 A series of tetrahydro-β-carboline tetrazole derivatives have been synthesized utilizing the Ugi-tetrazole reaction and were identified as potential antileishmanial chemotypes.
A series of tetrahydro-β-carboline tetrazole derivatives have been synthesized utilizing the Ugi-tetrazole reaction and were identified as potential antileishmanial chemotypes.
Abstract
A series of 2,3,4,9-tetrahydro-β-carboline tetrazole derivatives (14a–u) have been synthesized utilizing the Ugi multicomponent reaction and were identified as potential antileishmanial chemotypes. Most of the screened derivatives exhibited significant in vitro activity against the promastigote (IC50 from 0.59 ± 0.35 to 31 ± 1.27 μM) and intracellular amastigote forms (IC50 from 1.57 ± 0.12 to 17.6 ± 0.2 μM) of L. donovani, and their activity is comparable with standard drugs miltefosine and sodium stibogluconate. The most active compound 14t was further studied in vivo against the L. donovani/golden hamster model at a dose of 50 mg kg–1 through the intraperitoneal route for 5 consecutive days, which displayed 75.04 ± 7.28% inhibition of splenic parasite burden. Pharmacokinetics of compound 14t was studied in the golden Syrian hamster, and following a 50 mg kg–1 oral dose, the compound was detected in hamster serum for up to 24 h. It exhibited a large volume of distribution (651.8 L kg–1), high clearance (43.2 L h–1 kg–1) and long mean residence time (10 h).
Introduction
Leishmaniasis is one of the most neglected tropical human diseases caused by different species of genus Leishmania, a flagellate protozoan parasite transmitted by the bite of a tiny long (2–3 mm) insect vector, the phlebotomine sand fly.1 This disease may manifest itself in three basic clinical forms i.e. cutaneous (CL), mucocutaneous (MCL), and visceral leishmaniasis (VL) depending on the parasite species and the immune response of the host. Among them, visceral leishmaniasis (also known as kala-azar) is the most severe form of leishmaniasis, mainly caused by Leishmania donovani and is mainly endemic in Bangladesh, Ethiopia, India, Nepal, South Sudan and Sudan.2 VL often affects visceral organs such as the liver, spleen, and bone marrow and is usually fatal in more than 90% untreated cases.3 A recent World Health Organization (WHO) report indicates that 310 million people are at risk of contracting leishmaniasis, while 1.3 million new infections and 30 000 deaths take place annually.4 After infection in a mammalian body, the protozoa multiply within the phagolysosomes of macrophages as intracellular amastigotes where they cause dysfunction and the outcome of the infection depends on the production and/or secretion of immunosuppressive molecules including transforming growth factor (TGF)-β, interleukin (IL)-10, and prostaglandin E2 (PGE2).5 These molecules suppress host-protective microbicidal molecules, nitric oxide (NO), and reactive oxygen species (ROS) and cytokines interferon (IFN)-γ, IL-1, IL-12, and tumor necrosis factor-α (TNF-α).6
The present chemotherapy against leishmaniasis includes the first line drugs pentavalent antimonials (sodium stibogluconate and meglumine antimoniate), which has a long period of treatment associated with severe side effects including cardiac arrhythmia and pancreatitis.7 pentamidine and paromomycin drugs are restricted because they demonstrate renal, hepatic and pancreatic toxicity besides hypertension and dysglycemia.8 On the other hand, lipid formulation of amphotericin-B is highly effective but its high cost makes this unaffordable for poor people.9 Serendipitously discovered miltefosine is the first orally active drug having a long half-life (150–200 h) and due to its teratogenic effects, restricted for pregnant women.10 Thus, the chemotherapy against leishmaniasis is still inefficient; as a result, finding more effective and safer drugs for treating leishmaniasis remains desirable.
