Abstract
Purpose
Pterygium recurrence is a common complication of pterygium removal. Multiple surgical and medical approaches have been utilized to reduce recurrence rates. The present case series proposes a novel way to treat recurrent pterygia, by using the simple limbal epithelial transplantation (SLET) technique.
Observations
The cases of four patients who presented with recurrent pterygium were reviewed. In all four of the cases reported, the SLET procedure went without complication. There were no significant recurrences at each of the patient's most recent follow-up visits.
Conclusions and importance
This is the first report of SLET being used as a treatment modality for recurrent pterygium. Further studies are required to more reliably demonstrate the utility of the procedure in this clinical circumstance, but our results are encouraging that in select patients, this may be a viable option in treating aggressive recurrent pterygia.
Keywords: Simple limbal epithelial transplantation (SLET), Recurrent pterygium, Cornea, Conjunctiva, Stem cells
1. Introduction
Pterygia are fibrovascular growths extending from the conjunctiva onto the cornea. Indications for surgical excision include extensive growth onto the cornea, induced astigmatism and ocular irritation. One of the most common complications of pterygium surgery is recurrence. Rates of recurrence vary widely in the literature. The bare sclera approach leads to recurrence in 38–88% and has largely been abandoned.1 Conjunctival autograft with fibrin glue has become an increasingly popular technique and has lowered recurrence rates to 5.5–11.9%.1 Mitomycin C (MMC) has been used as adjunct therapy to further reduce recurrences.1,2
The current treatment paradigm for recurrent pterygia includes several options, though no option has proven impervious to failure. Techniques that employ repeat conjunctival autografting, use of amniotic membrane and application of MMC are some of the more common treatment options for recurrence and have demonstrated relatively good efficacy.3,4 Other less proven modalities include administration of subconjunctival anti-VEGF agents and injection of 5 fluorouracil (5-FU) into the pterygium.5, 6, 7, 8
Simple limbal epithelial transplantation (SLET) is a technique that was first described by Sangwan in 2012.9 It was presented in the context of treating unilateral limbal stem cell disease. The procedure involves harvesting limbal stem cells from the unaffected eye with healthy limbal stem cells. A variation of the technique has included harvesting the cells from the ipsilateral affected eye, if there is a healthy area of available limbal stem cells. The harvested stem cells are then transplanted onto the diseased area of cornea. Studies have since reported on the use of SLET for unilateral chemical burns, ocular surface squamous neoplasia and primary pterygium excision.9, 10, 11, 12
The following case series is the first to present SLET as a treatment option for surface reconstruction in eyes with recurrent pterygium (see Table 1).
Table 1.
Summary of pertinent demographics and results of the four patients presented in this case series.
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Gender | Male | Female | Female | Male |
| Age | 66 | 59 | 79 | 37 |
| Number of prior pterygia | 3 | 1 | 1 | 1 |
| Preexisting LSCD | No | No | Yes | Yes |
| Etiology of LSCD | – | – | Meibomian gland disease | Vernal kerato-conjunctivitis; blepharitis |
| Pre-operative BCVA | 20/80 + 1 | 20/30 | 20/400 | Counting fingers at 1 foot |
| Months of follow-up | 10 | 8 | 30 | 8 |
| Post-operative UVA | 20/60 | 20/40 | 20/200 | 20/40 |
| Post-operative BCVA | 20/25 | N/A | N/A | N/A |
| Post-operative complications | Symblepharon | Temporal pterygium (the excised pterygium was nasal and did not recur) | Superior neovascularization and LSCD | None |
| Post-Operative avastin | No | No | Yes | No |
1.1. Procedure
In the following four cases, one ophthalmologist (AS) performed the SLET procedure for cases of recurrent pterygium. While minor variations existed depending on the specific orientation of the pterygium, such as the sequence of particular steps or the site of the donor stem cells, the general steps of the procedure were essentially the same and are outlined here.
