Abstract
Positive symptoms of schizophrenia may be related to distortions in time perception. To examine this issue, we conducted a meta-analysis to determine whether positive symptoms are associated with deficits in time processing performance. MEDLINE and Web of Science were searched from January 1980 through March 2017, and all related articles and their references were scrutinized to find relevant studies. Studies were selected if they included participants with a diagnosis of schizophrenia, and reported data from behavioral measures of interval timing (e.g. duration discrimination and temporal order judgement). The results indicated that positive symptoms of schizophrenia are related with overestimation of interval timing (i.e., acceleration of the “internal clock”), and suggest that time perception may be associated with psychosis.
Keywords: Interval timing, Positive symptoms, Psychosis, Time perception, Internal clock
1. Introduction
There have been many attempts to identify cognitive/behavioral indicators linked to positive symptoms (delusions, hallucinations, and etc.) of schizophrenia (Bark et al., 2003; Bear et al., 2017; Graham-Schmidt et al., 2016; Giersch et al., 2016; Horga et al., 2014). Specifically, temporal information processes are essential to all aspects of behavior, such as walking, speaking and engaging in physical activity. Recently, disturbances of temporal information processes have been suggested to contribute to the generation of positive symptoms (Carroll et al., 2009; Gómez et al., 2014; Lošák et al., 2016; Stevenson et al., 2017; Ward et al., 2012). Therefore, attempts to integrate these behavioral manifestations deserve considerations.
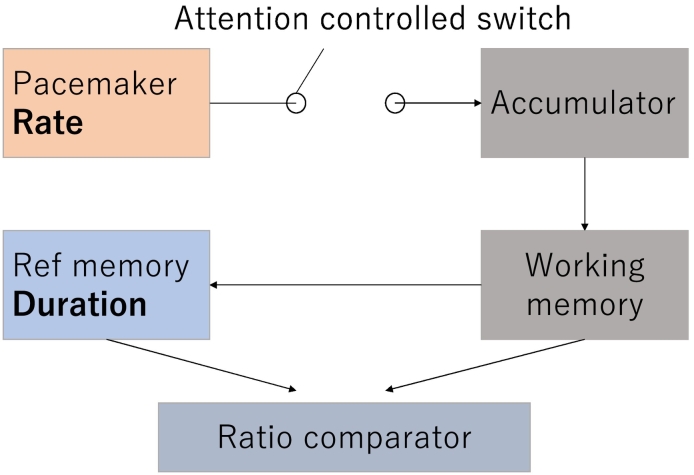
The nature of time perception is different from that of the other sensory modalities, e.g. visual and auditory processing, in that it is not paired to specific receptors, e.g. eyes and ears. It is generally agreed that attention and arousal may be important in temporal information processes (Merchant et al., 2013). Specifically, the pacemaker-accumulator model has been proposed as one of the main internal timing models (Treisman, 1963). This model is based on a multi-process theory that assumes three levels of timing processes common to animals and humans (Fig. 1; modified from Hartcher-O'Brien et al., 2016). The first level consists of a pacemaker and accumulator. The pacemaker generates pulses that are gated via a switch controlled by attention. In this level, a lack of attention leads to a deficiency in pulses in the accumulator. In the second level, the pulses reach the working memory module. The final level represents the decision mechanism. During this stage, the contents of the working memory module are compared to those of the reference memory of the pulse-duration on previous occasions, to which the subject responds accordingly (e.g., see Carroll et al., 2008; Hartcher-O'Brien et al., 2016).
Fig. 1.
The pacemaker accumulator model. Pulses are produced by the pacemaker and are gated via an attention-controlled switch. In this stage, the lack of attention leads to deficiency of pulses in the accumulator. The pulses reach working memory (or short term memory). The contents of working memory are compared with the reference memory (long term memory) which acquired pulse-duration in previous occasion and made decision how to response.
Modified from Hartcher-O'Brien et al., 2016 with permission from ELSEVIER LISENCE (License number: 4242350830031).
There are also some indications that altered time perception may be present in schizophrenia. Traditionally, the pathophysiology of schizophrenia has been associated with abnormalities in dopamine (DA) transmissions (e.g., Seeman et al., 2006), which in turn, have been linked to the speed of the internal clock (Cheng et al., 2007). In fact, several studies have demonstrated DA receptor agonists accelerate the internal clock, while antagonists decelerate it (e.g., Frederick and Allen, 1996; Meck, 2006).