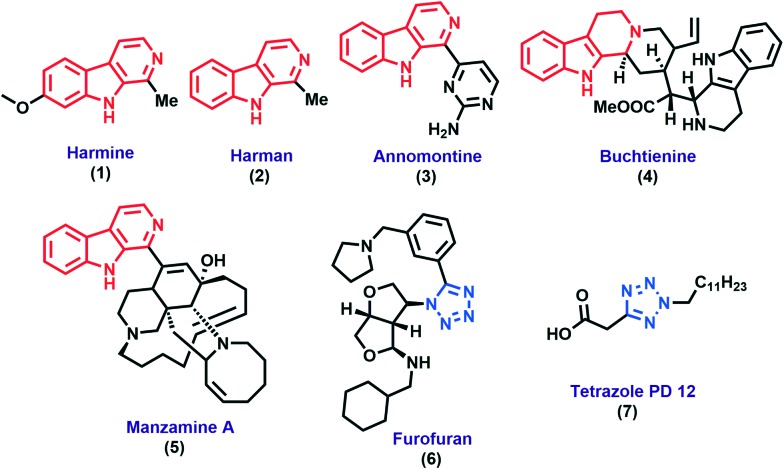
Nature is a rich source for the production of anti-infective agents, in which alkaloids have displayed potent antileishmanial activity for ages.11 A β-carboline prototype containing natural products and synthetic molecules has been reported for its antileishmanial activity, e.g. harmine (1), harman (2), annomontine (3), buchtienine (4), and manzamine-A (5) demonstrated significant antileishmanial activity (Fig. 1).12–14
Fig. 1. Some β-carboline based natural antileishmanial agents and tetrazole containing drugs.
On the other hand, tetrazole scaffolds have received great attention due to their wide range of biological activities,15–17and they are an important core of various modern drugs such as furofuran (6),18 tetrazole PD 12 (7)19etc.
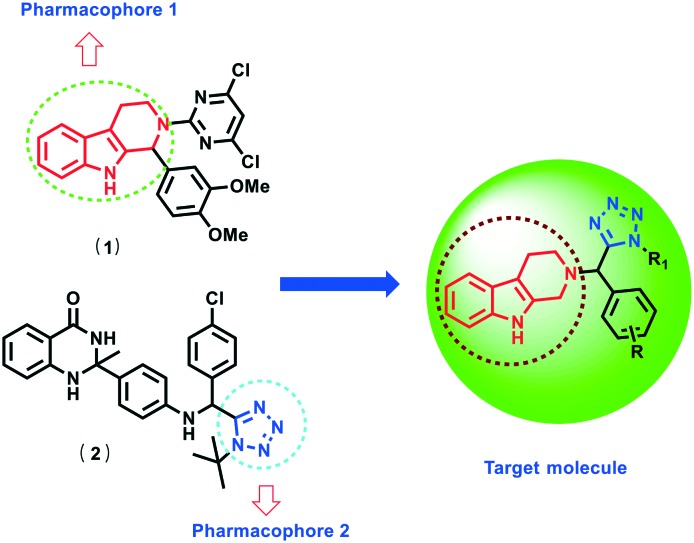
In continuation of our ongoing research on the anti-parasitic disease area, our research group had identified diverse β-carboline fused hybrid molecules as antileishmanial agents.20–24 Other groups across the world have also reported β-carboline based organic compounds as antileishmanial agents.25,26 Impressed by the biological activities of β-carboline based hybrid molecules, we have designed a hybrid framework containing two pharmacophore units, β-carboline and tetrazole nucleus (Fig. 2). In the present study, we synthesised tetrahydro-β-carboline–tetrazole hybrids and evaluated them as antileishmanial agents. The pharmacokinetics study and in silico prediction of the molecular properties of the compounds were also reported.
Fig. 2. Designing of a target molecule based on an active antileishmanial molecule.