The pterygium was excised in a standard fashion with the aid of a 64-Beaver blade, Westcott scissors and forceps. Mitomycin C 0.02% was applied for two to 3 min subconjunctivally. A conjunctival peritomy was performed on the same eye to expose the limbal sclera to permit stem cell harvesting. A crescent blade was used to shave a limbal strip of approximately 4 mm × 2 mm. The limbal tissue was then cut into approximately ten pieces with Vannas scissors. Amniotic membrane was used to cover the cornea as well as the bare sclera deep to the excised pterygium. Fibrin glue and 8-0 vicryl sutures held the amniotic membrane in place. The harvested limbal pieces were subsequently secured with glue over the amniotic membrane along the area of limbus and cornea involved by the pterygium. A bandage contact lens (BCL) was placed over the eye at the end of the surgery.
2. Findings
2.1. Case 1
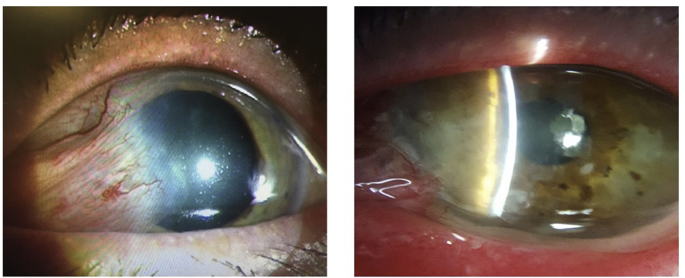
A 66-year-old male was referred for recurrent pterygium. He had undergone three pterygium excisions with conjunctival autografts (PECAs) in the left eye over the course of thirty years. Upon referral to our clinic, best-corrected visual acuity (BCVA) in the affected left eye was 20/200 + 1 pinholing to 20/80 + 1. The pterygium was nasal and measured 7.5 mm in height and extended 5 mm onto the cornea, partially covering the visual axis (Fig. 1a). Topography revealed 3.38 Diopters (D) of with-the-rule corneal astigmatism (axis 88°).
Fig. 1.
1a – Large recurrent pterygium with scarring extending paracentrally, close to the visual axis. 1b–10 months following a SLET procedure, there was no evidence of recurrence, though a small area of symblepharon had formed.
A SLET procedure was performed. At four months follow-up, the amnion was still in place with visible SLET pieces. There was some early neovascularization developing nasally and superonasally. The option of performing an avastin injection to promote regression of the neovascularization was discussed, but the patient preferred to wait and observe. At his most recent 10-month post-operative visit, vision had improved to 20/60 + 2 uncorrected and pinholed to 20/25. The amnion had still not yet fully dissolved. Corneal neovascularization was still present but appeared stable. There was an inferonasal area of symblepharon that had developed over his last few visits, but this was not felt to be a recurrence of the pterygium given its different location from the initial pathology (Fig. 1b).
2.2. Case 2
A 59-year-old female was referred for recurrent left pterygium, initially removed 25–30 years prior. BCVA was 20/30. The pterygium was nasal, measured 6 mm in height and extended 5 mm onto the cornea and was moderately inflamed. Topography revealed 2.81 D of with-the-rule corneal astigmatism (axis 100°).
At two months post-SLET, the amnion was still in place with visible pieces of SLET tissue. Uncorrected visual acuity (UCVA) was 20/70 and there was no evidence of recurrence. At eight months follow-up, vision had improved to 20/40 uncorrected. There was no evidence of pterygium recurrence nasally, though a new temporal pterygium was starting to develop.
2.3. Case 3
A 79-year-old female was referred to our clinic for management of recurrent pterygium in association with limbal stem cell disease (LSCD) related to Meibomian gland disease (MGD). She had a history of right PECA with tissue glue and sutures five years ago. Other ocular history included glaucoma, a right retinal laser retinopexy and hemifacial spasm. BCVA in the affected right eye was 20/400. Corneal examination revealed an extensive pterygium covering 270° of the corneal surface with obscuration of the visual axis. The pterygium was fleshy and associated with superior symblepharon. There was also extensive limbal stem cell disease. Corneal topography revealed significant irregular astigmatism.
