Abstract
Objective
To evaluate the clinical outcomes associated with anti-methicillin-resistant Staphylococcus aureus (MRSA) antimicrobials.
Methods
We reviewed a prospective database of 247 consecutive patients with clinically and microbiologically confirmed MRSA infections, hospitalized in 7 Japanese hospitals between April 2014 and March 2015, and treated with anti-MRSA pharmaceuticals. Survival was measured at 30 days. We examined the relationships between initial antimicrobial administered and survival and organ toxicity. HR and 95% CIs were calculated.
Results
Overall 30-day mortality was 12%. The lungs were infected in 105 (41%), skin and soft tissue in 73 (30%), and bones and joints in 21 (9%) patients. Bacteremia complicated the illness in 69 patients (28%). Among 5 pharmaceuticals, vancomycin was prescribed to 174 (71%), linezolid to 38 (16%), teicoplanin to 22 (9%), and daptomycin to 11 (5%) patients. Vancomycin tended to be associated with the lowest survival (HR=2.47; 95% CI=0.93–6.51; P=0.067), particularly in the lung-infected subgroup (HR=4.85; 95% CI=1.12–20.94; P=0.034) after adjustments for baseline illness severity. The incidence of renal dysfunction tended to be higher in patients with trough serum concentrations of vancomycin >15 mg/dL.
Conclusion
In this observational study reflecting real-world conditions, vancomycin was associated with higher 30-day mortality and incidence of kidney dysfunction than other anti-MRSA agents. The significance of the differences observed among antimicrobials other than vancomycin is uncertain.
Keywords: methicillin-resistant Staphylococcus aureus, vancomycin, linezolid, infectious mortality, renal dysfunction
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is one of the most common causes of fatal or near-fatal nosocomial infections of the lung, blood, and skin or soft tissues.1–4 The inordinately high mortality associated with MRSA, partially due to the gravity of the illness and the limited efficacy of antimicrobials available, is a source of major and widespread concern.
The appropriate choice, timing, and doses of antimicrobi-als against MRSA are major determinants of their efficacy, particularly when used to treat infections of the bloodstream or the lungs.5 A judicious choice of empiric therapy, before return of the results of microbiological cultures, is especially important.6–8 Vancomycin is used worldwide as first-line treatment of MRSA. However, well-designed, randomized trials comparing its efficacy with that of other antimicrobials are few. In addition, the availability of treatments, besides vancomycin, vary among countries and world regions, underscoring the importance of conducting scholarly, observational studies in various clinical settings to examine the relationship between choice of anti-MRSA agent and clinical efficacy, based on real-world clinical practice. Given the variations in the prescription of drug regimens in response to the publication of national or regional treatment guidelines,9–11 studies of the effectiveness of treatments against MRSA infections are certainly worthwhile.
Using data prospectively collected by a multicenter, real-world registry, we examined the treatment effects of various anti-MRSA drugs administered as recommended by professional practice guidelines to hospitalized patients presenting with microbiologically confirmed MRSA infections.9 We specifically compared the 30-day survival and development of organ dysfunctions associated with vancomycin, a standard antimicrobial widely recommended in the guidelines,9 with that associated with several other pharmaceuticals.
Study sample and methods
This observational study was conducted at 2 university-affiliated and 5 general teaching hospitals in Japan. The data were collected using 1) a prospective database maintained by the infection control teams of the participating hospitals, and 2) a review of medical records, with the approval of the Ethics Committee of Kyoto Medical Centre (#13-103), in accordance with the standards stated in the 1964 Declaration of Helsinki, and by the Ethics Committee of other hospitals, if requested. The Committee waived the signatures of informed consents by the patients whose records were reviewed, as this was a noninterventional, observational study with data management processed anonymously.
Patient selection
We reviewed the records of consecutively hospitalized patients, treated with any anti-MRSA drug for confirmed infections strictly due to MRSA, based on microbiological cultures and clinical evaluations. The MRSA detection systems varied among medical institutions (MicroScan Walk-Away® plus [Beckman Coulter, Inc., Brea, CA, USA]; n=4, VITEK® [ bioMérieux, Inc., Marcy-l’Étoile, France]; n=2, and BD-Phenix™ [Becton Dickinson, Franklin Lakes, NJ, USA]; n=1). The cultures, diagnoses of each infections focus, and the prescriptions of antimicrobials were the responsibility of the physicians caring for individual patients, in consultation, when necessary, with infectious disease specialists. The antimicrobials available to treat MRSA in Japan during the study period were vancomycin, teicoplanin, linezolid, daptomycin, and arbekacin (not used in this study). Authorization was obtained from the infection control team before initiating treatment with these drugs.
