Abstract
Two patients with an unmethylated MGMT promoter and IDH1 (R132H) wild-type recurrent glioblastoma were treated with crizotinib. Prolonged stabilization of the disease (17 months) was achieved in the first case. Interestingly, anaplastic lymphoma kinase (ALK) expression and c-MET protein overexpression was observed. Conversely, no response to crizotinib was obtained in the second case with MET protein overexpression and c-MET amplification but no ALK expression or ALK gene amplification. These case studies suggest that novel targeted ALK inhibitors may provide relevant clinical benefit in selected cases in which driver mutations are demonstrable.
KEYWORDS : ALK, crizotinib, glioblastoma, MET amplification, MET, targeted therapy
PRACTICE POINTS.
The prognostic of glioblastoma remains limited.
Novel targeted therapies have been recently developed for the treatment of glioblastoma.
C-MET overexpression and MET gain are frequently detected in glioblastoma but c-MET gene amplification is rare, observed in only 5% of all glioblastoma.
Anaplastic lymphoma kinase (ALK) overexpression and gain or amplification are seen in 18–48% of glioblastoma.
Two patients with ALK or c-Met expression but no ROS1 expression, unmethylated MGMT promoter recurrent glioblastoma were treated with crizotinib. To one of both, a prolonged stabilization of disease was observed after initiation of crizotinib.
These case studies suggest that patients with glioblastoma and ALK polysomy may derive clinically relevant benefit from novel targeted small molecular inhibitors such as crizotinib.
Case report
A 39-year-old man was presented with progressive headaches and vomiting and was found by brain MRI to have a left frontal intra-axial mass. A gross total tumor resection was performed. Histopathology and immunohistochemistry revealed a giant cell glioblastoma (GB) with p53 expression and without IDH1 mutated R132H expression. The MGMT gene promoter was nonmethylated.
The patient was treated with conventional radiotherapy, concomitant and adjuvant temozolomide (TMZ) for 6 cycles. Following completion of the 6th cycle of post-RT TMZ, the patient manifested recurrent disease by MRI only.
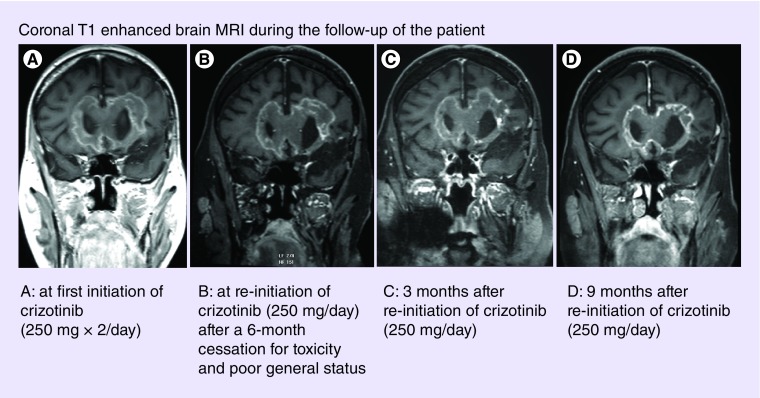
Single agent bevacizumab was initiated and administered until a second asymptomatic radiographic disease recurrence 10 months later. A second salvage therapy including fotemustine and bevacizumab was started. Three months subsequently, a third asymptomatic disease recurrence was observed. Treatment was changed to lomustine plus bevacizumab. A fourth asymptomatic recurrence was seen 5 months later, for which the patient received carboplatin and bevacizumab. However, subsequently the patient was presented with a rapid and severe clinical deterioration leading to an ECOG-Performance Status of 3 (previously 0) in a few weeks. Clear radiographic disease progression was evident by both T1 postgadolinium and T2/FLAIR MRI sequences (Figure 1). Methylprednisolone was introduced at 100 mg per day.
Figure 1. . Coronal T1 enhanced brain MRI during the follow-up of the patient.
