Abstract
Glioblastoma demonstrates imaging features of intratumor heterogeneity that result from underlying heterogeneous biological properties. This stems from variations in cellular behavior that result from genetic mutations that either drive, or are driven by, heterogeneous microenvironment conditions. Among all imaging methods available, only T1-weighted contrast-enhancing and T2-weighted fluid-attenuated inversion recovery are used in standard clinical glioblastoma assessment and monitoring. Advanced imaging modalities are still considered emerging techniques as appropriate end points and robust methodologies are missing from clinical trials. Discovering how these images specifically relate to the underlying tumor biology may aid in improving quality of clinical trials and understanding the factors involved in regional responses to treatment, including variable drug uptake and effect of radiotherapy. Upon validation and standardization of emerging MR techniques, providing information based on the underlying tumor biology, these images may allow for clinical decision-making that is tailored to an individual's response to treatment.
KEYWORDS : advanced imaging, glioblastoma, intratumor heterogeneity, subregional assessment, underlying biology
PRACTICE POINTS.
Progress in glioblastoma (GBM) management and monitoring has been disappointing, with the single major advancement resulting in an improved median survival of only 14 months and 26% 2-year survival with death inevitably being due to recurrence that is often local.
Gold standard for GBM diagnosis is via biopsy or open resection, two invasive approaches with the latter having a minor association with morbidity; both subject to sampling error as a result of intratumoral heterogeneity.
Current treatment involves maximum resection, via white light or fluorescence with 5-aminolevulinic acid, followed by radiotherapy (RT) with concomitant and adjuvant temozolomide (TMZ), with complete resections (<2% of contrast enhancement remaining) being associated with improved progression-free survival.
Underlying intrinsic and extrinsic biological features, including a heterogeneous tumor microenvironment, result in RT and TMZ having limited and varied effect, especially with cancer cells migrating through the tumor margin, which includes healthy and normal-appearing brain.
Cancer cells surviving treatment may evolve with conferred resistance to adjuvant TMZ.
Post-treatment monitoring of GBM relies on visual and manual analysis of conventional T1-weighted contrast enhancement and T2-weighted fluid-attenuated inversion recovery images, which demonstrate limited and nonspecific features of blood–brain barrier permeability and tissue water content, respectively.
These conventional imaging methods are unable to specifically identify treatment-induced changes in a timely manner, limiting the ability to distinguish pseudoprogression from true tumor progression.
Emerging imaging techniques that rely on specific underlying biological tumor properties lack validation and standardization, rendering them nonuseful for current clinical assessment of GBM.
Appropriate end points and methodologies for these emerging imaging methods need to be determined before they can be reliably included in clinical trials for GBM.
Advanced imaging studies, focusing on the nonenhancing invasive margin, are needed for assessing regional tumor response to treatment for further understanding of GBM invasion and recurrence, and improvement of patient management.
Glioblastoma (GBM) is the most common and aggressive malignant primary brain tumor in adults. Even under optimal treatment conditions, median survival is only 14 months with a 26% 2-year survival rate [1] and patient death results from inevitable tumor progression. Recurrence is often local and not easily detected using conventional MRI. Among all options available for treatment, extent of resection is the only variable that neurosurgeons can affect [2]. In spite of the incorporation of 5-aminolevulinic acid (5-ALA) improving maximal resection and 6-month progression-free survival (PFS) [3], poor drug delivery and relative radiotherapy (RT) resistance [4] contribute to poor prognosis. Though the progress in GBM management has been disappointing, with the single major advancement in the past decades being the incorporation of RT with concomitant and adjuvant temozolomide (TMZ) [1], there has been exponential growth in the advancements of MRI techniques for noninvasive characterization of GBM. In parallel, advancements in genomic tool development for molecular analysis on a genome-wide level have also been ongoing [5]. With this, work has begun for merging imaging information with that of the underlying biology driving intratumor heterogeneity of GBM.
Intratumor heterogeneity of GBM: what is it & why does it occur?
Tumor heterogeneity refers to biological differences between malignant cells in a same cancer, where variations in genetic alterations and phenotypic inconsistencies (including cellular metabolism, resilience and behavior) can drive tumor cell diversity within an individual tumor (intratumor heterogeneity) or among patients with the same tumor (intertumor heterogeneity) [6].
During tumor development, malignant cells evolve together with the extracellular matrix, microvasculature, stromal and immune cells [7]. Regional variations that arise from this coevolution are presumed to be a partnership of distinct ecosystems of cells, for which the microenvironment would be, in the primary instance, influenced by inconsistencies in vasculature and blood supply. The resulting microenvironment conditions would include subsequent regional inflammation, hypoxia and acidosis with limited glucose supply. This would set the scene for selective pressures, giving rise to regional cellular adaptations – a Darwinian concept of adaptive response. Heterogeneity, therefore, can arise through extrinsic mechanisms that confer functional differences to the malignant cells [8]. In a recent study, GBM cells isolated from two different microenvironments, the tumor core and margin, each demonstrated different phenotype and stem-cell signature while propagated within the same culture conditions [9]. Tumor cell diversity can also arise through genomic instability and differential response of clones to therapy, where cellular proliferation does not result in clusters of identical cells. Instead, many subclones are developed through complex spatial and temporal evolutionary trajectories [6,10,11].
What is the implication of intratumor heterogeneity on management of GBM?
Intratumor heterogeneity is a dynamic state, which is most likely the primary force-driving differential responses to therapy, development of treatment resistance and, ultimately, tumor recurrence [8,10,11,12]. RT and TMZ create a selective evolutionary pressure on GBM cells to which they are exposed. Microenvironment conditions that prevent treatment from having effect may result in surviving cells that proliferate to form clusters of cancer cells with conferred resistance, meaning that a single drug may not be enough for efficient treatment. Combined therapies appear to be the way forward; these would need to target trunk alterations of clonally dominant tumor drivers [13]. Problematically, passenger mutations can become drivers in response to environmental pressures caused by treatment [14].