Indeed, behavioral studies have reported overestimation (e.g. participants estimate the interval longer than objective time) in temporal durations ranging from milliseconds to several minutes in patients with schizophrenia (Jonson and Petzel, 1971; Volz et al., 2001; Carroll et al., 2009). The overestimation of time has been suggested to be caused by accelerated time processing (Droit-Volet and Meck, 2007).
Acceleration of the internal clock may be linked to the hypervigilance state that has been implicated in positive symptoms of schizophrenia (Lošák et al., 2016; Peterburs et al., 2013). Specifically, Dodgson and Gordon (2009) proposed that hypervigilance enhances responding biases and produces errors in cognitive processing, leading to the perception of a vague signal as a distinct one. Taken together, it is hypothesized that there is an association between positive symptoms and accelerated time perception, both linked to hypervigilance. Therefore, we conducted a meta-analysis to determine if severity of positive symptoms would be correlated with degree of acceleration of time perception in patients with schizophrenia.
2. Methods
2.1. Data sources
We conducted a literature review to search for articles that investigated timing abilities in schizophrenia. MEDLINE and Web of Science were searched through January 1980 to May 2017. We used two key words schizophrenia and positive symptoms in combination with any of the following terms: time discrimination, interval timing, and time perception. The reference list of identified studies was also hand-searched to obtain additional articles. Inclusion criteria were as follows: (1) published in English in a peer-reviewed journal; (2) included subjects with a primary diagnosis of schizophrenia on the basis of ICD (International Statistical Classification of Diseases and Related Health Problems) or DSM (Diagnostic and Statistical Manual of Mental Disorders) classification criteria; (3) reported behavioral measures of interval timing; and (4) conducted a correlation analysis between behavioral data and measures of positive symptom severity. Studies were excluded if correlational data concerning the timing ability of patients and positive symptom severity could not be extracted from the reported information.
The literature search yielded 100 < studies, 5 of which fulfilled the inclusion/exclusion criteria. Two articles reported on acceleration or deceleration in time perception (Waters and Jablensky, 2009; Peterburs et al., 2013), while three reported on the ability to detect temporal precision of duration discrimination (Carroll et al., 2008; Carroll et al., 2009; Stevenson et al., 2017). Multiple publications from the same investigators were examined to exclude duplication of subjects (Carroll et al., 2008). Finally, four studies (Carroll et al., 2009; Peterburs et al., 2013; Stevenson et al., 2017; Waters and Jablensky, 2009), containing a total of 101 individuals with schizophrenia, were included in the meta-analysis (See Table 1.).
Table 1.
Overview of studies on timing deficits in schizophrenia.
| Study | Sample size | Symptom scale | Task | Modality | Duration of the task | Findings |
|---|---|---|---|---|---|---|
| Carroll et al. (2009) | 28 | PANSS | Temporal bisection task | Audio | 3–6 s | Correlation between severity positive symptoms and temporal precision was slight almost negligible or low. |
| Peterburs et al. (2013) | 22 | PANSS | Anticipation movement task (AMT) | Visual | 3000 or 18,000 ms | Severity positive symptoms were associated with over estimation/under production of movement object time. |
| Stevenson et al. (2017) | 16 | SAPS | Simultaneity judgement task | Audio/visual | 0, ±10, ±20, ±50, ±80, and ±100 to 300 ms in 50 ms | Temporal precision associates with severity of hallucinations. |
| Waters and Jablensky. (2009) | 35 | FRS | Duration discrimination task | Audio | 1260–1440 ms | Subjects prone to overestimate were related to higher First Rank Symptoms scores. |
Note. PANSS = The Positive and Negative Syndrome Scale. SAPS = The Scale for the Assessment of Positive Symptoms. FRS = First Rank Symptom scale.
2.2. Data extraction
One of the four studies (Waters and Jablensky, 2009) examined the ability of subjects to compare the duration of a probe sensory stimulus with target stimulus previously stored in the working or reference memory (i.e., duration discrimination task). The second study (Carroll et al., 2009) used a temporal bisection task which required individuals to judge whether presented temporal stimuli were similar to the long or short standard durations previously presented. Another study (Stevenson et al., 2017) used a simultaneity judgement task to measure the smallest difference in duration between two intervals that could be detected by participants. The final study (Peterburs et al., 2013) included in the analysis examined the ability to anticipate how long it would take to move objects to reach a target position (i.e. anticipation movement task). In this study, if participants estimated the interval to be longer than objective time, the internal clock speed is assumed to be increased (Cheng et al., 2007).