Anti-MRSA treatment
We recorded the duration of initial administration of each anti-MRSA drug and their doses, total duration of therapy, substitution of other drugs, and time and causes of treatment discontinuation. In the case of glycopeptides, the initial trough serum concentration was measured 3 days after the onset of therapy. No standard protocol of drug administration was used by the participating institutions.
Concordance with the treatment guidelines was examined by cross-reference with the Japanese therapeutic guidelines for MRSA.9 When the initial therapeutic regimen administered for an infectious source was the first choice recommended by the guidelines (Table 1), it was classified as “concordant.”
Table 1.
First-choice antimicrobials recommended by the Japanese practice guidelines for the management of infections caused by MRSA
| Infectious focus | Choices of anti-MRSA pharmaceuticals |
|---|---|
| Lungs | Linezolid |
| Vancomycin | |
| Teicoplanin | |
| Blood | Daptomycin |
| Vancomycin | |
| Endocardium | Daptomycin |
| Vancomycin | |
| Skin and soft tissues | Daptomycin |
| Linezolid | |
| Vancomycin |
Note: Data from Niki.9
Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus.
Other baseline characteristics
We recorded the patient age, sex, Charlson score, acute physiology and chronic health evaluation (APACHE) II score on day 0, sequential organ failure assessment (SOFA) score on days 0, 2–3 and 5–7, definite or probable sources of infection, presence of bacteremia, and other characteristics, which, from previous publications,1–4,8 might have been related to outcome.
All-cause mortality was measured 30 days after the initiation of the anti-MRSBA pharmaceuticals, which was set as an appropriate time point to evaluate the outcomes of an acute infectious disease. The patient population was divided between survivors and nonsurvivors. Factors associated with fatal outcomes were evaluated in both groups and compared. Acute renal dysfunction was diagnosed as a >0.5 mg/dL serum creatinine concentration, or a >50% increase from baseline following the initiation of therapy. The eradication of microorganisms was evaluated at the end of therapy with each drug.
Sample size
Based on the known 15%–20% mortality associated with MRSA infections, we estimated that ≥200 patients were needed to perform meaningful multiple variable analyses.12
Statistical analysis
Continuous data are expressed as medians (interquartile ranges) and categorical data as counts (percentages). Demographic and clinical variables were compared among the anti-MRSA agents. Significant differences in medians and prevalence estimates were examined, using the χ2 and Kruskal–Wallis tests for categorical and continuous variables, respectively. Cox multiple variable regression analyses were used to evaluate the impact of initial anti-MRSA therapy on outcomes, including 30-day mortality and newly acquired renal dysfunction, nonadjusted or adjusted for potential confounders. The results were adjusted for disease severity, using age, APACHE II scores, bacteremia, and presence of pneumonia. We also verified the absence of multiple col-linearity of severity of illness and each covariate. HR and 95% CIs were calculated. With respect to the analysis of guideline concordance, the study sample was divided between concordant and nonconcordant groups. A P-value <0.05 was considered to indicate statistical significance. All analyses were performed using the SPSS software, version 24.0 (IBM Corporation, Armonk, NY, USA).
Results
Baseline characteristics
Since a single aminoglycoside is not considered an effective treatment of MRSA, 2 patients treated with albekacin alone were excluded and 245 patients were retained in the analysis. The Charlson, APACHE II, and SOFA scores on the first day of MRSA treatment were 3 (1–4), 12 (8–12), and 2 (0–6), respectively (Table 2). Confirmed or suspected pneumonia was the most frequent source of infection in 105 patients (42.9%), followed by skin and soft tissue in 73 (29.8%), and bones and joints in 21 (8.6%) patients. The disease was complicated by bacteremia in 69 (28.2%) patients. The minimum inhibitory concentration of vancomycin against the target MRSA was >2 mg/L in all but a single patient.
Table 2.
