Abstract
Background
Evaluation of task-evoked cortical responses during movement has been limited in individuals with bilateral cerebral palsy (CP), despite documented alterations in brain structure/function and deficits in motor control.
Objective
To systematically evaluate cortical activity associated with lower extremity tasks, and relate activation parameters to clinical measures in CP.
Methods
28 ambulatory participants (14 with bilateral CP and 14 with typical development) completed five motor tasks (non-dominant ankle dorsiflexion, hip flexion and leg cycling as well as bilateral dorsiflexion and cycling) in a block design while their sensorimotor cortex was monitored using functional near infrared spectroscopy (fNIRS), in addition to laboratory and clinical measures of performance.
Results
Main effects for group and task were found for extent of fNIRS activation (number of active channels; p < 0.001 and p = 0.010, respectively), magnitude of activation (sum of beta values; p < 0.001 for both), and number of active muscles (p = 0.001 and p < 0.001, respectively), but no group by task interactions. Collectively, subgroups with CP and especially those with greater impairments, showed higher extent and magnitude of cortical sensorimotor activation as well as higher amounts of concurrent activity in muscles not required for task performance. Magnitude of fNIRS activation during non-dominant dorsiflexion correlated with validated measures of selective control (r = −0.60, p = 0.03), as well as mobility and daily activity (r = −0.55, p = 0.04 and r = −0.52, p = 0.05, respectively) and self-reported gait function (r = −0.68, p = 0.01) in those with CP.
Conclusions
The association between higher activity in the sensorimotor cortex and decreased selectivity in cortical organization suggests a potential neural mechanism of motor deficits and target for intervention.
Keywords: Brain, Hemodynamic response, Electromyography, Selective motor control, Cerebral palsy
Highlights
-
•
First fNIRS comparison of a range of lower extremity tasks in children with and without bilateral CP.
-
•
FNIRS showed a greater amount and extent of activation of sensorimotor cortices in CP.
-
•
Greater activation correlated with a greater number of muscles involved in the task.
-
•
fNIRS results correlated to clinical measures of motor control and function.
1. Introduction
Cerebral palsy (CP) is the most prevalent child-onset motor disorder and is caused by a non-progressive brain injury early in life (Graham et al., 2016; Sanger et al., 2006). In the case of bilateral lower extremity motor impairment, substantial research has been done in describing and quantifying motor behavior. It has been shown that this population demonstrates difficulty with selective voluntary motor control (Sanger et al., 2006; Fowler & Goldberg, 2009), manifesting as atypical synergistic movements within and across limbs (Fowler et al., 2009; Thelen et al., 2003). Studies have demonstrated greater difficulty with isolated distal movements compared to proximal movements (Fowler et al., 2010; Lim, 2015), increased co-contraction across joints (Chen et al., 2003), and impaired reciprocal activation (Chen et al., 2003).
Damage to the sensorimotor cortex and the corticospinal pathways are postulated to be a primary contributor to impaired selective voluntary motor control (Fowler et al., 2009; Cahill-Rowley & Rose, 2014; Sukal-Moulton et al., 2014a), with sensory cortex responses to external stimuli (Wingert et al., 2010; Kurz et al., 2014a) or sensory pathways (Hoon Jr. et al., 2009) in particular being implicated in bilateral CP. Transcranial magnetic stimulation (TMS) and magnetic resonance imaging (MRI) investigations in unilateral CP have indicated the capacity of the less damaged hemisphere to maintain ipsilateral projections in order to influence control of the paretic arm in unilateral CP (Eyre et al., 2007; Vandermeeren et al., 2009). In contrast, far less data have been reported in bilateral CP. Using TMS, a lateral shift in the location of motor representations, or “hot spots”, for individual lower extremity segments was shown (Kesar et al., 2012; Maegaki et al., 1999), in addition to overlapping and sometimes bilaterally projecting cortico-muscular connections to upper and lower extremities (Wittenberg, 2009). One child with bilateral, asymmetrical CP showed a large area of fMRI cortical activation during an ankle dorsiflexion task (Phillips et al., 2007).
Brain imaging techniques enable researchers to decipher the mechanisms by which alterations in sensory and motor pathways may affect underlying lower extremity motor control in CP, with MRI having the greatest potential to evaluate both superficial and deeper brain structures (Wingert et al., 2010; Hilderly et al., 2016). However, with MRI, researchers are often limited to either structural or resting state connectivity measures (Burton et al., 2009), or small amplitude mostly single joint movements in supine during functional imaging measures (Phillips et al., 2007; Hilderly et al., 2016). Furthermore, obtaining high quality images is not always possible in children with CP due to exaggerated startle responses and/or involuntary movements which become increasingly more problematic in children with greater degrees of neurological involvement. Mobile brain imaging techniques such as functional near infrared spectroscopy (fNIRS) move with the head and body, allowing for the study of a wide range of motor tasks in a more naturalistic setting and in a broader clinical population.
FNIRS is a non-invasive neuroimaging method that uses low-levels of red to near-infrared light to measure task-evoked changes in oxygenated and deoxygenated hemoglobin (Hb) concentrations on the cortical surface. These measurements are made by placing a head cap containing light emitters and detectors on the scalp overlying specific brain regions, which allows recording of optical density measures that are converted to relative changes in Hb concentrations, specifically increases in oxygenated hemoglobin (HbO) and decreases in deoxygenated hemoglobin (HbR) during functional tasks. This technology has been used with healthy adults during a number of motor tasks including stepping (Huppert et al., 2013; Koenraadt et al., 2014), gait (Miyai et al., 2001; Miyai et al., 2003; Suzuki et al., 2008), and balance tasks (Karim et al., 2013; Karim et al., 2012).
In unilateral CP, fNIRS has been used to assess brain activation during hand movements (Tian et al., 2008; Tian et al., 2010; Khan et al., 2010; Decampos et al., 2016), and to evaluate pre-frontal cortical activation in bilateral CP during a throwing task (Chaudhary et al., 2014). With respect to the lower extremity, one study evaluated fNIRS activation during gait in four children with bilateral CP compared to eight children with typical development (TD). The results showed higher levels of activation across sensorimotor and superior parietal lobules and greater variability in gait patterns in CP (Kurz et al., 2014b). Because of the small sample size, a direct correlation between cortical activity and gait variability was seen only with combined data from both groups. Understanding the relationship between increased brain activation and specific motor control deficits in CP is challenging when measured during a complex task such as gait, which involves coordination of multiple joints, reciprocal activity across legs, as well as dynamic postural control, all of which may be impaired in CP.
The goal of the current study was to systematically evaluate cortical activity and potential neural mechanisms associated with a range of simple to incrementally more complex lower extremity tasks including distal (ankle dorsiflexion) and proximal (hip flexion) single joint movements, coordinated unilateral and bilateral multi-joint tasks (single and bilateral cycling), and synchronous (bilateral dorsiflexion) as well as reciprocal (bilateral cycling) tasks performed in an upright position. FNIRS measures were related to clinical scales and simultaneous lower limb electromyography (EMG) measures to elucidate potential neural mechanisms underlying functional and performance differences. Our primary hypothesis was that individuals with CP would demonstrate higher magnitude and more widespread extent of brain activation (Kurz et al., 2014b) that would be related to a greater degree of task performance impairment as measured by EMG, a lower extremity selective motor control test and clinical scales assessing gait function, mobility and daily activity. Secondary hypotheses included: 1) a dominant hemisphere would still be identifiable in all groups contralateral to the dominant lower limb as indicated by group differences in an activation laterality index, even though the brain injury in CP is considered bilateral; 2) the center of activity would be more laterally located in CP compared to TD for unilateral tasks due to the high prevalence of midline brain injuries in the patient group and reorganization that may consequently occur more laterally (Maegaki et al., 1999); and 3) contrasts between tasks (unilateral, bilateral, single joint, multiple joint) would reveal differential responses across groups with respect to relative task difficulty due to underlying differences in motor coordination.