Results and discussion
Chemistry
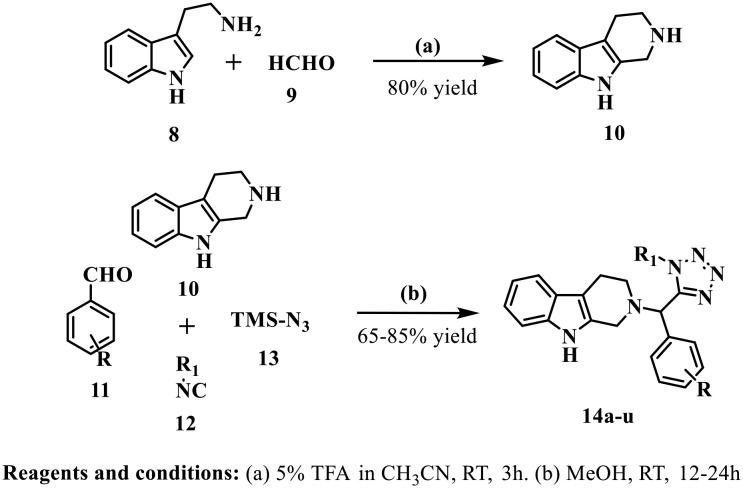
2,3,4,9-Tetrahydro-1H-pyrido[3,4-b]indole (10), an intermediate for the synthesis of the final compound, was obtained by Pictet–Spengler cyclization of the commercially available tryptamine (8) and formaldehyde (9).27 The final compounds were obtained via the simple and efficient Ugi four component reaction using 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (10) as the amine partner, aldehyde (11), isocyanide (12) and azidotrimethylsilane (TMS-N3) (13) in anhydrous methanol as solvent at room temperature for 12–24 h in good to excellent yield. The detailed synthetic route of 2,3,4,9-tetrahydro-β-carboline–tetrazoles (14a–u) is outlined in Scheme 1. The chemical structures of all synthesized derivatives were characterized by 1H-NMR, 13C-NMR, HRMS and IR spectroscopy.
Scheme 1. Synthesis of tetrahydro-β-carboline–tetrazole derivatives.
Biological assay
In vitro antileishmanial activity
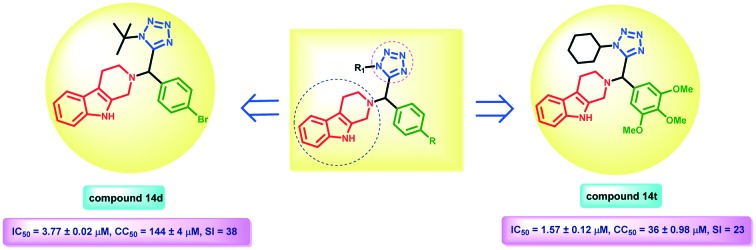
In an endeavor to discover novel antileishmanial β-carboline tetrazole hybrids, a series of 21 compounds have been synthesized and screened for their antileishmanial activity. The in vitro activity results are summarized in Table 1. All candidates were evaluated for their in vitro activity against the WHO reference strain (MHOM/IN/80/Dd8) and murine macrophages (expressing firefly luciferase reporter gene) for extracellular promastigote and intracellular amastigote forms of L. donovani, respectively. The in vitro cytotoxicity assay was performed using mouse macrophage cells (J-774A.1cell line), which were procured from the NCCS, Pune, India. Standard antileishmanial drugs, SSG and miltefosine, were included in the study as positive control drugs. Biological screening results indicate that the promastigote form of the parasite is more sensitive to the tetrazole hybrids than the amastigote form. As evident from Table 1, 18 derivatives showed potent to moderate activity against promastigote with IC50 values ranging from 0.59 ± 0.35 to 31 ± 1.27 μM. 13 compounds show high to moderate activity against amastigote with IC50 = 1.57 ± 0.12 to 17.6 ± 0.2 μM compared to the standard drug. The rest of the compounds were found to be almost inactive with IC50 values ranging from ≥25 to ≥100 μM. In the present study, our main aim was to investigate the impact of the substituent of the phenyl ring (R) and tetrazole ring (R1) on the activity; however, no obvious trend of activity with respect to the substituent was observed. Initially, we have synthesized derivative 14a with R1 as tert-butyl and R as the unsubstituted phenyl ring, which showed moderate anti-promastigote (IC50 = 4.07 ± 0.21 μM) activity and 1.5 times better anti-amastigote (IC50 = 5.41 ± 0.13 μM) activity than the standard drug with a significant SI (27). The para substituted