Characteristics of entire study sample and of each treatment group
| Variable | All patients (n=245) |
Anti-MRSA pharmaceuticals
|
|||
|---|---|---|---|---|---|
| Vancomycin (n=174) |
Linezolid (n=38) |
Daptomycin (n=11) |
Teicoplanin (n=22) |
||
| Age, years | 71 (61–79) | 71 (60–78) | 74 (65–79) | 70 (65–74) | 65 (53–82) |
| Men | 176 (71.8) | 121 (69.5) | 26 (68.4) | 11 (100) | 18 (81.8) |
| APACHE II | 12 (8–20) | 11 (8–19) | 15 (9–23) | 11 (7–12) | 12 (8–16) |
| Charlson score | 3 (1–4) | 3 (1–5) | 2 (1–4) | 2 (0–3) | 2 (0–3) |
| History of | |||||
| Diabetes mellitus* | 90 (36.7) | 75 (43.1) | 6 (15.8) | 3 (27.3) | 6 (27.3) |
| End-stage renal disease | 43 (17.6) | 34 (19.5) | 5 (13.2) | 1 (9.1) | 3 (13.6) |
| Cancer | 67 (27.3) | 40 (23.0) | 16 (42.1) | 4 (36.4) | 6 (27.3) |
| Liver disease | 23 (9.4) | 16 (9.2) | 4 (10.5) | 2 (18.2) | 0 |
| Infectious source | |||||
| Bacteraemiaa | 69 (28.2) | 56 (32.2) | 6 (15) | 2 (18.2) | 5 (22.7) |
| Lung* | 105 (42.9) | 72 (41.4) | 29 (76.3) | 1 (9.1) | 3 (13.6) |
| Skin and soft tissue* | 73 (29.8) | 50 (28.7) | 5 (13.2) | 7 (63.6) | 11 (50.0) |
| Bone and joint | 21 (8.6) | 14 (8.0) | 2 (5.3) | 2 (18.2) | 3 (13.6) |
| Othersb | 38 (13.5) | 28 (16.1) | 5 (13.2) | 1 (9.1) | 4 (18.2) |
| SOFA score | |||||
| Day 0* | 2 (0–6) | 2 (0–6) | 4 (2–7) | 2 (1–7) | 0 (0–3) |
| Days 2–3 (n=244) | 2 (0–5) | 2 (0–5) | 3 (1–6) | 1 (0–6) | 0 (0–4) |
| Days 5–7 (n=243) | 2 (0–4) | 1 (0–4) | 3 (0–5) | 1 (0–2) | 0 (0–3) |
| Intensive care unit admission* | 83 (33.9) | 55 (31.6) | 20 (52.6) | 7 (63.6) | 1 (4.5) |
| Mechanical ventilation* | 58 (23.7) | 38 (21.8) | 13 (34.2) | 6 (54.5) | 1 (4.5) |
| Days of initial therapy | 11 (7–16) | 12 (7–17) | 8 (7–13) | 11 (8–17) | 10 (6–13) |
| Change in MRSA therapy* | 66 (26.9) | 38 (21.8) | 3 (7.8) | 3 (27.2) | 6 (27.2) |
| Change or discontinuation of antimicrobial for adverse effect | 17 (6.9) | 11 (6.4) | 5 (13.2) | 0 | 1 (4.5) |
| Newly acquired renal dysfunction | 35 (14.3) | 30 (17.2) | 3 (7.9) | 0 | 2 (9.1) |
| 30-day mortality, % | 12.2 | 14.4 | 7.9 | 9.1 | 4.5 |
Notes: Unless specified otherwise, the values are medians (interquartile ranges) or numbers (%) of observations.
Infectious source unidentified in 24 patients;
includes 4 infective endocarditis and 4 meningitis.
P<0.05.
Abbreviations: APACHE II, acute physiology and chronic health evaluation; MRSA, methicillin-resistant Staphylococcus aureus; SOFA, sequential organ failure assessment.
Drug therapy
Vancomycin was the first antimicrobial administered in 174 patients (71.0%), followed by linezolid in 38 (15.5%), teicoplanin in 22 (8.9%), and daptomycin in 11 (4.5%) patients. Within 30 days, 25 of 174 (14.4%) patients treated with vancomycin died, in contrast with 1 of 22 patients (4.5%) who received teicoplanin, 3 of 38 patients (7.9%) who received linezolid, and 1 of 11 patients (9.1%) who received daptomycin, corresponding to a 7.0% 30-day mortality in the group treated with antimicrobials other than vancomycin. The rate of microorganism eradication was similar among the various treatment groups and nearly identical in the 174 patients treated with vancomycin compared with the 71 patients treated with other antimicrobials (data not shown). The baseline SOFA score was significantly higher in patients treated with linezolid or daptomycin on day 0 (Table 2). However, after the initiation of antimicrobial therapy, the SOFA scores on days 2–3 and days 5–7 were similar among the treatment groups. Acute renal dysfunction was diagnosed in 30 patients (17.2%) treated with vancomycin, 2 patients (9.1%) treated with teicoplanin, 3 patients (7.9%) with linezolid, and no patient treated with daptomycin. Supplemental drugs with and without anti-MRSA properties were administered to 16 and 21 patients, respectively, without differences in mortality among the treatment groups (no supplemental drug: 12%; supplemental drug with anti-MRSA properties, including clindamycin, minocycline and trimethoprim-sulfamethoxazole: 13%; supplemental drug without anti-MRSA properties, including β-lactams and quinolones: 14% [P=0.56]). Twenty-seven patients had a history of MRSA infection. The mortality of patients with (4%) and without (13%) histories of MRSA infection was not significantly different (P=0.25).