2. Materials and methods
2.1. Participants
A total of 28 participants completed the study, including 14 (5 males) with bilateral CP, GMFCS Levels I–III, and 14 (9 males) age-matched with TD. Participants were included if they were at least 5 years old, able to understand and follow simple directions for performing a repetitive task, and agreed to not drink caffeine or alcohol for 24 h before assessments to avoid associated alterations in blood flow dynamics. Exclusion criteria included any health condition or diagnosis other than CP that would affect the ability to maintain attention or move a body part repetitively for short periods of time, surgery within a year, or botulinum toxin injections within 6 months. The study was approved by the Institutional Review Board of the National Institutes of Health Clinical Center (protocol #13-CC-0110) and all participants (or their parents, as applicable) completed informed consent and assent.
A complete history and physical examination was performed by a pediatric physiatrist, followed by a number of standardized clinical assessments for those with CP, including the Gross Motor Function Classification System (GMFCS) (Palisano et al., 2008) and Hypertonia Assessment Tool (HAT) (Jethwa et al., 2010). Furthermore, the Selective Control Assessment of the Lower Extremity (SCALE) (Fowler et al., 2009), AbilLOCO (Caty et al., 2008), and PEDI-CAT (version 2.5) (Haley et al., 2005) were completed for correlation to fNIRS and electromyography (EMG) activity. The SCALE evaluates the ability to move each joint of the lower extremity independently, with each of 5 joints receiving a score of 0, 1, or 2 (best). For example, the SCALE score at the ankle involves dorsiflexing, plantarflexing, and then dorsiflexing the ankle again to a timed count, while the examiner observes for movement occurring at other joints on that limb or on the opposite leg. Parents of or adult participants with CP completed the AbilLOCO questionnaire about difficulty performing ambulation tasks and the PEDI-CAT which yields summary scores for Mobility and Daily Activity, with higher scores indicating greater function. A structural magnetic resonance image (MRI, T1 and T2 weighted) was completed on each participant, and evaluated by a neuroradiologist to provide more information about the type and extent of brain injuries to aid in interpretation of NIRS data. The Edinburg Handedness Inventory was completed by all participants with an additional question about lower extremity preferences to determine which leg to test.
2.2. Setup
Setup for each participant included placement of the fNIRS optodes (CW6, TechEn, Milford, MA) on the scalp regions that overlay the sensorimotor cortical areas (Fig. 1A). The hair was carefully parted to align with optode placement as described previously (Sukal-Moulton et al., 2014b). The optode arrangement (8 sources and 16 detectors, with inter-optode distance ranging between 22.4 and 36.5 mm) was centered on Cz and covered bilateral sensorimotor areas of the brain, as defined by the International 10/20 System. The CW6 fNIRS system uses 690 nm and 830 nm wavelengths emitted from the source optodes, which are frequency encoded for decomposition at each of the detectors. Power for each wavelength at the source optodes were regularly checked between participants and adjusted according to the manual specifications.
Fig. 1.
Probe design and setup. A. Probe layout viewed from the top down. White circles represent light sources, dark gray circles represent detectors, and the gray lines between them represent channels. The probe is 13 cm in width and 6 cm in height in total, and centered about Cz. B. Example of the probe registered to a participant-specific MRI to show where it sits on the actual scalp using AtlasViewer (Cooper et al., 2012). C. Participant seated in a semi-recumbent position for a dorsiflexion task. The black and white hat shields the detectors from the light of the motion capture cameras in the room, and infrared cables are supported by an overhead track. The hip flexion task position included a sling which supported the flexing leg from the same overhead track as the cables. For the cycling tasks, a stationary cycle was secured to the plinth using a strap while the lower segment of the plinth was fully horizontal. D. Schematic of trials, with 15 second task blocks (n = 8), interspersed with rest periods.
Three-dimensional locations for each of the optodes as well as the nasion, inion, and pre-auricular points for each participant were recorded using reflective markers and a motion capture system (Vicon Motion Systems Ltd., Oxford, UK). To confirm that channels on all participants corresponded to the same underlying brain regions, the coordinates of optodes were imported into AtlasViewer and standardized to the Colin27 atlas (Fig. 1B), translating their original coordinates into a common space. For each optode, an average location and standard deviations in x, y, and z were calculated. The results showed an average standard deviation of optode placement of 4 mm between participants, with the higher values found on the lateral portions of the scalp. Also, no relationship was found between head size and optode placement variations. Co-registration to subject-specific MRIs was not performed due to limitations in applying standard normalization processes to participants with brain lesions.
In addition, EMG electrodes (Delsys Trigno, Natick, MA) were placed according to SENIAM guidelines (Hermens et al., 2000) over the following muscles bilaterally: rectus femoris (RF), vastus lateralis (VL), semimembranosus (SM), tibialis anterior (TA), and medial gastrocnemius (MG). Data were synchronized between the EMG and fNIRS data acquisition computers using a mouse button press trigger pulse which was split to analog inputs on both systems at the start of a trial (Sukal-Moulton et al., 2014b). fNIRS data were collected at 50 Hz with triggers logged at the beginning of each task block in the fNIRS software, and EMG data were collected at 1080 Hz and then were baseline corrected, rectified, low pass filtered at 4 Hz using a second order Butterworth filter,
For all tasks, the optode cables were supported by a length-adjustable cord suspended from an overhead track to maximize participant comfort and minimize motion artifacts associated with small movements of the body during tasks (Fig. 1C).
2.3. Motor tasks
Tasks were completed in a semi-randomized fashion to minimize set-up changes, and included: non-dominant ankle dorsiflexion, bilateral ankle dorsiflexion, non-dominant hip flexion with the leg supported by an overhead sling, non-dominant single leg cycling and bilateral cycling. For all tasks, participants were positioned with their trunk well supported on a plinth in a semi-recumbent position and completed 8 task blocks of 15-s each, interspersed with a variable rest period of 25 to 35 s (Fig. 1D). They were cued to move at 1 Hz with audio (Van de Winckel et al., 2013) and visual prompts customized for this experiment. Cycling tasks were completed using a MOTOmed (Reck, Reckstraße, Germany) cycle with no resistance, using a counterweight for unilateral cycling. Participants were given rest breaks in between tasks.
2.4. fNIRS data processing and analysis
Light intensities in the fNIRS signals were analyzed using a set of open source Matlab functions found in the NIRS toolbox (Huppert, 2016). Using this toolbox, a number of processing steps were completed. First, optical density was converted to oxygenated and deoxygenated hemoglobin using the modified Beer Lambert Law. A first-level canonical general linear model (Huppert, 2016; Barker et al., 2013) was then used to estimate the statistical response of each fNIRS light emitter-detector (channel) to the functional task. In brief, as first described in Barker et al., (2013), an autoregressive whitening filter was iteratively computed and applied to the data and linear regressor model in order to reduce the effect of serially-correlated noise. A robust linear regression using the Huber bisquare weighting was applied to estimate the model. The regression coefficients (beta values) and t statistical effects were estimated for each fNIRS channel and task condition. This procedure was previously shown to correct the high false-discovery rate problems that result from motion and non-task coupled superficial physiological noise in fNIRS analysis (Barker et al., 2013). For all analyses, the sum of oxygenated and deoxygenated hemoglobin, or the total hemoglobin (HbT) was used to incorporate both aspects of neurovascular coupling and for its robustness against artifact from the pial vein (Gagnon et al., 2012). Statistical estimates used Benjamini-Hochberg controlling procedures for comparisons across all fNIRS emitter-detector pairs and task conditions and the false discovery rate (FDR) corrected p-values (denoted as the q-value) were used.