Therapy of GBM requires, in addition to target tumor cells being in cycle, tissue oxygenation. This, in turn, requires adequate blood supply. Though angiogenesis is a feature of GBM, the new circulation systems are usually tortuous, leaky and chaotic, creating an environment of inconsistent blood supply with resultant regional hypoxia and interstitial hypertension [15,16], in spite of increased angiogenesis. Hypoxia reduces the effects of radiation-induced DNA damage of GBM cells (the oxygen-fixation hypothesis) [17] and recurrence often occurs within a few centimeters of the primary lesion [18]. Additionally, the go or grow hypothesis [19] states that the migrating phenotype is different to that of the proliferative one, where invading cells are not proliferative and, therefore, not affected by RT. The heterogeneously leaky microvasculature also results in increased interstitial fluid pressures (IFP) and nonuniform drug delivery [16,20], with intrinsic cellular properties also playing a role [21]. Furthermore, secondary somatic mutations emerge as result of therapies, contributing to changes in the tumor clonal architecture [13] and activation of genes of drug resistance.
Conventional imaging for monitoring intratumor heterogeneity of GBM: why is it not enough?
Currently, conventional imaging (Table 1), including T1-weighted and T2-weighted fluid-attenuated inversion recovery (FLAIR), are used for surgical planning and monitoring of GBM. These conventional sequences, though widely available, are limited with their representation of regional biological features driving tumor pathology (Figure 1). T2-weighted FLAIR has been, since 2006, included in tumor characterization to identify regions of surrounding edema of GBM [22]. It does not, however, differentiate between pure vasogenic and infiltrative edema, which is a GBM feature.
Table 1. . Conventional MRI modalities for assessment of glioblastoma.
| Modality | Biological properties visualized | Limitations |
|---|---|---|
| T1-weighted | Anatomy; BBB permeability/disruption; compromised vessel integrity | Lacks biological specificity; does not clearly identify tumor ‘bulk’ boundaries; the ‘necrotic core’ component may not exclusively possess necrotic tissue as implied |
| T2-weighted | Anatomy; tissue water content/edema | Lacks biological specificity; limited identification of invasive/noninvasive tissue; does not accurately differentiate pure vasogenic from infiltrative edema |
| T2-FLAIR | Anatomy; improved visualization of edema with less ‘noise’ from the ventricles. | Does not show full extent of margin; does not accurately differentiate pure vasogenic from infiltrative edema |
BBB: Blood–brain barrier; FLAIR: Fluid-attenuated inversion recovery.
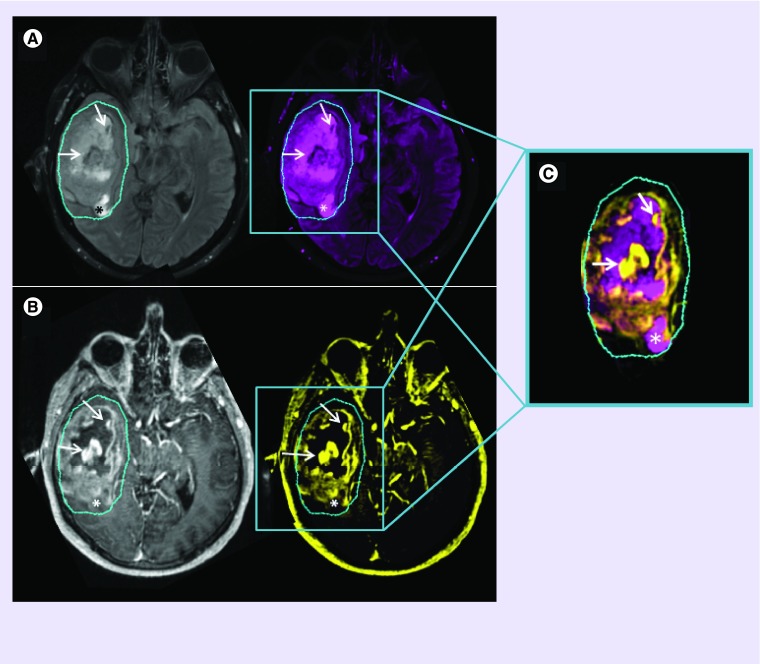
Figure 1. . Conventional MRI.
T2-weighted FLAIR images (A) are used to non-specifically assess edema surrounding the T1-weighted contrast-enhancement (B). Though signal hyper- or hypo-intensities may indicate certain biological scenarios, one cannot be certain of the eco-biology within ROIs. Combined ROIs (C) of low T2-FLAIR with high T1-post-contrast signal (arrows) can imply absence of edema, as a result of tumor and/or inflammatory cell presence, with compromised BBB integrity and leaky vasculature; ROIs of increased T2-FLAIR with low T1-post contrast signal (*) can imply regional edema, resulting from an adjacent inflammatory response or from an absence of tumor cells (possible necrosis), with a probable intact BBB and preserved vascular integrity. Contrary to what may be expected, a low signal on a T1-post-contrast image does not necessarily imply a reduced or compromised blood supply- information of this nature would only be obtainable with perfusion imaging.
BBB: Blood–brain barrier; FLAIR: Fluid attenuated inversion recovery; ROI: Region of interest.
Increased or new T1-weighted contrast enhancement does not necessarily imply tumor recurrence. The damage to epithelial cells along with local tissue inflammation caused by cytotoxic or radiation therapy is hypothesized to result in the increased edema on T2-weighted and increased or even new contrast enhancement on T1-weighted images [23], mimicking tumor progression. Though pseudoprogression was described in the late 1980s [24], its detailed mechanisms driving tissue damage, along with the inflammatory responses, are still not well understood. Conversely, pseudoresponse, which involves the decrease in T1-weighted contrast enhancement following treatment, is likely the result of normalization of abnormally permeable and leaky tumor vasculature, mimicking tumor response to therapy. The Response Assessment in Neuro-Oncology (RANO) criteria suggest that these features should persist at least 4 weeks before true response can be considered [25], further rationalizing the use of complementary imaging modalities for more prompt characterization of post-treatment tumor behavior.
Advanced imaging for monitoring intratumor heterogeneity of GBM: can one see the biology?
Advanced imaging methods (Table 2) allow for the assessment of GBM based on regional biological properties found within and surrounding the tumor. These include, but are not limited to, diffusion-weighted (DWI), diffusion tensor (DTI), diffusional kurtosis (DKI), perfusion-weighted (PWI), MR spectroscopic (1H-MRS) and positron emission tomographic (PET) imaging. Though explored by various groups for some years, these emerging techniques require further investigation, validation and standardization.
