6 prior to infection were associated with higher risk of pneumonia requiring hospitalization,(Yende et al., 2005) suggesting that the altered serum cytokine profile of psoriasis patients may contribute to their risk of serious infection. Though such proinflammatory cytokines are known to be involved in the mediation of normal and protective systemic inflammatory responses to pathogens, overproduction of particular cytokines including TNF may also contribute to the promotion of bacterial invasion and initiation of pneumonia, in particular.(Cundell et al., 1995; Mason et al., 1997; White et al., 1986)
Our findings add to the scant literature that exists regarding the association between psoriasis and infection and are consistent with smaller population-based studies in the Netherlands (Wakkee et al., 2011) and Taiwan (Kao et al., 2014) that also found increased risks of serious infection and hospitalized pneumonia, respectively, among patients with psoriasis. To our knowledge, ours is the first study to examine the risk of opportunistic infection and the second to evaluate the risk of herpes zoster across an entire cohort of patients with psoriasis regardless of treatment status. The novelty and a major strength of our study lies in its larger size and inclusion of risk factors for infection such as BMI, smoking and drinking status, and vaccination history that were not available in prior studies as well as use of a nested cohort of psoriasis patients with information on BSA involvement by psoriasis, thus allowing us to directly evaluate the effect of psoriasis severity on the serious infection outcome. Additional strengths of our study include the use of a large population-based psoriasis cohort with validly identified psoriasis diagnosis that is representative of the general population in the UK and, thus, likely generalizable to other Western countries.
Some limitations of our study include potential misclassification of psoriasis severity particularly in the full THIN cohort that used therapy as a surrogate measure of disease severity. In THIN, systemic medications for psoriasis are often prescribed by specialists or consultants and are not always recorded by general practitioners (GPs). Nevertheless, prior studies evaluating treatment patterns among patients with psoriatic disease in THIN indicate that the prevalence of oral systemic treatment in THIN approximates that of population-based estimates of moderate-to-severe psoriasis,(Ogdie et al., 2014; Ogdie et al., 2013) suggesting that use of systemic therapy is a reasonable indicator of more severe psoriasis. While compared to our analysis in the full THIN cohort the risk of serious infection among those with ≥ 3% BSA affected by psoriasis in the nested iHOPE cohort was attenuated, it was still significantly increased and supported our primary finding in THIN that the risk of serious infection is greatest among those with moderate-to-severe disease. Notably, the association between serious infection and moderate-to-severe psoriasis in the iHOPE cohort was largely driven by the moderate psoriasis group. Thus, the true risk of serious infection among patients with moderate-to-severe psoriasis may be underestimated in the iHOPE cohort. Misclassification of the infection outcomes and hospitalizations is also possible, though likely to be non differential which, if anything, would bias our results towards the null. While we accounted for numerous potential confounders in our analyses (BMI, smoking and drinking status, vaccination history), which had not been included in prior studies, additional unmeasured or unknown confounders (e.g., ethnicity or region of origin as a risk factor for tuberculosis) may still exist. Lastly, ascertainment bias may be considered though this is unlikely to explain our results as patients with and without psoriasis had information collected in a similar manner, and our sensitivity analyses limiting the study population to those patients who had seen their GP at least once yearly yielded results similar to the primary analyses.
In conclusion, our findings suggest that psoriasis is associated with an increased risk of serious infection, and more severe psoriasis, whether defined by treatment pattern or by BSA involvement, is a predictor of greater serious infection risk. Considering that the incidence rates for upper and lower respiratory infections were among the highest of the infection types evaluated in patients with psoriasis, it is particularly relevant for GPs and dermatologists to ensure flu and pneumonia vaccination, especially among those who have more severe psoriasis or are receiving immunosuppressive therapy, consistent with the British Association of Dermatologists guidelines,(Smith et al., 2017) Advisory Committee on Immunization Practices recommendations,(Kim et al., 2015; Strikas et al., 2015) and the National Psoriasis Foundation Medical Board’s recommendations to vaccinate adult patients on systemic immunosuppressive therapy for psoriasis.(Wine-Lee et al., 2013) Psoriasis patients with moderate-to-severe disease are also at increased risk of developing opportunistic infections and herpes zoster, but this risk appears to be limited to those patients receiving immunosuppressive therapies. Therefore, GPs and dermatologists should also consider herpes zoster vaccination with the new non-live vaccine for psoriasis patients who will be or are already receiving immunosuppressive therapies. Future studies will be important to further characterize the risk of various infections among patients with psoriasis, compare the risk of infection associated with psoriasis to that of other chronic diseases, and delineate the pathophysiologic mechanisms that contribute to the increased risk of infections associated with psoriasis and its therapies.