2.5. Evaluation of hemodynamic response and task performance (primary hypothesis)
To evaluate differences in the brain activity between groups (TD, GMFCS I, GMFCS II, and GMFCS III), channels were reversed across the midline in participants whose tested, non-dominant lower limb was on the right so that all results are reported as if all participants were performing a left-sided task during unilateral conditions for ease of interpretation. Therefore, the assumed active hemisphere during a unilateral tasks was always the right side of the probe. The NIRS toolbox was used to test the effect of group on the entire probe (covering both left and right hemispheres) and post-hoc testing between group pairs was performed over the whole probe and across the 4 ROIs.
The number of fNIRS channels that were significantly active (q < 0.05) and the sum of beta values for all active channels (Huppert, 2016) were determined for each hemisphere and across hemispheres as an indication of the extent and magnitude of brain activity, respectively, for each participant.
Task performance was evaluated relative to the muscles required to complete the task. Within and across joint cross correlations of specific muscle pairs quantified the extent to which mirror movements, co-activation with the antagonist, or activation in synergy patterns were present (for details of the muscle pairs evaluated for each task, see Table 2). Mirroring correlations were done only for unilateral tasks because it is defined as unwanted EMG activity in the same muscle on the opposite side, and can only be measured during intended unilateral tasks.
Table 2.
Summary of group effects across task.
| Test | Unilateral dorsiflexion | Bilateral dorsiflexion | Unilateral hip flexion | Unilateral cycling | Bilateral cycling |
|---|---|---|---|---|---|
| Group comparisons HbT | |||||
| TD compared to GMFCS I | q < 0.001⁎ | q < 0.001⁎ | q < 0.001⁎ | q < 0.001⁎ | q < 0.001⁎ |
| TD compared to GMFCS II | q = 0.183 | q < 0.001⁎ | q < 0.001⁎ | q < 0.001⁎ | q < 0.001⁎ |
| TD compared to GMFCS III | q < 0.001⁎ | q < 0.001⁎ | q < 0.001⁎ | q < 0.001⁎ | q < 0.001⁎ |
| GMFCS I compared to GMFCS II | q = 0.051 | q = 0.544 | q = 0.740 | q = 0.081 | q = 0.673 |
| GMFCS I compared to GMFCS III | q < 0.001⁎ | q < 0.001⁎ | q < 0.001⁎ | q < 0.001⁎ | q < 0.001⁎ |
| GMFCS II compared to GMFCS III | q < 0.001⁎ | q < 0.001⁎ | q < 0.001⁎ | q < 0.001⁎ | q < 0.001⁎ |
| Active fNIRS channels | F=7.10, p=0.002⁎ | F=1.42, p=0.263 | F=2.50, p=0.095 | F=3.85, p=0.024⁎ | F=1.44, p=0.257 |
| Sum of beta values | F=8.69, p=0.001⁎ | F=7.56, p=0.001⁎ | F=3.10, p=0.054 | F=15.24, p<0.001⁎ | F=12.06, p<0.001⁎ |
| EMG mirroring correlations |
d-TA and n-TA |
d-TA and n-TA |
d-RF and n-RF |
d-RF and n-RF |
d-RF and n-RF |
| F=8.29, p=0.001⁎ | F=0.07, p=0.976 | F=7.88, p=0.001⁎ | F=6.94, p=0.002⁎ | F=15.37, p<0.001⁎ | |
|
d-VL and n-VL |
|||||
| F=13.45, p<0.001⁎ |
|||||
| EMG co-contraction correlations |
n-TA and n-MG |
n-TA and n-MG |
n-RF and n-SM |
n-RF and n-SM |
n-RF and n-SM |
| F = 1.26, p = 0.310 | F = 0.36, p = 0.786 | F = 4.21, p = 0.019⁎ | F = 3.17, p = 0.042⁎ | F = 6.35, p = 0.003⁎ | |
|
d-TA and d-MG |
d-RF and d-SM |
||||
| F = 0.48, p = 0.702 |
F = 10.94, p < 0.001⁎ |
||||
| EMG synergy correlations |
n-TA and n-SM |
n-TA and n-SM |
n-RF n-TA |
n-RF and n-MG |
n-TA and n-SM |
| F = 6.74, p = 0.002⁎ | F = 7.54, p = 0.001⁎ | F = 10.37, p < 0.001⁎ | F = 5.21, p = 0.007⁎ | F = 2.28, p = 0.107 | |
|
d-TA and d-SM |
d-TA and d-SM |
||||
| F = 12.40, p < 0.001⁎ | F = 4.53, p = 0.013⁎ | ||||
|
n-RF and n-MG |
|||||
| F = 15.13, p < 0.001⁎ | |||||
|
d-RF and d-MG |
|||||
| F = 12.75, p < 0.001⁎ |
|||||
| Correlation: active fNIRS and active EMG channels | r = 0.47, p = 0.019⁎ | r = 0.47, p = 0.016⁎ | r = 0.31, p = 0.183 | r = 0.25, p = 0.221 | r = 0.41, p = 0.040⁎ |
| Correlation: sum of fNIRS beta and active EMG channels | r = 0.54, p = 0.004⁎ | r = 0.51, p = 0.009⁎ | r = 0.47, p = 0.031⁎ | r = 0.38, p = 0.061 | r = 0.37, p = 0.061 |
HbT = Total hemoglobin; TD = typical development; GMFCS = Gross Motor Function Classification System; EMG mirroring = activation of the same muscle on the contralateral side; co-contraction = activation of agonist and antagonist concurrently; synergy = simultaneous activity in muscles of hip flexion, knee flexion, and dorsiflexion; n = non-dominant; d = dominant; RF = rectus femoris; TA = tibialis anterior; MG = medial gastrocnemius; SM = semimebranosus.
indicates q < 0.05 or p < 0.05.
Individual muscles were considered to be significantly active during the task if the task activation exceeded 3 standard deviations from baseline (range of possible active muscles for each task was 0–10). Correlations were performed between the number of active fNIRS channels and the number of active muscles, as well as between the sum of beta values across both hemispheres and the number of active muscles to determine if the extent and magnitude of brain activity was related to activity observed in the peripheral neuromotor system.
To evaluate the relationship of selective voluntary motor control of the tested limb and measures of brain activation, the SCALE limb score on the non-dominant side was correlated with both the active fNIRS channels and the sum of the beta values on the contralateral hemisphere for the unilateral dorsiflexion and hip flexion tasks. These two tasks are similar to SCALE test items which evaluate motions at single joints. To evaluate the relationship of functional scales to brain activation, PEDI-CAT scores, and AbilLOCO logit scores were each correlated with the active fNIRS channels and sum of beta values across both hemispheres for all tasks.
2.6. Evaluation of dominant hemisphere (secondary hypothesis #1)
Within the NIRS toolbox, a contrast was performed to evaluate group differences in activation between right and left hemispheres. On an individual participant level, laterality indices were calculated using the difference in beta values of significantly active channels (q < 0.05) between hemispheres divided by the sum. This index quantifies the overall balance in activation between contralateral and ipsilateral hemispheres relative to the task being performed and has been widely used in previous studies (Koeda et al., 2013).
2.7. Location of maximal activity (secondary hypothesis #2)
For all unilateral tasks, the center of activation was calculated with a weighted average of the beta values of significantly active (q < 0.05) channels to determine the medial-lateral location of highest activity on the right and left hemispheres independently, similar to calculations done by Khan et al., (2011)
2.8. Comparison between tasks (secondary hypothesis #3)
To address the last hypothesis, the NIRS toolbox was used to further evaluate contrasts between task types, including the difference between bilateral and unilateral tasks (dorsiflexion and cycling), and between a focal, single joint task (unilateral dorsiflexion) and a multi-joint complex task (unilateral cycling).
2.9. Statistical analyses
Analysis of variance (ANOVA) procedures were used to evaluate the effect of group (4-levels: TD or CP GMFCS Levels I, II, III) and task (5 levels: unilateral dorsiflexion, bilateral dorsiflexion, unilateral hip flexion, unilateral cycling, bilateral cycling) for each of the following dependent variables: number of active fNIRS channels, sum of beta values for active fNIRS channels, correlation coefficients for each muscle pair, laterality index, and center of activation. Significant main or interaction effects were explored further using post-hoc tests with Bonferroni corrections. To relate brain activation extent and magnitude to the muscle activation ratios, SCALE scores and functional scales, Pearson's r correlations or Spearman non-parametric correlations were performed depending on normality of the data.