halogens on R demonstrate variable activity, e.g. fluoro (14b), chloro (14c), and bromo (14d) derivatives have shown anti-promastigote (IC50 = 2.71 ± 1.34 μM, IC50 = 3.45 ± 0.42 μM, and IC50 = 1.58 ± 0.37 μM) activity and anti-amastigote (IC50 = 17.5 ± 1.27 μM, IC50 = 14.15 ± 0.05 μM, and IC50 = 3.77 ± 0.02 μM) activity, respectively. The strongly electron withdrawing nitro group on R for 14f showed excellent anti-promastigote activity (IC50 = 0.59 ± 0.35 μM) and potent anti-amastigote activity (IC50 = 3.78 ± 0.20 μM) with no toxicity for the J-774A.1 cell line (CC50 = 139.5 ± 1.5 μM). However, the isomeric meta-nitro derivative 14e was less active against the promastigote (IC50 = 4.91 ± 0.32 μM) and exhibited comparable activity against the amastigote (IC50 = 3.86 ± 0.11 μM) with lower toxicity (CC50 = 149.5 ± 1.5 μM) than compound 14f. The cyano group containing compound 14g displayed better anti-promastigote activity with IC50 = 2.82 ± 0.28 μM and poorer anti-amastigote (IC50 = 16.4 ± 0.1 μM) activity than miltefosine. Compound 14h having an electron donating para-methoxy substituent showed potent anti-promastigote (IC50 = 1.14 ± 0.28 μM) activity, as well as anti-amastigote (IC50 = 4.32 ± 0.33 μM) activity; nonetheless, increasing the number of methoxy substituents to dimethoxy for 14i and trimethoxy for 14j was less favorable resulting in a concomitant decrease in the antileishmanial activity (see Table 1). The para-isopropyl group containing derivative 14k displayed potent anti-promastigote (IC50 = 3.56 ± 0.08 μM) activity but was found to be less active for the amastigote form (IC50 = 17.6 ± 0.2 μM). Interestingly, replacement of tert-butyl of R1 by cyclohexyl was found beneficial in only a few cases, e.g.14m (R = 4-Cl), 14s (R = 3,4 di-OMe) and 14t (R = 3,4,5 tri-OMe), which were found to be more active with an enhanced selectivity index than their corresponding tert-butyl analogs. Compounds 14l, 14o, 14p and 14r were found to be active against the promastigote, but in the case of the amastigote, they were almost inactive as well as toxic. Unexpectedly, derivative 14u containing a hydroxyl substituent was found to be inactive. Similarly, compounds 14n and 14q were found to be almost inactive as well as toxic. Compound 14t was found to be the most active in the series having IC50 = 1.57 ± 0.12 μM. Even after extensive biological screening, it was very tough to conclude any clear relation between the biological activity and the substituent given the limited number of analogues. Compounds 14a, 14d, 14e, 14f, 14g, 14h, 14m, 14s, and 14t were found to be the most active among all the synthesized derivatives with no toxicity for the murine macrophage J-774A.1 cell line.
Table 1. In vitro antileishmanial activity of tetrahydro-β-carboline tetrazoles (14a–u) against L. donovani and cytotoxicity against the J774A.1 cell line.