Table 2. . Emerging MRI modalities for assessing intratumor heterogeneity in glioblastoma.
| Modality | Biological properties visualized | Limitations† |
|---|---|---|
| DWI | Signal drop in regions of free-moving (Gaussian) diffusion | Should not be assessed without corresponding T2-weighted or proton density image as the T2-signal can affect DWI signal (T2-shinethrough); nonquantifiable |
| ADC | Quantifiable; high signal may indicate: cystic lesions, edema, low cellularity, necrosis; low signal may indicate: cytotoxic edema, cellularity. | Cannot differentiate tumor cells from inflammatory cells |
| DTI | White matter integrity based on infiltration, displacement or disruption; with this, one can distinguish tumor bulk from the surrounding margin | Provides details solely pertaining to isotropic and anisotropic movement of 1H (protons). Limited microenvironment information |
| T1-weighted DCE | BBB disruption, blood vessel permeability, ‘leaky’ vasculature | Model-dependent: based on either flow-limited or permeability limited conditions. Model details can be found in work by Tofts et al. [49] |
| T2*-weighted DSCI | Regional‡ rCBV | Due to BBB disruption, T2*-signal drops with recirculation of blood. As the rCBV model assumes an intact BBB, leakage correction is essential to account for damaged and leaky BBB in GBM |
| 1H-MRS CSI | Metabolic changes; microenvironment: high Cho = membrane turnover (cellularity); low NAA = damaged neurons; lactate and lipids = anaerobic, acidic conditions | Improved spectra resolution comes at the cost of increased acquisition time (longer time in the scanner); scanning time further increases when obtaining 3D data |
†A limitation relevant to these imaging modalities, the emerging ones in particular, is a lack of validation and standardization.
‡Though various metrics can be derived using T2*-weighted imaging, only rCBV is focused on as it is the one most analyzed, showing greatest promise in terms of potential standardization [55].
ADC: Apparent diffusion coefficient; BBB: Blood–brain barrier; Cho: Choline; DCE: Dynamic contrast enhanced; DSCI: Dynamic susceptibility contrast imaging; DTI: Diffusion tensor imaging; DWI: Diffusion-weighted imaging; GBM: Glioblastoma; 1H-MRS CSI: Magnetic resonance spectroscopy chemical shift imaging; NAA: N-acetylaspartate; rCBV: Relative cerebral blood volume.
• Diffusion imaging
Diffusion imaging essentially provides information pertaining to the nature of water molecule movement within biological tissue. As the biology of the edematous invasive margin is different to that of the tumor bulk [9], the development of imaging techniques for GBM characterization, that also focus on the margin as a region of interest, is paramount for the incorporation of advanced imaging in assessment criteria of GBM.
DTI is a modification of DWI that is sensitive to the anisotropic diffusion of water along axonal fibers, where one can decompose the diffusion tensor into isotropic (p-) and anisotropic (q-) components. By assessing the disruption of white matter (identified as a decrease in q) with adjacent edema (identified as an increase in p), DTI has allowed for differentiation of the tumor bulk from the invasive margin [26,27], which can extend beyond the T2-weighted FLAIR abnormality (Figure 2). The underlying biology observed with DTI beyond that of white matter integrity is not well described, making it a technique that is limited in providing detailed information of the microenvironment. DTI has, however, been used to identify patterns of recurrence [28] and white matter invasion in GBM [29], with improved delineation of the invasive margin [30]. Interestingly, DTI-derived characteristics of white matter invasion have also been associated with IDH1 status, where IDH1-positive tumors, associated with improved prognosis, have demonstrated patterns of minimal invasion surrounded by intact white matter [31].
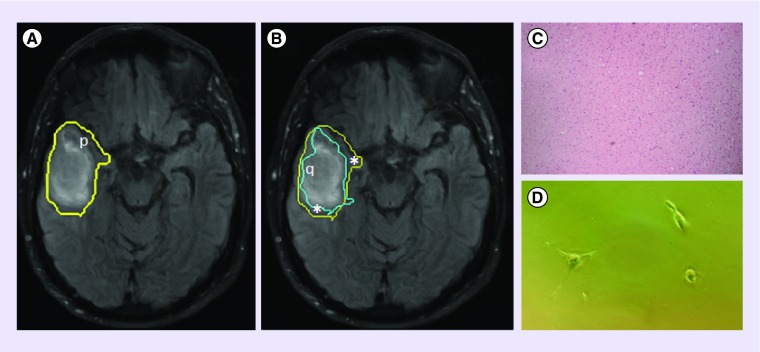
Figure 2. . T2-weighted FLAIR image of a GBM lesion with a superimposed delineation of the p-abnormality, which extends beyond FLAIR enhancement (A).
This, in conjunction with the q-abnormality (B), can be used to differentiate the tumor bulk (q) from the margin (*), which has a normal †histological appearance (C), but which harbors tumor ††cells (D). Regions of p- and q- abnormality were obtained using the p- and q- components of the diffusion tensor, as described by [30].
†Tissue samples obtained using 5-ALA fluorescence-guided resection, allowing access to normal-appearing tissue, stained with H&E, 10× magnification.
††Margin cells were plated in vitro and observed 4DIV.
5-ALA: 5-aminolevulinic acid; DIV: Days in vitro; FLAIR: Fluid-attenuated inversion recovery; GBM: Glioblastoma; H&E: Hematoxylin and eosin stain.
The apparent diffusion coefficient (ADC) measures motional processes, like flow and diffusion. However, when assessing mean ADC values of entire lesions, results can be ambiguous as different treatment strategies provide different microenvironments. Inconsistencies in study results can be attributed to biological intratumor heterogeneity, where an entire lesion is composed of various subregions, each possessing characteristic quantitative values, recapitulating cellular and molecular phenomena. The entire lesion, however, is quantitatively represented as an average of values from all subregions with both a skewed and large distribution (Figure 3).
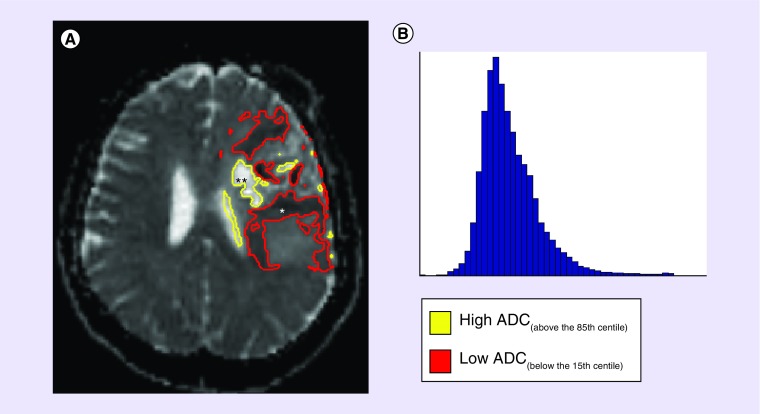
Figure 3. . A pre-treatment ADC scan of GBM (A) can show marked variation of low (*) and high (**) ADC values, which can regionally represent various biological scenarios- low ADC: high tumor or inflammatory cell presence, ischemia or a combination of both; high ADC: vasogenic edema, limited tumor and/or inflammatory cells, necrosis or a combination of any.