For all statistical tests performed outside of the NIRS toolbox using SPSS (version 24), p < 0.05 was considered significant. For any tests done using NIRS toolbox, an FDR corrected p-value, q < 0.05, was used as a threshold for significance.
3. Results
3.1. Participant characteristics and clinical features
All participants (Table 1) with CP had spasticity in at least one lower extremity as evaluated by the HAT. One had dystonia in the right leg, 3 in the left leg, and 6 bilaterally. Unilateral dystonia, when present, was on the more impaired leg.
Table 1.
Participant characteristics.
| ID | Age (years) | Hand | GMFCS | AbilLOCO logit (SE) | SCALE (L|R) | PEDI-CAT (daily activity|mobility) | HAT (dystonia) | Etiology |
|---|---|---|---|---|---|---|---|---|
| CP01 | 22.8 | L | III | −0.19 (0.69) | 4|3 | 55|61 | B | B PVL |
| CP02 | 12.6 | R | II | 2.64 (0.67) | 6|3 | 63|66 | N/A | B PVL |
| CP03 | 13.6 | R | III | −0.70 (0.71) | 5|3 | 56|58 | B | B PVL |
| CP04 | 21.2 | R | II | 2.64 (0.67) | 6|7 | 66|65 | N/A | B PVL |
| CP05 | 15.3 | L | I | 2.22 (0.65) | 4|6 | 58|65 | N/A | Cortical disorganization; agenesis of corpus callosum |
| CP06 | 13.4 | R | II | 3.09 (0.72) | 7|5 | 66|67 | R | Encephalitis; MRI without abnormalities |
| CP07 | 12.8 | L | I | 3.62 (0.80) | 7|5 | 70|71 | N/A | Symmetrical colpocephaly; B PVL |
| CP08 | 10.3 | L | II | 1.45 (0.63) | NT | 48|61 | B | B PVL |
| CP09 | 13.8 | L | I | 4.31 (0.97) | 4|8 | 59|67 | B | Left PVL, hypoplasia of corpus callosum |
| CP10 | 15.4 | R | III | −2.27 (0.72) | 4|3 | 56|62 | L | B PVL |
| CP11 | 32.9 | R | I | 3.62 (0.80) | 7|7 | 66|68 | L | B PVL |
| CP12 | 10.5 | R | II | 1.07 (0.63) | 2|3 | 55|64 | B | B PVL |
| CP13 | 9.0 | L | II | 1.09 (0.71) | 2|6 | 53|62 | L | B Cystic PVL |
| CP14 | 42.8 | R | II | 2.34 (0.70) | 5|5 | 58|64 | B | B PVL |
| CP summary | 17.6 ± 9.6 |
6 L 8 R |
I n = 4 II n = 7 III n = 3 |
1.78 ± 1.84 | 4.88 ± 1.72 | 61.8 ± 5.53 | ||
| TD01 | 12.9 | R | ||||||
| TD02 | 10.8 | R | ||||||
| TD03 | 13.6 | R | ||||||
| TD04 | 15.3 | R | ||||||
| TD05 | 12.2 | R | ||||||
| TD06 | 12.8 | R | ||||||
| TD07 | 23.6 | R | ||||||
| TD08 | 22.6 | R | ||||||
| TD09 | 13.8 | R | ||||||
| TD10 | 24.2 | R | ||||||
| TD11 | 11.8 | R | ||||||
| TD12 | 9.8 | R | ||||||
| TD13 | 11.3 | R | ||||||
| TD14 | 45.8 | R | ||||||
| TD summary | 17.2 ± 9.6 | 14 R | N/A | N/A | N/A | N/A | N/A |
CP = cerebral palsy; TD = typical development; GMFCS = Gross Motor Functional Classification System; SE = standard error; SCALE = Selective Control Assessment of the Lower Extremity; HAT = Hypertonia Assessment Tool, indicating dystonia in the lower extremity; R = right; L = left; B = bilateral; PVL = periventricular leukomalacia; N/A = non applicable.
Because light transmission can be decreased by darker hair and skin color, these characteristics (Orihuela-Espina et al., 2010) are also reported. Overall, 31% of participants self-reported having black hair, 42% brown, 19% blonde, and 8% red. Participants also self-reported skin type as: 23% sunburn often, 31% sunburn usually, 23% sunburn rarely and tan usually, 19% sunburn rarely and tan often, and 4% sunburn extremely rarely.
3.2. Evaluation of hemodynamic response and task performance
Supporting our primary hypothesis, visual differences between tasks and groups can be appreciated in activation maps (Fig. 2), and in the statistical results in Table 2.
Fig. 2.
Total hemoglobin (HbT) activation maps by group and task. Significantly active channels (q < 0.05) are in solid lines, and the colorbar represents the value of the t-statistic. Positive values denote increases in HbT relative to baseline, and negative values indicate that signal was greater during baseline than task. The right hemisphere is contralateral to the task limb for the lower extremity non-dominant cycling (D), hip flexion (C), and dorsiflexion tasks (A). The orientation of the probe (Fig. 1) is centered around Cz with contralateral and ipsilateral hemispheres denoted by ‘I' and ‘C' in the typically developing unilateral, non-dominant dorsiflexion task (A).
Across all tasks, there was a significant effect of group when evaluating HbT activation, with clear differentiation between groups except for the comparisons between GFMCS I and II, although trends towards differences in unilateral and bilateral dorsiflexion were seen. Evaluation of the number of active fNIRS channels, a measure of activation extent, revealed a main effect of group for unilateral dorsiflexion and unilateral cycling (Table 2), with post-hoc tests showing GMFCS level III had more active channels than TD. Evaluation of the sum of beta values, a measure of activation magnitude, revealed a main effect of group for all tasks except unilateral hip flexion (Table 2). Post-hoc comparisons showed that the GMFCS level III had higher values than all other groups except for bilateral dorsiflexion where they were only higher than the TD group. A summary of ROI comparisons completed in the NIRS Toolbox between groups for all tasks is available in Supplementary Table S1. Similar trends were seen in this analysis, where the comparison between GFMCS I and II was less frequently significant across tasks, and TD was more similar to mild CP (GMFCS I and II) for unilateral dorsiflexion. Nearly all significant comparisons resulted from the more impaired group having higher activation than the less impaired comparison.
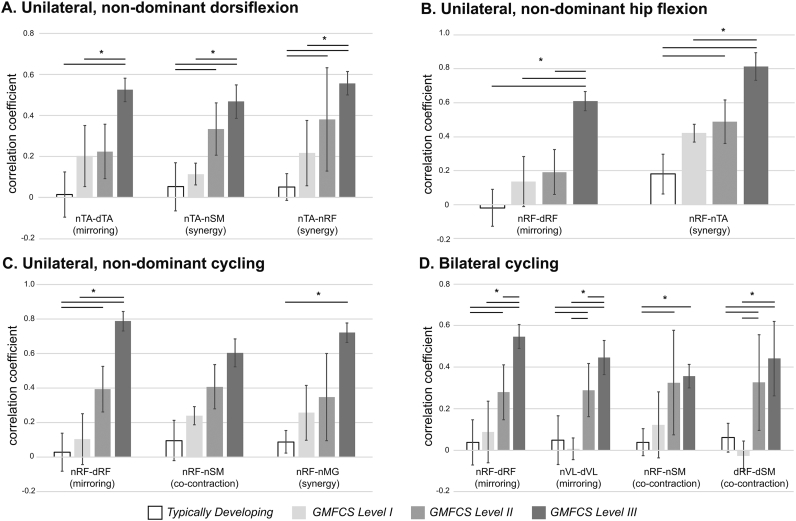
There were a number of muscle pairs that demonstrated differences between groups. Mirroring, or activation of analogous muscles on both lower extremities was seen in all tasks except for bilateral dorsiflexion where activation of both analogous muscles was the expected response, and was present in both proximal (RF, VL) and distal (TA) muscles. Co-contraction of the knee flexors and extensors was seen in both cycling tasks. Finally, synergistic patterns were seen in unilateral proximal tasks with dorsiflexors active during hip flexion, and knee extensors and plantarflexors during unilateral cycling. Hip and knee flexors were activated during unilateral and bilateral dorsiflexion. In all significant comparisons, the strength of correlation increased in a nearly linear fashion from TD through increasing GMFCS levels in CP (Supplementary Fig. S1). Finally, significant correlations were found between extent and magnitude of fNIRS activity and the number of active EMG channels for several of the tasks (Fig. 3).