| ||||||
| Entry | R | R1 | IC50 ± SEM
a
(μM) |
CC50 (μM) on J774A.1 cell line | SI b | |
| Anti-promastigote | Anti-amastigote | |||||
| 14a | 4-H | tert. Butyl | 4.07 ± 0.21 | 5.41 ± 0.13 | 146.5 ± 3.5 | 27 |
| 14b | 4-F | tert. Butyl | 2.71 ± 1.34 | 17.5 ± 1.27 | 48.8 ± 0.70 | 3 |
| 14c | 4-Cl | tert. Butyl | 3.45 ± 0.42 | 14.15 ± 0.05 | 36.95 ± 1.5 | 2 |
| 14d | 4-Br | tert. Butyl | 1.58 ± 0.37 | 3.77 ± 0.02 | 144 ± 4 | 38 |
| 14e | 3-NO2 | tert. Butyl | 4.91 ± 0.32 | 3.86 ± 0.11 | 149.5 ± 1.5 | 39 |
| 14f | 4-NO2 | tert. Butyl | 0.59 ± 0.35 | 3.78 ± 0.20 | 139.5 ± 1.5 | 37 |
| 14g | 4-CN | tert. Butyl | 2.82 ± 0.28 | 16.4 ± 0.1 | 149.5 ± 2.5 | 9 |
| 14h | 4-OMe | tert. Butyl | 1.14 ± 0.28 | 4.32 ± 0.33 | 41.95 ± 1.48 | 10 |
| 14i | 3,4 di-OMe | tert. Butyl | 5.71 ± 0.03 | 15.95 ± 0.05 | 138.5 ± 3.5 | 8 |
| 14j | 3,4,5 tri-OMe | tert. Butyl | 23.5 ± 0.96 | ≥50 | ND c | ND |
| 14k | 4-Isopropyl | tert. Butyl | 3.56 ± 0.08 | 17.6 ± 0.2 | 130 | 7 |
| 14l | 4-F | Cyclohexyl | 14.5 ± 0.84 | ≥25 | ND | ND |
| 14m | 4-Cl | Cyclohexyl | 2.89 ± 0.13 | 2.08 ± 0.11 | 35.5 ± 0.28 | 17 |
| 14n | 2-Cl | Cyclohexyl | ≥100 | ≥50 | ND | ND |
| 14o | 4-Br | Cyclohexyl | 16.5 ± 0.56 | ≥50 | ND | ND |
| 14p | 4-NO2 | Cyclohexyl | 31 ± 1.27 | ≥50 | ND | ND |
| 14q | 4-CN | Cyclohexyl | ≥100 | ≥50 | ND | ND |
| 14r | 4-OMe | Cyclohexyl | 20.35 ± 2.33 | ≥50 | ND | ND |
| 14s | 3,4 di-OMe | Cyclohexyl | 1.20 ± 0.16 | 3.31 ± 0.19 | 41.9 ± 0.14 | 13 |
| 14t | 3,4,5 tri-OMe | Cyclohexyl | 2.81 ± 0.08 | 1.57 ± 0.12 | 36 ± 0.98 | 23 |
| 14u | 4-OH | Cyclohexyl | NA d | NA | ND | ND |
| Miltefosine e | 1.05 ± 0.2 | 8.4 ± 2.1 | 12.42 ± 3.2 | 1.48 | ||
| SSG f | 947 ± 8.5 | 46.70 ± 2.8 | 297.38 ± 10.2 | 6.38 | ||
aIC50 (μM): concentration corresponding to 50% growth inhibition of the parasite. IC50 and CC50 values are the average of three independent assays expressed as average ± standard error.
bSelectivity index (SI): IC50 values of cytotoxic activity/IC50 values of the anti-amastigote antileishmanial activity.
cND: not determined.
dNA: not available.
eMiltefosine: used as the standard.
fSSG: sodium stibogluconate: used as the standard.
In continuation of the SAR study of β-carboline core compounds, it was revealed for earlier synthesised compounds20–22,26 that their activity was due to the substitution at C-1 of the β-carboline ring system. Meanwhile in the case of our study, most of the compounds (14d, 14e, 14f, 14m, 14s, 14t) have shown potent in vitro antileishmanial activity with the unsubstituted C-1 position of the saturated β-carboline ring.
To optimise the antileishmanial activity of 2,3,4,9-tetrahydro β-carboline and the effect of substituted or unsubstituted phenyl at C-1 of 2,3,4,9-tetrahydro β-carboline on the activity, we further synthesise C-1 substituted β-carboline and its activity will be discussed in our next project.
In vivo antileishmanial activity
On the basis of the in vitro potency results, we selected those compounds having SI = >5 for further evaluation; accordingly, the in vivo drug response potency measurements of those compounds were performed in the golden hamster model (splenic parasitic burden) at a dosage of 50 mg kg–1 through the intraperitoneal (IP) route for 5 consecutive days. All experiments were performed in compliance with the relevant laws and the Institutional Animal Ethics Committee (IAEC) guidelines approved by the National Laboratory Animal Center (NLAC) of CSIR-CDRI, Lucknow. Male hamsters weighing 40–45 g were used in the study, housed at 23 ± 2 °C, with 60 to 63% humidity and fed with standard rodent pellets and fresh drinking water. Miltefosine was used as the standard drug for this study. Compound 14t showed promising inhibition (75.04 ± 7.28%) against the Leishmania parasite, and the rest of the compounds showed poor inhibition. Although compound 14t in the IP route showed promising activity, due to the limitation of IP administration, we further screened 14t through the oral route at a dosage of 100 mg kg–1 for 5 consecutive days, showing moderate inhibition (50.85 ± 8.67%) (Table 2).