Studies assessing distribution of ADC with histogram analyses (B), although providing interesting quantitative data, give no information pertaining to the spatial distribution of ADC throughout the lesion.
ADC: Apparent diffusion coefficient; GBM: Glioblastoma.
Without taking into account what other imaging modalities could contribute to the picture, increased and decreased ADC values, as in vitro studies show [32], may represent different events within different treatment settings. In pretreatment scans (Figure 3), regions of low ADC may be representative of: first, densely packed tumor cells that restrict extracellular water movement, or second, regional ischemia resulting in faulty cellular ion efflux; high ADC could represent vasogenic edema, resulting from a disrupted blood–brain barrier (BBB); regions with less tumor cells, allowing extracellular water to move freely; or necrotic tissue. Postcytotoxic treatment scans may show regionally decreased ADC resulting from cytotoxic edema, during which cells swell due to ion imbalances caused by cell wall damage; or recurrent tumor. Increased ADC after cytotoxic therapy, also an early sign of possible pseudoprogression [33], can indicate either cell death or necrosis, where one finds increased water motility in extracellular spaces; or an inflammatory response with subsequent peritumoral edema. With inflammation, however, comes an inflammatory cell response, which may contribute to possible decreases in regional ADC, further warranting caution when using ADC to assess and monitor GBM. In an antiangiogenic therapy setting, histogram studies vary. One has found low ADC to correlate with better survival patterns [34]. A subsequent analysis associated high ADC with increased levels of extracellular matrix protein gene expression [35], believed to enhance tumor invasion by promoting collagen-enriched matrices for rapid migration [36]. Other studies have, conversely, found an association of low ADC with poor outcome [37] in recurrent GBM. The latter comes from a multicenter study and is in agreement with more specific histological analyses of regional ADC demonstrating an inverse correlation between tumor cell density and ADC [38]. Low ADC has also been suggested for use during image-guided biopsies of tumor-containing regions in areas of FLAIR abnormality [39].
Changes in regional ADC are apparent before changes in conventional imaging features [40]. Functional diffusion maps (fDM), which take the intratumoral heterogeneity of ADC into account, produce regional color maps according to whether ADC-values have increased, decreased or remained unchanged. Studies have used fDMs to predict responses to therapy as early as 3 weeks from start of chemo-RT [41,42].
Diffusional measurements, though widely explored, still offer a range of metrics that have not yet been fully explored. For example, DKI [43], a spin-off from DTI and histogram analysis, is a statistic that quantifies non-Gaussian properties of lesions, where a value of kurtosis serves as a marker of diffusional heterogeneity. Though explored as a measure of heterogeneity during grading [44], DKI has not yet been applied to assessing subregional heterogeneity in GBM. Though diffusion imaging has shown promise in various aspects of assessment, GBMs are spatially heterogeneous tumors, where regions of high cellularity can be found adjacent to areas of necrosis and edema, highlighting the importance of a multimodal imaging approach for improved regional GBM characterization.
• Perfusion imaging
PWI dynamic susceptibility contrast imaging (DSCI) measures of relative cerebral blood volume (rCBV) correlate with regional tumor vascularity [45,46] and cellular proliferation [47]. As with diffusion, perfusion can vary significantly with changes in cell density, edema and necrosis, and various biological situations can be presumed during regional assessment [48]. Within the ‘necrotic core’ of contrast-enhanced images (Figure 4), regional low rCBV would presumably represent a cell population that was unable to adapt to a hypoxic and/or acidic environment. A poorly perfused, yet cellular, region may be indicative of an adaptive cell niche (Figure 4). This niche is of importance as it implies the evolution of a cell population resistant to regular causes of necrosis or apoptosis – these may be resistant to current chemo-RT. Dynamic contrast enhancement (DCE) MRI relies on compartment-based tracer kinetic models, where certain parameters can be used to describe BBB integrity and leakiness of blood vessels [49,50]. These parameters can be used to indirectly describe biological properties, including relative IFP. However, most work, with the exception of one [50], has focused primarily on summary statistics, such as the median or mean, of DCE parameters without taking into account the regional heterogeneity of GBM. As with ADC, extreme values within PWI niches result in mean values that do not necessarily represent what is happening within the entire lesion. As mentioned, resistance to drug delivery is caused, at least in part, by impaired microvasculature and lymphatic system function, leading to increased IFP [16], making DCE another potential perfusion imaging method for characterizing GBM based on regional IFPs, which may be responsible for regional inadequate drug uptake in both the tumor bulk and the nonenhancing invasive margin.
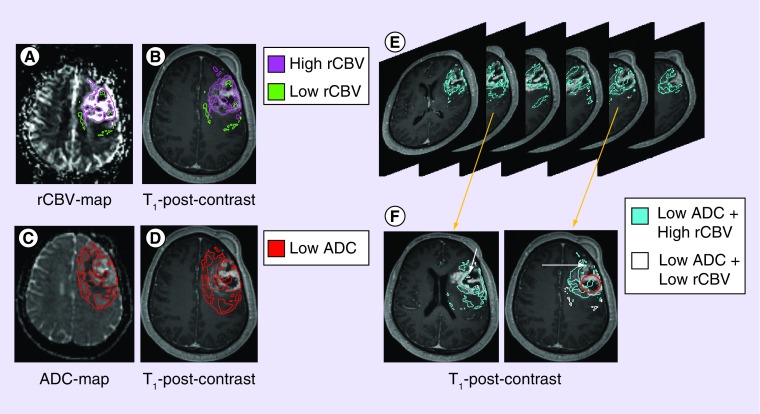
Figure 4. . ADC and rCBV can give better indications of what may be happening within sub-regions of GBM.
rCBV-maps (A) provide clues pertaining to the microenvironment that surrounds cells. Superimposing these sub-regions to T1-post-contrast (B) images provides details pertaining to where these ecosystems may be in relation to the tumor ‘bulk’ (T1-enhancing region)- either within the ‘necrotic core’, within the ‘bulk’, or just outside. Combining this with regional low ADC (C) and assessing its location on T1-post-contrast images (D) can also give spatial information relative to tumor ‘bulk’. As one scans through images of combined ADC and rCBV ROIs (E), further scenarios can be presumed (F): in the ‘necrotic core’, cellular regions with adequate perfusion suggest a population residing in regions of adequate oxygen and nutrient supply (O); cellular regions with lowered blood volume (arrow), however, suggest a population adapted to a hypoxic and acidic microenvironment conditions. Note how these advanced methods also suggest that the conventional ‘necrotic core’ may harbor cells and possess regional blood supply.