Supplementary Fig. S1.
Task performance. There was a significant effect of the independent variable of group on the ANOVA for each of the dependent variables shown here. *indicates p < 0.05 for the post-hoc comparisons between groups with Bonferroni correction for multiple comparisons. Significant EMG correlation findings include evidence of higher correlation in muscles related to mirroring (activation of the same muscle on the contralateral side), co-contraction (activation of agonist and antagonist concurrently), and synergy (simultaneous activity in muscles of hip flexion, knee flexion, and dorsiflexion). n = non-dominant; d = dominant; RF = rectus femoris; TA = tibialis anterior; MG = medial gastrocnemius; SM = semimebranosus; GMFCS = Gross Motor Functional Classification System. Error bars represent one standard deviation.
Fig. 3.
Relationship between brain and muscle activity. The activity in the recorded muscles measured by EMG and sensorimotor cortex measured by fNIRS recorded shows that a higher number of significantly active fNIRS channels was related to a higher number of significantly active muscles. TD = typical development; GMFCS = Gross Motor Functional Classification System; EMG = electromyography; fNIRS = functional near infrared spectroscopy.
The SCALE total limb score for the tested leg was correlated to the magnitude of activity in the contralateral hemisphere during unilateral dorsiflexion (r = −0.60, p = 0.031), but not hip flexion (r = −0.53, p = 0.078). The average score for the hip on the tested leg was 1.15 ± 0.38 and dorsiflexion was 0.69 ± 0.48 (two-tailed t-test comparison between hip and ankle SCALE t = 3.21, p = 0.008). There was also a relationship between magnitude of activation across the whole sensorimotor area for unilateral dorsiflexion and the mobility section of the PEDI-CAT (rho = −0.55, p = 0.042). The AbilLOCO score was correlated with magnitude and extent of activity with hip flexion (r = −0.63, p = 0.042 and r = −0.714, p = 0.009, respectively) and the magnitude of activation with unilateral dorsiflexion (rho = −0.678, p = 0.008). No other correlations, including for the number of active fNIRS channels for any task, were found with any of the clinical measures.
3.3. Evaluation of dominant hemisphere (secondary hypothesis #1)
No significant effect of group was noted in the ANOVA analyses for laterality index with any of the tasks. A few differences emerged when ROI contrasts were analyzed for contralateral and ipsilateral hemispheres using the NIRS toolbox (Huppert, 2016). The contralateral hemisphere was more active for GMFCS level III during hip flexion, unilateral and bilateral cycling (q < 0.001 for all). The ipsilateral hemisphere was more active for GMFCS level III for unilateral and bilateral dorsiflexion (q < 0.001), and for GMFCS level I during hip flexion (q = 0.02) and unilateral cycling (q = 0.03).
3.4. Location of maximal activity (secondary hypothesis #2)
A significant effect of group was found for the location of highest activity on both ipsilateral (F = 4.95, p = 0.009) and contralateral (F = 3.26, p = 0.041) hemispheres for unilateral dorsiflexion and in the contralateral hemisphere (F = 4.28, p = 0.020) for unilateral hip flexion. On average, activation for the CP groups was more laterally located in the contralateral hemispheres than the TD group. There was no effect of group for either hemisphere during unilateral cycling (ipsilateral F = 0.83, p = 0.493; contralateral F = 1.95, p = 0.153).
3.5. Comparisons between tasks (secondary hypothesis #3)
There was a significant main effect of both group and task for number of active NIRS channels (group F = 11.77, p < 0.001; task F = 3.51, p = 0.010), sum of beta values across both hemispheres (group F = 42.56, p < 0.001; task F = 7.85, p < 0.001), and number of active muscles (group F = 5.56, p = 0.001; task F = 7.40, p < 0.001), but no group by task interaction (active fNIRS F = 0.43, p = 0.949; sum of beta F = 1.82, p = 0.054; active EMG F = 1.12, p = 0.355). Post-hoc comparisons revealed only a few between-task differences that were present across the whole cohort in the study (p < 0.05): unilateral cycling required more activation (sum of beta) than unilateral dorsiflexion (Fig. 4B); unilateral cycling required a greater extent of activity (number of active fNIRS channels) than bilateral cycling (Fig. 4C); and bilateral cycling had more active muscles than any other task (p < 0.05). Only GMFCS II showed a significant difference between unilateral and bilateral dorsiflexion, with more ipsilateral activity (Fig. 4A).
Fig. 4.
Contrast between tasks within groups. Significantly different channels (q < 0.05) are in solid lines, and the colorbar represents the value of the t-statistic. Negative values (blue colors) indicate that the second task listed showed more activity, and positive values (red colors) indicate the first task listed showed more activity. The overall q value when comparing across all channels is listed below each groups' activity map.
4. Discussion
Using fNIRS, we investigated the dynamic localized changes in total hemoglobin associated with performance of specific motor tasks relative to rest to evaluate the magnitude and extent of cortical activity associated with each. Despite the anatomic location of the foot and ankle deep in the central sulcus which tests the limits of the depth of the fNIRS light transmission, we were able to demonstrate activation even in the isolated dorsiflexion tasks in all groups. Across tasks, greater and largely undesirable EMG activity was present in the groups with CP, demonstrating a lack of efficiency or fluency in movements consistent with previous reports (Damiano et al., 2000; Ikeda et al., 1998; Poon & Hui-Chan, 2009; Alves-Pinto et al., 2016; Arpin et al., 2013) and corresponding to an increase in the extent and intensity of activity measured on the cortical surface, even in cases where there may be damage to and consequently loss of neurons in those areas.
The tasks were chosen to be easy enough for all participants to complete in comparable ways with respect to extent and timing of the movements. The relative simplicity of the tasks was demonstrated by low levels of activation for the TD group in both the fNIRS channels (lower beta values and fewer significantly active channels) and the muscles active during the tasks. As expected, fewer channels were active in the focal, distal joint task (unilateral dorsiflexion) compared to bilateral multi-joint activities such as cycling, as has been shown in adults (Promjunyakul et al., 2015). Although higher activation would be expected in the tasks involving more joints, the unilateral cycling task showed higher levels of cortical activity than bilateral cycling. This can be explained by the novelty of the unilateral task compared to cycling or other common reciprocal leg movements practiced during walking or other play or exercise activities, the increased postural control demands to stabilize the trunk for unilateral efforts, or the influence of subcortical or spinal mechanisms such as central pattern generators contributing to the bilateral cycling activity that might need to be inhibited when only using one leg (Kautz et al., 2006). In addition, there is evidence in typically developing young adults for very limited cortical activity, and on average some decreases (as seen in our study), in HbT over the motor cortex during continual cycling (Rupp et al., 2013).
