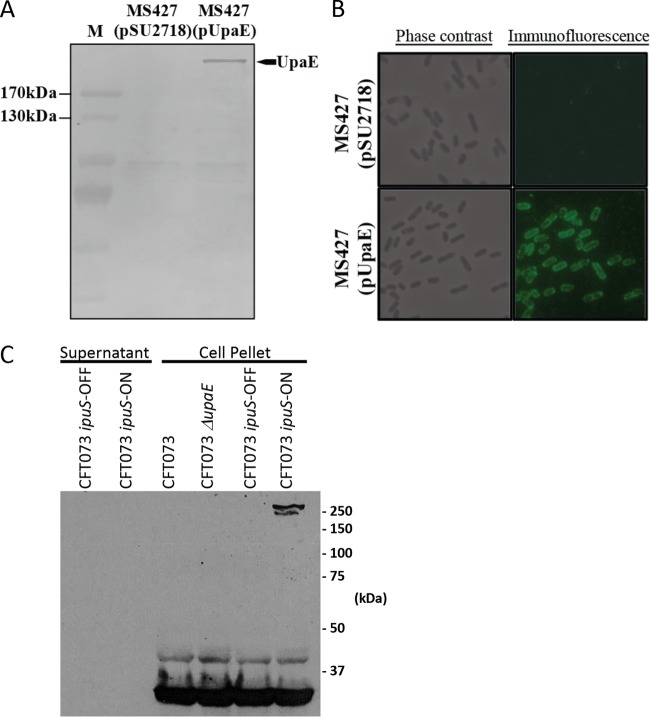
FIG 5 .
Expression and surface localization of UpaE in E. coli K-12. (A) Western blot analysis of whole-cell lysates prepared from MS427(pSU2718) vector control and MS427(pUpaE). A band corresponding to UpaE (271 kDa) was detected in MS427(pUpaE) but not in the MS427(pSU2718) control. Lane M refers to molecular weight markers; the 170-kDa and 130-kDa proteins are indicated. (B) Phase-contrast and immunofluorescence microscopy using specific antisera against UpaE. Positive reactions indicating the surface localization of UpaE were detected in MS427(UpaE) (bottom) but not in the MS427(pSU2718) vector control (top). (C) Western blot analysis of pelleted cells solubilized in crack buffer or concentrated 10-ml TCA precipitations of culture supernatants. Protein detection was performed using a polyclonal antiserum raised to an UpaE-MBP fusion protein. A band consistent with the predicted 271-kDa size of UpaE is present only in the cellular fraction of phase locked-ON cells.