ADC: Apparent diffusion coefficient; GBM: Glioblastoma; rCBV: Relative cerebral blood volume.
Following chemo-RT, increased perfusion measures can occur prior to changes in T1-contrast enhancement [51]. PWI measures have been utilized for differentiating pseudoprogression from true recurrence [52,53] and studies have found certain parameters, including rCBV [47,51], to change in the nonenhancing margin of GBM [54]. This makes standardized PWI [49,55] a potential tool for future analyses of, not only the enhancing tumor bulk, but also the nonenhancing component that is left behind postsurgery.
• Spectroscopic imaging
Multivoxel MRS, or chemical shift imaging (CSI), reveals changes in tissue metabolism. Metabolic data have been used not only independently but also as complementary techniques to improve tumor characterization in combination with parameters of DWI [56,57] and PWI [58,59]. CSI also provides additional information for better understanding of the microenvironment, such as increased cellular density, identified as an increase in levels of Choline (Cho) [56]; neuronal damage, identified as a decrease in N-acetylaspartate (NAA) levels; and tumor metabolism with associated hypoxia and acidosis, identified as increased lactate and lipid production [60]. Studies looking at margin delineation [61] and characterization using CSI have reported increased Cho/NAA ratio in the nonenhancing component of GBM [62,63,64], where an increase of this ratio coincides with an increase in degree of invasion [65]. Conversely, studies have correlated Cho/NAA ratio with tumor cell proliferation [61,66]. This conflict in findings, where the similar results are reported to correlate with proliferation and invasion, two cellular processes reported to carry different phenotypic characteristics in GBM, highlights the need for caution when relying on MRS CSI for regional characterization of these tumors. Nonetheless, this imaging approach demonstrates potential, especially when combining this modality with other advanced imaging methods.
• Positron emission tomography
Using a tracer to functionally image tumor metabolic activity, PET has been used in studies of GBM treatment effects. 18F-flurodeoxyglucose (FDG) PET, which, until recently, has been the most widely used, allows one to image regional glucose metabolism, but is of limited value as normal brain naturally demonstrates high glucose uptake. Though amino acid (AA) PET, including C-methionine (MET) and 18F-fluoroethyltyrosine (FET), have shown potential in assessing effects of RT [67], including identification of true progression [68], it appears to be less effective than DWI under direct comparison [69]. 3′-Deoxy-3′-18F-fluorothymidine (FLT), in addition to MET, is a useful biomarker of cellular proliferation in GBM, with the regional heterogeneity of uptake correlating to with MIB-1 labeling index [70]. This, in turn, correlates with FLT transport across the BBB [71]. Though contrast with FLT is better than with FDG [72], uptake is BBB-determined, which poses a problem in GBM analysis, whose BBB is characteristically leaky around contrast-enhancing components. Effects of RT have been examined using FLT. These findings, however, have largely involved animal studies [73]. To assess potential malignancy based on cellular membrane metabolism, 18F-fluorocholine (CHO PET) has gathered attention in GBM monitoring, with increased CHO uptake being characteristic of high-grade gliomas [74]. To assess response to antiangiogenic therapies, 18F-fluciclatide has been used to visualize regional integrin, which plays a key role in angiogenesis [75]. Furthermore, hypoxia markers, such as 18F-fluoromisonidazole (FMISO), continue to be investigated for predicting response to RT [76] and bevacizumab. As drug delivery in tumors is heterogeneous [20], PET tracers for specific drugs with positron emitters would produce images of tracer uptake that are representative of regional drug uptake, making PET an imaging tool of significant potential.
Conclusion & future perspective
The complex dynamics of biological systems in GBM result in heterogeneous and inconsistent imaging features. It is becoming evident, however, that regional variations within GBM result in specific imaging characteristics. Very little is known of the molecular and genomic properties found within separate niches in GBM, but having an idea of the microenvironment they inhabit, could provide details of the adaptive strategies undertaken for cell survival. Therefore, a valuable metric for future GBM analysis could involve assessment of MRI-defined ‘habitat’ changes in response to therapy. This would be beneficial in personalized treatment, such as the implementation of boost RT dosing in regions of high risk.
Though the potential appears promising, the use of advanced imaging as surrogate measures of tumor biology carries limitations. In addition to interobserver variability, limitations include enhancing variability, measurement variability, as well as false positives and negatives [77]. This highlights the need for future refinement in the assessment criteria with a particular focus on standardization [49,55] of imaging characteristics along with clearer understanding of underlying biological properties, whether they are at the cellular, molecular or genetic level. This would potentially minimize errors caused by intrinsic parametric variation, and enhance the accuracy of assessing response to treatment [22] in a multicenter setting. Determining the added value of assessing nonenhancing tumor progression, as a measure of clinically relevant and treatment-related change, and incorporating into the assessment criteria, may too be a way forward. This will most likely be achieved with identification of appropriate end points in multicenter trials. Additionally, a shift to 3D assessment [25] will improve the understanding of regional spatial dynamics. Furthermore, assessing regional volumes, as opposed to areas, would preserve statistical power of results while using a smaller patient cohort.
As future directions for GBM management probably involve multimodal therapy directed at multiple subregions, the advantages obtained with a detailed understanding of regional imaging properties, and the underlying ecobiology, will contribute to improved strategies for personalized medicine of GBM.
Footnotes
Financial & competing interests disclosure
SJ Price is funded by a Clinician Scientist Award from the National Institute for Health Research. Research conducted by Stephen J Price is supported by the Cambridge Biomedical Research Centre and Cancer Research UK. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]; • The major single advancement in GBM treatment over the past years, incorporating RT with concomitant and adjuvant temozolomide.
