Abstract
Hemangioblastoma is a rare benign neoplasm, accounting for less than 2% of all primitive brain tumors. It may arise sporadically in a solitary form, or associated with Von Hippel–Lindau (VHL) disease with multiple tumors. Surgery is the mainstay treatment, but management is challenging in case of recurrent and/or multiple tumors. VHL protein is defective in both forms of hemangioblastoma, leading to the accumulation of hypoxia-inducible factor, stimulating angiogenesis via VEGF and PDGF mainly. Here, we report a 37-year-old woman's case with recurrent and rapidly progressive VHL-associated hemangioblastomas, causing severe disability. She was treated 24 months with pazopanib, a multityrosine kinase inhibitor (TKI) targeting VEGF and PDGF-β pathways. Despite moderate radiological changes, progressive improvement in her clinical condition persisting over 3 years was observed. Inhibiting angiogenesis is a therapeutic option that may improve the quality of life and the autonomy of VHL patients disabled with multiple hemangioblastomas.
KEYWORDS : hemangioblastoma, pazopanib, Von Hippel–Lindau disease
PRACTICE POINTS.
Diagnosis of Von Hippel–Lindau (VHL) disease is often based on clinical criteria. Patients with a family history, and either CNS hemangioblastoma, pheochromocytoma or clear cell renal carcinoma allows to establish the diagnosis. Our patient had previous familial history of VHL syndrome, and present with multiple CNS hemangioblastomas.
In case of absence of family history, patient must have two or more CNS hemangioblastomas, or one CNS hemangioblastoma and one visceral tumor to meet the diagnostic criteria.
CNS Hemangioblastoma is a benign tumor which may be sporadic or associated with VHL disease.
Dysfunctional VHL tumor suppressor protein leads to accumulation of the transcription factor HIF which in turn up regulates VEGF and PDGF.
Pazopanib is a multityrosine kinase inhibitor targeting VEGF and PDGF among others.
Pazopanib seems to exert antitumor effects on tumors holding VHL defective cells such as renal cell carcinoma, soft tissue sarcoma and hemangioblastoma.
Presentation of case: setting & patient details/history initial diagnosis/assessment
We report the case of a 37-year-old female for whom the VHL diagnosis was suspected in the context of familial history. No genetic testing was performed to confirm VHL diagnosis as the patient did not wish to. First clinical symptoms appeared in 1997, with headache and neck pain. The work-up revealed a tumor extending in the right cerebellum. A complete recovery was achieved after the removal of the tumor, and pathological evaluation confirmed the diagnosis of hemangioblastoma.
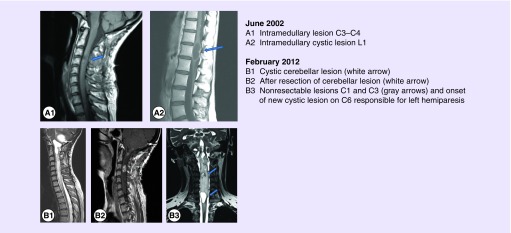
The patient was free of symptoms until 2002 when she developed proportional right hemiparesis with Brown Sequard syndrome. The spinal MRI showed several lesions of the spinal cord at C3–C4, C6–C7 (Figure 1A1) and L1 (Figure 1A2). All hemangioblastomas were partially removed, allowing the progressive recovery of neurological function.
Figure 1. . Illustrative examples of the hemangioblastomas arising from 2002 to 2012.
After a 10-year period with indolent slow progression of residual tumors, the patient presented in early 2012 with the rapid recurrence of intracranial hypertension. Brain MRI (February 2012) showed the cystic progression of a known hemangioblastoma in the inferolateral right cerebellum (Figure 1B1). This lesion was partially removed (Figure 1B2).
Rapidly after this procedure, patient had a novel neurological deterioration with the onset of bilateral paresis predominantly on the left side. These symptoms were explained by a rapid increase in size of multiple lesions, notably in the cerebellum, at C3 and C6–C7 level (Figure 1B3). In June 2012, the C6–C7 cystic lesion was completely removed but the lesion in C3 was not resectable. The C3 lesion was classified as unresectable because of the size and location. The expected postsurgical deficits were too significant. In fact even if the major vascularization comes from the anterior spinal artery, the embolization is not possible due to numerous perforant vessels.
The evolution of the disease went quite rapidly with numerous disseminated nodules. Postoperative course was complicated with severe motor deficits with a grade B tetraplegia according to the American Spinal Cord Injury classification (ASIA) predominant in the left side.
