Abstract
We present the first cirrhotic patient who underwent liver transplantation (LT) and presented a hepatic artery thrombosis of the graft due to Aspergillus fumigatus, within the first month of LT. This culminated in graft loss, re-transplant with multiple biliary and infectious complications. To our knowledge, this is a case report of an early hepatic artery thrombosis due to Aspergillus fumigatus in an infection-free patient.
Keywords: Aspergillus fumigatus, Hepatic artery thrombosis, Liver transplantation
1. Introduction
Early hepatic artery thrombosis (E-HAT) is a major cause of graft loss and mortality after liver transplantation (LT) [2], [3], with an incidence between 2.5% and 6% in adults and 15–20% in the pediatric population [1], with a graft survival of 52%, 36.6% and 27.4% at 1, 3, and 5 years respectively,
Invasive Aspergillosis (IA) due to Aspergillus fumigatus is one of the causes of Invasive Fugal Infection (IFI)with a mortality rate of 40–90% [4]. LT recipients present an important risk of developing such infections, in some cases with an incidence of 10–43% [5]. Diagnosis is difficult due to unspecific symptoms and in some cases requiring microscopic identification of septate hyphae on tissue biopsy [6].
There are few reported cases of IA in LT patients, consisting of cases of pulmonary Aspergillosis by A. Lentulus [7], and IFI of the abdominal cavity and solid bodies [8]. This is a case report of an E-HAT due to Aspergillus in an infection-free LT patient.
2. Case
A 54-year old male patient with a medical history of two years of liver cirrhosis due to HCV infection (CHILD B AND MELD 16), with hepatic encephalopathy grade II-III, grade II oesophageal varices, moderate ascites and resolved primary peritonitis. The patient was added to the Pre-transplant Protocol for LT in April 2005 and four months later an orthotropic LT was performed from a 32-year old deceased donor with O blood group, and an ischemia time of 5 h counting as his day 0. Transfusion was required during the surgery, consisting of 12 units of red blood cells, due to the occurrence of a hepatic subcapsular hematoma during LT.
After the surgical procedure, the patient remained in the Intensive Care Unit for 3 days, with a total of 17 days of hospitalization. During his hospitalization the patient persisted with elevated transaminases. An endoscopic retrograde cholangiopancreatography (ERCP) and liver biopsy were performed, demonstrating a permeable bile duct and a moderate acute graft rejection. This was managed with 500 mg/day of methylprednisolone during a 3-day period. Later, the patient showed stabilization of his bilirubin and liver enzymes levels and was discharged with immunosuppressive treatment including Tacrolimus and Prednisolone.
Ten days after discharge, the patient presented marked jaundice and adynamia (AST 133 U/L, ALT 150 U/L, BT 11.5 mg/dL, DB 5, 5 mg/dL, Serum Creatinine 1.34 mg/dL). Another ERCP was performed which showed evidence of a biliary duct obstruction; this was managed with a stent.
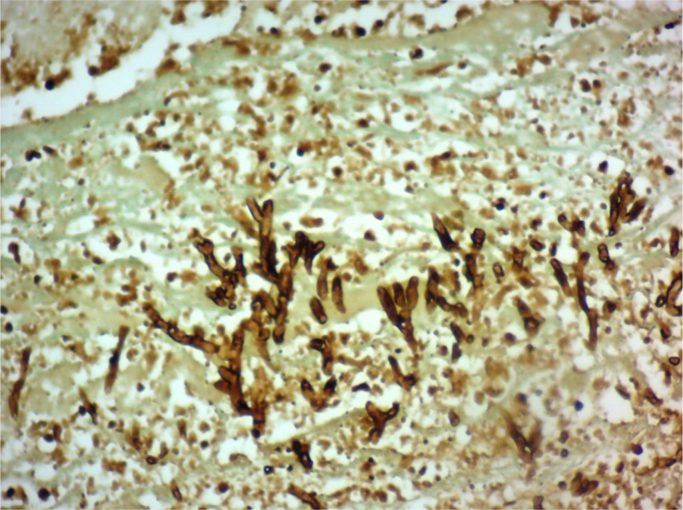
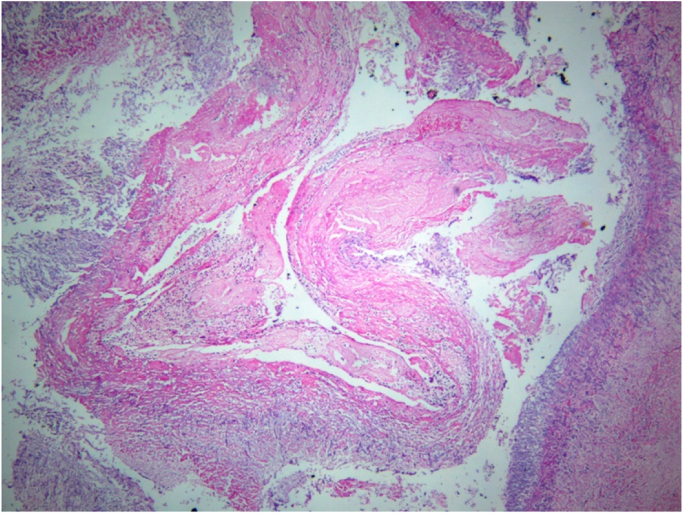
In addition, a Doppler ultrasound of the liver and bile ducts was performed which showed an absence of flow in the hepatic artery. A liver re-transplantation was carried out on September 21st, 2005 from a cadaveric donor. During the surgery, a Hepatic Artery Thrombosis (HAT) and pseudoaneurysm were found. The affected segment of the hepatic artery was removed and sent to pathology where a fibrin thrombus was found occluding the vascular lumen and within which there were abundant septate hyphae with ramifications at an angle of 45 degrees, corresponding to Aspergillus (Fig. 1, Fig. 2), the patients sample was culture in Sabouraud agar and Mycosel agar at 28 °C temperature. The definivite culture results reported isolation of Aspergillus fumigatus in Sabouraud agar, and no growth in Mycosel. Chest x-ray showed no signs of Pulmonary Aspergillosis.