Table 2. In vivo antileishmanial activity of some compounds against the L. donovani/golden hamster model.
| Percent inhibition ± SD |
||||
| Entry | Treatment results |
Route of adminstration | Dose | |
| 7th day | 28th day | |||
| 14a | 4.05 ± 2.12 | 3.79 ± 1.46 | IP | 50 mg kg–1 |
| 14d | 33.68 ± 7.80 | 53.06 ± 10.42 | IP | 50 mg kg–1 |
| 14e | 4.83 ± 3.34 | 2.92 ± 0.14 | IP | 50 mg kg–1 |
| 14f | 20.41 ± 3.99 | 19.62 ± 8.50 | IP | 50 mg kg–1 |
| 14t | 75.04 ± 7.28 | 88.92 ± 8.56 | IP | 50 mg kg –1 |
| 14t | 50.85 ± 8.67 | 77.45 ± 6.83 | Oral | 100 mg kg –1 |
| Miltefosine | 98.1 ± 1.2 | Oral | 30 mg kg–1 | |
Pharmacokinetics study
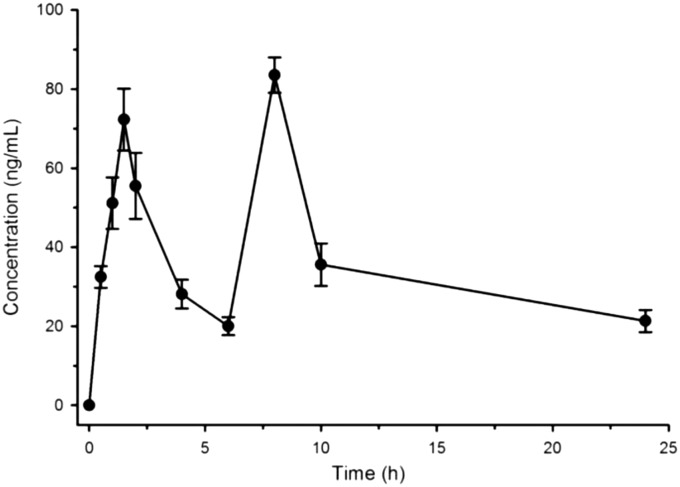
Encouraged by the in vitro and in vivo activity of compound 14t, we have performed the in vivo pharmacokinetic study for 14t. The animals used in this study tolerated the treatment, as no peculiarities in their behaviour were observed. Chromatographic separations and quantification of the compounds in serum was achieved by a rapid, sensitive and validated liquid chromatography–tandem mass spectrometry method using electrospray ionization. Following a 50 mg kg–1 oral dose, the compound was monitored for up to 24 h (Fig. 3). Based on the multiple peak phenomenon in the PK profile of any drug, enterohepatic recirculation is mostly responsible for the multiple (two) peak phenomenon. It occurs by biliary excretion and intestinal reabsorption, sometimes with hepatic conjugation and intestinal deconjugation. Enterohepatic recirculation leads to prolonged elimination half-life of the drugs. In the present study, long mean residence time and the two peaks in the PK profile of compound 14t indicate its probable enterohepatic recirculation;28 the serum concentration–time profile was subjected to non-compartmental analysis using Phoenix WinNonlin (version 6.3; Certara Inc, Missouri, USA), and the calculated pharmacokinetic parameters are shown in Table 3. The volume of distribution (Vd/F) of 14t (651.8 ± 59.8 L kg–1) was higher than the total blood volume (0.087 L kg–1) of the hamster, indicating extra vascular distribution. Its clearance (43.2 ± 4.3 L h–1 kg–1) is also higher than the hepatic blood flow (0.39 L h–1) of the hamster, indicating extra hepatic elimination.