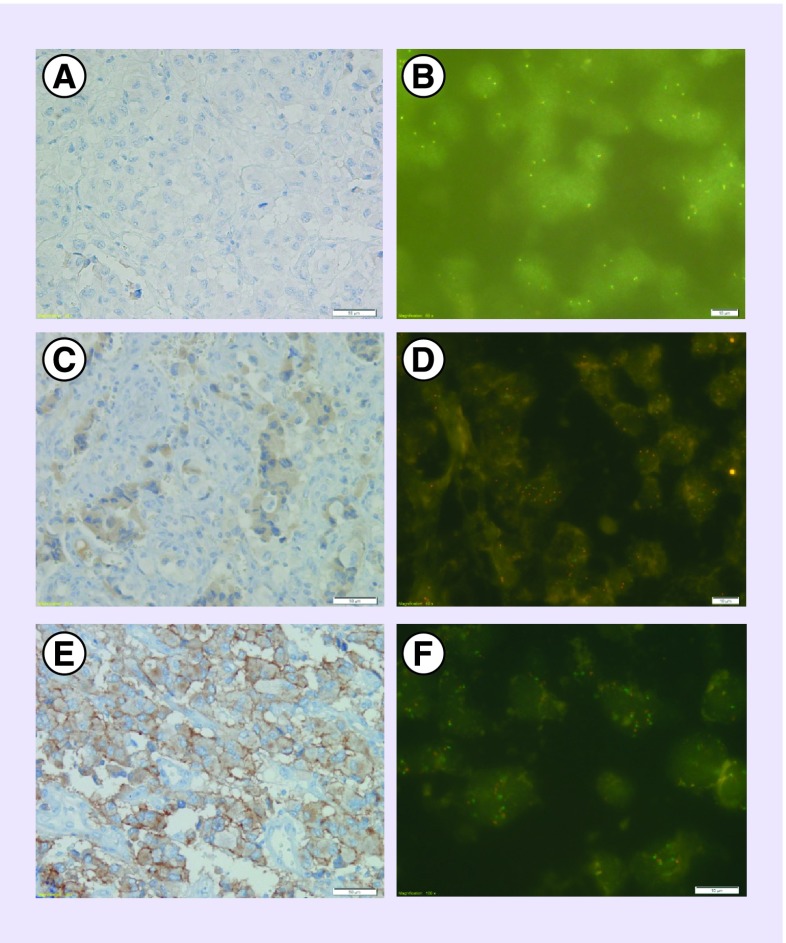
Additional molecular analyses of the original tumor were then performed and demonstrated weak expression of anaplastic lymphoma kinase (ALK) protein in 25% of the tissue as well as polysomy of chromosome 2 (ALK locus) in 53% of neoplastic nuclei. MET or HGFR (HGF Receptor) analysis showed weak to moderate expression of protein in 70 and 20% of the tumor cells, respectively. Polysomy of chromosome 7 (c-MET locus) was revealed by FISH in 84.5% of tumor nuclei wherein 43% of nuclei demonstrated five or more MET copies and 36% of nuclei had six or more copies. The ratio gene/centromere was equal to one. ROS1 expression was not observed, and no amplification or gain of gene was observed by FISH (Figure 2). The V600E BRAF mutation was not observed (Table 1).
Figure 2. . ROS1, ALK, c-MET immunohistochemical and FISH analysis for the first patient.
Table 1. . Association between crizotinib sensibility and molecular phenotypes.
| Patient no. 1, man | Patient no. 2, woman | |
|---|---|---|
| Diagnostic age | 39-year-old | 58-year-old |
| MGMT promoter | Nonmethylated | Nonmethylated |
| IDH1 gene | Wild-type | Wild-type |
| ROS1 protein/gene | No expression | No expression |
| No gain or polysomy | No gain or polysomy | |
| ALK protein/gene | Weak expression 25% | No expression |
| Polysomy chromosome 2.53% and gain in 13% | No polysomy no gain no amplification | |
| MET | Weak + moderate expression 70 and 20%, respectively | Moderate + strong expression 20 and 80%, respectively |
| Polysomy of chromosome 7 and low amplification 36% | High amplification with clusters in 70% | |
| Crizotinib sensibility | Strong benefit with stability | No benefit |
| Treatment at full dose: 2 months; break: 4 months, reduced dose: 12 months | Treatment during 4 months | |
Bevacizumab was therefore discontinued and crizotinib (250 mg twice daily) was initiated after discussion with the patient and his family and after obtaining written consent.