In stark contrast to the typically developing cohort, groups with CP demonstrated more widespread areas of activation in all tasks at both the cortical and muscle levels, which is consistent with a preliminary investigation of cortical activity during walking (Kurz et al., 2014b). This may be the result of the tasks being more difficult to execute for those with CP due to differences in strength, previous motor practice or sensory input to the nervous system (Kurz et al., 2014a; Kurz et al., 2014c; Kurz et al., 2015). However, the most relevant contributor to increased recruitment of muscles in participants with CP is likely their deficits in selective voluntary motor control (Fowler & Goldberg, 2009; Fowler et al., 2010; Cahill-Rowley & Rose, 2014; Sukal-Moulton et al., 2014a), which can be explained by the location, greater extent, amount of activity in the brain leading to co-activation of two or more muscles. More lateral activity was recorded on the sensorimotor cortices during single joint movements, consistent with measures of corticomotor excitability using TMS (Kesar et al., 2012; Maegaki et al., 1999). This could be evidence of neural reorganization or the mechanical result of expanded lateral ventricles given the common etiology of periventricular leukomalacia in our study cohort. Ankle movements, as expected, showed higher prevalence of motor control deficits than the hip (Fowler et al., 2010). During ankle dorsiflexion, the magnitude of cortical activity correlated with an impairment-specific measure, the SCALE limb score, in addition to being predictive of mobility in two participant-reported functional scales. This suggests that overflow and amplification of activity observed in the sensorimotor cortex has important implications for functional mobility for those with bilateral CP. The lack of a relationship between unilateral hip flexion and selectivity measures also reflects the greater prevalence of distal versus proximal involvement in bilateral CP. The SCALE score tends to be higher at the hip (Fowler et al., 2010), with more potential for subcortical areas of the brain that are not measured by fNIRS to be responsible for activity at this joint (Cahill-Rowley & Rose, 2014). Those with GMFCS III tended to favor the contralateral hemisphere during non-dominant hip tasks but the ipsilateral hemisphere during ankle tasks, which again may relate to and provide insights into the greater deficits in selective control in distal versus proximal activities seen in CP.
Some particularly unique differences were seen between TD and CP groups in both types of cycling. Those with TD required very little activity for either task with less activity seen in bilateral versus unilateral cycling, even though more joints are involved. It is possible that oscillating circuits at the spinal level were largely involved in this task with some higher level inhibition needed to suppress the tendency to cycle bilaterally. Participants in the groups with CP showed larger amounts of cortical activity along with higher levels of muscle activity and co-contraction, which were consistent with findings of another study of cycling in adults with CP (Alves-Pinto et al., 2016). The two tasks were also more similar in CP in the level of brain activation as seen in Fig. 4C. One explanation for this difference between those with and without CP is that while unilateral cycling was likely a novel activity for all participants (TD and CP), bilateral cycling may have also been relatively novel as well to those with greater impairment. Additionally, children with bilateral CP often have difficulty with inter-limb reciprocal coordination needed for asynchronous bilateral activities (Damiano et al., 2017) that is more pronounced in those with greater involvement. Because cycling is used as an intervention in clinics and in research studies (Siebert et al., 2010; Damiano et al., 2017), it would also be interesting to evaluate if a cycling intervention would reduce cortical activity and increase the contrast between unilateral and bilateral cycling as seen in TD.
In contrast to previous studies showing more pronounced ipsilateral control of the more impaired limb in hemiplegia (Eyre et al., 2007; Friel et al., 2013), CP groups with significant differences between hemispheres in this study showed higher levels of activity in the contralateral hemisphere to the moving limb during the novel task of unilateral cycling, but higher ipsilateral activity during unilateral dorsiflexion. This may indicate that the mechanisms of re-organization following bilateral injury may be dependent on the location and extent of the injury, or simply are more variable than those following unilateral injury.
This study has a number of limitations, some of which are due to the limitations of the technology. NIRS does not allow for investigation of neural activity in structures below the level of the cortex, eliminating the possibility to evaluate activation in structures such as thalamus, brain stem nuclei, or spinal cord, which are all likely candidates for structural and functional differences in CP compared to TD. The influence of systemic task related changes in blood flow in the scalp cannot be entirely ruled out in this study, but the relationship between cortical and peripheral measures suggests a neural rather than cardiovascular mechanism. Further development is also needed regarding co-registration of subject-specific MRI to the NIRS probe in injury cases. While the group results are illuminating, the precision for evaluating small individual differences in activation is still limited but has much potential for designing and evaluating personalized treatment prescriptions that are informed by the type or extent of brain activity associated with tasks important for an individual's goals. Evaluation of other task types is warranted, including unilateral tasks in both extremities to determine if there are differences in laterality between dominant and non-dominant limbs in this population as has been well documented in unilateral CP.
In conclusion, the overflow of unwanted muscle activity noted in the lower extremities of participants with CP was largely explained by the overflow of neural activity in the sensorimotor cortex, pointing to inefficient cortical organization as a potential and even primary culprit in the lack of selective voluntary motor control in the lower extremities. More effective rehabilitation strategies that progressively train more precise motor control aiming to reduce unwanted intralimb synergistic movements or improve interlimb coordination are needed (Damiano et al., 2017). Further, greater use of techniques such as fNIRS or EEG in combination with EMG measures are recommended for inclusion as outcome measures to determine whether the intervention succeeded first at the level of impairment, with improvement in functional activity and participation in those with bilateral CP the ultimate goal. It is clear that there are differences across patients (Weinstein et al., 2018) and differences in how they respond to therapies based on available motor pathways (Kuhnke et al., 2008), so the use of techniques such as fNIRS to provide prognostic information about response to intervention would be a dramatic step forward.
The following are the supplementary data related to this article.
Statistical test (q-value) for the HbT comparison between Regions of Interest across groups.
Acknowledgements
We gratefully acknowledge the time and efforts of our participants and their families. This study was funded by the Intramural Research Program of the NIH Clinical Center (Protocol #13-CC-0110). Chris Stanley, Andy Gravunder, Laurie Ohlrich, Cristiane Zampieri-Gallagher, and Sara Sadeghi of the Functional and Applied Biomechanics Section provided valuable support for participant recruitment and data collection.