Fig. 3. Mean serum concentration–time profile of 14t after the 50 mg kg–1 oral dose in golden Syrian hamsters (n = 5). The bar represents the SEM.
Table 3. Pharmacokinetic parameters of 14t after the 50 mg kg–1 oral dose in golden Syrian hamsters a .
| Parameters | Estimate | |
| C max (ng mL–1) | 1 | 76.2 ± 5.4 |
| 2 | 83.5 ± 4.0 | |
| t max (h) | 1 | 1.6 ± 0.1 |
| 2 | 8.0 ± 0.0 | |
| AUClast (ng h–1 mL–1) | 844.5 ± 54.5 | |
| MRT (h) | 10.2 ± 2.0 | |
| Cl/F (L h–1 kg–1) | 43.2 ± 4.3 | |
| Vd/F (L kg–1) | 651.8 ± 59.8 |
aEach value represents the average for five hamsters dosed orally (50 mg kg–1); the values are mean ± SEM.
Molecular properties
The molecular properties of any compound play a crucial role in absorption, distribution, metabolism, excretion and toxicity (ADMET). In silico prediction of the molecular properties of tetrahydro-β-carboline tetrazole compounds was done by Molinspiration Cheminformatics (; http://www.molinspiration.com). Those compounds that follow Lipinski's “rule of five” parameters such as milog P (≤5), molecular weight (≤500), number of hydrogen bond acceptors (≤5) and number of hydrogen bond donors (≤10) did not suffer from a bioavailability problem.29 This rule is used as a filter for the prediction of drug-like properties of a molecule. In our synthesised compound series, some compounds did not satisfy one of the Lipinski's parameters i.e., (a) in case of 14k, 14m, 14n, and 14o, their milog P value was greater than 5, and (b) for 14t, its molecular weight was greater than 500 (Table 4). So, from the in silico prediction of molecular properties, it was concluded that most of the compounds might not have any bioavailability problems.
Table 4. Predicted in silico molecular properties of the antileishmanial compounds.
| Entry | Mol. wt | Milog P | HBA a | HBD b | Entry | Mol. wt | Milog P | HBA a | HBD b |
| 14a | 386.50 | 4.02 | 1 | 6 | 14j | 476.58 | 3.56 | 1 | 9 |
| 14b | 404.49 | 4.18 | 1 | 6 | 14k | 428.58 | 5.53 | 1 | 6 |
| 14c | 420.95 | 4.70 | 1 | 6 | 14l | 430.53 | 4.90 | 1 | 6 |
| 14d | 465.40 | 4.83 | 1 | 6 | 14m | 446.99 | 5.42 | 1 | 6 |
| 14e | 385.50 | 3.12 | 1 | 6 | 14o | 491.44 | 5.55 | 1 | 6 |
| 14f | 385.50 | 3.15 | 1 | 6 | 14p | 411.53 | 3.87 | 1 | 6 |
| 14g | 399.50 | 3.55 | 1 | 7 | 14r | 442.57 | 4.80 | 1 | 7 |
| 14h | 416.53 | 4.08 | 1 | 7 | 14s | 472.59 | 4.39 | 1 | 8 |
| 14i | 446.56 | 3.67 | 1 | 8 | 14t | 502.62 | 4.37 | 1 | 9 |
| Miltefosine | 407.58 | 0.12 | 1 | 5 |
aHydrogen bond acceptor.
bHydrogen bond donor.