Following 2 months of crizotinib therapy, clinical improvement was observed and the steroid dose could be reduced (prednisone 20 mg per day). Brain MRI showed stable disease. Adverse events ascribed to crizotinib were observed manifested as grade 3 thrombocytopenia and hepatotoxicity and required an interruption of crizotinib. Prednisone was maintained at 20 mg per day during this period. After a 4-month-cessation therapy, toxicities resolved and crizotinib (250 mg/day) was then reinitiated, after discussion with the patient and his family, notwithstanding only minimal clinical and MRI progression (Figure 1). Following 3 months at reduced dose crizotinib therapy, a significant clinical improvement (personal autonomy and cognitive improvement) was observed as was the ECOG-PS (1). Additionally, prednisone was reduced to 10 mg per day. The patient remained independent in activities of daily living and with stable radiographic disease during the succeeding 9 months (Figure 1). The patient died of status epilepticus 12 months after the reintroduction of reduced dose crizotinib. The neurological condition was stable prior to the status epilepticus.
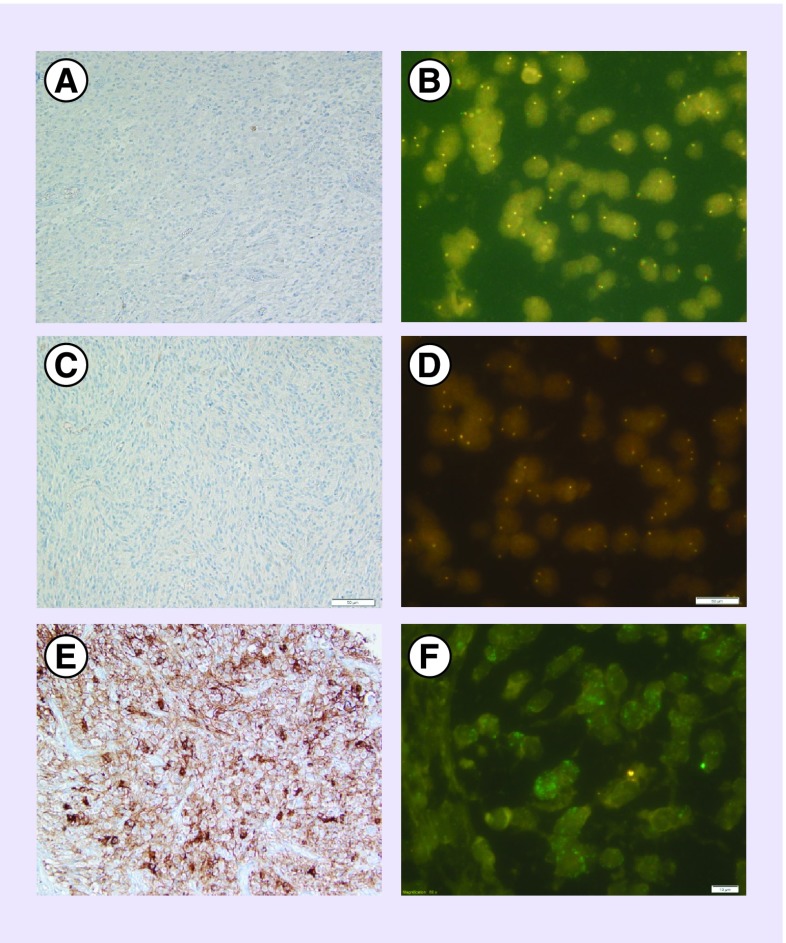
A 58-year-old woman was diagnosed with a right fronto-insular glioblastoma manifesting as partial left hemicorporal sensory-motor seizures and progressive headaches. A large but incomplete resection was performed. Histopathology and immunohistochemistry revealed a GB without IDH1 R132H expression. The MGMT gene promoter was unmethylated. Additional molecular analysis demonstrated strong expression of MET in 100% of tumor cells coupled with a high amplification of the gene (clusters) in 70% of tumor cells. Contrary to the first patient, the tumor did not express ALK and no amplification or gain of gene was observed by FISH. ROS1 expression was not observed, and no amplification or gain of gene was observed by FISH (Figure 3). The V600E BRAF mutation was not observed (Table 1).
Figure 3. . ROS1, ALK, c-MET immunohistochemical and FISH analysis for the second patient.