A SLET procedure was performed in October 2015. At three months post-SLET, superior LSCD was noted with mild neovascularization of the superior cornea, extending downward over the pupillary axis. There was no recurrence of the pterygium. A series of three corneal bevacizumab injections was administered once a month between six to ten months post-SLET to promote regression of this vascularization. Following the injections, the vascularization had indeed regressed significantly. Due to extensive central corneal scarring and thinning, however, a combined surgery with penetrating keratoplasty (PKP) and cataract extraction was performed ten months after the initial SLET procedure. At the three-week post-operative visit, the epithelium was completely healed, suggestive of a healthy limbal stem cell population.
At the patient's most recent clinic visit, corresponding to 19 months post-PKP and 30 months post-SLET, the corneal transplant was clear with no signs of pterygium recurrence and only mild epitheliopathy at the site of prior LSCD. Uncorrected visual acuity was 20/200-1. Vision at this time was still limited by irregular astigmatism from the PKP and an epiretinal membrane.
2.4. Case 4
A 37 year-old male presented with significant visual loss due to double-headed kissing pterygia covering the majority of his left cornea. Prior ocular history included pterygium excision six years prior with conjunctival autograft and adjunctive use of MMC. The pterygia were associated with dense scarring and fairly diffuse LSCD, secondary to chronic vernal keratoconjunctivitis and blepharitis. Presenting visual acuity was counting fingers at 1 foot.
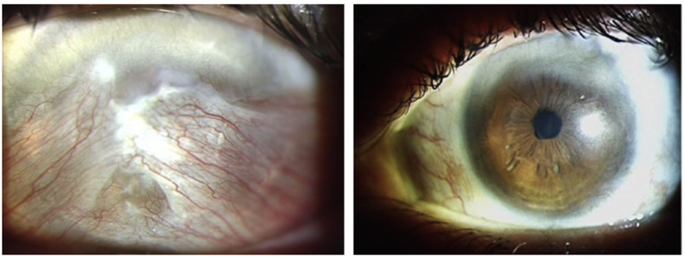
The patient underwent SLET with MMC. The option of taking stem cells from his unaffected right eye was proposed to the patient, but he was understandably hesitant to have any surgical intervention on his primary seeing eye. Two clock hours of viable limbal stem cells were identified in the ipsilateral eye, and the limbal stem cells were harvested from this area. Eight months post-operatively, the cornea was clear and the vision had dramatically improved to 20/40 and there were no signs of any recurrence (Fig. 2).
Fig. 2.
2a – Double-headed recurrent kissing pterygium in a patient with prior pterygium surgery six years earlier. 2b – Post-operatively, the cornea was clear and there were no signs of recurrent pterygium in this aggressive case.
3. Discussion
It has been proposed that limbal stem cell disease is the inciting event in pterygium formation13, 14, 15. Limbal stem cells represent the barrier to potential conjunctivalization of the cornea, and if they are damaged, fibrovascular proliferation onto the cornea, as is seen in pterygia, becomes possible.
Conventional methods of treating recurrent pterygia include adjunctive use of MMC, repeat conjunctival autografting and use of amniotic membrane if autologous conjunctiva is unavailable for repeat harvesingt.3,4 More aggressive pterygia are sometimes targeted with 5-FU and subconjunctival anti-VEGF, though these treatments are less proven.5, 6, 7, 8
Several studies have investigated the role of limbal stem cell transplantation as part of primary pterygium surgery. Conjunctival limbal autograft (CLAG) was first reported as a treatment option for aggressive and recurrent pterygia in 2000.16 It has since been compared to simple excision with adjunctive MMC, and results demonstrated equal or fewer recurrences in the CLAG group.17,18 In a study of 42 eyes undergoing CLAG, only two of 42 eyes showed pterygium recurrence at 18 months.19
While CLAG is a well-studied surgical option in limbal stem cell disease, the procedure entails dissection of a large limbal area and poses a risk of further stem cell disease at the donor site. SLET is a newer procedure which requires the harvesting of less limbal stem cell tissue and theoretically reduces the risk of iatrogenic stem cell disease; furthermore, it allows for the harvesting of limbal stem cells from the same eye.