References
- Alves-Pinto A., Blumenstein T., Turova V., Lampe R. Altered lower leg muscle activation patterns in patients with cerebral palsy during cycling on an ergometer. Neuropsychiatr. Dis. Treat. 2016;12:1445–1456. doi: 10.2147/NDT.S98260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpin D.J., Stuberg W., Stergiou N., Kurz M.J. Motor control of the lower extremity musculature in children with cerebral palsy. Res. Dev. Disabil. 2013;34(4):1134–1143. doi: 10.1016/j.ridd.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Barker J.W., Aarabi A., Huppert T.J. Autoregressive model based algorithm for correcting motion and serially correlated errors in fNIRS. Biomed. Opt. Express. 2013;4(8):1366–1379. doi: 10.1364/BOE.4.001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H., Dixit S., Litkowski P., Wingert J.R. Functional connectivity for somatosensory and motor cortex in spastic diplegia. Somatosens. Mot. Res. 2009;26(4):90–104. doi: 10.3109/08990220903335742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill-Rowley K., Rose J. Etiology of impaired selective motor control: emerging evidence and its implications for research and treatment in cerebral palsy. Dev. Med. Child Neurol. 2014;56(6):522–528. doi: 10.1111/dmcn.12355. [DOI] [PubMed] [Google Scholar]
- Caty G.D., Arnould C., Thonnard J.L., Lejeune T.M. ABILOCO-Kids: a Rasch-built 10-item questionnaire for assessing locomotion ability in children with cerebral palsy. J. Rehabil. Med. 2008;40(10):823–830. doi: 10.2340/16501977-0267. [DOI] [PubMed] [Google Scholar]
- Chaudhary U., Hall M., Gonzalez J., Elbaum L., Bloyer M., Godavarty A. Motor response investigation in individuals with cerebral palsy using near infrared spectroscopy: pilot study. Appl. Opt. 2014;53(3):503–510. doi: 10.1364/AO.53.000503. [DOI] [PubMed] [Google Scholar]
- Chen C.L., Wu C.Y., Wong A.M., Cheng P.T., Hong W.H., Chen H.C. Correlation of polyelectromyographic patterns and clinical motor manifestations in children with cerebral palsy. Am. J. Phys. Med. Rehabil. 2003;82(8):627–635. doi: 10.1097/01.PHM.0000078180.72129.5C. [DOI] [PubMed] [Google Scholar]
- Cooper R.J., Caffini M., Dubb J. Validating atlas-guided DOT: a comparison of diffuse optical tomography informed by atlas and subject-specific anatomies. NeuroImage. 2012;62(3):1999–2006. doi: 10.1016/j.neuroimage.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano D.L., Martellotta T.L., Sullivan D.J., Granata K.P., Abel M.F. Muscle force production and functional performance in spastic cerebral palsy: relationship of cocontraction. Arch. Phys. Med. Rehabil. 2000;81(7):895–900. doi: 10.1053/apmr.2000.5579. [DOI] [PubMed] [Google Scholar]
- Damiano D.L., Stanley C.J., Ohlrich L., Alter K.E. Task-specific and functional effects of speed-focused elliptical or motor-assisted cycle training in children with bilateral cerebral palsy: randomized clinical trial. Neurorehabil. Neural Repair. 2017;31(8):736–745. doi: 10.1177/1545968317718631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decampos A.C., Sukal-Moulton T., Damiano D.L. 2016. Functional Near-Infrared Spectroscopy (fNIRS) - A Promising Tool to Investigate Brain Activity During Motor Tasks in Individuals With Cerebral Palsy. International Conference on Cerebral Palsy and other Childhood-onset Disabilities; 1–4 June 2016. (Stockholm, Sweeden) [Google Scholar]
- Eyre J.A., Smith M., Dabydeen L. Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system? Ann. Neurol. 2007;62(5):493–503. doi: 10.1002/ana.21108. [DOI] [PubMed] [Google Scholar]
- Fowler E.G., Goldberg E.J. The effect of lower extremity selective voluntary motor control on interjoint coordination during gait in children with spastic diplegic cerebral palsy. Gait Posture. 2009;29(1):102–107. doi: 10.1016/j.gaitpost.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Fowler E.G., Staudt L.A., Greenberg M.B., Oppenheim W.L. Selective Control Assessment of the Lower Extremity (SCALE): development, validation, and interrater reliability of a clinical tool for patients with cerebral palsy. Dev. Med. Child Neurol. 2009;51(8):607–614. doi: 10.1111/j.1469-8749.2008.03186.x. [DOI] [PubMed] [Google Scholar]
- Fowler E.G., Staudt L.A., Greenberg M.B. Lower-extremity selective voluntary motor control in patients with spastic cerebral palsy: increased distal motor impairment. Dev. Med. Child Neurol. 2010;52(3):264–269. doi: 10.1111/j.1469-8749.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- Friel K.M., Chakrabarty S., Martin J.H. Pathophysiological mechanisms of impaired limb use and repair strategies for motor systems after unilateral injury of the developing brain. Dev. Med. Child Neurol. 2013;55(Suppl. 4):27–31. doi: 10.1111/dmcn.12303. [DOI] [PubMed] [Google Scholar]
- Gagnon L., Yucel M.A., Dehaes M. Quantification of the cortical contribution to the NIRS signal over the motor cortex using concurrent NIRS-fMRI measurements. NeuroImage. 2012;59(4):3933–3940. doi: 10.1016/j.neuroimage.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham H.K., Rosenbaum P., Paneth N. Cerebral palsy. Nat. Rev. Dis. Primers. 2016;2:15082. doi: 10.1038/nrdp.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley S.M., Raczek A.E., Coster W.J., Dumas H.M., Fragala-Pinkham M.A. Assessing mobility in children using a computer adaptive testing version of the pediatric evaluation of disability inventory. Arch. Phys. Med. Rehabil. 2005;86(5):932–939. doi: 10.1016/j.apmr.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Hermens H.J., Freriks B., Disselhorst-Klug C., Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000;10(5):361–374. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- Hilderly A.J., Morgan B.R., Lee W., Fehlings D., Taylor M.J., Wright V. American Academy of Cerebral Palsy and Developmental Medicine; September, 2016. 2016. Neural activation associated with lower limb movements in children with hemiplegic cerebral palsy. (Hollywood, FL) [Google Scholar]
- Hoon A.H., Jr., Stashinko E.E., Nagae L.M. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev. Med. Child Neurol. 2009;51(9):697–704. doi: 10.1111/j.1469-8749.2009.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert T.J. Commentary on the statistical properties of noise and its implication on general linear models in functional near-infrared spectroscopy. Neurophotonics. 2016;3(1) doi: 10.1117/1.NPh.3.1.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert T., Schmidt B., Beluk N., Furman J., Sparto P. Measurement of brain activation during an upright stepping reaction task using functional near-infrared spectroscopy. Hum. Brain Mapp. 2013;34(11):2817–2828. doi: 10.1002/hbm.22106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A.J., Abel M.F., Granata K.P., Damiano D.L. Quantification of cocontraction in spastic cerebral palsy. Electromyogr. Clin. Neurophysiol. 1998;38(8):497–504. [PubMed] [Google Scholar]
- Jethwa A., Mink J., Macarthur C., Knights S., Fehlings T., Fehlings D. Development of the Hypertonia Assessment Tool (HAT): a discriminative tool for hypertonia in children. Dev. Med. Child Neurol. 2010;52(5):e83–e87. doi: 10.1111/j.1469-8749.2009.03483.x. [DOI] [PubMed] [Google Scholar]
- Karim H., Schmidt B., Dart D., Beluk N., Huppert T. Functional near-infrared spectroscopy (fNIRS) of brain function during active balancing using a video game system. Gait Posture. 2012;35(3):367–372. doi: 10.1016/j.gaitpost.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim H., Fuhrman S.I., Sparto P., Furman J., Huppert T. Functional brain imaging of multi-sensory vestibular processing during computerized dynamic posturography using near-infrared spectroscopy. NeuroImage. 2013;74:318–325. doi: 10.1016/j.neuroimage.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautz S.A., Patten C., Neptune R.R. Does unilateral pedaling activate a rhythmic locomotor pattern in the nonpedaling leg in post-stroke hemiparesis? J. Neurophysiol. 2006;95(5):3154–3163. doi: 10.1152/jn.00951.2005. [DOI] [PubMed] [Google Scholar]