The patient was initially treated with conventional radiotherapy and concomitant and adjuvant TMZ for 3 cycles. She then developed worsening and progressive left hemiparesis resulting in an ECOG-PS of 2. Brain MRI confirmed progression. Corticosteroids were initiated (Medrol 32 mg per day). Following the initial molecular analysis, crizotinib (250 mg twice daily) was initiated. Before drug treatment a discussion with the patient and family was initiated that included alternative treatments and the experimental nature of crizotinib for this indication. Also shared with the family was a soon to open French clinical trial (ACSE NCT02034981) in patients with recurrent high grade gliomas and MET amplified tumors that would be treated in an experimental manner with crizotinib.
Treatment was well tolerated without adverse events for 4 months at which time a second MRI revealed disease progression. Furthermore there was evidence of clinical deterioration with an ECOG-PS of 2. The treatment was changed to bevacizumab plus lomustine leading to a prolonged response and permitting cessation of corticosteroids.
Discussion
GB is the most common and aggressive malignant primary brain tumor in adults with a median overall survival of one year [1,2]. As GB is fatal despite multimodality treatment, new therapies for GB are an unmet need in neuro-oncology. Emerging data in the molecular characterization of GB have identified new rare but clinically relevant and actionable molecular alterations that are potentially druggable with targeted therapies. In the current case report, the tyrosine kinase receptors of the insulin receptor superfamily, ALK and MET, were investigated. Both are expressed in many cancers and appear to have a role in modulating mitosis, cell migration, tumor cell survival [3–5] and angiogenesis [6–8]. Additionally, ALK is highly expressed in the nervous system during development [9] whereas c-MET regulates embryonic development and the immune response [3].
C-MET overexpression is detected in 29 to 88% of GB [10–12], but c-MET gene amplification has been observed in only 4–5% of all GB [13,14]. In a study of 70 GB, MET gain was present in 72%. In the majority of cases, the level of MET gain was low (2.7–5.0 copies). In this cohort, MET gain was more frequent in patients older than 40 years (54 vs 19%). The frequency of gain of MET was similar when comparing (IDH1 wild-type) and secondary GB (IDH1 mutated) respectively 47 and 44% [15]. Moreover, MET gain was more frequent in astrocytoma versus oligodendroglioma (38 vs 16%) and the appearance of MET gain appears to be associated with astrocytic progression [15]. C-MET overexpression has also been associated with multifocal lesions by MRI [10]. In the cohort of study by Pierscianek, MET gain was associated with significantly shorter patient survival and worse prognosis [15]. Overexpression or amplification of c-MET has been reported to be associated with a worse prognosis in most cancers as in glioblastoma [3,10,15–17]. It may be that c-MET expression affects prognosis due to the association with resistance to chemoradiation in GB [10,18].
Less data are available in the literature regarding ALK in GB. ALK overexpression and gain/amplification are seen in up to 18% of GB by immunohistochemistry and up to 48% by FISH, respectively [19,20]. The expression of ALK is more frequent in high-grade glioma as compared with low-grade glioma [5,19,21]. Oncogenic activation of the tyrosine kinase receptors c-ros oncogene 1 (ROS1) is rarely observed in glioblastoma [22,23]. ROS1 and ALK domains are partially homologous and consequently both are targeted by crizotinib.
Crizotinib is an orally available 1st generation ATP-competitive dual inhibitor of ALK and MET. Crizotinib has clinical activity in ALK translocation positive tumors and was approved for treating ALK positive nonsmall cell lung cancer [24,25]. Several additional ALK inhibitors are being tested in clinical trials, and of these, two with improved CNS penetration have been approved (ceritinib and alectinib). The role of crizotinib is currently being evaluated in several tumor types all of which have been demonstrated to express ALK, c-MET or ROS, all targets of crizotinib (ACsé trial: NCT02034981).
Generally after first recurrence of GB and with salvage therapy, 6-month progression-free survival varies from 17.5 to 52% and overall survival (OS) varies from 6 to 10 months. Survival postbevacizumab progression is very limited with an OS of 3 4.6 months regardless of subsequent treatment [1,2]. Nonetheless our first patient clearly benefited from crizotinib notwithstanding several prior clinical and MRI progressions.
The first patient required a 4-month cessation of crizotinib due to toxicity. The limited progression during this 4-month period without treatment and on a stable dose of corticosteroids cannot be clearly explained. Possibly there was a prolonged response effect of ALK inhibition despite interruption of therapy. We hypothesize that crizotinib has not only a cytostatic action but also a cytolytic action. De facto crizotinib induce cellular cycle stand by and cell death.
