Abstract
Background
Posttraumatic Stress Disorder (PTSD) is associated with a number of negative physical and mental health consequences. Fear conditioning plays an important mechanistic role in PTSD, and PTSD patients also show deficits in safety signal learning. Sleep, particularly REM sleep, is linked to improved safety learning and extinction processes in animal models and healthy humans. No studies have examined the link between REM sleep and safety signal learning or extinction memory in clinical populations.
Methods
This study examined the relationship between REM sleep, safety signal learning, and extinction processes in veterans with PTSD (n = 13). Patients' overnight sleep was characterized in the laboratory via polysomnography (PSG). The next day, participants underwent a fear conditioning paradigm during which they acquired fear toward a visual cue. This testing session also included a visual cue that became a safety signal (CS-). Following conditioning, the veterans' sleep was monitored overnight again, after which they underwent extinction training. Following a third night of sleep, extinction recall and safety recall were tested. Bivariate correlations examined the relationship between the slope of safety signal learning and subsequent REM sleep, as well as the relationship between REM sleep and subsequent extinction recall and safety recall on the last day of testing.
Results
Veterans learned to differentiate the CS+ and the CS- on the first day of testing. Veterans who underwent safety learning more quickly on the first day of testing showed more efficient REM sleep that night (r = .607, p = .028). On the second day of testing, the patients successfully underwent extinction learning. Patients with a higher percentage of REM sleep on the last night of the study showed more safety recall early on the last day of testing (r = .688, p = .009).
Conclusion
To our knowledge, this was the first study to examine the relationship between objective sleep and fear-potentiated startle performance in veterans with PTSD. Study methods were well tolerated by participants, supporting feasibility of the experimental design. Results indicated REM sleep was associated with both initial safety learning and subsequent safety recall. Taken together with previous studies in healthy controls, these preliminary results provide additional evidence suggesting REM sleep could play a mechanistic role in the maintenance of PTSD and thus identify a modifiable biological process to target in treatment of PTSD. These findings should be replicated in larger samples.
Keywords: Posttraumatic stress disorder, Sleep, Fear conditioning, Safety learning
1. Introduction
Approximately 5.2 million adults suffer from posttraumatic stress disorder (PTSD) in the United States in a given year (Kessler et al., 2005). PTSD is associated with a number of negative physical (Boscarino, 2008) and mental health (Kilpatrick et al., 2003) consequences. Over the past few decades, researchers have developed evidence-based pharmacological and psychosocial interventions for PTSD (Foa et al., 2008). Despite these advances, not all PTSD patients respond, or respond fully, to treatment (Schottenbauer et al., 2008). Understanding the physiological processes involved in the development and maintenance of PTSD symptoms will aid in understanding this differential response to treatment. Additionally, a greater understanding of the mechanisms underlying treatment response will inform the development of more effective interventions.
One example of an important mechanism in PTSD is fear conditioning. Fear conditioning plays a crucial role in PTSD, as patients with PTSD retain conditioned fear to cues associated with trauma long after the traumatic event has passed (Milad et al., 2009; Wessa and Flor, 2007). Extinction of conditioned fear is necessary for PTSD symptom reduction, either through the natural course of recovery or in response to exposure-based therapies for PTSD (Foa et al., 2007). Additionally, it is critical for patients to retain extinction over time to remain free of PTSD symptoms long-term. A growing body of evidence also implicates impaired safety signal learning in PTSD. Patients with PTSD are often unable to distinguish threatening from safe environments, or have difficulty inhibiting the fear response even in the presence of safety signals (e.g., feeling anxious in response to hearing a helicopter even after having returned home from combat). Laboratory studies using fear conditioning paradigms have observed impairments in safety signal learning in PTSD (Acheson et al., 2015; Jovanovic et al., 2012). Importantly, impaired safety learning is hypothesized to contribute to hypervigilance symptoms of PTSD (Acheson et al., 2012), and successful safety learning is an additional mechanism in response to exposure-based treatments (Foa et al., 2007).
Recent research with healthy humans shows that sleep, particularly REM sleep, serves an important role in the acquisition and recall of extinction memories. The importance of REM sleep to fear inhibition learning may be critical for patients with PTSD, because some of the most ubiquitous and distressing symptoms of PTSD are insomnia and nightmares (Neylan et al., 1998). These sleep difficulties are associated with disruptions in sleep architecture, particularly in REM sleep (Germain, 2013). Previous studies using fear-potentiated startle (FPS) paradigms in healthy human control participants link REM sleep disruption to difficulties retaining extinction over time (Straus et al., 2017a), and other studies show associations between REM sleep consolidation and safety learning and retention (Marshall et al., 2014). Together, these studies strongly suggest a likely link between REM sleep and extinction memory and/or safety signal learning in PTSD. However, little is known about the association between sleep and extinction and/or safety signals in clinical populations, and no studies to date have examined the relationship between REM sleep and extinction processes or safety signal learning in patients with PTSD. Examination of this relationship in PTSD will demonstrate the feasibility of examining these parameters in a PTSD sample as well as add to the literature suggesting that sleep disturbance is a mechanism in the development and maintenance of PTSD.
This study examined the relationship between REM sleep, safety signal learning, extinction, and safety recall processes in military veterans with PTSD (n = 13). Patients' overnight sleep was characterized in the lab (Night 1). The next day, they underwent an FPS conditioning session that included learning of safety signals. Following this, their sleep was monitored overnight again (Night 2), after which they underwent an extinction learning session 24 h after fear conditioning. After a third night of sleep monitoring (Night 3), extinction recall and safety recall were tested. Analyses examined the relationship between safety signal learning and subsequent changes in REM sleep, as well as the relationship between REM sleep and extinction recall. We hypothesized: 1) greater safety signal learning would be associated with less disrupted REM sleep on Night 2, 2) greater REM sleep disruption on Night 3 would be associated with more impaired extinction recall on the final day of testing, and 3) REM sleep on Night 3 would be associated with better safety recall on the final day of testing. Thus, we planned to provide preliminary evidence that sleep disruption may play an important mechanistic role in daytime fear-related symptom severity in PTSD patients.