MATERIALS & METHODS
Study design and data source
We conducted a population-based cohort study using THIN, an electronic medical records database in the UK. We performed an additional cohort study only for the serious infection outcome using the iHOPE cohort, which is a nested cohort of patients within THIN.(Langan et al., 2012; Seminara et al., 2011; Yeung et al., 2013) THIN is broadly representative of the general UK population and contains information on medical diagnoses, treatment, and select laboratory data for over 12 million individuals covering 5.7% of the population. THIN has been widely used for epidemiologic research and has been validated for the study of psoriasis and other diagnoses.(Lewis et al., 2007; Meropol and Metlay, 2012; Seminara et al., 2011) In the UK, the majority of patients are registered with a GP who serves as the primary contact for all aspects of the patient’s care and records data on diagnoses, prescriptions, and laboratory results in the electronic medical record. Data in this study were collected prospectively between 1994 and January 2014. This study was conducted according to the Declaration of Helsinki and reported according to the Strengthening the Reporting of Observational Studies in Epidemiology(von Elm et al., 2007) statement. The study was granted exempt status by the University of Pennsylvania Institutional Review Board and approved by THIN’s scientific review committee. Data were de-identified and, thus, patient consent was not possible/waived.
Study population and time of observation
THIN cohort
All patients with psoriasis aged 18–89 at the start date were included in the study. Diagnoses in THIN are recorded using the Read diagnostic code scheme,(Chisholm, 1990) and prescriptions are recorded using codes from the UK Prescription Pricing Authority.(Garcia Rodriguez and Perez Gutthann, 1998) Patients were identified to have psoriasis (i.e., exposed) if they had received at least one Read code for psoriasis, as previously validated in THIN.(Seminara et al., 2011) Moderate-to-severe psoriasis was defined by the presence of a prescription code for therapies used to treat moderate-to-severe psoriasis (i.e., phototherapy including ultraviolet B or psoralen and ultraviolet A [PUVA], methotrexate, cyclosporine, oral retinoids, etanercept, infliximab, adalimumab, or ustekinumab).
Each patient with psoriasis was matched with up to five randomly-selected patients without psoriasis (i.e., unexposed with no history of a Read code for psoriasis) who were seen in the same practice and who had a date of observation within 180 days of the start date of the patient with psoriasis. We excluded patients with human immunodeficiency virus (HIV), malignancy (excluding non-melanoma skin cancer), or solid organ or liquid transplant prior to cohort entry (Figure S1A).
The start date for follow-up began on the latest of the following: date when the patient’s practice began using THIN software, 180 days after patient registration in the practice, and date of psoriasis diagnosis for the exposed or the closest corresponding visit date for the unexposed. Censoring occurred when patients developed the outcome of interest, died, transferred out of THIN, or reached the end of the study.
Incident Health Outcomes and Psoriasis Events cohort (iHOPE)
The iHOPE cohort was created by randomly sampling patients in THIN who were alive and aged 25 to 64 years at the time of sampling, had received at least one Read code for psoriasis in the two years prior to sampling, and were registered in a practice with an Additional Information Services (AIS) contract (i.e., an agreement to complete questionnaires in exchange for compensation). Surveys with face and content validity designed by experts in epidemiology, dermatology, and primary care were sent to GPs of the sampled psoriasis patients to verify their psoriasis diagnosis and classify their disease extent via the National Psoriasis Foundation classification system into mild (limited disease with < 3% BSA affected), moderate (scattered disease with 3 to 10% BSA affected), or severe (extensive disease with > 10% BSA affected). The exposed group consisted of patients with psoriasis Read codes whose psoriasis diagnosis and amount of skin involvement were verified by their GPs. Patients with moderate and severe psoriasis in the iHOPE cohort were combined into a single moderate-to-severe psoriasis group resulting in two disease severity categories (mild and moderate-to-severe) and, thus, enabling more direct comparisons with the mild and moderate-to-severe psoriasis groups that were identified by treatment pattern in the full THIN cohort. The unexposed comparison group was constructed by matching each psoriasis patient to up to 10 randomly-selected patients without psoriasis Read codes who were from the same practice, in the same age category, and alive and actively registered with at least one GP visit within two years prior to sampling. We excluded patients with HIV, malignancy (excluding non-melanoma skin cancer), or solid organ or liquid transplant prior to cohort entry (Figure S1B).