The respective roles of ALK, ROS1 and c-MET in the response to crizotinib in GB is unclear. Similar to the current case report, a previous case documented a rapid and prolonged clinical and radiological improvement when treated with crizotinib. In this patient with a recurrent MET gene amplified GB the expression of ALK was not reported [26]. In our second case c-MET protein overexpression was detected and was associated with high amplification of the MET gene but there was no benefit observed when treated with crizotinib. Interestingly ALK was not expressed nor was there evidence of gene amplification or gain observed. There was no evidence of a mutation in the kinase domain of ROS1 in our second case.
The main difference between our two cases of glioblastoma likely was the expression of the ALK, that probably explains the two differing patterns of response to crizotinib. We hypothesize that MET expression and MET amplification are not sufficient alone to determine a response to crizotinib. The MET gene may not be an oncogenic driver in GB. Indeed, although MET is important in the promotion of gliomagenesis, isolated dysfunction of MET may not be sufficient without corresponding loss of the ALK gene. In our second case, the inhibition of MET had no effect possibly because other genes like AKT or ERK are constitutively activated.
In conclusion, these case studies suggest that patients with GB and ALK polysomy may derive clinically relevant benefit from novel targeted small molecular inhibitors such as crizotinib. The respective roles of ALK and c-MET genes as predictors of response to crizotinib in GB need to be defined.
Footnotes
Ethical conduct of research
According to French law, no IRB/Ethical Committee approval is required for the publication of case reports. Nonetheless permission was obtained from the patient and family and they were informed of the manuscript.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest
- 1.Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma – are we there yet? Neuro Oncol. 2013;15(1):4–27. doi: 10.1093/neuonc/nos273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Rhun E, Rhun EL, Taillibert S, Chamberlain MC. The future of high-grade glioma: where we are and where are we going. Surg. Neurol. Int. 2015;6(Suppl. 1):S9–S44. doi: 10.4103/2152-7806.151331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu W, Fu Y, Xu S, et al. c-Met expression is associated with time to recurrence in patients with glioblastoma multiforme. J. Clin. Neurosci. 2011;18(1):119–121. doi: 10.1016/j.jocn.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Wallace GC, 4th, Dixon-Mah YN, Vandergrift WA, 3rd, et al. Targeting oncogenic ALK and MET: a promising therapeutic strategy for glioblastoma. Metab. Brain Dis. 2013;28(3):355–366. doi: 10.1007/s11011-013-9401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mesaros EF, Ott GR, Dorsey BD. Anaplastic lymphoma kinase inhibitors as anticancer therapeutics: a patent review. Expert Opin. Ther. Pat. 2014;24(4):417–442. doi: 10.1517/13543776.2014.877890. [DOI] [PubMed] [Google Scholar]; • A review on the ALK inhibitors as therapeutic option.
- 6.Sennino B, Ishiguro-Oonuma T, Wei Y, et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov. 2012;2(3):270–287. doi: 10.1158/2159-8290.CD-11-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu KV, Chang JP, Parachoniak CA, et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell. 2012;22(1):21–35. doi: 10.1016/j.ccr.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Belle E, Witzenbichler B, Chen D, et al. Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor: the case for paracrine amplification of angiogenesis. Circulation. 1998;97(4):381–390. doi: 10.1161/01.cir.97.4.381. [DOI] [PubMed] [Google Scholar]
- 9.Grzelinski M, Steinberg F, Martens T, Czubayko F, Lamszus K, Aigner A. Enhanced antitumorigenic effects in glioblastoma on double targeting of pleiotrophin and its receptor ALK. Neoplasia. 2009;11(2):145–156. doi: 10.1593/neo.81040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong D-S, Song S-Y, Kim D-H, et al. Prognostic significance of c-Met expression in glioblastomas. Cancer. 2009;115(1):140–148. doi: 10.1002/cncr.23972. [DOI] [PubMed] [Google Scholar]; • An evaluation of the expression of c-MET protein in a cohort of 62 glioblastomas.