Fig. 1.
Hematoxylin and Eosin 40X10. Panoramic view of hepatic artery with thrombus due to septate hyphae at angle 45 degrees, corresponding to Aspergillus spp.
Fig. 2.
Gomori Stain 20X10. Septate hyphae ramifications growing in thrombus of hepatic artery, compatible with Aspergillus spp.
Following the re-transplant, the patient required a 41-day hospitalization with 75 mg/day of Amphotericin B as antifungal management for 36 days and antibiotic treatment for bacteremia due to Acinetobacter baumannii and Klebsiella pneumonia with Ciprofloxacin. Voriconazole was not used in our patient since at the time Amphotericin B was the antifungal drug available in our institution. The patient then returned to his city of origin with normal renal function.
3. Discussion
Aspergillus species are widespread in the environment, growing on plants, soil, dust and in indoor environments [9]. They are mainly opportunistic pathogens and although the main port of entry is the respiratory tract, other means of entry have been described [9], [10].
Among the factors affecting the net state of immunosuppression in transplanted patients, the nature of the immunosuppressive therapy represents an important risk factor for IA [11], which may have been the case of our patient who received a high dosage of corticosteroid treatment for graft rejection days prior to E-HAT.
Although it is generally believed that E-HAT is a surgical complication as a result of technical difficulties with the reconstruction of the hepatic artery, studies have shown nonsurgical factors associated with E-HAT, such as donor age and a defective unbalanced haemostatic system secondary to cirrhosis that can turn into hypercoagulation when the system is challenged [2]. Other studies have shown CMV infection as an associated risk factor in Late HAT due to a rapid procoagulant response that follows the infection of endothelial cells by CMV and may be responsible for the hypercoagulability and reduced fibrinolysis that leads to HAT [12].
Despite the fact that in the majority of cases it is not possible to identify the triggering factor for the HAT, in our case report, we describe a patient who required multiple blood transfusions which, in addition to risk factors inherent to liver cirrhosis and LT, promoted coagulation and platelet activation [3], [13].
Whether the infection is community acquired or hospital acquired, Aspergillus fumigatus is the most common species of IA, due to its ubiquitousness in the environment and its virulence factors [14]. Although rare, post-operative IA may arise after colonization of surgical sites from Aspergillus spores [15], and since aspergillus has a rapid growth rate, around 2 mm/h [16], the onset of the disease may occur days after the surgical procedure. Nevertheless, it is unclear if elevated Aspergillus spore levels in the operating room represent an important risk factor [15].
Infection arises from inhalation of conidia, with contaminating sources including air filters and other defects in the ventilation systems [17], yet most cases of IA arise sporadically and a specific source point is never found [14]. Recent studies have demonstrated that high counts of Aspergillus conidia in air correlate with the appearance of new cases of IA [18], which is important in order to prove an environmental source of an outbreak and nosocomial acquisition of the infection. In our report, construction work in the hospital was being carried during the surgical act of the LT, which has been reported to be a probable cause in half of Aspergillus conidia outbreaks in hospitals [19]. Yet at the time being, no microbiological assessment of the operating room was performed.
Acknowledgements
None.
Acknowledgments
Conflict of interest
The authors of this manuscript have no conflicts of interest.