2. Methods
2.1. Participants
Veterans were recruited through the VA San Diego Healthcare System (VASDHS) to participate in the current study. The VA Internal Review Board as well as the University of California, San Diego's Human Research Protections Program approved all recruitment, consent and testing materials for this study. Compensation was provided for in-person eligibility screening ($10) as well as participation in the testing phase of the study (up to $290 for completing the baseline week and all three nights in the laboratory).
Inclusion criteria were: 1) Veteran with a primary diagnosis of PTSD; 2) 18–50 years old; and 3) literate in English. Exclusion criteria were: 1) A history of mania and/or psychosis; 2) substance use disorder during the prior 6 months; 3) untreated sleep disorder other than insomnia and nightmares; 4) change in type and/or dosage of psychotropic medication in preceding 2 months; 5) history of severe TBI (i.e., reported loss of consciousness for 6 h or more); 6) colorblindness; and 7) nonresponder to psychophysiological startle testing (see below).
Forty-four (44) phone screens were conducted on initial recruitment outreach, which yielded 26 baseline assessment visits. Of these, 6 participants (23.1%) were ineligible due to being non-responders on the startle threshold testing (see below). Five participants (19.2%) voluntarily withdrew from the study prior to the sleep lab phase. Therefore, 15 participants were enrolled and participated in at least one in-lab sleep study. Of these, one participant was ruled out after Night 1 due to untreated Obstructive Sleep Apnea, and one additional participant withdrew after Night 2, leaving 13 participants with complete data sets, which included 11 men and 2 women. See Table 1 for demographics of the final study sample.
Table 1.
Demographics of the study sample.
| Mean | SD | |
|---|---|---|
| Age | 31.88 | 7.67 |
| N | % | |
| Male | 11 | 84.6 |
| Female | 2 | 15.4 |
| Race | ||
| American Indian or Alaskan Native | 0 | 0.0 |
| Asian | 0 | 0.0 |
| Black or African American | 1 | 7.7 |
| Caucasian | 11 | 84.6 |
| Native Hawaiian or Other Pacific Islander | 1 | 7.7 |
| Ethnicity | ||
| Hispanic | 5 | 38.5 |
| Non-Hispanic | 8 | 61.5 |
| Military Branch | ||
| Army | 4 | 30.8 |
| Navy | 3 | 23.1 |
| Marine Corps | 6 | 46.2 |
| Air Force | 0 | 0.0 |
| Education | ||
| HS Grad | 0 | 0.0 |
| Some College | 11 | 84.6 |
| Completed Bachelor's Degree | 1 | 7.7 |
| Completed Master's Degree | 1 | 7.7 |
2.2. Procedures
Initial screening of participants took place via telephone. Those not excluded for obvious violations of eligibility received an in-person screen. The in-person screening appointment included a series of measures to confirm eligibility, including the Clinician Administered PTSD scale (CAPS) to establish current PTSD diagnosis and individual modules from the Structured Clinical Interview for DSM-IV (SCID-IV) to rule out exclusionary psychiatric diagnoses (see Measures below). Informed consent was signed at the beginning of this first in-person meeting. Participants also underwent a Startle Threshold Testing session, which consisted of presentation of 16 acoustic startle probes (in the absence of any visual cue) to examine baseline startle reactivity. Veterans who did not meet the criterion of responding to at least 75% (12 of 16) of the startle probes were deemed nonresponders and excluded from participation in the study. Those not excluded based on these measures underwent one week of baseline sleep assessment at home, during which they filled out Sleep Diaries and wore a wrist actigraph to characterize baseline sleep.
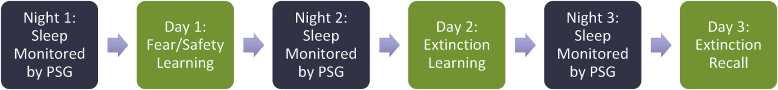
Testing Phase: After the baseline week, participants were brought to the laboratory, where they spent three consecutive nights with their sleep monitored by polysomnography. They also underwent a conditioning paradigm with established reliability in the literature (Acheson et al., 2013; Norrholm et al., 2006, 2011, 2013). See Fig. 1 for study timeline. Participants underwent an adaptation night (Night 1) to adjust to sleeping in the laboratory environment. This night was also used to screen for exclusionary sleep disorders (e.g., sleep apnea). Night 1 was followed by fear/safety acquisition (described below) on Day 1. After a second night in the laboratory (Night 2), participants underwent extinction learning on Day 2. Participants then spent a third night of sleep in the laboratory (Night 3), followed by extinction recall testing on Day 3. See Fig. 2 for a diagram showing the FPS testing procedures for each session.
Fig. 1.
Timeline of testing phase.