Pathology description
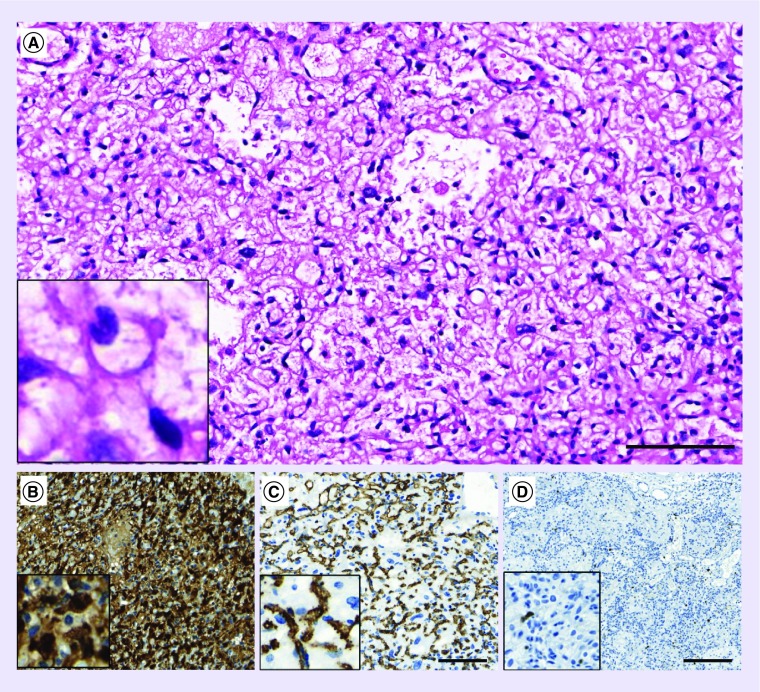
Both tumor samples (2002 and 2012) showed histological and phenotypic features of a grade I hemangioblastoma, with polygonal stromal cells frequently harboring vacuolated cytoplasma and of a rich network of capillary structures (Figure 2A). Immunostaining for S100 marked stroma cells (Figure 2B), whereas immunostaining for CD34 confirmed the presence of a dense capillary network (Figure 2C). Proliferation index (Ki67) was low (around 1%, Figure 2D).
Figure 2. . Pathology description.
Hematoxylin and Eosin (H/E) staining (A) or immunostaining (B–D) were performed on formalin-fixed and paraffin-embedded histological sections.
(A) Tumor proliferation is characterized by stromal cells situated in a dense capillary network. (B) Immunostaining demonstrates strong and diffuse S100 positivity in stroma cells. (C) The presence of a dense capillary network is illustrated by the endothelial marker CD34 staining. (D) Only few proliferating cells are detected by immunostaining for Ki67. Scale bars for A–C: 100 μm, for D: 200 μm.
Treatment & outcome
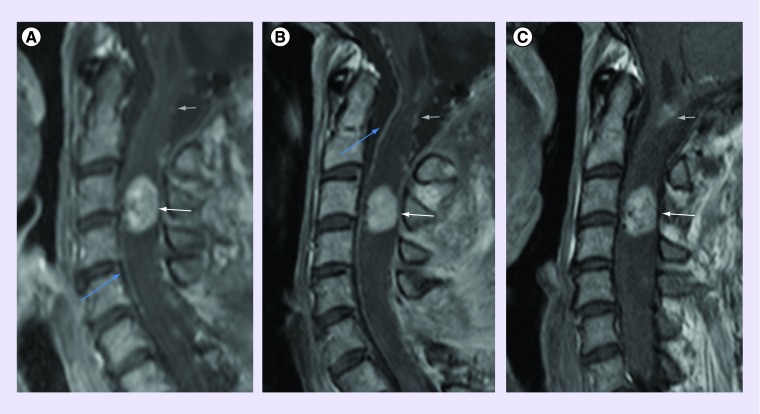
Considering the clinical degradation with multiple rapidly evolving lesions and the absence of surgical options, pazopanib was introduced in June 2012. Patient received daily 800 mg orally on 28 day cycles. Steroids were progressively tapered down and ultimately stopped. Pazopanib was overall well tolerated with typical toxicities such as grade 2 partial alopecia, hair depigmentation and grade I diarrhea that were well accepted by the patient. Five months after pazopanib initiation, first signs of improvement in motricity were observed in the right hand and foot, leading to the uninterrupted administration of pazopanib for 23 months. Along this period, the patient noted a complete recovery on the right side, and a slow but continuous partial recovery on the left side. After 16 months, she was able to walk alone for 50 m, with a complete autonomy for personal care and duties at home. A complete radiological work-up (brain and spinal cord MRI) performed in April 2014 did not show significant changes in the size of the target lesions (despite some slight changes in contrast enhancement) and did not reveal any new lesion. No other neurological deficit occurred meanwhile and the clinical condition is now stable 1 year after pazopanib interruption. In this specific case, the outcome before pazopanib was characterized by a continuous multifocal progression for approximately 1 year. The introduction of pazopanib had an immediate effect on tumor growth. This strongly suggests a cytotoxic effect and not only a staggering growth pattern. In addition, although no significant shrinkage of the lesion was observed (RANO criteria), we noticed a reshaping of the lesion (Figure 3).