The choice of initial therapy was concordant with the professional practice guidelines in 217, and nonconcordant in 28 patients (bacteremias of known origin were counted twice). The 30-day mortality was similar in the concordant (12.9%) and the nonconcordant (7.1%) groups, while the rate of substitution of other anti-MRSA agents was significantly higher in the nonconcordant (39.2%) than in the concordant (13.4%) group (P<0.01).
Factors associated with mortality
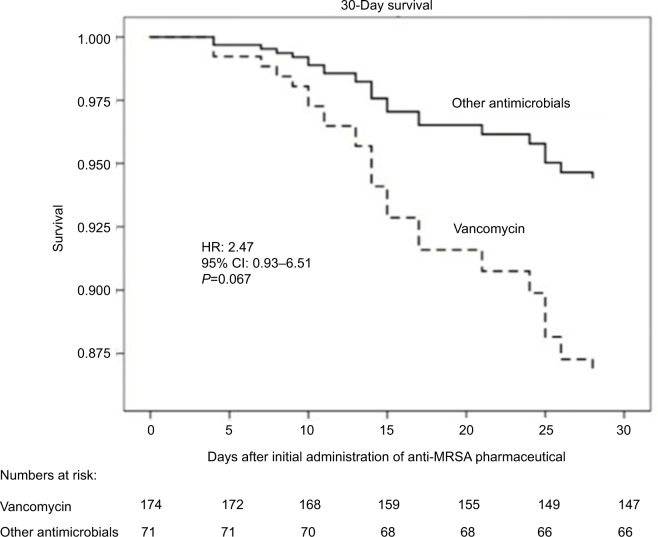
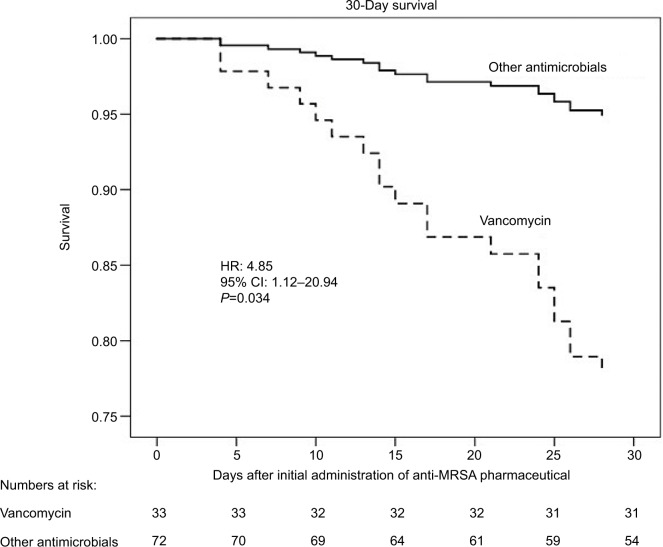
Unadjusted comparisons revealed that the 30 nonsurvivors were older, presented with higher Charlson, APACHE II, and SOFA scores, and with a higher prevalence of bacteremia than the 215 survivors. There was a trend (P=0.11) toward a significant relationship between 30-day mortality and vancomycin therapy. By Cox multiple variable regression analysis adjusted for APACHE II score and bacteremia, a trend toward shorter survival (adjusted HR=2.47; 95% CI=0.93–6.51; P=0.067) was observed in the vancomycin-treated group compared with the other groups (Figure 1; Table 3). Among 105 patients presenting with lung infections, the survival of 33 patients treated with vancomycin was significantly shorter (adjusted HR=4.85; 95% CI=1.12–20.94; P=0.034) than the 72 patients treated with other antimicrobials (Figure 2). Furthermore, in the subgroup presenting with lung infections, the survival of 29 patients treated with linezolid tended to be longer than that of 76 patients treated with other antimicrobials (Figure S1).
Figure 1.
Survival of vancomycin versus non-vancomycin treatment groups at 30 days in the overall sample.