- 2.Jackson RJ, Fuller GN, Abi-Said D, et al. Limitations of stereotactic biopsy in the initial management of gliomas. Neuro Oncol. 2001;3(3):193–200. doi: 10.1093/neuonc/3.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen H-J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre Phase III trial. Lancet Oncol. 2006;7(5):392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 4.Deacon J, Peckham MJ, Steel GG. The radioresponsiveness of human tumours and the initial slope of the cell survival curve. Radiother. Oncol. 1984;2(4):317–323. doi: 10.1016/s0167-8140(84)80074-2. [DOI] [PubMed] [Google Scholar]
- 5.Diehn M, Nardini C, Wang DS, et al. Identification of noninvasive imaging surrogates for brain tumor gene-expression modules. Proc. Natl Acad. Sci. USA. 2008;105(13):5213–5218. doi: 10.1073/pnas.0801279105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burrell R a, McGranahan N, Bartek J, Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501(7467):338–345. doi: 10.1038/nature12625. [DOI] [PubMed] [Google Scholar]
- 7.Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501(7467):346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 8.Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21(3):283–296. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piccirillo SGM, Dietz S, Madhu B, et al. Fluorescence-guided surgical sampling of glioblastoma identifies phenotypically distinct tumour-initiating cell populations in the tumour mass and margin. Br. J. Cancer. 2012;107(3):462–468. doi: 10.1038/bjc.2012.271. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrates that, with the use of 5-ALA, fluorescence-guided resection can be used to identify normal-appearing brain-harboring GBM cells.
- 10.Sottoriva A, Spiteri I, Piccirillo SGM, et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc. Natl Acad. Sci. USA. 2013;110(10):4009–4014. doi: 10.1073/pnas.1219747110. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• By assessing multiple intratumor fragments, this group found distinct GBM subtypes within the same tumor, with a hierarchy of mitotic clones coexisting within the same fragment.
- 11.Patel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A study that sequels Sottoriva et al. (2013), intratumor single-cell analyses identified inherently variable expression of transcriptional programs related to tumor behavior and microenvironment.
- 12.Piccirillo SGM, Spiteri I, Sottoriva A, et al. Contributions to drug resistance in glioblastoma derived from malignant cells in the sub-ependymal zone. Cancer Res. 2014;75(1):194–202. doi: 10.1158/0008-5472.CAN-13-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher R, Pusztai L, Swanton C. Cancer heterogeneity: implications for targeted therapeutics. Br. J. Cancer. 2013;108(3):479–485. doi: 10.1038/bjc.2012.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yap TA, Gerlinger M, Futreal PA, Pusztai L, Swanton C. Intratumor heterogeneity: seeing the wood for the trees. Sci. Transl. Med. 2012;4(127):127ps10. doi: 10.1126/scitranslmed.3003854. [DOI] [PubMed] [Google Scholar]
- 15.Gillies RJ, Schornack PA, Secomb TW, Raghunand N. Causes and effects of heterogeneous perfusion in tumors. Neoplasia. 1999;1(3):197–207. doi: 10.1038/sj.neo.7900037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukumura D, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J. Cell. Biochem. 2007;101(4):937–949. doi: 10.1002/jcb.21187. [DOI] [PubMed] [Google Scholar]
- 17.Ewing D. The oxygen fixation hypothesis: a reevaluation. Am. J. Clin. Oncol. 1998;21(4):355–361. doi: 10.1097/00000421-199808000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Gebhardt BJ, Dobelbower MC, Ennis WH, Bag AK, Markert JM, Fiveash JB. Patterns of failure for glioblastoma multiforme following limited-margin radiation and concurrent temozolomide. Radiat. Oncol. 2014;9(1):130. doi: 10.1186/1748-717X-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garay T, Juhász É, Molnár E, et al. Cell migration or cytokinesis and proliferation? Revisiting the “go or grow” hypothesis in cancer cells in vitro . Exp. Cell Res. 2013;319(20):3094–3103. doi: 10.1016/j.yexcr.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu. Rev. Biomed. Eng. 1999;1:241–263. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- 21.Piccirillo SGM, Colman S, Potter NE, et al. Genetic and functional diversity of propagating cells in glioblastoma. Stem Cell Reports. 2015;4(1):7–15. doi: 10.1016/j.stemcr.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellingson BM, Wen PY, van den Bent MJ, Cloughesy TF. Pros and cons of current brain tumor imaging. Neuro Oncol. 2014;16(Suppl. 7):vii2–vii11. doi: 10.1093/neuonc/nou224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 24.Fiegler W, Langer M, Scheer M, Kazner E. Reversible computed tomographic changes following brain tumor irradiation induced by the “early-delayed reaction” after radiation. Radiologe. 1986;26(4):206–209. [PubMed] [Google Scholar]
- 25.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]; •• Highlights limitations to response assessment criteria in neurooncology with recommendations for improvement.
- 26.Price SJ, Peña A, Burnet NG, et al. Tissue signature characterisation of diffusion tensor abnormalities in cerebral gliomas. Eur. Radiol. 2004;14(10):1909–1917. doi: 10.1007/s00330-004-2381-6. [DOI] [PubMed] [Google Scholar]
- 27.Peña A, Green HAL, Carpenter TA, Price SJ, Pickard JD, Gillard JH. Enhanced visualization and quantification of magnetic resonance diffusion tensor imaging using the p:q tensor decomposition. Br. J. Radiol. 2006;79(938):101–109. doi: 10.1259/bjr/24908512. [DOI] [PubMed] [Google Scholar]
- 28.Price SJ, Jena R, Burnet NG, Carpenter TA, Pickard JD, Gillard JH. Predicting patterns of glioma recurrence using diffusion tensor imaging. Eur. Radiol. 2007;17(7):1675–1684. doi: 10.1007/s00330-006-0561-2. [DOI] [PubMed] [Google Scholar]
- 29.Price SJ, Burnet NG, Donovan T, et al. Diffusion tensor imaging of brain tumours at 3T: a potential tool for assessing white matter tract invasion? Clin. Radiol. 2003;58(6):455–462. doi: 10.1016/s0009-9260(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 30.Price SJ, Jena R, Burnet NG, et al. Improved delineation of glioma margins and regions of infiltration with the use of diffusion tensor imaging: an image-guided biopsy study. AJNR Am. J. Neuroradiol. 2006;27(9):1969–1974. [PMC free article] [PubMed] [Google Scholar]; • Demonstrates, with use of image-guided biopsy, that the invasive margin can be identified by decomposing the diffusion tensor into its isotropic (p) and anisotropic (q) components.