If a study included two sets of empirical data and correlation analyses, we included the data that used longer than a 1 s duration (supra-second) on the basis of the assumption that the use of longer duration data would be more effective than shorter duration data in terms of detecting variability in the internal clock.
2.3. Data synthesis
One study reported Spearman's rank correlation coefficients, while another used Kendall's rank correlation coefficients. We transformed Spearman's and Kendall's correlation coefficients to Pearson's coefficients using transformation functions (Croux and Dehon, 2010) on the assumption that the variables followed a normal distribution after applying an adequate statistical transformation (e.g., Box-Cox transformation). Another study conducted a partial correlation analysis. We treated the partial correlation as if it was a simple Pearson's correlation analysis, which tends to be a conservative approach for the assessment of correlational significance.
A meta-analysis was conducted with the R (version 3.2.3; R Core Team, 2016) Meta package (Schwarzer et al., 2015). Pooled data from the four studies suggested a low to large association between performance on timing tasks vs. total psychotic symptom severity, as assessed by Positive symptom scores from the Positive and Negative Syndrome Scale (PANSS), Scale for the Assessment of Positive Symptoms (SAPS), or First Rank Symptom scale (Waters and Jablensky, 2009).
3. Results
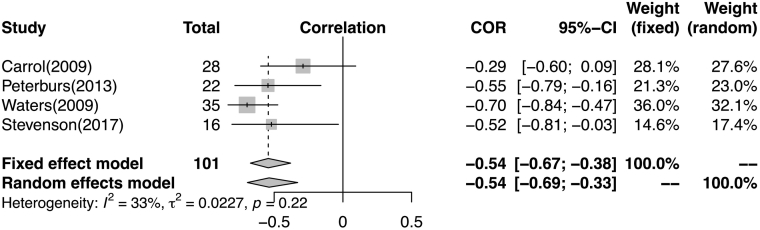
The meta-analysis revealed a moderate correlation between severity of positive symptoms and performance on timing tasks (Fig. 2). Correlation coefficient was −0.54[−0.69; −0.33] in the random effects model (I2 = 0.328, γ2 = 0.022, p = 0.216). Visual inspection of peak funnel plots did not reveal potential publication bias or other gross abnormalities.
Fig. 2.
Forest plot of standardized correlation coefficient between timing task performance and positive symptoms.
4. Discussion
In spite of the small total number of subjects included in the meta-analysis, we found a link between positive symptoms of schizophrenia and the acceleration and precision of timing. This finding may not be a result of random error, but represents an association between timing task performances and positive symptoms.
A small degree of heterogeneity was found across the studies included in this meta-analysis. This is attributable to one study (Carroll et al., 2009) that reported small correlation coefficients between temporal precision and severity of positive symptoms. That study used outpatients whose positive symptoms were not severe (with an average PANSS score of 64), probably yielding the heterogeneity.
Findings from the studies included in this meta-analysis are consistent with the concept that accelerated time perception are linked to hypervigilance (McCarthy-Jones and Longden, 2016), which may underlie the generation of positive symptoms. Hypervigilance is a mental state in which attention to external stimuli is exaggerated (Tiemann et al., 2012). The relationship between positive symptoms and dysregulation of the internal clock, observed in this study, is consistent with a role for hypervigilance in mediating these two mental events.
Altered DA transmissions have been suggested to cause positive psychotic symptoms in several psychiatric illnesses, particularly schizophrenia (Seeman et al., 2006). As discussed above, DA transmissions also regulate the speed of the internal clock (Frederick and Allen, 1996). Therefore, it is possible that dysregulation of DA activities may produce positive symptoms via modulation of the internal clock, which deserves further investigations.
The present results may have some clinical implications. Specifically, as the change in time perception has been associated with psychotic states, its measurement may add to an objective evaluation of psychosis, and possibly, early interventions (Cellard et al., 2007; de Montalembert et al., 2016; Horton et al., 2014; Reed and Randell, 2014). Accordingly, Penney et al. (2005) reported interval timing deficits in people who were at high risk for developing schizophrenia. Taken together, further efforts should be directed to determining the neural substrates underlying the altered time perception in psychotic conditions.
Limitations of this study include reproducibility problem due to the small number of trials (only 4 studies were included in this meta-analysis), suggesting a need for further study.
5. Conclusions
In this meta-analysis, positive symptoms of schizophrenia were found to be related to acceleration of timing. Further examinations of factors regulating this association may help understand the neural mechanisms underlying the pathophysiology of psychosis.