Note: After adjustment for age, APACHE II score, bacteremia, and pneumonia, the risk of dying tended to be higher in the vancomycin-treated group than in the groups treated with other pharmaceuticals.
Abbreviations: APACHE II, acute physiology and chronic health evaluation; MRSA, methicillin-resistant Staphylococcus aureus.
Table 3.
Outcome of Cox regression analysis of clinical outcomes; comparison of vancomycin versus other antimicrobials
| Variable | Cox model
|
|||||
|---|---|---|---|---|---|---|
| Unadjusted
|
Adjusted
|
|||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Vancomycin versus non-vancomycin | ||||||
| 30-day mortality | 2.28 | 0.88–5.93 | 0.09 | 2.47 | 0.93–6.51 | 0.06 |
| Newly acquired renal dysfunction | 2.65 | 1.02–6.83 | 0.04 | 1.99 | 0.76–5.18 | 0.15 |
Figure 2.
Thirty-day survival of vancomycin versus non-vancomycin treatment groups presenting with lung infections.
Note: Among patients with lung infections and after adjustment for baseline severity of the illness, survival in the vancomycin-treated group was significantly lower than in the group treated with other drugs.
Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus.
Drugs concordant with the practice guidelines recommendations were initially administered to 217 patients (89%). While this concordance was associated with infrequent changes in the initial drug regimen, we observed no significant relationship with the 30-day mortality (Table 4).
Table 4.
Concordance of initial anti-MRSA therapy with practice guidelines
| Variable | Concordance, n=217 | Nonconcordance, n=28 | P-value |
|---|---|---|---|
| Anti-MRSA pharmaceutical | <0.01 | ||
| Vancomycin | 169 (97.1) | 5 (2.9) | |
| Teicoplanin | 4 (18.2) | 18 (81.8) | |
| Linezolid | 35 (92.1) | 3 (7.9) | |
| Daptomycin | 9 (81.8) | 2 (18.2) | |
| Drug substitution after initial treatment | 29 (13.4) | 11 (39.2) | <0.01 |
| Improvement in SOFA score | |||
| Day 0 to days 2–3 (n=244) | 138 (63.9) | 17 (60.7) | 0.74 |
| Days 2–3 to days 5–7 (n=243) | 148 (68.8) | 21 (75.0) | 0.50 |
| Newly acquired renal dysfunction | 31 (14.3) | 4 (14.2) | 1.00 |
| 30-day mortality, % | 12.9 | 7.1 | 0.38 |
Note: Unless specified otherwise, the values are numbers (%) of observations.
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; SOFA, sequential organ failure assessment.
Trough concentrations of vancomycin and 30-day outcomes
Within the 30 days of follow-up, 215 patients survived (87.8%) and 30 (12.2%) died. In the nonadjusted model, the incidence of renal dysfunction was significantly higher in patients treated with vancomycin, though not in the adjusted model (Table 3). In the sensitivity analysis based on the initial trough concentrations of vancomycin, the 30-day mortality was similar in both groups. The incidence of acute kidney dysfunction paralleled the increase in initial trough concentrations, suggesting that an initial trough concentration >15 mg/dL is a predictor of newly acquired renal dysfunction (Table S1).
Discussion
Main study findings
This analysis identified an association between the use of initial antimicrobial therapy and mortality of patients presenting with definite or probable MRSA infections. Vancomycin, the most prevalent antimicrobial in this study, was associated with nonsurvival after adjustment for severity of illness. In addition, the measurement of high trough concentrations of vancomycin tended to be associated with an increased incidence of acute renal dysfunction developing after the initiation of antimicrobial therapy.
Several randomized trials and meta-analyses comparing vancomycin with other antimicrobials administered in various clinical settings and for different infectious diseases have been published. A recent, systematic review and meta-analysis compared vancomycin, as the established anti-MRSA agent, with six other antimicrobials effective against MRSA. In a Bayesian network meta-analysis of 27 randomized trials, line-zolid was more effective and safer than vancomycin, specifi-cally for the treatment of complicated nosocomial, soft tissue infections and for ventilator-associated pneumonia.13 Another meta-analysis of 8 studies comparing directly the efficacy and safety of vancomycin with linezolid for all MRSA infections also found a significantly greater treatment efficacy conferred by linezolid (OR=1.77; 95% CI=1.22–2.56).14 In addition, an analysis of variables associated with clinical success, using data from a randomized trial, found that treatment with linezolid was more likely to be successful (OR=1.55; 95% CI=1.01–2.34).15 The results of our study, which confirmed the efficacy of antimicrobials other than vancomycin as initial treatment of MRSA infections, of the lung in particular, are concordant with the results of these meta-analyses.