Figure 3. . C3-C4 target lesion evolution under therapy.
Intramedullary contrast-enhancing lesion at the level of C3–C4 (white arrow) with associated myelopathy (A), February 2012 baseline MRI (sag T1Gd), which remains stable at follow-up (9 months later (B) and 15 months later (C)). Note the presence of the other known lesion at the level of C1 (gray arrow), the postoperative changes at the level of C6 (only partially displayed), and the anterior spinal artery (blue arrow).
Discussion & conclusion
VHL disease is an inherited syndrome characterized by germline mutations or deletions of the VHL gene, leading to a protean clinical pattern with different benign and malignant tumors, such as renal cell carcinoma (RCC), retinal hemangioblastoma, pheochromocytoma, CNS hemangioblastoma and rare benign tumors of the pancreas, middle ear or epididymis. VHL gene codes for a tumor suppressor protein, and individuals with this syndrome are born with one defective allele, while the second allele should be damaged in somatic cells by mutation, deletion or silencing to induce tumor growth.
The role of VHL in the regulation of key cellular processes and angiogenesis is well established [1,2]. The molecular consequences of defective VHL gene in both alleles are therefore tremendous and cannot be the scope of this case report. Briefly, VHL targets oxygen-sensitive alpha subunits of the hypoxia inducible factor (HIF) for HIF-1a isoform, HIF-2a (which both can trigger angiogenic mechanisms [3]) and HIF-3a. A defective VHL protein (similarly as hypoxic conditions) is not able any more to promote proteasome degradation of HIF-1α, which accumulates and binds HIF-1β in the cytoplasm. Then, the HIF-1α/HIF-1β heterodimer translocates to the nucleus, playing a critical role as a transcription factor. This ultimately induces the production of several growth factors such as VEGF, PDGF-β and TGF-α, promoting both tumor cell growth and neoangiogenesis.
The latter observations and report of a previous response to pazopanib [4] provided a strong rationale for targeting these growth factors signaling pathways and/or angiogenesis in VHL-associated hemangioblastomas not manageable by surgery [5], as in RCC [6]. However, reported results using EGFR tyrosine kinase inhibitors [7], antiangiogenic agents [8,9] and thalidomide [10] were rather disappointing. A recent review on the role of several molecules (e.g., semaxanib, vatalanib, sunitinib and bevacizumab) for the treatment of VHL-associated hemangioblastoma did not identify a molecule with reproducible benefit, although some modest clinical impact was observed in individual cases [11].
Pazopanib is a potent multityrosine kinase inhibitor targeting, among others, VEGF receptor, PDGF receptor, FGF receptor and cytokine receptor (c-Kit). This compound has been approved for the first-line treatment of metastatic renal cell carcinoma [12–14] and for the second-line treatment of soft tissue sarcoma [15]. More recently, a comparative study between pazopanib with sunitinib for the treatment of RCC favored pazopanib in terms of safety and quality-of-life profiles with similar progression-free and overall survivals [16].
Such efficacy and safety characteristics in RCC harboring same molecular abnormalities as CNS hemangioblastoma were the rational for choosing pazopanib for the patient reported here. The safety profile was indeed compatible with long-term administration. Although minimal radiological changes were noted on targeted tumors, no other lesion was appearing in a 3-year period. More importantly, the clinical benefit was impressive, in the context of a rapidly growing multifocal tumor before the introduction of pazopanib. Similar observations have been reported by others for one patient with VHL-associated CNS hemangioblastoma treated with the same pazopanib schedule (800 mg/day) [4].
Overall, the major clinical benefit reported here and by others [4] for two patients with severe and progressive disability due to VHL-associated CNS hemangioblastoma are supporting the further investigation of pazopanib as a high-value palliative care in this devastating disease.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Li L, Kaelin WG., Jr New insights into the biology of renal cell carcinoma. Hematol. Oncol. Clin. North Am. 2011;25(4):667–686. doi: 10.1016/j.hoc.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Focuses primarily on the most common form of RCC, clear-cell renal carcinoma, noting some recent advances in the other histologic subtypes.