References
- 1.Feltracco P., Barbieri S., Cillo U., Zanus G., Senzolo M., Ori C. Perioperative thrombotic complications in liver transplantation. World J. Gastroenterol. 2015;21(26):8004–8013. doi: 10.3748/wjg.v21.i26.8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mourad M.M., Liossis C., Gunson B.K., Mergental H., Isaac J., Muiesan P. Etiology and management of hepatic artery thrombosis after adult liver transplantation. Liver Transplant. 2014;20:713–723. doi: 10.1002/lt.23874. [DOI] [PubMed] [Google Scholar]
- 3.Silva M.A., Jambulingam P.S., Gunson B.K., Mayer D., Buckels J.A.C., Mirza D.F. Hepatic artery thrombosis following orthotopic liver transplantation: A 10-year experience from a single centre in the United Kingdom. Liver Transplant. 2006;12:146–151. doi: 10.1002/lt.20566. [DOI] [PubMed] [Google Scholar]
- 4.Pemán J., Salavertb M. Epidemiología general de la enfermedad fúngica invasora. Enferm. Infecc. Microbiol. Clin. 2012;30(2):90–98. doi: 10.1016/j.eimc.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Akamatsu N., Sugawara Y., Kaneko J., Tamura S., Makuuchi M. Preemptive treatment of fungal infection based on plasma ( 1 fi 3) b - D-Glucan levels after liver transplantation. Infection. 2007;35(5):346–351. doi: 10.1007/s15010-007-6240-7. [DOI] [PubMed] [Google Scholar]
- 6.Zhou T., Xue F., Han L.Z., Xi Z.F., Li Q.G. Invasive fungal infection after liver transplantation: risk factors and significance of immune cell function monitoring. J. Dig. Dis. 2011;12:467–475. doi: 10.1111/j.1751-2980.2011.00542.x. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida H., Seki M., Umeyama T., Urai M., Kinjo Y. Invasive pulmonary aspergillosis due to Aspergillus lentulus: successful treatment of a liver transplant patient. J. Infect. Chemother. 2015;21(6):479–481. doi: 10.1016/j.jiac.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Jafarian A., Kasraianfard A., Nassiri-toosi M. Revision liver transplant for persistent infection and localized aspergillosis after hepatic artery thrombosis. Exp. Clin. Transplant. 2014;4:381–383. doi: 10.6002/ect.2013.0129. [DOI] [PubMed] [Google Scholar]
- 9.Paulussen C., Hallsworth J.E., Nierman W.C., Alvarez-p S., Hamill P.G., Blain D. Ecology of aspergillosis: insights into the pathogenic potency of Aspergillus fumigatus and some other Aspergillus species. Microb. Biotechnol. 2016;0(0):1–27. doi: 10.1111/1751-7915.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denning D.W. Invasive Aspergillosis. Clin. Infect. Dis. 1998;26:781–803. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 11.Baddley J.W. Clinical risk factors for invasive aspergillosis. Med. Mycol. 2011;49:7–12. doi: 10.3109/13693786.2010.505204. (March 2010) [DOI] [PubMed] [Google Scholar]
- 12.Gunsar F., Rolando N., Pastacaldi S., Patch D., Raimondo M.L., Davidson B. Late hepatic artery thrombosis after orthotopic liver transplantation. Liver Transplant. 2003;9(6):605–611. doi: 10.1053/jlts.2003.50057. [DOI] [PubMed] [Google Scholar]
- 13.Duffy J.P., Hong J.C., Farmer D.G., Ghobrial R.M., Yersiz H., Hiatt J.R. Vascular complications of orthotopic liver transplantation: experience in more than 4200 patients. Am. Coll. Surg. 2009;208(5):896–903. doi: 10.1016/j.jamcollsurg.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 14.Gregg K.S., Kauffman C.A. Invasive Aspergillosis: epidemiology, clinical aspects, and treatment. Semin Respir. Crit. Care Med. 2015;36(5):662–672. doi: 10.1055/s-0035-1562893. [DOI] [PubMed] [Google Scholar]
- 15.Torres-narbona M., Mun P., Jensen J., Pela T. Post-surgical invasive aspergillosis: an uncommon and under-appreciated entity*. J. Infect. 2010;60:162–167. doi: 10.1016/j.jinf.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Rhodes J.C. Aspergillus fumigatus: growth and virulence. Med Mycol. 2006;44(s1):77–81. doi: 10.1080/13693780600779419. [DOI] [PubMed] [Google Scholar]
- 17.Gibert P., Brudieu E., Timsit J.F., Foroni L., Thie´baut-Bertrand C., Allenet B. Invasive aspergillosis: drug-dispensing systems as a source of filamentous fungal contamination in high- risk units? J. Hosp. Infect. 2012;82:293–296. doi: 10.1016/j.jhin.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Guinea J., García de Viedma D., Pela´ez T., Escribano P., Muñoz P., F., Meis J. Molecular epidemiology of Aspergillus fumigatus: an in-depth genotypic analysis of isolates involved in an outbreak of invasive Aspergillosis. J. Clin. Microbiol. 2011;49(10):3498–3503. doi: 10.1128/JCM.01159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vonberg R., Gastmeier P. Nosocomial aspergillosis in outbreak settings. J. Hosp. Infect. 2006;63:246–254. doi: 10.1016/j.jhin.2006.02.014. [DOI] [PubMed] [Google Scholar]