Among various anti-MRSA drugs, the in vitro bactericidal potency of daptomycin against Gram-positive pathogens is the highest. Studies comparing the efficacy of daptomycin versus vancomycin have also suggested a superiority of daptomycin in specific settings. A randomized trial comparing the efficacy of daptomycin with that of glycopeptides supports the use of the former as first-line treatment for complicated skin and soft tissue infections.16 Higher clinical and microbiological successes were observed with daptomycin versus vancomycin and teicoplanin in 194 randomized patients, especially when patients were ≥65 years of age. Other small studies have suggested a higher clinical success or longer survival associated with daptomycin in complicated dermatologic infections17 or complicated bacteremia due to MRSA.18 These data suggest a contribution to a higher efficacy in the non-vancomycin group in this study. Due to the small numbers of patients treated with drugs other than vancomycin, these drugs could not be directly compared. One recent study observed that the addition of β-lactam antimicrobials might improve the outcome of patients treated with anti-MRSA drugs.19 However, in this study, only a few patients received combinations of drugs with and without anti-MRSA properties, and the mortality was similar among all groups compared.
The low efficacy of vancomycin might be explained in part by its pharmacologic and pharmacokinetic properties. Current practice guidelines recommend the monitoring and maintenance of its trough serum concentrations at 15–20 μg/mL to optimize its efficacy and limit the risk of renal insufficiency or ototoxicity.9–11,20 Keeping the blood concentrations of the drug within this narrow target range is challenging, particularly in critically ill patients. Previous studies have observed that these targets were reached by fewer than one fourth of patients.21 This is concordant with our own observation of initial trough measurements of vancomycin kept at the target level in only 21% of our patients. It is also noteworthy that we found no relationship between these trough concentrations of the drug and 30-day mortality. The undesirable effects on renal function at the target or higher trough levels22 may have contributed to a higher incidence of renal dysfunction, SOFA scores, and mortality in the vancomycin group. This is consistent with the results of previous meta-analyses, which found significant increases in the incidence of nephrotoxicity caused by vancomycin prescribed for pneumonia14,23 or bacteremia.18
Previous studies have suggested that the acute severity of illness represented by the APACHE II score is the most significant predictor of death of patients presenting with MRSA bacteremia.24,25 These analyses, however, did not include the effects of treatment on clinical outcomes. In contrast, we examined the effects of antimicrobial therapy after adjustment for the APACHE II severity score, to analyze accurately the effects of drug therapy on the 30-day outcomes.
Study limitations
A first limitation of our study is its observational design. Although we adjusted our statistical analyses for the severity of the disease, interactions of several unexpected factors may have influenced outcomes. Second, the diagnosis of MRSA infection, as well as the choices, timings, and doses of each anti-MRSA drugs were left to the decision of the primary physicians, instead of being systematically guided by protocols or by recommendations from infectious disease specialists. Delays in suspecting the presence of MRSA and whether the drug was administered empirically or objectively might have considerably influenced the clinical outcomes. In turn, the data, reflecting the real-world management could provide useful information to assess current clinical situations and to consider future comparative studies. Third, we could not compare linezolid, daptomycin, and teicoplanin with each other because of the small numbers of observations. Direct, randomized comparisons of the efficacy and safety of these 3 drugs, between linezolid and daptomycin in particular, are lacking due to the small numbers, precluding the recommendation of a single drug as an optimal choice for the treatment of MRSA. A large study is needed to compare these drugs directly. Finally, our results cannot be extrapolated to other clinical settings, where new anti-MRSA drugs are available. Further studies should compare new pharmaceuticals, including ceftaroline, ceftobiprole, tedizolid, telavancin, dalbavancin, and oritavancin.26,27
Conclusion
In conclusion, this study examined current real-world practices in the treatment of infections caused by MRSA, as well as the relationship between choice of anti-MRSA drugs and 30-day mortality adjusted for the baseline severity of illness. Vancomycin was associated with a higher incidence of renal dysfunction and a lower 30-day survival in patients presenting with lung infections. Randomized studies to validate these observations are warranted.
Supplementary materials
Thirty-day survival of linezolid versus non-linezolid treatment groups presenting with lung infections.
Note: Among patients with lung infections and after adjustment for age, a trend was observed toward a higher survival in the linezolid-treated group compared with the group treated with other drugs.
Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus.
Table S1.