- 31.Price SJ, Boonzaier NR, Lupson V, Larkin T. BrainLab Neurosurgery Award 196 IDH-1 mutated glioblastomas have a less invasive phenotype than IDH-1 wild type glioblastomas: a diffusion tensor imaging study. Neurosurgery. 2014;61(Suppl. 1):225. [Google Scholar]
- 32.Matsumoto Y, Kuroda M, Matsuya R, et al. In vitro experimental study of the relationship between the apparent diffusion coefficient and changes in cellularity and cell morphology. Oncol. Rep. 2009;22(3):641–648. doi: 10.3892/or_00000484. [DOI] [PubMed] [Google Scholar]
- 33.Asao C, Korogi Y, Kitajima M, et al. Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. Am. J. Neuroradiol. 2005;26(6):1455–1460. [PMC free article] [PubMed] [Google Scholar]
- 34.Pope WB, Lai A, Mehta R, et al. Apparent diffusion coefficient histogram analysis stratifies progression-free survival in newly diagnosed bevacizumab-treated glioblastoma. Am. J. Neuroradiol. 2011;32(5):882–889. doi: 10.3174/ajnr.A2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pope WB, Mirsadraei L, Lai A, et al. Differential gene expression in glioblastoma defined by ADC histogram analysis: relationship to extracellular matrix molecules and survival. Am. J. Neuroradiol. 2012;33(6):1059–1064. doi: 10.3174/ajnr.A2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huijbers IJ, Iravani M, Popov S, et al. A role for fibrillar collagen deposition and the collagen internalization receptor endo180 in glioma invasion. PLoS ONE. 2010;5(3):1–12. doi: 10.1371/journal.pone.0009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pope WB, Qiao XJ, Kim HJ, et al. Apparent diffusion coefficient histogram analysis stratifies progression-free and overall survival in patients with recurrent GBM treated with bevacizumab: a multi-center study. J. Neurooncol. 2012;108(3):491–498. doi: 10.1007/s11060-012-0847-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugahara T, Korogi Y, Kochi M, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J. Magn. Reson. Imaging. 1999;9(1):53–60. doi: 10.1002/(sici)1522-2586(199901)9:1<53::aid-jmri7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 39.Barajas RF, Phillips JJ, Parvataneni R, et al. Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR Imaging. Neuro Oncol. 2012;14(7):942–954. doi: 10.1093/neuonc/nos128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chenevert TL, Stegman LD, Taylor JM, et al. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J. Natl Cancer Inst. 2000;92(24):2029–2036. doi: 10.1093/jnci/92.24.2029. [DOI] [PubMed] [Google Scholar]
- 41.Moffat BA, Chenevert TL, Meyer CR, et al. The functional diffusion map: an imaging biomarker for the early prediction of cancer treatment outcome. Neoplasia. 2006;8(4):259–267. doi: 10.1593/neo.05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamstra DA, Galbán CJ, Meyer CR, et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J. Clin. Oncol. 2008;26(20):3387–3394. doi: 10.1200/JCO.2007.15.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 2005;53(6):1432–1440. doi: 10.1002/mrm.20508. [DOI] [PubMed] [Google Scholar]
- 44.Raab P, Hattingen E, Franz K, Zanella FE, Lanfermann H. Cerebral gliomas: diffusional kurtosis imaging analysis of microstructural differences. Radiology. 2010;254(3):876–881. doi: 10.1148/radiol.09090819. [DOI] [PubMed] [Google Scholar]
- 45.Sugahara T, Korogi Y, Kochi M, et al. Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am. J. Roentgenol. 1998;171(6):1479–1486. doi: 10.2214/ajr.171.6.9843274. [DOI] [PubMed] [Google Scholar]
- 46.Aronen HJ, Pardo FS, Kennedy DN, et al. High microvascular blood volume is associated with high glucose uptake and tumor angiogenesis in human gliomas. Clin. Cancer Res. 2000;6(6):2189–2200. [PubMed] [Google Scholar]
- 47.Price SJ, Green HAL, Dean AF, Joseph J, Hutchinson PJ, Gillard JH. Correlation of MR relative cerebral blood volume measurements with cellular density and proliferation in high-grade gliomas: an image-guided biopsy study. AJNR Am. J. Neuroradiol. 2011;32(3):501–506. doi: 10.3174/ajnr.A2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gatenby RA, Grove O, Gillies RJ. Quantitative imaging in cancer evolution and ecology. Radiology. 2013;269(1):8–15. doi: 10.1148/radiol.13122697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J. Magn. Reson. Imaging. 1999;10(3):223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 50.Rose CJ, Mills SJ, O'Connor JPB, et al. Quantifying spatial heterogeneity in dynamic contrast-enhanced MRI parameter maps. Magn. Reson. Med. 2009;62(2):488–499. doi: 10.1002/mrm.22003. [DOI] [PubMed] [Google Scholar]
- 51.Blasel S, Franz K, Ackermann H, Weidauer S, Zanella F, Hattingen E. Stripe-like increase of rCBV beyond the visible border of glioblastomas: site of tumor infiltration growing after neurosurgery. J. Neurooncol. 2011;103(3):575–584. doi: 10.1007/s11060-010-0421-4. [DOI] [PubMed] [Google Scholar]
- 52.Hu LS, Baxter LC, Smith KA, et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from post-treatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion. AJNR Am. J. Neuroradiol. 2009;30(3):552–558. doi: 10.3174/ajnr.A1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cha J, Kim ST, Kim H-J, et al. Differentiation of tumor progression from pseudoprogression in patients with post-treatment glioblastoma using multiparametric histogram analysis. AJNR Am. J. Neuroradiol. 2014;35(7):1309–1317. doi: 10.3174/ajnr.A3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lehmann P, Vallée J-N, Saliou G, et al. Dynamic contrast-enhanced T2*-weighted MR imaging: a peritumoral brain oedema study. J. Neuroradiol. 2009;36(2):88–92. doi: 10.1016/j.neurad.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Bedekar D, Jensen T, Schmainda KM. Standardization of relative cerebral blood volume (rCBV) image maps for ease of both inter- and intrapatient comparisons. Magn. Reson. Med. 2010;64(3):907–913. doi: 10.1002/mrm.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta RK, Cloughesy TF, Sinha U, et al. Relationships between choline magnetic resonance spectroscopy, apparent diffusion coefficient and quantitative histopathology in human glioma. J. Neurooncol. 2000;50(3):215–226. doi: 10.1023/a:1006431120031. [DOI] [PubMed] [Google Scholar]