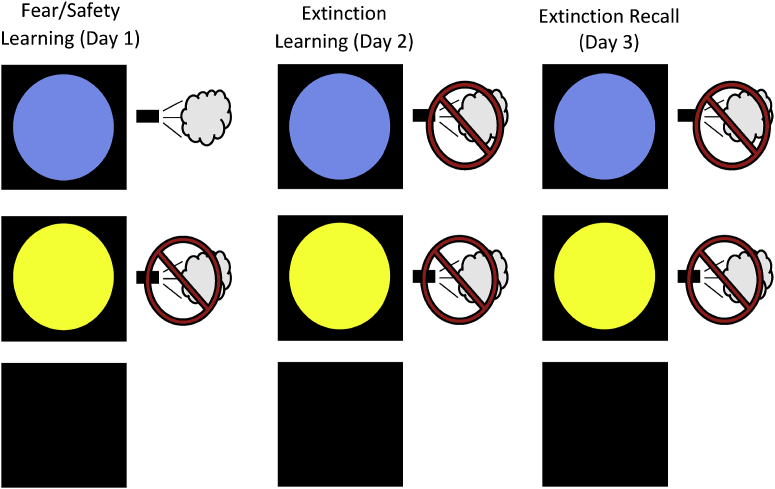
Fig. 2.
Fear Potentiated Startle Testing Procedures. Day 1 of testing (Fear/Safety Learning) included 8 presentations of the CS+ (blue circle), paired with an air puff to the throat 75% of the time. Day 1 also included 8 presentations of the CS- without being paired with the air puff, and 8 Noise Alone (NA) trials. Day 2 (Extinction Learning) included 16 presentations of the CS+, 16 presentations of the CS-, and 16 NA trials. No air puffs were administered during this session. Day 3 (Extinction Recall) included 8 CS+, 8 CS-, and 8 NA trials. As on the day before, no air puffs were administered. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Testing sessions were conducted 1 h following participants' habitual wake time. Participants' habitual wake times ranged from 5am to 8am and thus testing sessions ranged from 6am to 9am. For all sessions, participants were seated in a lounge chair in a sound-attenuated room. Visual cues were presented via LCD monitor. Sensors were fitted to measure blink reflex, which was used as the operational measure of the conditioned fear response, safety learning, extinction, and extinction recall.
Fear/Safety Acquisition: During this phase, participants underwent a fear potentiated startle (FPS) procedure to acquire conditioned fear to a visual cue (the conditioned stimulus, or CS+; (Acheson et al., 2013; Acheson et al., 2015; Glenn et al., 2017; Norrholm et al., 2011; Norrholm et al., 2013; Norrholm et al., 2006). We conducted FPS acquisition and extinction as previously described (Acheson et al., 2013). In brief, the unconditioned stimulus (US) was a 500 ms puff of air delivered to the throat. FPS acquisition began with an acclimation period during which 70 db of broad band background noise, followed by six 108 db, 40 ms acoustic startle probes. Following the acclimation period, 3 trial types were presented: 2 conditioned stimulus (CS) trials and one no-stimulus trial. For each CS trial, 1 of 2 possible shapes were presented on the LCD monitor, for 6 s. Between 4 and 5 s after the onset of the shape, a startle probe was delivered to assess fear conditioning. One of these shapes (a blue circle, serving as CS+) was paired with an air puff to the throat 75% of the time. The second shape (yellow circle) was never paired with the air puff and thus served as the safety signal (CS-). Overall, the FPS acquisition phase consisted of 24 trials, including 8 CS + trials, 8 CS- trials, and 8 Noise Alone (NA) trials during which the startle probe was presented in the absence of any visual cue. Stimulus presentation was block randomized with the constraint of two trials of each type (CS+, CS−, and NA) per block. FPS was measured by eyeblink magnitude in response to the acoustic stimuli presented in the presence/absence of the CS+.
Extinction Learning: After spending a second night in the laboratory and 24 h after Fear/Safety Acquisition, participants underwent a standard extinction procedure that consisted of 72 trials (16 CS+, 16 CS-, 16 NA). Visual cues and startle probes were presented as in the Fear/Safety Acquisition phase, but no air puffs were delivered. The CS+ was presented during this testing session repeatedly without the air puff. Participants therefore had the opportunity to “un-learn” the association between the CS+ the US (i.e., the conditioned response was extinguished).
Extinction/Safety Recall: This phase was conducted 24 h after the Extinction learning session, after Night 3 (see Fig. 1). It consisted of 24 trials (8 CS+, 8 CS-, 8 NA). During this session, both the CS+ and CS- were presented repeatedly, and no cues were paired with the air puff.
2.3. Measures
Clinician Administered PTSD Scale, DSM-5 version (CAPS-5): The CAPS is a semistructured interview corresponding to DSM-5 criteria for PTSD (Weathers et al., 2015). It was recently adapted to correspond to DSM-5 symptoms of PTSD and is widely considered to be the gold standard PTSD assessment (Weathers et al., 2001). For this study, the CAPS was the primary method used to establish PTSD diagnosis.
Structured Clinical Interview for DSM-IV (SCID): The SCID is a clinician-administered diagnostic interview based on DSM-IV criteria (First et al., 2012). For this study, individual modules of the SCID were used to rule out exclusionary Axis I disorders, including psychotic symptoms, manic symptoms, and substance abuse/dependence during the prior 6 months (see above).
In addition to the measures above, participants also completed several self-report questionnaires to characterize clinical symptoms, including the Patient Health Questionnaire, 9-item version (PHQ-9; Kroenke et al., 2001), the PTSD Checklist, stressor-specific version (PCL-S; Weathers et al., 1993), the Insomnia Severity Index (ISI; Batien, Vallières and Morin, 2001), the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989) and Addendum for PTSD (PSQI-A; Germain et al., 2005). Participants also completed a standard interview regarding recent medication use. When this was unclear, the PI verified this information using the patient's medical record.
2.3.1. Sleep assessments
Sleep Diaries: The sleep diary included typical subjective measures (such as bed time, wake time, sleep latency, number and duration of awakenings, total time in bed) and two calculated variables (total sleep time and sleep efficiency). Frequency and intensity of nightmares were also assessed. Daily sleep diaries are commonly used in sleep research (Carney et al., 2012), and we have used this one in several studies. These data were not used for investigation of the main study aims, but instead were used to further characterize baseline sleep in this sample.