Relationship between highest trough vancomycin concentrations and clinical outcomes
| Highest trough concentrations of vancomycin (mg/dL) | Patients | New renal dysfunction | 30-day mortality |
|---|---|---|---|
| 0–4.9 | 0 | 0 | 0 |
| 5.0–9.9 | 11 (6.3) | 0 | 3 (27.3) |
| 10.0–14.9 | 31 (17.8) | 2 (6.5) | 2 (6.5) |
| 15.0–19.9 | 40 (22.9) | 10 (25.0) | 7 (17.5) |
| 20.0–24.9 | 32 (18.3) | 6 (18.8) | 2 (6.3) |
| ≥25.0 | 28 (16.1) | 9 (32.1) | 6 (21.4) |
| Not measured | 32 (18.3) | 3 (9.4) | 5 (15.6) |
| Total | 174 (100) | 30 (17.2) | 25 (14.4) |
Note: Unless specified otherwise, the values are numbers (%) of observations.
Acknowledgments
The study was conducted at Kyoto Medical Centre. We thank Rodolphe Ruffy, MD, an English-speaking physician (www.cardiocript.com), who reviewed our manuscript for style and language. This work is supported, in part, by a Grant-in Aid for Promoting Research for Evidence-Based Medicine, National Hospital Organization (H26-EBM[observational]-02).
Footnotes
Disclosure
Nobuaki Shime, MD, PhD, received a lecturer fee from Pfizer Pharmaceutical Inc. The authors report no other conflicts of interest in this work.
References
- 1.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36(1):53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 2.Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26(2):166–174. doi: 10.1086/502522. [DOI] [PubMed] [Google Scholar]
- 3.Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):273–279. doi: 10.1086/512627. [DOI] [PubMed] [Google Scholar]
- 4.Reed SD, Friedman JY, Engemann JJ, et al. Costs and outcomes among hemodialysis-dependent patients with methicillin-resistant or methicillin-susceptible Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol. 2005;26(2):175–183. doi: 10.1086/502523. [DOI] [PubMed] [Google Scholar]
- 5.Gemmell CG, Edwards DI, Fraise AP, Gould FK, Ridgway GL, Warren RE, Joint Working Party of the British Society for Joint Working Party of the British Society for Antimicrobial Chemotherapy, Hospital Infection Society and Infection Control Nurses Association Guidelines for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the UK. J Antimicrob Chemother. 2006;57(4):589–608. doi: 10.1093/jac/dkl017. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118(1):146–155. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 7.Leibovici L, Drucker M, Konigsberger H, et al. Septic shock in bacteremic patients: risk factors, features and prognosis. Scand J Infect Dis. 1997;29(1):71–75. doi: 10.3109/00365549709008668. [DOI] [PubMed] [Google Scholar]
- 8.Guilarde AO, Turchi MD, Martelli CM, Primo MG. Staphylococcus aureus bacteraemia: incidence, risk factors and predictors for death in a Brazilian teaching hospital. J Hosp Infect. 2006;63(3):330–336. doi: 10.1016/j.jhin.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Niki Y, Japanese Association for infectious disease Practical guidelines for the management and treatment of infections caused by MRSA, the 2nd ed. Kansenshogaku Zasshi. 2014;88(5):597–668. Japanese. [PubMed] [Google Scholar]
- 10.Liu C, Bayer A, Cosgrove SE, et al. Infectious Diseases Society of America Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 11.Gould FK, Brindle R, Chadwick PR, et al. MRSA Working Party of the British Society for Antimicrobial Chemotherapy Guidelines (2008) for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the United Kingdom. J Antimicrob Chemother. 2009;63(5):849–861. doi: 10.1093/jac/dkp065. [DOI] [PubMed] [Google Scholar]
- 12.Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54(5):621–629. doi: 10.1093/cid/cir895. [DOI] [PubMed] [Google Scholar]
- 13.Bally M, Dendukuri N, Sinclair A, Ahern SP, Poisson M, Brophy J. A network meta-analysis of antibiotics for treatment of hospitalised patients with suspected or proven meticillin-resistant Staphylococcus aureus infection. Int J Antimicrob Agents. 2012;40(6):479–495. doi: 10.1016/j.ijantimicag.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 14.An MM, Shen H, Zhang JD, Xu GT, Jiang YY. Linezolid versus vancomycin for meticillin-resistant Staphylococcus aureus infection: a meta-analysis of randomised controlled trials. Int J Antimicrob Agents. 2013;41(5):426–433. doi: 10.1016/j.ijantimicag.