The start date for follow-up was defined by the GP survey sampling date (November 2008 to September 2010). Censoring occurred when patients developed the outcome (serious infection), died, transferred out of THIN, or reached the end of the study.
Outcome and covariate definitions
Three infection outcome categories were evaluated separately using the full THIN cohort: serious (i.e., hospitalized) infection, opportunistic infection, and herpes zoster. Only the serious infection outcome was also evaluated using the iHOPE cohort; the other infection outcomes were not common enough to assess reliably in the smaller iHOPE cohort. The serious infection outcome was defined by at least one Read code for a prespecified set of infections modified from Patkar et al.(Patkar et al., 2009) followed by Read code for hospitalization within 30 days.(Meropol and Metlay, 2012) The opportunistic infection outcome was defined by at least one Read code for any of the following infections: actinomycosis/nocardia, aspergillosis, BK virus, cryptococcus, cytomegalovirus, mucormycosis, other mycoses (blastomycosis, coccidioidomycosis, histoplasmosis, and paracoccidioidomycosis), pneumocystis, progressive multifocal leukoencephalopathy, tuberculosis, and toxoplasmosis. The herpes zoster outcome was defined by at least one Read code for herpes zoster. Covariates assessed were potential confounders or risk factors for infection including age, sex, presence of comorbid disease including asthma/chronic obstructive pulmonary disease (COPD), cardiovascular disease (CVD), chronic kidney disease (CKD), congestive heart failure (CHF), dementia, depression, diabetes, inflammatory bowel disease (IBD), liver disease, osteoarthritis (OA), psoriatic arthritis (PsA), rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE), inhaled or systemic corticosteroid use within 90 days of start date, infection or any hospitalization within 30 days of start date, flu and pneumonia vaccination status, smoking and drinking history, body mass index (BMI), and Townsend deprivation index. See Table S3 for variable code lists. Patients were classified as having a comorbid disease if they had received a diagnostic code for any of the comorbid conditions of interest before the study start date. Smoking and drinking status, BMI, and Townsend deprivation index were determined from the data closest in time to the start date.
Statistical analysis
Patients with psoriasis (overall, mild, and moderate-to-severe) were compared to patients without psoriasis using standardized differences whereby a standardized difference ≥ 0.1 was considered to indicate a significant difference between groups.(Austin, 2009) Incidence rates of the various infection outcomes among patients with and without psoriasis were reported descriptively. Cox proportional hazards regression was used to compare the rates of infection in the overall, mild, and moderate-to-severe psoriasis groups to that in the unexposed group. A purposeful selection modeling approach was used to build the multivariable model.(Bursac et al., 2008) All covariates with significant imbalance between exposed and unexposed groups were included in the multivariable model as potential confounders. Nonsignificant covariates (P > 0.05) were eliminated from the multivariable model if their removal did not change the hazard ratio estimates of the exposure variable by more than 10%. Effect modification by age and sex were also evaluated for each outcome. Log-log survival plots were examined to test the proportional hazards assumption.
We performed multiple sensitivity analyses to further assess the robustness of our primary analyses in the full THIN cohort as follows: i) multiple imputation for missing data; ii) limited analyses to only those patients who had been seen at least once yearly by their GP to minimize ascertainment bias; iii) excluded patients with IBD or RA, both of which have been suggested to be associated with increased risk of infection; iv) excluded patients with psoriatic arthritis to assess the relationship between skin-only disease and the outcomes; v) excluded patients with prior history of the infections of interest in order to assess incident infection risk; and vi) excluded patients who had received immunosuppressive psoriasis treatments (methotrexate, cyclosporine, any biologic) in an effort to minimize medication effects on the risk of infection. All statistical analyses were performed with Stata (Version 13, StataCorp, College Station, TX, USA). Statistical significance was determined by two sided P values at P<0.05.
Supplementary Material
Acknowledgments
FUNDING/SUPPORT
This study was supported by an unrestricted grant from Pfizer Inc. to the trustees of the University of Pennsylvania (Gelfand). Dr. Takeshita was supported by a Dermatology Foundation Career Development Award and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grant K23-AR068433. Dr. Ogdie is supported by NIAMS grant K23-AR063764. Dr. Gelfand is supported by NIAMS grant K24-AR064310.