Actigraphy: Actigraphy is the most commonly used validated objective sleep assessment outside of a laboratory context (Ancoli-Israel et al., 2003). Respironics Actiwatch 2 and Actiware software were used to calculate total sleep time, wake after sleep onset, and sleep efficiency. As with the Sleep Diaries, actigraphy data were not used for investigation of main study aims, but were used to provide objective estimates of participants' sleep continuity from the baseline week.
Polysomnography (PSG): During the Testing Phase of the study (see above), participants' sleep was monitored with a standard overnight polysomnogram, including EEG, EOG, and EMG. On Night 1, additional monitors screened for sleep apnea and periodic leg movements. Sleep recordings were scored for sleep stages (i.e. N1, N2, N3, REM) by a single scorer and according to standardized procedures (Iber, 2007). Our three REM sleep variables (See Data Analysis below) were determined by this scoring.
2.3.2. Psychophysiological assessments
Blink Reflex was measured during Fear/Safety Acquisition, Extinction Learning, and Extinction Recall via two small EMG cup electrodes (Ag/AgCl) placed below and lateral to the left eye over the orbicularis oculi muscle, referenced to an electrode placed over the left mastoid. EMG data were recorded at a sampling rate of 1 kHz, amplified (0.5 mV electrode input was amplified to 2500 mV signal output), band-pass filtered (100–1000 Hz), rectified, and then smoothed with a 5-point rolling average. EMG files were visually inspected and startle responses were differentiated from voluntary blinks by ensuring that onset of the startle peak occurred within 30–70 ms after the startle probe.
2.4. Analytic plan
Descriptive statistics were used to characterize demographics and clinical symptoms (i.e., CAPS, PCL-S, PHQ-9). Descriptive statistics were also used for the Sleep Diary, Actigraphy, and PSG to characterize sleep.
Prior to data analysis, physiological data were scored according to our research group's previously-established procedures (see Straus et al., 2017a). CS+ and CS- trials were averaged within each session into blocks of two trials each. NA trials within a session were averaged to acquire a baseline startle response. This baseline was then subtracted from the respective CS+ and CS- block within each session, creating scores representing potentiated startle above baseline for each CS type within each block. Thus, there were four blocks for the CS+ and CS- during the acquisition session; eight blocks for the CS+ and CS- for the extinction session; and four blocks for the CS+ and CS- during the recall session. Participants who did not complete all components of the testing phase of the study were dropped from final analyses.
Fear Conditioning, Safety Learning, and Extinction Data Analysis: The Day 1 Fear/Safety Acquisition session was analyzed by using a priori comparisons based on previous studies using the same paradigm (e.g., Straus et al., 2017a), which served as validity checks confirming the FPS paradigm worked as intended. Paired samples t-tests to examine the response to the CS+ and CS- at the beginning of the testing session (Block 1) relative to the end of the session (Block 4). Then, paired samples t-tests were used to compare the response to the CS + to the CS- at each block. It was hypothesized that response would not differ by cue early in the testing session, but response to the CS + would be greater than the CS- at the end of the session due to participants distinguishing the threat cue versus safety cue. As in our previous work (Straus et al., 2017a), for the extinction learning session on Day 2, Blocks 1–2, Blocks 3–4, Blocks 5–6, and Blocks 7–8 were averaged together to create variables representing early, early-middle, late-middle, and late extinction learning. Then, paired samples t-tests were used to examine response to the CS+ and CS- at the beginning of the testing session relative to the end of the session. Finally, paired samples t-tests were used to compare the response to the CS + to the CS- at each of the time points (early, early-middle, late-middle, and late). It was hypothesized that participants would distinguish the cues early in the testing session due to the learning the previous day, but that response to both cues would be attenuated late in the testing session after multiple presentations of both cues without the air puff. Parallel paired samples t-tests tests were performed to examine participants' responses on the recall session on Day 3. It was hypothesized that participants would not respond differently by cue at the beginning of this session due to extinction learning the previous day.
REM Sleep and FPS Analyses: REM sleep was measured based on three variables: 1) REM Percent (i.e., REM sleep duration divided by total sleep duration), 2) REM Efficiency (i.e., REM minutes divided by the total duration of all REM periods), and 3) REM Latency (i.e., duration of non-REM sleep before the first REM onset). These variables were chosen a priori based on their use in previous studies (see Marshall et al., 2014; Straus et al., 2017a).
To examine the effect of safety learning on subsequent REM sleep, a difference score was created based on the blink response to the CS- above baseline on Block 1 of the Acquisition session in comparison to Block 2 (Block 1 – Block 2) to create a continuous variable representing the speed of safety learning, with large numbers indicating rapid safety learning. Bivariate correlation was then used to examine the relationship between this score and REM latency, REM efficiency, and REM percent on the subsequent night (Night 2).
To examine the relationship between REM sleep and extinction recall on the final morning of testing, an extinction recall index was computed comparing the CS + responses above baseline at recall with the maximal CS + responses above baseline during conditioning, based on other studies using this paradigm (e.g., Milad et al., 2009; Acheson et al., 2013). The following equation was used: 100 − 100(CS + response during the first block of recall/maximum CS + block across acquisition phase). For example, if the participant's EMG response to the CS+ was 300 after baseline subtraction at the first block of the extinction recall session, and reached 600 after baseline subtraction during acquisition, the extinction retention index would be 100–100 (300/600) = 50. Bivariate correlations were then used to explore associations between this index and REM efficiency, REM percent and REM latency on the previous night (Night 3).