- 2.Kaelin WG., Jr New cancer targets emerging from studies of the Von Hippel–Lindau tumor suppressor protein. Ann. NY Acad. Sci. 2010;1210:1–7. doi: 10.1111/j.1749-6632.2010.05781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Highlights the various targetable pathways in VHL disease.
- 3.Flamme I, Krieg M, Plate KH. Up-regulation of vascular endothelial growth factor in stromal cells of hemangioblastomas is correlated with up-regulation of the transcription factor HRF/HIF-2alpha. Am. J. Pathol. 1998;153(1):25–29. doi: 10.1016/s0002-9440(10)65541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim BY, Jonasch E, Mccutcheon IE. Pazopanib therapy for cerebellar hemangioblastomas in von Hippel–Lindau disease: case report. Target Oncol. 2012;7(2):145–149. doi: 10.1007/s11523-012-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Report of oral pazopanib resulting in significant neurologic improvement and radiologic tumor volume reduction in CNS hemangioblastomas associated with VHL disease.
- 5.Lonser RR, Glenn GM, Walther M, et al. Von Hippel–Lindau disease. Lancet. 2003;361(9374):2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]; • This article provides an overview of the clinical aspects, management and treatment options for von Hippel–Lindau disease.
- 6.Heng DY, Bukowski RM. Anti-angiogenic targets in the treatment of advanced renal cell carcinoma. Curr. Cancer Drug Targets. 2008;8(8):676–682. doi: 10.2174/156800908786733450. [DOI] [PubMed] [Google Scholar]
- 7.Rogers LR, Lorusso P, Nadler P, et al. Erlotinib therapy for central nervous system hemangioblastomatosis associated with von Hippel–Lindau disease: a case report. J. Neurooncol. 2011;101(2):307–310. doi: 10.1007/s11060-010-0244-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omar AI. Bevacizumab for the treatment of surgically unresectable cervical cord hemangioblastoma: a case report. J. Med. Case Rep. 2012;6(1):238. doi: 10.1186/1752-1947-6-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aiello LP, George DJ, Cahill MT, et al. Rapid and durable recovery of visual function in a patient with von Hippel–Lindau syndrome after systemic therapy with vascular endothelial growth factor receptor inhibitor su5416. Ophthalmology. 2002;109(9):1745–1751. doi: 10.1016/s0161-6420(02)01159-4. [DOI] [PubMed] [Google Scholar]
- 10.Sardi I, Sanzo M, Giordano F, et al. Monotherapy with thalidomide for treatment of spinal cord hemangioblastomas in a patient with von Hippel–Lindau disease. Pediatr. Blood Cancer. 2009;53(3):464–467. doi: 10.1002/pbc.22065. [DOI] [PubMed] [Google Scholar]
- 11.Capitanio JF, Mazza E, Motta M, et al. Mechanisms, indications and results of salvage systemic therapy for sporadic and von Hippel–Lindau related hemangioblastomas of the central nervous system. Crit. Rev. Oncol. Hematol. 2013;86(1):69–84. doi: 10.1016/j.critrevonc.2012.10.001. [DOI] [PubMed] [Google Scholar]; •• A comprehensive report on multitarget tyrosine kinase inhibitors investigated in sporadic- and VHL-associated hemangioblastomas.
- 12.Zagzag D, Krishnamachary B, Yee H, et al. Stromal cell-derived factor-1alpha and CXCRexpression in hemangioblastoma and clear cell-renal cell carcinoma: von Hippel–Lindau loss-of-function induces expression of a ligand and its receptor. Cancer Res. 2005;65(14):6178–6188. doi: 10.1158/0008-5472.CAN-04-4406. [DOI] [PubMed] [Google Scholar]
- 13.Sternberg CN, Hawkins RE, Wagstaff J, et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. Eur. J. Cancer. 2013;49(6):1287–1296. doi: 10.1016/j.ejca.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a Phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043) J. Clin. Oncol. 2009;27(19):3126–3132. doi: 10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]; • EORTC Phase II trial showing inocuity and efficacy of pazopanib in relapsed, advanced STS.
- 15.Van Der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled Phase 3 trial. Lancet. 2012;379(9829):1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]; •• EORTC Phase III trial showing efficacy of pazopanib for patients with metastatic nonadipocytic soft-tissue sarcoma after previous chemotherapy.
- 16.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N. Engl. J. Med. 2013;369(8):722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]; •• Phase III randomized trial showing pazopanib and sunitinib has similar efficacy, with safety and quality-of-life profiles favoring pazopanib.