We are indebted to Sean Hennessy, PharmD, PhD for his intellectual contributions and review of the manuscript. We also thank Suzette Baez Vanderbeek, MPH for her administrative support.
Abbreviations
- BMI
body mass index
- BSA
body surface area
- CHF
congestive heart failure
- CI
confidence interval
- CKD
chronic kidney disease
- COPD
chronic obstructive pulmonary disease
- CVD
cardiovascular disease
- HR
hazard ratio
- IBD
inflammatory bowel disease
- iHOPE
Incident Health Outcomes and Psoriasis Events
- OA
osteoarthritis
- PsA
psoriatic arthritis
- PUVA
psoralen and ultraviolet A
- RA
rheumatoid arthritis
- SLE
systemic lupus erythematosus
- THIN
The Health Improvement Network
Footnotes
PREVIOUS OR PLANNED MEETING PRESENTATION
Abstracts of portions of the data contained in this manuscript were presented at the International Federation of Psoriasis Associations’ 4th World Psoriasis & Psoriatic Arthritis Conference (July 2015), the Society for Investigative Dermatology 2016 Annual Meeting (May 2016), and the 7th International Dermato-Epidemiology Association Congress and Keratinocyte Carcinoma Consortium (September 2016).
CONFLICT OF INTEREST
Dr. Takeshita receives a research grant (to the Trustees of the University of Pennsylvania) from Pfizer Inc. for work that is unrelated to this manuscript and received payment for continuing medical education work related to psoriasis that was supported indirectly by Eli Lilly and Novartis. Dr. Ogdie has served as a consultant for Bristol-Myers Squibb, Novartis, Pfizer Inc., and Takeda, receiving honoraria; and is a co-investigator on a research grant (to the Trustees of the University of Pennsylvania) from Pfizer Inc. Dr. Gelfand served as a consultant for Coherus (DSMB), Dermira, Janssen Biologics, Merck (DSMB), Novartis Corp, Regeneron, Sanofi, and Pfizer Inc., receiving honoraria; and received research grants (to the Trustees of the University of Pennsylvania) from Abbvie, Janssen, Novartis Corp, Regeneron, Sanofi, Celgene, and Pfizer Inc.; and received payment for continuing medical education work related to psoriasis that was supported indirectly by Eli Lilly and Abbvie. Dr. Gelfand is a co-patent holder of resiquimod for treatment of cutaneous T cell lymphoma.
ROLES OF SPONSORS
Funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K. Br J Dermatol. 2010;163:586–92. doi: 10.1111/j.1365-2133.2010.09941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arican O, Aral M, Sasmaz S, Ciragil P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273–9. doi: 10.1155/MI.2005.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PC. Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Observational Research. Commun Stat Simul Comput. 2009;38:1228–34. [Google Scholar]
- Azfar RS, Seminara NM, Shin DB, Troxel AB, Margolis DJ, Gelfand JM. Increased risk of diabetes mellitus and likelihood of receiving diabetes mellitus treatment in patients with psoriasis. Arch Dermatol. 2012;148:995–1000. doi: 10.1001/archdermatol.2012.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm J. The Read clinical classification. BMJ. 1990;300:1092. doi: 10.1136/bmj.300.6732.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–8. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- Garcia Rodriguez LA, Perez Gutthann S. Use of the UK General Practice Research Database for pharmacoepidemiology. Br J Clin Pharmacol. 1998;45:419–25. doi: 10.1046/j.1365-2125.1998.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand JM, Dommasch ED, Shin DB, Azfar RS, Kurd SK, Wang X, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411–8. doi: 10.1038/jid.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–41. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537–41. doi: 10.1001/archderm.141.12.1537. [DOI] [PubMed] [Google Scholar]
- Hollox EJ, Huffmeier U, Zeeuwen PL, Palla R, Lascorz J, Rodijk-Olthuis D, et al. Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet. 2008;40:23–5. doi: 10.1038/ng.2007.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao LT, Lee CZ, Liu SP, Tsai MC, Lin HC. Psoriasis and the risk of pneumonia: a population-based study. PloS One. 2014;9:e116077. doi: 10.1371/journal.pone.0116077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DK, Bridges CB, Harriman KH Centers for Disease Control and Prevention, Advisory Committee on Immunization Practices, Advisory Committee on Immunization Practices Adult Immunization Work Group. Advisory committee on immunization practices recommended immunization schedule for adults aged 19 years or older--United States, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:91–2. [Google Scholar]
- Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003–2004. J Am Acad Dermatol. 2009;60:218–24. doi: 10.1016/j.jaad.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan SM, Seminara NM, Shin DB, Troxel AB, Kimmel SE, Mehta NN, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556–62. doi: 10.1038/jid.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16:393–401. doi: 10.1002/pds.1335. [DOI] [PubMed] [Google Scholar]
- Mason CM, Dobard E, Summer WR, Nelson S. Intraportal lipopolysaccharide suppresses pulmonary antibacterial defense mechanisms. J Infect Dis. 1997;176:1293–302. doi: 10.1086/514125. [DOI] [PubMed] [Google Scholar]
- Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000–6. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meropol SB, Metlay JP. Accuracy of pneumonia hospital admissions in a primary care electronic medical record database. Pharmacoepidemiol Drug Saf. 2012;21:659–65. doi: 10.1002/pds.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogdie A, Alehashemi S, Love TJ, Jiang Y, Haynes K, Hennessy S, et al. Validity of psoriatic arthritis and capture of disease modifying antirheumatic drugs in the health improvement network. Pharmacoepidemiol Drug Saf. 2014;23:918–22. doi: 10.1002/pds.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogdie A, Langan S, Love T, Haynes K, Shin D, Seminara N, et al. Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatology. 2013;52:568–75. doi: 10.1093/rheumatology/kes324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar NM, Curtis JR, Teng GG, Allison JJ, Saag M, Martin C, et al. Administrative codes combined with medical records based criteria accurately identified bacterial infections among rheumatoid arthritis patients. J Clin Epidemiol. 2009;62:321–7. 7e1–7. doi: 10.1016/j.jclinepi.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara NM, Abuabara K, Shin DB, Langan SM, Kimmel SE, Margolis D, et al. Validity of The Health Improvement Network (THIN) for the study of psoriasis. Br J Dermatol. 2011;164:602–9. doi: 10.1111/j.1365-2133.2010.10134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CH, Jabbar-Lopez ZK, Yiu ZZ, Bale T, Burden AD, Coates LC, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628–36. doi: 10.1111/bjd.15665. [DOI] [PubMed] [Google Scholar]
- Strikas RA Centers for Disease Control and Prevention, Advisory Committee on Immunization Practices, Advisory Committee on Immunization Practices Child/Adolescent Immunization Work Group. Advisory committee on immunization practices recommended immunization schedules for persons aged 0 through 18 years--United States, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:93–4. [PMC free article] [PubMed] [Google Scholar]
- Suarez-Farinas M, Li K, Fuentes-Duculan J, Hayden K, Brodmerkel C, Krueger JG. Expanding the psoriasis disease profile: interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J Invest Dermatol. 2012;132:2552–64. doi: 10.1038/jid.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- Wakkee M, de Vries E, van den Haak P, Nijsten T. Increased risk of infectious disease requiring hospitalization among patients with psoriasis: a population-based cohort. J Am Acad Dermatol. 2011;65:1135–44. doi: 10.1016/j.jaad.2010.08.036. [DOI] [PubMed] [Google Scholar]
- Wan J, Wang S, Haynes K, Denburg MR, Shin DB, Gelfand JM. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi: 10.1136/bmj.f5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JC, Nelson S, Winkelstein JA, Booth FV, Jakab GJ. Impairment of antibacterial defense mechanisms of the lung by extrapulmonary infection. J Infect Dis. 1986;153:202–8. doi: 10.1093/infdis/153.2.202. [DOI] [PubMed] [Google Scholar]
- Wine-Lee L, Keller SC, Wilck MB, Gluckman SJ, Van Voorhees AS. From the Medical Board of the National Psoriasis Foundation: Vaccination in adult patients on systemic therapy for psoriasis. J Am Acad Dermatol. 2013;69:1003–13. doi: 10.1016/j.jaad.2013.06.046. [DOI] [PubMed] [Google Scholar]
- Yende S, Tuomanen EI, Wunderink R, Kanaya A, Newman AB, Harris T, et al. Preinfection systemic inflammatory markers and risk of hospitalization due to pneumonia. Am J Respir Crit Care Med. 2005;172:1440–6. doi: 10.1164/rccm.200506-888OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung H, Takeshita J, Mehta NN, Kimmel SE, Ogdie A, Margolis DJ, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149:1173–9. doi: 10.1001/jamadermatol.2013.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.