To our knowledge, no previous studies have examined relationships between REM sleep and safety recall. Therefore, we conducted some additional exploratory analyses to investigate this in our sample. To examine the relationship between REM sleep and safety recall on the final morning of testing, a difference score was based on blink response to the CS- above baseline on Block 1 of the Recall session in comparison to Block 2 (Block 1 – Block 2). The result was a continuous variable with large numbers indicating rapid safety recall. Bivariate correlation was then used to examine the relationship between REM latency, REM efficiency, and REM percent on the previous night (Night 3) and this measure of the slope of safety recall at the subsequent Recall session.
3. Results
Clinical Symptoms: Table 2 shows a summary of clinical symptom data. Mean total CAPS was indicative of moderately severe PTSD symptoms (M = 42.85, SD = 12.22). On the PCL-S, the average score was above the clinically significant threshold that has been suggested for veteran populations at VA clinics (M = 55.68, SD = 16.29; Yeager, Magruder, Knapp, Nicholas, Frueh, 2008). The average score on the PHQ-9 was indicative of moderately severe depression (M = 14.31, SD = 6.94).
Table 2.
Clinical characteristics of the study sample.
| N | % | Cutoff Score | |
|---|---|---|---|
| Criterion A Trauma Type | |||
| Combat | 10 | 76.9 | – |
| Military Sexual Trauma | 2 | 15.4 | – |
| Other | 1 | 7.7 | – |
| Mean | SD | ||
| CAPS-5 | 42.85 | 12.22 | 25 |
| PCL-S | 55.78 | 16.29 | 50 |
| PHQ-9 | 14.31 | 6.94 | 5 |
| Insomnia Severity Index | 14.77 | 5.63 | 8 |
| PSQI Global Score | 11.86 | 3.74 | 5 |
| PSQI Addendum for PTSD | 10.77 | 4.59 | 4 |
| Sleep Diaries (baseline week) | |||
| Time in Bed (minutes) | 488.56 | 73.82 | – |
| Total Sleep Time (minutes) | 368.47 | 90.89 | 360 |
| Sleep Latency (minutes) | 42.06 | 25.47 | 30 |
| Wake After Sleep Onset (minutes) | 77.26 | 53.53 | 30 |
| Sleep Efficiency (%) | 74.79 | 12.10 | 85 |
| Total Number of Nightmares | 8.20 | 7.24 | – |
| Average Nightmare Intensity | 5.15 | 1.92 | – |
| Actigraphy (baseline week) | |||
| Total Sleep Time (minutes) | 361.54 | 46.29 | 360 |
| Wake After Sleep Onset (minutes) | 47.07 | 19.54 | 30 |
| Sleep Efficiency (%) | 75.62 | 9.90 | 30 |
| Polysomnography (average over 3 nights) | |||
| Total Sleep Time (minutes) | 388.03 | 54.27 | 360 |
| Sleep Latency (minutes) | 17.90 | 18.52 | 30 |
| Wake After Sleep Onset (minutes) | 28.51 | 26.56 | 30 |
| Sleep Efficiency (%) | 89.85 | 6.20 | 85 |
Sleep Summary Data: Table 2 shows a summary of the sleep measures. The average score on the ISI was suggestive of moderate insomnia (M = 14.77, SD = 5.63). On the PSQI and PSQI Addendum for PTSD, average scores were considerably above the threshold indicating clinically significant sleep disturbance (PSQI: M = 11.86, SD = 3.74; PSQI-A: M = 10.77, SD = 4.59). Sleep diaries and actigraphy data from the baseline week showed clinically significant insomnia symptoms, with a wide variability between participants, replicating previous studies showing high variability of sleep in PTSD patients (Straus et al., 2015). On in-lab PSG, Total Sleep Time averaged ∼6.5 h (SD = 54.27 min), and mean Sleep Efficiency was just under 90% (SD = 6.5).
3.1. Fear conditioning and extinction testing
Fear Acquisition: Paired samples t-tests revealed participants did not respond differently to the CS+ and the CS- during the first block (t = 0.04, p = .973), but by the last block they responded more anxiously to the CS + than they did to the CS- (t = 2.36, p = .034), suggesting they learned to differentiate between the “threat signal” and the “safety signal” (Fig. 3).
Fig. 3.
EMG startle potentiation compared to baseline, by testing session and stimulus type. Data points represent difference scores of the EMG startle response to the CS + or CS- with the average EMG response to Noise Alone trials subtracted out. Fear/Safety Learning took place on Day 1, Extinction Learning took place on Day 2, and Extinction Recall took place on Day 3.
Extinction Learning and Recall: Participants showed greater startle potentiation to the CS + than they did to the CS- during early extinction learning (t = 2.26, p = .042), though by late extinction learning there was no difference in response between the two cues (t = 1.24, p = .242). Hence by the end of the testing session, participants learned that the “dangerous” cue was now safe, and thus no longer discriminated between the two cues. Discrimination remained low during the recall test on Day 3 (CS + vs. CS- at block 1: t = 1.36, p = .198 and block 4 t = 0.57, p = .581), and CS + responding was significantly lower than CS + responding during cue recall on Day 2. Additionally, startle potentiation to the CS+ was significantly smaller on Block 1 of the recall session on Day 3 in comparison to Block 1 during the extinction learning session on Day 2 (t = 2.41, p = .03), supporting that extinction recall was successful.
3.2. Safety learning, extinction, and REM sleep analyses
Safety and Fear Acquisition and Subsequent REM Sleep: There was a significant relationship between speed of safety learning and subsequent REM efficiency on Night 2 (r = 0.607, p = .028). Speed of safety learning was not significantly associated with REM latency (r = 0.168, p = .583) or REM percent on Night 2 (r = 0.22, p = .474).