- 11.Hirose Y, Kojima M, Sagoh M, et al. Immunohistochemical examination of c-Met protein expression in astrocytic tumors. Acta Neuropathol. (Berl.) 1998;95(4):345–351. doi: 10.1007/s004010050809. [DOI] [PubMed] [Google Scholar]
- 12.Nabeshima K, Shimao Y, Sato S, et al. Expression of c-Met correlates with grade of malignancy in human astrocytic tumours: an immunohistochemical study. Histopathology. 1997;31(5):436–443. doi: 10.1046/j.1365-2559.1997.3010889.x. [DOI] [PubMed] [Google Scholar]
- 13.Chi AS, Batchelor TT, Dias-Santagata D, et al. Prospective, high-throughput molecular profiling of human gliomas. J. Neurooncol. 2012;110(1):89–98. doi: 10.1007/s11060-012-0938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smolen GA, Sordella R, Muir B, et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc. Natl Acad. Sci. USA. 2006;103(7):2316–2321. doi: 10.1073/pnas.0508776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierscianek D, Kim Y-H, Motomura K, et al. MET gain in diffuse astrocytomas is associated with poorer outcome. Brain Pathol. Zurich Switz. 2013;23(1):13–18. doi: 10.1111/j.1750-3639.2012.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; • An evaluation of the role of the gain of MET in a cohort of 264 gliomas.
- 16.Koochekpour S, Jeffers M, Rulong S, et al. Met and hepatocyte growth factor/scatter factor expression in human gliomas. Cancer Res. 1997;57(23):5391–5398. [PubMed] [Google Scholar]
- 17.Arrieta O, Garcia E, Guevara P, et al. Hepatocyte growth factor is associated with poor prognosis of malignant gliomas and is a predictor for recurrence of meningioma. Cancer. 2002;94(12):3210–3218. doi: 10.1002/cncr.10594. [DOI] [PubMed] [Google Scholar]
- 18.Lal B, Xia S, Abounader R, Laterra J. Targeting the c-Met pathway potentiates glioblastoma responses to gamma-radiation. Clin. Cancer Res. 2005;11(12):4479–4486. doi: 10.1158/1078-0432.CCR-05-0166. [DOI] [PubMed] [Google Scholar]
- 19.Stylianou DC, Auf der Maur A, Kodack DP, et al. Effect of single-chain antibody targeting of the ligand-binding domain in the anaplastic lymphoma kinase receptor. Oncogene. 2009;28(37):3296–3306. doi: 10.1038/onc.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kulig K, McLendon RE, Locke SC, et al. MET and ALK in glioblastoma multiforme (GBM): Comparison of IHC and FISH. J. Clin. Oncol. 2012;30(Suppl.) Abstract 2021. [Google Scholar]
- 21.Wellstein A. ALK receptor activation, ligands and therapeutic targeting in glioblastoma and in other cancers. Front. Oncol. 2012;2:192. doi: 10.3389/fonc.2012.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charest A, Wilker EW, McLaughlin ME, et al. ROS fusion tyrosine kinase activates a SH2 domain-containing phosphatase-2/phosphatidylinositol 3-kinase/mammalian target of rapamycin signaling axis to form glioblastoma in mice. Cancer Res. 2006;66(15):7473–7481. doi: 10.1158/0008-5472.CAN-06-1193. [DOI] [PubMed] [Google Scholar]
- 23.Davare MA, Saborowski A, Eide CA, et al. Foretinib is a potent inhibitor of oncogenic ROS1 fusion proteins. Proc. Natl Acad. Sci. USA. 2013;110(48):19519–19524. doi: 10.1073/pnas.1319583110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDermott U, Iafrate AJ, Gray NS, et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res. 2008;68(9):3389–3395. doi: 10.1158/0008-5472.CAN-07-6186. [DOI] [PubMed] [Google Scholar]
- 25.Christensen JG, Zou HY, Arango ME, et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol. Cancer Ther. 2007;6(12 Pt 1):3314–3322. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- 26.Chi AS, Batchelor TT, Kwak EL, et al. Rapid radiographic and clinical improvement after treatment of a MET-amplified recurrent glioblastoma with a mesenchymal–epithelial transition inhibitor. J. Clin. Oncol. 2012;30(3):e30–e33. doi: 10.1200/JCO.2011.38.4586. [DOI] [PubMed] [Google Scholar]; • The first case report with a prolonged response to crizotinib.