Author contributions
NU and TS wrote and revised the manuscript. KM conducted statistical analyses and revised it. All authors approved submission of the final draft.
Funding
This study was funded by Kakenhi (Nos. 18K13363, 17K10321) from Japan Society for the Promotion of Science, Intramural Research Grants (29-1, 30-1, 30-8) for Neurological and Psychiatric Disorders, National Center of Neurology and Psychiatry, and AMED under Grant Number 18dk0307081.
Conflict of interest
All authors report no conflict interest.
References
- Bark N., Revheim N., Huq F., Khalderov V., Ganz Z.W., Medalia A. The impact of cognitive remediation on psychiatric symptoms of schizophrenia. Schizophr. Res. 2003;63:229–235. doi: 10.1016/s0920-9964(02)00374-2. [DOI] [PubMed] [Google Scholar]
- Bear A., Fortgang R.G., Bronstein M.V., Cannon T.D. Mistiming of thought and perception predicts delusionality. Proc. Natl. Acad. Sci. 2017;114(40):10791–10796. doi: 10.1073/pnas.1711383114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll C.A., Boggs J., O'Donnell B.F., Shekhar A., Hetrick W.P. Temporal processing dysfunction in schizophrenia. Brain Cogn. 2008;67:150–161. doi: 10.1016/j.bandc.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll C.A., O'Donnell B.F., Shekhar A., Hetrick W.P. Timing dysfunctions in schizophrenia span from millisecond to several-second durations. Brain Cogn. 2009;70:181–190. doi: 10.1016/j.bandc.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Cellard C., Tremblay S., Lehoux C., Roy M.A. Processing spatial-temporal information in recent-onset schizophrenia: the study of short-term memory and its susceptibility to distraction. Brain Cogn. 2007;64(3):201–207. doi: 10.1016/j.bandc.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Cheng R.K., Ali Y.M., Meck W.H. Ketamine “unlocks” the reduced clock-speed effects of cocaine following extended training: evidence for dopamine-glutamate interactions in timing and time perception. Neurobiol. Learn. Mem. 2007;88:149–159. doi: 10.1016/j.nlm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Croux C., Dehon C. Influence functions of the Spearman and Kendall correlation measures. Stat. Methods Appl. 2010;19:497–515. [Google Scholar]
- Dodgson G., Gordon S. Avoiding false negatives: are some auditory hallucinations an evolved design flaw? Behav. Cogn. Psychother. 2009;37:325–334. doi: 10.1017/S1352465809005244. [DOI] [PubMed] [Google Scholar]
- Droit-Volet S., Meck W.H. How emotions colour our perception of time. Trends Cogn. Sci. 2007;11(12):504–513. doi: 10.1016/j.tics.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Frederick D.L., Allen J.D. Effects of selective dopamine D1- and D2-agonists and antagonists on timing performance in rats. Pharmacol. Biochem. Behav. 1996;53:759–764. doi: 10.1016/0091-3057(95)02103-5. [DOI] [PubMed] [Google Scholar]
- Giersch A., Lalanne L., Isope P. Implicit timing as the missing link between neurobiological and self disorders in schizophrenia? Front. Hum. Neurosci. 2016;10:303. doi: 10.3389/fnhum.2016.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez J., Jesús Marín-Méndez J., Molero P., Atakan Z., Ortuño F. Time perception networks and cognition in schizophrenia: a review and a proposal. Psychiatry Res. 2014;220(3):737–744. doi: 10.1016/j.psychres.2014.07.048. [DOI] [PubMed] [Google Scholar]
- Graham-Schmidt K.T., Martin-Iverson M.T., Holmes N.P., Waters F.A.V. When one's sense of agency goes wrong: absent modulation of time perception by voluntary actions and reduction of perceived length of intervals in passivity symptoms in schizophrenia. Conscious. Cogn. 2016;45:9–23. doi: 10.1016/j.concog.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Hartcher-O'Brien J., Brighouse C., Levitan C.A. A single mechanism account of duration and rate processing via the pacemaker–accumulator and beat frequency models. Curr. Opin. Behav. Sci. 2016;8:268–275. doi: 10.1016/j.cobeha.2016.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horga G., Schatz K.C., Abi-Dargham A., Peterson B.S. Deficits in predictive coding underlie hallucinations in schizophrenia. J. Neurosci. 