Bivariate correlations revealed no significant associations between REM efficiency on Night 3 (r = 0.17, p = .596), REM percent (r = 0.09, p = .793), nor REM Latency (r = −0.32, p = .318), and the extinction retention index.
With regard to safety recall, there was a significant relationship between REM percent on Night 3 and the slope of subsequent safety response (r = 0.69, p = .009). Neither REM latency (r = 0.168, p = .583) nor REM percent on Night 3 (r = 0.22, p = .474) was associated with the slope of safety recall.
4. Discussion
Though research in healthy humans has shown critical links between REM sleep and fear and safety learning, this was the first study to investigate this relationship in patients with PTSD. This small preliminary study (n = 13) conducted in veterans, investigated this relationship using in-lab sleep assessment and a classical conditioning FPS paradigm over a three-day period. Of note, only one participant voluntarily withdrew from the sleep lab portion of the study, which represents an attrition rate comparable to our previous studies in healthy controls (Straus et al., 2017a) and supports the feasibility of using this experimental design in patients with PTSD. Additionally, findings from this preliminary study parallel findings in healthy human controls. Recent studies in healthy human participants showed safety learning is related to REM sleep (Marshall et al., 2014; Menz et al., 2016), which then has implications for subsequently differentiating between threat and safety. Here, we replicate that basic finding in patients with PTSD, in that worse safety learning at the acquisition session on Day 1 was associated with less REM efficiency that night. Additionally, this study showed patients with PTSD who exhibited lower REM percent on Night 3 showed more subsequent difficulties with safety signal recall. Together, these findings represent the first translation of the healthy control data showing a link between safety signals and REM sleep into a PTSD sample and further validate the hypothesis that REM sleep disturbances may play a potential role in maintaining daytime PTSD symptoms (Straus et al., 2017b; Germain, 2013).
The associations between safety learning and REM sleep found in this study are understandable given the hypothesized links between sleep and the biological processes involving fear and safety learning. Fear conditioning involves activation of central stress responses, involving the limbic system (Phillips and LeDoux, 1992) as well as the noradrenergic system, which is, in turn, associated with disrupted REM sleep (Raskind et al., 2007). In the current study, less efficient safety signal learning was associated with less efficient REM sleep the following night, which supports the hypothesis that alterations in limbic activity and catecholamine levels is associated with both safety learning performance and fragmented REM sleep. In addition to activation of limbic and noradrenergic pathways, studies have also shown inhibition of fear, such as safety signal learning, involves activation of frontal brain regions such as the vmPFC to serve as a “top-down” process for modulating fear response (Jovanovic and Norrholm, 2011). Studies in healthy control participants show sleep disruption results in increased activation in the amygdala as well as decreased connectivity the frontal regions needed to modulate expression of that fear response (van der Helm et al., 2011; Nieuwenhuis and Takashima, 2011; Lerner et al., 2017). In this study, REM sleep disruption was associated with impaired ability to recall safety signal learning on the last day of testing. Though we did not measure these brain systems or neurotransmitters directly in this study, the results are in line with what would be expected given the neurophysiological links between sleep and the fear system (Spoormaker et al., 2010, 2012; Lerner et al., 2017). Direct causality cannot be confirmed due to the small study sample and associational design; however, these data support the notion sleep disruption could play a critical role in the development and/or maintenance of daytime PTSD symptoms.
Contrary to other studies in PTSD samples, participants in our study did not show deficits in extinction recall. Also, contrary to our hypotheses, no relationship was found between REM sleep consolidation and reactivity to the threat signal (CS+), either during the extinction learning session or during the extinction recall session the following day. Many studies show extinction recall deficits in PTSD compared to controls (e.g., Milad et al., 2008), and other prior work in animals (e.g., Fu et al., 2007; Datta & O'Malley, 2013) and healthy human control participants (Straus et al., 2017a; Spoormaker et al., 2010, Spoormaker et al., 2012) have shown fragmentation of REM sleep is associated with impaired recall of extinguished fear. These results were not duplicated in this study. There are a variety of possible explanations for these null results. Notably, this study did not compare PTSD patients to control participants, which limits the interpretation of normative versus impaired extinction processes. Additionally, studies in animals or healthy human control participants often directly manipulate sleep by use of total sleep deprivation (Straus et al., 2017a), or selective REM sleep deprivation (Spoormaker et al., 2012) and compare findings to a control group who did not undergo the sleep manipulation. This type of design allows for the ability to determine the causal effects of sleep disruption on fear and extinction parameters. This study did not directly manipulate REM sleep. Future studies could use a manipulation to either disrupt REM sleep (e.g., via REM deprivation) or consolidate REM sleep overnight (e.g., via administration of a pharmacological agent such as prazosin), and examine the relationship between REM sleep and extinction processes in PTSD patients. Perhaps the most important consideration for these null results, though, is the small size of this study. The question about the relationship between REM sleep and extinction processes in PTSD patients remains open. Larger studies may be needed to detect these relationships.
Though this study establishes important links between REM sleep disturbance and safety learning processes in patients with PTSD, there are a few limitations worth noting. First, this was a small exploratory study. Some analyses were planned and based on previous literature, while others were exploratory and meant to inform future studies. The small size and exploratory nature of this study resulted in limited power to examine relationships between sleep and daytime fear learning. This study did not find hypothesized links between REM sleep and extinction processes. As noted above, the small size of this study results in a lack of statistical power to detect effects. Future research should scale up and re-examine potential relationships between REM sleep and fear/extinction parameters. Second, this study only examined behavioral responses. Future studies should include neuroimaging or other biological sampling to examine hypothesized links between fear learning, neural networks, and neurotransmitters. Third, due to recruitment concerns and limitations regarding statistical power in this small sample, we did not comprehensively control for psychotropic use, psychiatric comorbidities (e.g., depression, panic symptoms), or medical comorbidities that may have affected sleep continuity during the in-lab portion of this study or performance during startle testing. Future studies could limit participation to medication-free, medically healthy participants, or control for medication use, psychiatric symptoms, and medical comorbidities for a more “pure” test of study hypotheses (although one should note that would also reduce generalizability of the findings). Despite these limitations, this study represents the first published study to examine the link between REM sleep and fear inhibition in patients with PTSD. Results extend previous research and add to the literature suggesting sleep disturbance interferes with safety learning, underscoring the argument sleep disruption is an important factor in the development and maintenance of daytime PTSD symptoms.