Only one other study has looked at using SLET as part of pterygium management12.. Ten eyes underwent SLET as part of primary pterygium excision. The results were encouraging, as there were no recurrences at eight months nor any sight-related complications.
The present case series is the first to report on the use of SLET for recurrent pterygium. The study by Bogantes et al. looked only at primary pterygium excision.12 The technique was also slightly different than the one used in our series, as a second layer of amniotic membrane was used on top of the stem cells, in contrast to the above cases in which a BCL was placed over the stem cells, as described by Sangwan's initial technique.9 Both techniques have been reported and there is no evidence to suggest superiority of one method over the other.
In terms of pterygium treatment, SLET's utility may be greatest in cases of the recurrent ones. PECA is a relatively less invasive and involved procedure than SLET and typically leads to fairly low pterygium recurrence rates. It is in the more aggressive and recurrent cases, however, where SLET might be considered as the more appropriate option; in such cases, removal of the pterygium may not be enough, and the limbal stem cell barrier might need to be re-populated.
While SLET was effective at preventing significant recurrence in these eyes, it is not completely surprising that corneal neovascularization did recur to a degree in two of the patients. These are aggressive pterygia that have had significant recurrence, and though the goal is to shut down any further recurrence, perhaps reducing the tempering the degree of recurrence is the best result in some of these patients.
Subconjunctival bevacizumab effectively prompted regression of these vessels in one patient, to the point that the ocular surface was sufficiently primed for a successful PKP. The use of anti-VEGF inhibitors can thus be a helpful adjunct in these cases, though observation and close monitoring is also an option.
The efficacy of anti-VEGF injections in preventing pterygium recurrence has been debated. While some studies have shown short-term regression of pterygia, both primary and recurrent, following anti-VEGF injection (Lavruc and Olup, 2012), a meta-analysis concluded that while bevacizumab is safe and well-tolerated, subconjunctival usage did not have a significant effect on reducing pterygium recurrence.5,6 More recent studies, however, have concluded that anti-VEGF injections, particularly subtenon ranibizumab, may have therapeutic utility in pterygium treatment, effectively preventing regression in up to 50% of cases.7
There are several limitations of this study that should be acknowledged. While we attribute a great deal of the success of these cases to the SLET procedure, the adjunctive use of MMC makes it difficult to firmly conclude that it was the SLET and not the MMC that limited the recurrences. MMC is itself a modality used in treating recurrent pterygia. It was not felt, however, that the aggressive cases presented here would have responded sufficiently if only the MMC was used, given some of these patients had already failed MMC treatment.
The obvious limitation of this study is that it is a series of only four cases. Importantly, however, the purpose to be conveyed is that SLET is a viable surgical technique for recurrent pterygium that may help reduce further recurrences. Our results are encouraging that SLET may develop into an important part of the treatment algorithm in aggressive pterygia. Further studies are needed to more reliably assess the outcomes of this procedure in terms of long-term recurrences and complications.
4. Conclusions
SLET is a novel surgical option for the treatment of recurrent pterygia. The procedure addresses a key pathological process in pterygium development and should be considered in aggressive and recurrent cases.
Patient consent
Written consent to publish this case has not been obtained. This report does not contain any personal identifying information.
Funding
None.
Conflicts of interest
The following authors have no financial disclosures: (ZM, TB, AEL, NS, DSR).
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship."
Acknowledgements
None.