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Shorr AF, Zilberberg MD, Micek ST, Kollef MH. Outcomes associated with bacteremia in the setting of methicillin-resistant Staphylococcus aureus pneumonia: a retrospective cohort study. Crit Care. 2015;19:312. doi: 10.1186/s13054-015-1029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quist SR, Fierlbeck G, Seaton RA, Loeffler J, Chaves RL. Comparative randomised clinical trial against glycopeptides supports the use of daptomycin as first-line treatment of complicated skin and soft-tissue infections. Int J Antimicrob Agents. 2012;39(1):90–91. doi: 10.1016/j.ijantimicag.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Kauf TL, McKinnon P, Corey GR, et al. An open-label, pragmatic, randomized controlled clinical trial to evaluate the comparative effectiveness of daptomycin versus vancomycin for the treatment of complicated skin and skin structure infection. BMC Infect Dis. 2015;15:503. doi: 10.1186/s12879-015-1261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rehm SJ, Boucher H, Levine D, et al. Daptomycin versus vancomycin plus gentamicin for treatment of bacteraemia and endocarditis due to Staphylococcus aureus: subset analysis of patients infected with methicillin-resistant isolates. J Antimicrob Chemother. 2008;62(6):1413–1421. doi: 10.1093/jac/dkn372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Truong J, Veillette JJ, Forland SC. Outcomes of vancomycin plus a β-lactam versus vancomycin only for treatment of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2018;62(2) doi: 10.1128/AAC.01554-17. pii: e01554-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49(3):325–327. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 21.Forstner C, Dungl C, Tobudic S, Mitteregger D, Lagler H, Burgmann H. Predictors of clinical and microbiological treatment failure in patients with methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia: a retrospective cohort study in a region with low MRSA prevalence. Clin Microbiol Infect. 2013;19(7):E291–E297. doi: 10.1111/1469-0691.12169. [DOI] [PubMed] [Google Scholar]
- 22.Davies SW, Guidry CA, Petroze RT, Hranjec T, Sawyer RG. Vancomycin and nephrotoxicity: just another myth? J Trauma Acute Care Surg. 2013;75(5):830–835. doi: 10.1097/TA.0b013e3182a74b70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Zou Y, Xie J, et al. Linezolid versus vancomycin for the treatment of suspected methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a systematic review employing meta-analysis. Eur J Clin Pharmacol. 2015;71(1):107–115. doi: 10.1007/s00228-014-1775-x. [DOI] [PubMed] [Google Scholar]
- 24.Mylotte JM, Aeschlimann JR, Rotella DL. Staphylococcus aureus bacteremia: factors predicting hospital mortality. Infect Control Hosp Epidemiol. 1996;17(3):165–168. doi: 10.1086/647264. [DOI] [PubMed] [Google Scholar]
- 25.Mylotte JM, Tayara A. Staphylococcus aureus bacteremia: predictors of 30-day mortality in a large cohort. Clin Infect Dis. 2000;31(5):1170–1174. doi: 10.1086/317421. [DOI] [PubMed] [Google Scholar]
- 26.Holmes NE, Howden BP. What’s new in the treatment of serious MRSA infection? Curr Opin Infect Dis. 2014;27(6):471–478. doi: 10.1097/QCO.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 27.Cosimi RA, Beik N, Kubiak DW, Johnson JA. Ceftaroline for severe methicillin-resistant Staphylococcus aureus infections: a systematic review. Open Forum Infect Dis. 2017;4(2):ofx084. doi: 10.1093/ofid/ofx084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Thirty-day survival of linezolid versus non-linezolid treatment groups presenting with lung infections.
Note: Among patients with lung infections and after adjustment for age, a trend was observed toward a higher survival in the linezolid-treated group compared with the group treated with other drugs.
Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus.
Table S1.
Relationship between highest trough vancomycin concentrations and clinical outcomes
| Highest trough concentrations of vancomycin (mg/dL) | Patients | New renal dysfunction | 30-day mortality |
|---|---|---|---|
| 0–4.9 | 0 | 0 | 0 |
| 5.0–9.9 | 11 (6.3) | 0 | 3 (27.3) |
| 10.0–14.9 | 31 (17.8) | 2 (6.5) | 2 (6.5) |
| 15.0–19.9 | 40 (22.9) | 10 (25.0) | 7 (17.5) |
| 20.0–24.9 | 32 (18.3) | 6 (18.8) | 2 (6.3) |
| ≥25.0 | 28 (16.1) | 9 (32.1) | 6 (21.4) |
| Not measured | 32 (18.3) | 3 (9.4) | 5 (15.6) |
| Total | 174 (100) | 30 (17.2) | 25 (14.4) |
Note: Unless specified otherwise, the values are numbers (%) of observations.