- 57.Khayal IS, Crawford FW, Saraswathy S, et al. Relationship between choline and apparent diffusion coefficient in patients with gliomas. J. Magn. Reson. Imaging. 2008;27(4):718–725. doi: 10.1002/jmri.21288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am. J. Neuroradiol. 2003;24(10):1989–1998. [PMC free article] [PubMed] [Google Scholar]
- 59.Lupo JM, Cha S, Chang SM, Nelson SJ. Analysis of metabolic indices in regions of abnormal perfusion in patients with high-grade glioma. AJNR Am. J. Neuroradiol. 2007;28(8):1455–1461. doi: 10.3174/ajnr.A0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McLean MA, Cross JJ. Magnetic resonance spectroscopy: principles and applications in neurosurgery. Br. J. Neurosurg. 2009;23(1):5–13. doi: 10.1080/02688690802491673. [DOI] [PubMed] [Google Scholar]
- 61.Guo J, Yao C, Chen H, et al. The relationship between CHO/NAA and glioma metabolism: implementation for margin delineation of cerebral gliomas. Acta Neurochir. (Wien.) 2012;154(8):1361–1370. doi: 10.1007/s00701-012-1418-x. discussion 1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Di Costanzo A, Scarabino T, Trojsi F, et al. Multiparametric 3T MR approach to the assessment of cerebral gliomas: tumor extent and malignancy. Neuroradiology. 2006;48(9):622–631. doi: 10.1007/s00234-006-0102-3. [DOI] [PubMed] [Google Scholar]
- 63.Bieza A, Krumina G. Magnetic resonance study on fractional anisotropy and neuronal metabolite ratios in peritumoral area of cerebral gliomas. Medicina (B. Aires) 2012;48(10):497–506. [PubMed] [Google Scholar]
- 64.Wijnen JP, Idema AJS, Stawicki M, et al. Quantitative short echo time 1H MRSI of the peripheral edematous region of human brain tumors in the differentiation between glioblastoma, metastasis, and meningioma. J. Magn. Reson. Imaging. 2012;36(5):1072–1082. doi: 10.1002/jmri.23737. [DOI] [PubMed] [Google Scholar]
- 65.Croteau D, Scarpace L, Hearshen D, et al. Correlation between magnetic resonance spectroscopy imaging and image-guided biopsies: semiquantitative and qualitative histopathological analyses of patients with untreated glioma. Neurosurgery. 2001;49(4):823–829. doi: 10.1097/00006123-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 66.McKnight TR, Lamborn KR, Love TD, et al. Correlation of magnetic resonance spectroscopic and growth characteristics within Grades II and III gliomas. J. Neurosurg. 2007;106(4):660–666. doi: 10.3171/jns.2007.106.4.660. [DOI] [PubMed] [Google Scholar]
- 67.Xiangsong Z, Weian C. Differentiation of recurrent astrocytoma from radiation necrosis: a pilot study with 13N-NH3 PET. J. Neurooncol. 2007;82(3):305–311. doi: 10.1007/s11060-006-9286-y. [DOI] [PubMed] [Google Scholar]
- 68.Mehrkens JH, Pöpperl G, Rachinger W, et al. The positive predictive value of O-(2-[18F]fluoroethyl)-L-tyrosine (FET) PET in the diagnosis of a glioma recurrence after multimodal treatment. J. Neurooncol. 2008;88(1):27–35. doi: 10.1007/s11060-008-9526-4. [DOI] [PubMed] [Google Scholar]
- 69.Kim YH, Oh SW, Lim YJ, et al. Differentiating radiation necrosis from tumor recurrence in high-grade gliomas: assessing the efficacy of 18F-FDG PET, 11C-methionine PET and perfusion MRI. Clin. Neurol. Neurosurg. 2010;112(9):758–765. doi: 10.1016/j.clineuro.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 70.Price SJ, Fryer TD, Cleij MC, et al. Imaging regional variation of cellular proliferation in gliomas using 3′-deoxy-3′-[18F]fluorothymidine positron-emission tomography: an image-guided biopsy study. Clin. Radiol. 2009;64(1):52–63. doi: 10.1016/j.crad.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 71.Jacobs AH, Thomas A, Kracht LW, et al. 18F-fluoro-L-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J. Nucl. Med. 2005;46(12):1948–1958. [PubMed] [Google Scholar]
- 72.Chen W, Cloughesy T, Kamdar N, et al. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J. Nucl. Med. 2005;46(6):945–952. [PubMed] [Google Scholar]
- 73.Chandrasekaran S, Hollander A, Xu X, et al. 18F-fluorothymidine-PET imaging of glioblastoma multiforme: effects of radiation therapy on radiotracer uptake and molecular biomarker patterns. ScientificWorldJournal. 2013;2013:796029. doi: 10.1155/2013/796029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kwee SA, Ko JP, Jiang CS, Watters MR, Coel MN. Solitary brain lesions enhancing at MR imaging: evaluation with fluorine 18 fluorocholine PET. Radiology. 2007;244(2):557–565. doi: 10.1148/radiol.2442060898. [DOI] [PubMed] [Google Scholar]
- 75.Battle MR, Goggi JL, Allen L, Barnett J, Morrison MS. Monitoring tumor response to antiangiogenic sunitinib therapy with 18F-fluciclatide, an 18F-labeled αVbeta3-integrin and αV beta5-integrin imaging agent. J. Nucl. Med. 2011;52(3):424–430. doi: 10.2967/jnumed.110.077479. [DOI] [PubMed] [Google Scholar]
- 76.Shibahara I, Kumabe T, Kanamori M, et al. Imaging of hypoxic lesions in patients with gliomas by using positron emission tomography with 1-(2-[18F] fluoro-1-[hydroxymethyl]ethoxy)methyl-2-nitroimidazole, a new 18F-labeled 2-nitroimidazole analog. J. Neurosurg. 2010;113(2):358–368. doi: 10.3171/2009.10.JNS09510. [DOI] [PubMed] [Google Scholar]
- 77.Provenzale JM, Ison C, Delong D. Bidimensional measurements in brain tumors: assessment of interobserver variability. AJR Am. J. Roentgenol. 2009;193(6):W515–W522. doi: 10.2214/AJR.09.2615. [DOI] [PubMed] [Google Scholar]