References
- 1.Hacioglu D., Erdol H. Developments and current approaches in the treatment of pterygium. Int Ophthalmol. 2017;37:1073–1081. doi: 10.1007/s10792-016-0358-5. [DOI] [PubMed] [Google Scholar]
- 2.Akinci A., Zilelioglu O. Comparison of limbal-conjunctival autograft and intraoperative 0.02% mitomycin-C for treatment of primary pterygium. Int Ophthalmol. 2007;27(5):281–285. doi: 10.1007/s10792-007-9034-0. [DOI] [PubMed] [Google Scholar]
- 3.Katircoglu Y.A., Altiparmak U., Goktas S.E., Cakir B., Singar E., Ornek F. Comparison of two techniques for the treatment of recurrent pterygium: amniotic membrane vs conjunctival autograft combined with mitomycin C. Semin Ophthalmol. 2015;20(5–6):321–327. doi: 10.3109/08820538.2013.874468. [DOI] [PubMed] [Google Scholar]
- 4.Kenyon K.R., Wagoner M.D., Hettinger M.E. Conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology. 1985;92:1461. doi: 10.1016/s0161-6420(85)33831-9. [DOI] [PubMed] [Google Scholar]
- 5.Hu Q, Qiao Y, Nie X, Cheng X, Ma Y. Bevacizumab in the Treatment of Pterygium: a Meta-analysis. [DOI] [PubMed]
- 6.Lavric A., Olup B.D. Efficiency of subconjunctival bevacizumab on pterygium. Vestnik Zdravinski. 2012;(0):82. [Google Scholar]
- 7.Rose L., Byrd J.M., Qaseem Y. Subtenon injections of ranibizumab arrest growth in early recurrent pterygium. Eye Contact Lens. 2017;43(6):399–405. doi: 10.1097/ICL.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 8.Said D.G. Intra-lesional 5 fluorouracil for the management of recurrent pterygium. Eye. 2013;27(10):1123–1129. doi: 10.1038/eye.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sangwan V.S., Basu S., MacNeil S., Balasubramanian D. Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96:931–934. doi: 10.1136/bjophthalmol-2011-301164. [DOI] [PubMed] [Google Scholar]
- 10.Mittal V., Jain R., Mittal R., Vashist U., Narang P. Successful management of severe unilateral chemical burns in children using simple limbal epithelial transplantation (SLET) BJO (Br J Ophthalmol) 2016;100(8) doi: 10.1136/bjophthalmol-2015-307179. [DOI] [PubMed] [Google Scholar]
- 11.Mittal V., Narang P., Menon V., Mittal R., Honavar S. Primary simple limbal epithelial transplantation along with excisional biopsy in the management of extensive ocular surface squamous neoplasia. Cornea. 2016;35(12):165–1652. doi: 10.1097/ICO.0000000000000953. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez-Bogantes E., Amescua G., Navas A. Minor ipsilateral simple limbal epithelial transplantation (mini-SLET) for pterygium treatment. Br J Ophthalmol. 2015;99(12):1598–1600. doi: 10.1136/bjophthalmol-2015-306857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwok L.S., Coroneo M.T. A model for pterygium formation. Cornea. 1994;13(3):219–224. doi: 10.1097/00003226-199405000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Coroneo MT, Di Girolamo N, Wakefield D. The pathogenesis of pterygia. Curr Opin Ophthalmol. 199;10(4):282–288. [DOI] [PubMed]
- 15.Chu D., Reed M. Pterygium excision with mini-conjunctival graft: a new surgical approach. Invest Ophthalmol Vis Sci. 2003;44 E-Abstract 1330. [Google Scholar]
- 16.Gris O., Guell J., del Campo Z. Limbal-conjunctival autograft transplantation for the treatment of recurrent pterygium. Ophthalmology. 2000;107(2):270–273. doi: 10.1016/s0161-6420(99)00041-x. [DOI] [PubMed] [Google Scholar]
- 17.Akinci A., Zilelioglu O. Comparison of limbal-conjunctival autograft and intraoperative 0.02% mitomycin-C for treatment of primary pterygium. Int Ophthalmol. 2007;27(5):281–285. doi: 10.1007/s10792-007-9034-0. [DOI] [PubMed] [Google Scholar]
- 18.Melek I., Zghal I., Chebbi A. Conjunctival limbal autograft versus simple excision with intraoperative mitomycin C in pterygium surgery: a comparative study. J Fr Ophthalmol. 2013;36(3):230–235. doi: 10.1016/j.jfo.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Mahdy S., Bhatia J. Treatment of primary pterygium: role of limbal stem cells and conjunctival autograft transplantation. Eur J Ophthalmol. 2009;19(5):729–732. doi: 10.1177/112067210901900507. [DOI] [PubMed] [Google Scholar]