2014;34(24):8072–8082. doi: 10.1523/JNEUROSCI.0200-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton L.E., Smith A.A., Haas G.L. The nature and timing of social deficits in child and adolescent offspring of parents with schizophrenia: preliminary evidence for precursors of negative symptoms? Res. 2014;159(1):27–30. doi: 10.1016/j.schres.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonson J., Petzel T. Temporal orientation and time estimation in chronic schizophrenics. J. Clin. Psychol. 1971;27:194–196. doi: 10.1002/1097-4679(197104)27:2<194::aid-jclp2270270210>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Lošák J., Hüttlová J., Lipová P., Mareček R., Bareš M., Filip P., Žůbor J., Ustohal L., Vaníček J., Kašpárek T. Predictive motor timing and the cerebellar vermis in schizophrenia: an fMRI study. Schizophr. Bull. 2016;42(6):1517–1527. doi: 10.1093/schbul/sbw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy-Jones S., Longden E. Auditory verbal hallucinations in schizophrenia and post-traumatic stress disorder: common phenomenology, common cause, common interventions? Front. Psychol. 2016;6:1–12. doi: 10.3389/fpsyg.2015.01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck W.H. Frontal cortex lesions eliminate the clock speed effect of dopaminergic drugs on interval timing. Brain Res. 2006;1108:157–167. doi: 10.1016/j.brainres.2006.06.046. [DOI] [PubMed] [Google Scholar]
- Merchant H., Harrington D.L., Meck W.H. Neural basis of the perception and estimation of time. Ann. Rev. Neurosci. 2013;36:313–336. doi: 10.1146/annurev-neuro-062012-170349. [DOI] [PubMed] [Google Scholar]
- de Montalembert M., Coulon N., Cohen D., Bonnot O., Tordjman S. Time perception of simultaneous and sequential events in early-onset schizophrenia. Neurocase. 2016;22(4):392–399. doi: 10.1080/13554794.2016.1205098. [DOI] [PubMed] [Google Scholar]
- Penney T.B., Meck W.H., Roberts S.A., Gibbon J., Erlenmeyer-Kimling L. Interval-timing deficits in individuals at high risk for schizophrenia. Brain Cogn. 2005;58:109–118. doi: 10.1016/j.bandc.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Peterburs J., Nitsch A.M., Miltner W.H.R., Straube T. Impaired representation of time in schizophrenia is linked to positive symptoms and cognitive demand. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0067615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer, Carpenter, Ru¨cker . 2015. Meta-Analysis with R. [Google Scholar]
- Seeman P., Johannes S., Jiang-Fan C., Henry S., Perreault M., McKnight G.S., Roder J.C., Re'mi Q., Patricia B., Srivastava L.K., Kazuhiko Y., Dabid W., Tomiki S. Psychosis pathways converge via D2High dopamine receptors. Synapse. 2006;60:319–346. doi: 10.1002/syn.20303. [DOI] [PubMed] [Google Scholar]
- Stevenson R.A., Park S., Cochran C., McIntosh L.G., Noel J.P., Barense M.D., Ferber S., Wallace M.T. The associations between multisensory temporal processing and symptoms of schizophrenia. Schizophr. Res. 2017;179:97–103. doi: 10.1016/j.schres.2016.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemann L., Schulz E., Winkelmann A., Ronel J., Henningsen P., Ploner M. Behavioral and neuronal investigations of hypervigilance in patients with fibromyalgia syndrome. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman M. Temporal discrimination and the indifference interval: implications for a model of the “internal clock”. Psychol. Monogr. Gen. Appl. 1963;77:1–31. doi: 10.1037/h0093864. [DOI] [PubMed] [Google Scholar]
- Volz H.P., Nenadic I., Gaser C., Rammsayer T.H., Häger F., Sauer H. Time estimation in schizophrenia: an fMRI study at adjusted levels of difficulty. Neuroreport. 2001;12:313–316. doi: 10.1097/00001756-200102120-00026. [DOI] [PubMed] [Google Scholar]
- Ward R.D., Kellendonk C., Kandel E.R., Balsam P.D. Timing as a window on cognition in schizophrenia. Neuropharmacology. 2012;62(3):1175–1181. doi: 10.1016/j.neuropharm.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters F., Jablensky A. Time discrimination deficits in schizophrenia patients with first-rank (passivity) symptoms. Psychiatry Res. 2009;167(1–2):12–20. doi: 10.1016/j.psychres.2008.04.004. [DOI] [PubMed] [Google Scholar]