- Kesar T.M., Sawaki L., Burdette J.H. Motor cortical functional geometry in cerebral palsy and its relationship to disability. Clin. Neurophysiol. 2012;123(7):1383–1390. doi: 10.1016/j.clinph.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan B., Tian F., Behbehani K. Identification of abnormal motor cortex activation patterns in children with cerebral palsy by functional near-infrared spectroscopy. J. Biomed. Opt. 2010;15(3) doi: 10.1117/1.3432746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan B., Chand P., Alexandrakis G. Spatiotemporal relations of primary sensorimotor and secondary motor activation patterns mapped by NIR imaging. Biomed. Optics Express. 2011;2(12):3367–3386. doi: 10.1364/BOE.2.003367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeda M., Takahashi H., Matsuura M., Asai K., Okubo Y. Cerebral responses to vocal attractiveness and auditory hallucinations in schizophrenia: a functional MRI study. Front. Hum. Neurosci. 2013;7:221. doi: 10.3389/fnhum.2013.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenraadt K.L., Roelofsen E.G., Duysens J., Keijsers N.L. Cortical control of normal gait and precision stepping: an fNIRS study. NeuroImage. 2014;85(Pt 1):415–422. doi: 10.1016/j.neuroimage.2013.04.070. [DOI] [PubMed] [Google Scholar]
- Kuhnke N., Juenger H., Walther M., Berweck S., Mall V., Staudt M. Do patients with congenital hemiparesis and ipsilateral corticospinal projections respond differently to constraint-induced movement therapy? Dev. Med. Child Neurol. 2008;50(12):898–903. doi: 10.1111/j.1469-8749.2008.03119.x. [DOI] [PubMed] [Google Scholar]
- Kurz M.J., Heinrichs-Graham E., Arpin D.J., Becker K.M., Wilson T.W. Aberrant synchrony in the somatosensory cortices predicts motor performance errors in children with cerebral palsy. J. Neurophysiol. 2014;111(3):573–579. doi: 10.1152/jn.00553.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz M.J., Wilson T.W., Arpin D.J. An fNIRS exploratory investigation of the cortical activity during gait in children with spastic diplegic cerebral palsy. Brain Dev. 2014;36(10):870–877. doi: 10.1016/j.braindev.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz M.J., Becker K.M., Heinrichs-Graham E., Wilson T.W. Neurophysiological abnormalities in the sensorimotor cortices during the motor planning and movement execution stages of children with cerebral palsy. Dev. Med. Child Neurol. 2014;56(11):1072–1077. doi: 10.1111/dmcn.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz M.J., Heinrichs-Graham E., Becker K.M., Wilson T.W. The magnitude of the somatosensory cortical activity is related to the mobility and strength impairments seen in children with cerebral palsy. J. Neurophysiol. 2015;113(9):3143–3150. doi: 10.1152/jn.00602.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H. Correlation between the selective control assessment of lower extremity and pediatric balance scale scores in children with spastic cerebral palsy. J. Phys. Ther. Sci. 2015;27(12):3645–3649. doi: 10.1589/jpts.27.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegaki Y., Maeoka Y., Ishii S. Central motor reorganization in cerebral palsy patients with bilateral cerebral lesions. Pediatr. Res. 1999;45(4 Pt 1):559–567. doi: 10.1203/00006450-199904010-00016. [DOI] [PubMed] [Google Scholar]
- Miyai I., Tanabe H.C., Sase I. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. NeuroImage. 2001;14(5):1186–1192. doi: 10.1006/nimg.2001.0905. [DOI] [PubMed] [Google Scholar]
- Miyai I., Yagura H., Hatakenaka M., Oda I., Konishi I., Kubota K. Longitudinal optical imaging study for locomotor recovery after stroke. Stroke. 2003;34(12):2866–2870. doi: 10.1161/01.STR.0000100166.81077.8A. [DOI] [PubMed] [Google Scholar]
- Orihuela-Espina F., Leff D.R., James D.R., Darzi A.W., Yang G.Z. Quality control and assurance in functional near infrared spectroscopy (fNIRS) experimentation. Phys. Med. Biol. 2010;55(13):3701–3724. doi: 10.1088/0031-9155/55/13/009. [DOI] [PubMed] [Google Scholar]
- Palisano R.J., Rosenbaum P., Bartlett D., Livingston M.H. Content validity of the expanded and revised Gross Motor Function Classification System. Dev. Med. Child Neurol. 2008;50(10):744–750. doi: 10.1111/j.1469-8749.2008.03089.x. [DOI] [PubMed] [Google Scholar]
- Phillips J.P., Sullivan K.J., Burtner P.A., Caprihan A., Provost B., Bernitsky-Beddingfield A. Ankle dorsiflexion fMRI in children with cerebral palsy undergoing intensive body-weight-supported treadmill training: a pilot study. Dev. Med. Child Neurol. 2007;49(1):39–44. doi: 10.1017/s0012162207000102.x. [DOI] [PubMed] [Google Scholar]
- Poon D.M., Hui-Chan C.W. Hyperactive stretch reflexes, co-contraction, and muscle weakness in children with cerebral palsy. Dev. Med. Child Neurol. 2009;51(2):128–135. doi: 10.1111/j.1469-8749.2008.03122.x. [DOI] [PubMed] [Google Scholar]
- Promjunyakul N.O., Schmit B.D., Schindler-Ivens S.M. A novel fMRI paradigm suggests that pedaling-related brain activation is altered after stroke. Front. Hum. Neurosci. 2015;9:324. doi: 10.3389/fnhum.2015.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp T., Jubeau M., Millet G.Y. Muscle, prefrontal, and motor cortex oxygenation profiles during prolonged fatiguing exercise. Adv. Exp. Med. Biol. 2013;789:149–155. doi: 10.1007/978-1-4614-7411-1_21. [DOI] [PubMed] [Google Scholar]
- Sanger T.D., Chen D., Delgado M.R. Definition and classification of negative motor signs in childhood. Pediatrics. 2006;118(5):2159–2167. doi: 10.1542/peds.2005-3016. [DOI] [PubMed] [Google Scholar]
- Siebert K.L., Demuth S.K., Knutson L.M., Fowler E.G. Stationary cycling and children with cerebral palsy: case reports for two participants. Phys. Occup. Ther. Pediatr. 2010;30(2):125–138. doi: 10.3109/01942630903578399. [DOI] [PubMed] [Google Scholar]
- Sukal-Moulton T., Krosschell K.J., Gaebler-Spira D.J., Dewald J.P. Motor impairments related to brain injury timing in early hemiparesis. Part II: abnormal upper extremity joint torque synergies. Neurorehabil. Neural Repair. 2014;28(1):24–35. doi: 10.1177/1545968313497829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukal-Moulton T., de Campos A.C., Stanley C.J., Damiano D.L. Functional near infrared spectroscopy of the sensory and motor brain regions with simultaneous kinematic and EMG monitoring during motor tasks. J. Vis. Exp. 2014;94 doi: 10.3791/52391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Miyai I., Ono T., Kubota K. Activities in the frontal cortex and gait performance are modulated by preparation. An fNIRS study. NeuroImage. 2008;39(2):600–607. doi: 10.1016/j.neuroimage.2007.08.044. [DOI] [PubMed] [Google Scholar]
- Thelen D.D., Riewald S.A., Asakawa D.S., Sanger T.D., Delp S.L. Abnormal coupling of knee and hip moments during maximal exertions in persons with cerebral palsy. Muscle Nerve. 2003;27(4):486–493. doi: 10.1002/mus.10357. [DOI] [PubMed] [Google Scholar]
- Tian F., Delgado M.R., Clegg N.J., Romero M.I., Liu H. Vol. 2008. 2008. Investigation of the Motor Cortex Function in Children with Cerebral Palsy Using Functional Near-infrared Spectroscopic Imaging. [Google Scholar]
- Tian F., Delgado M.R., Dhamne S.C. Quantification of functional near infrared spectroscopy to assess cortical reorganization in children with cerebral palsy. Opt. Express. 2010;18(25):25973–25986. doi: 10.1364/OE.18.025973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Winckel A., Klingels K., Bruyninckx F. How does brain activation differ in children with unilateral cerebral palsy compared to typically developing children, during active and passive movements, and tactile stimulation? An fMRI study. Res. Dev. Disabil. 2013;34(1):183–197. doi: 10.1016/j.ridd.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Vandermeeren Y., Davare M., Duque J., Olivier E. Reorganization of cortical hand representation in congenital hemiplegia. Eur. J. Neurosci. 2009;29(4):845–854. doi: 10.1111/j.1460-9568.2009.06619.x. [DOI] [PubMed] [Google Scholar]
- Weinstein M., Green D., Rudisch J. Understanding the relationship between brain and upper limb function in children with unilateral motor impairments: A multimodal approach. Eur. J. Paediatr. Neurol. 2018;22(1):143–154. doi: 10.1016/j.ejpn.2017.09.012. [DOI] [PubMed] [Google Scholar]
- Wingert J.R., Sinclair R.J., Dixit S., Damiano D.L., Burton H. Somatosensory-evoked cortical activity in spastic diplegic cerebral palsy. Hum. Brain Mapp. 2010;31(11):1772–1785. doi: 10.1002/hbm.20977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg G.F. Motor mapping in cerebral palsy. Dev. Med. Child Neurol. 2009;51(Suppl. 4):134–139. doi: 10.1111/j.1469-8749.2009.03426.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical test (q-value) for the HbT comparison between Regions of Interest across groups.