Acknowledgments and disclosures
Writing of this manuscript was supported by NIMH 1F31MH106209-01A1 (to LDS), the Veterans Affairs Center of Excellence for Stress and Mental health, and the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs.
We thank William Perrine, RPSGT; and Elisa Tsan for their technical support.
SBN's work has been supported by the Department of Veterans Affairs and the National Institutes of Health. VBR's work has been funded by National Institutes of Health, the Department of Defense, Navy Bureau of Medicine and Surgery, and the Department of Veterans Affairs. In the last 2 years, she has received funding from Johnson & Johnson and is a consultant for Sunovion Pharmaceuticals. DTA's work has been funded by the Brain and Behavior Research Foundation (National Alliance for Research on Schizophrenia and Depression), the Department of Defense, and the University of California–San Diego Clinical and Translational Research Institute. SPAD's work has been funded by the National Institutes of Health, the Department of Defense, the National Science Foundation, and the National Health and Medical Research Council (Australia). Within the last 2 years, he has received compensation as Secretary Treasurer and President of the Sleep Research Society and sat on an Advisory Board for Arena Pharmaceuticals.
References
- Acheson D., Feifel D., de Wilde S., Mckinney R., Lohr J., Risbrough V. The effect of intranasal oxytocin treatment on conditioned fear extinction and recall in a healthy human sample. Psychopharmacology. 2013;229(1):199–208. doi: 10.1007/s00213-013-3099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson D., Geyer M., Baker D., Nievergelt C., Yurgil K., Risbrough V., Team M.-I. Conditioned fear and extinction learning performance and its association with psychiatric symptoms in active duty Marines. Psychoneuroendocrinology. 2015;51:495–505. doi: 10.1016/j.psyneuen.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson D.T., Gresack J.E., Risbrough V.B. Hippocampal dysfunction effects on context memory: possible etiology for posttraumatic stress disorder. Neuropharmacology. 2012;62(2):674–685. doi: 10.1016/j.neuropharm.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S., Cole R., Alessi C., Chambers M., Moorcroft W., Pollak C. The role of actigraphy in the study of sleep and circadian rhythms. American Academy of Sleep Medicine Review Paper. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Bastien C.H., Vallières A., Morin C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Boscarino J.A. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and prevention. Psychosom. Med. 2008;70(6):668. doi: 10.1097/PSY.0b013e31817bccaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse D.J., Reynolds C.F., Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr. Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carney C.E., Buysse D.J., Ancoli-Israel S., Edinger J.D., Krystal A.D., Lichstein K.L. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S., O'Malley M.W. Fear extinction memory consolidation requires potentiation of pontine-wave activity during REM sleep. J. Neurosci. 2013;33(10):4561–4569. doi: 10.1523/JNEUROSCI.5525-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B. Administration Booklet: American Psychiatric Pub; 2012. Structured Clinical Interview for DSM-iv Axis I Disorders (SCID-i), Clinician Version. [Google Scholar]
- Foa E., Hembree E., Rothbaum B.O. Oxford University Press; 2007. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences Therapist Guide. [Google Scholar]
- Foa E.B., Keane T.M., Friedman M.J., Cohen J.A., editors. Effective Treatments for PTSD: Practice Guidelines from the International Society for Traumatic Stress Studies. Guilford Press; 2008. [Google Scholar]
- Fu J., Li P., Ouyang X., Gu C., Song Z., Gao J. Rapid eye movement sleep deprivation selectively impairs recall of fear extinction in hippocampus-independent tasks in rats. Neuroscience. 2007;144(4):1186–1192. doi: 10.1016/j.neuroscience.2006.10.050. [DOI] [PubMed] [Google Scholar]
- Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am. J. Psychiatr. 2013;170(4):372–382. doi: 10.1176/appi.ajp.2012.12040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A., Hall M., Krakow B., Shear M.K., Buysse D.J. A brief sleep scale for posttraumatic stress disorder: Pittsburgh Sleep Quality Index Addendum for PTSD. J. Anxiety Disord. 2005;19(2):233–244. doi: 10.1016/j.janxdis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Glenn D.E., Acheson D.T., Geyer M.A., Nievergelt C.M., Baker D.G., Risbrough V.B. Fear learning alterations after traumatic brain injury and their role in development of posttraumatic stress symptoms. Depress. Anxiety. 2017;34(8):723–733. doi: 10.1002/da.22642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C. American Academy of Sleep Medicine; 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. [Google Scholar]
- Jovanovic T., Kazama A., Bachevalier J., Davis M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 2012;62(2):695–704. doi: 10.1016/j.neuropharm.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T., Norrholm S.D. Neural mechanisms of impaired fear inhibition in posttraumatic stress disorder. Front. Behav. Neurosci. 2011;5:44. doi: 10.3389/fnbeh.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Chiu W.T., Demler O., Merikangas K.R., Walters E.E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatr. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D.G., Ruggiero K.J., Acierno R., Saunders B.E., Resnick H.S., Best C.L. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: results from the National Survey of Adolescents. J. Consult. Clin. Psychol. 2003;71(4):692. doi: 10.1037/0022-006x.71.4.692. [DOI] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B. The phq-9. J. Gen. Intern. Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner I., Lupkin S.M., Sinha N., Tsai A., Gluck M.A. Baseline levels of rapid eye movement sleep may protect against excessive activity in fear-related neural circuitry. J. Neurosci. 2017;37(46):11233–11244. doi: 10.1523/JNEUROSCI.0578-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall A.J., Acheson D.T., Risbrough V.B., Straus L.D., Drummond S.P. Fear conditioning, safety learning, and sleep in humans. J. Neurosci. 2014;34(35):11754–11760. doi: 10.1523/JNEUROSCI.0478-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menz M.M., Rihm J.S., Büchel C. REM sleep is causal to successful consolidation of dangerous and safety stimuli and reduces return of fear after extinction. J. Neurosci. 2016;36(7):2148–2160. doi: 10.1523/JNEUROSCI.3083-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M.R., Orr S.P., Lasko N.B., Chang Y., Rauch S.L., Pitman R.K. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J. Psychiatr. Res. 2008;42(7):515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M.R., Pitman R.K., Ellis C.B., Gold A.L., Shin L.M., Lasko N.B. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatr. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylan T.C., Marmar C.R., Metzler T.J., Weiss D.S., Zatzick D.F., Delucchi K.L. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Sleep. 1998;155(7) doi: 10.1176/ajp.155.7.929. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis I.L., Takashima A. The role of the ventromedial prefrontal cortex in memory consolidation. Behav. Brain Res. 2011;218(2):325–334. doi: 10.1016/j.bbr.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Norrholm S.D., Jovanovic T., Olin I.W., Sands L.A., Bradley B., Ressler K.J. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol. Psychiatr. 2011;69(6):556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm S.D., Jovanovic T., Smith A.K., Binder E., Klengel T., Conneely K. Differential genetic and epigenetic regulation of catechol-O-methyltransferase is associated with impaired fear inhibition in posttraumatic stress disorder. Front. Behav. Neurosci. 2013;7 doi: 10.3389/fnbeh.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm S.D., Jovanovic T., Vervliet B., Myers K.M., Davis M., Rothbaum B.O. Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learn. Mem. 2006;13(6):681–685. doi: 10.1101/lm.393906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R.G., LeDoux J.E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106(2):274. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Raskind M.A., Peskind E.R., Hoff D.J., Hart K.L., Holmes H.A., Warren D. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol. Psychiatr. 2007;61(8):928–934. doi: 10.1016/j.biopsych.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Schottenbauer M.A., Glass C.R., Arnkoff D.B., Tendick V., Gray S.H. Nonresponse and dropout rates in outcome studies on PTSD: review and methodological considerations. Psychiatry. 2008;71(2):134–168. doi: 10.1521/psyc.2008.71.2.134. [DOI] [PubMed] [Google Scholar]
- Spoormaker V., Sturm A., Andrade K., Schröter M., Goya-Maldonado R., Holsboer F. The neural correlates and temporal sequence of the relationship between shock exposure, disturbed sleep and impaired consolidation of fear extinction. J. Psychiatr. Res. 2010;44(16):1121–1128. doi: 10.1016/j.jpsychires.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Spoormaker V.I., Schröter M.S., Andrade K.C., Dresler M., Kiem S.A., Goya-Maldonado R. Effects of rapid eye movement sleep deprivation on fear extinction recall and prediction error signaling. Hum. Brain Mapp. 2012;33(10):2362–2376. doi: 10.1002/hbm.21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus L.D., Acheson D.T., Risbrough V.B., Drummond S.P. Sleep deprivation disrupts recall of conditioned fear extinction. Biol. Psychiatr.: Cognitive Neuroscience and Neuroimaging. 2017;2(2):123–129. doi: 10.1016/j.bpsc.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus L.D., Drummond S., Nappi C.M., Jenkins M.M., Norman S.B. Sleep variability in military-related PTSD: a comparison to primary insomnia and healthy controls. J. Trauma Stress. 2015;28(1):8–16. doi: 10.1002/jts.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus L.D., Drummond S.P., Risbrough V.B., Norman S.B. Springer; 2017. Sleep Disruption, Safety Learning, and Fear Extinction in Humans: Implications for Posttraumatic Stress Disorder. Current Topics in Behavioral Neuroscience. [DOI] [PubMed] [Google Scholar]
- van der Helm E., Yao J., Dutt S., Rao V., Saletin J.M., Walker M.P. REM sleep depotentiates amygdala activity to previous emotional experiences. Curr. Biol. 2011;21(23):2029–2032. doi: 10.1016/j.cub.2011.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers F.W., Blake D.D., Schnurr P.P. US Department of Veterans Affairs. National Center for PTSD; 2015. Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) [Google Scholar]
- Weathers F.W., Keane T.M., Davidson J.R. Clinician-Administered PTSD Scale: a review of the first ten years of research. Depress. Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Weathers F., Litz B., Herman D., Huska J., Keane T. Paper Presented at the Annual Convention of the International Society for Traumatic Stress Studies, San Antonio, TX. October 1993. The PTSD checklist (PCL): reliability, validity, and diagnostic utility. [Google Scholar]
- Wessa M., Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am. J. Psychiatr. 2007;164(11):1684–1692. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- Yeager D.E., Magruder K.M., Knapp R.G., Nicholas J.S., Frueh B.C. Performance characteristics of the posttraumatic stress disorder checklist and SPAN in Veterans Affairs primary care settings. Gen. Hosp. Psychiatr. 2007;29(4):294–301. doi: 10.1016/j.genhosppsych.2007.03.004. [DOI] [PubMed] [Google Scholar]