Experimental
Chemistry: general procedure
All reagents were commercially available from Sigma Aldrich and were used without further purification. Chromatography was carried out on silica gel (100–200 mesh). All reaction progress was routinely monitored by TLC on pre-coated silica gel aluminium plates. Melting points were taken in open capillaries using melting point apparatus containing silicon oil and are uncorrected. Infrared spectra were recorded using a FTIR spectrometer and are recorded in terms of the frequency of absorption (cm–1). 1H NMR and 13C NMR spectra were recorded using a 400 MHz (all signals are reported in ppm with the internal chloroform signal at 7.28 ppm as the standard) spectrometer and a 100 MHz (all signals are reported in ppm with the internal chloroform signal at 77.21 ppm as the standard) spectrometer respectively with tetramethylsilane (TMS) as the internal standard. Coupling constants (J) are reported in hertz (Hz). Multiplicities are reported as follows: singlet (s), doublet (d), triplet (t), multiplet (m), and broad singlet (bs). Electrospray ionization mass spectra (ESIMS) were recorded using a Thermo Lcq Advantage Max-IT. High resolution mass spectra (HRMS) were recorded as ESI-HRMS using a Q-TOF, LC-MS/MS mass spectrometer. The purity of the final compounds was determined by analytical HPLC, which was carried out using a Water/ACN HPLC system (model pump: 515, detector PDA-2998). HPLC analysis conditions: Merck C18 (5.0 μM), 4.6 × 250 mm column and a flow rate of 0.8 mL min–1. All biologically evaluated compounds are ≥95% pure.
General procedure (GP) for the synthesis of compounds 14a–u
To a round bottomed flask, the stirred methanolic (1 M) solution of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (10) (1 equiv.) and the corresponding benzaldehyde (1 equiv.) was added followed by addition of tert-butyl or cyclohexyl isocyanide (1 equiv.) and TMSN3 (2 equiv.). This reaction mixture was allowed to run for 12–24 h under a N2 atmosphere at room temperature. The progress of the reaction was monitored by TLC. After the completion of the reaction, the solvent was evaporated under reduced pressure. Then, the crude reaction mixture was diluted with DCM (20 mL) and washed with brine (10 mL). The aqueous layer was extracted with DCM (2 × 10 mL). The combined organic layer was dried over anhydrous Na2SO4 and evaporated to dryness, and the corresponding desired products (14a–u) were obtained by column chromatography using 100–200 mesh silica gels.
2-((1-(tert-Butyl)-1H-tetrazol-5-yl)(phenyl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (14a)
According to GP, 14a was obtained by the reaction of methanolic (1 M, 3 mL) solution of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 516 mg, 3 mmol), benzaldehyde (1 equiv., 318 mg, 3 mmol), tert-butyl isocyanide (1 equiv., 249.4 mg, 3 mmol) and TMSN3 (2 equiv., 691 mg, 3 mmol) for 12 h with 66% yield (0.66 mmol, 255 mg) as a white solid; m.p. = 210–213 °C; IR (KBr), νmax: 669, 757, 1215, 1584, 3020, 3368 cm–1; HPLC-PDA: tr = 21.63 min (% area = 95.33%); 1H NMR (400 MHz, CDCl3) δ: 7.71 (bs, 1H, HNH), 7.50–7.44 (m, 3H, HAr), 7.40–7.37 (t, J = 5.00 Hz, 3H, HAr), 7.26 (s, 1H, HAr), 7.14–7.06 (m, 2H, HAr), 5.69 (s, 1H, HCH), 4.15 (d, J = 15.10 Hz, 1H, HCH2), 3.71 (d, J = 14.72 Hz, 1H, HCH2), 3.14–3.08 (m, 1H, HCH2), 3.02–2.96 (m, 1H, HCH2), 2.80–2.77 (t, J = 5.72 Hz, 2H, HCH2), 1.70 (s, 9H, H3×CH3) ppm; 13C NMR (100 MHz, CDCl3): 154.37 (C-5), 136.07 (C–Ar), 135.53 (C–Ar), 131.58 (C–Ar), 129.81 (C–Ar), 128.67 (C–Ar), 127.19 (C–Ar), 121.37 (C–Ar), 119.30 (C–Ar), 117.87 (C–Ar), 110.67 (C–Ar), 108.16 (C–Ar), 63.87 (C–C(Me)3), 61.60 (C–CH), 48.38 (C–CH2), 46.80 (C–CH2), 30.28 (C–CH3), 21.26 (C–CH2) ppm; HRMS (EI) calcd. for [C23H26N6 + H]+ 387.2297; found 387.2290.
2-((1-(tert-Butyl)-1H-tetrazol-5-yl)(4-fluorophenyl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (14b)
According to GP, 14b was obtained by the reaction of methanolic (1.0 M, 3.0 mL) solution of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 533 mg, 3.1 mmol), 4-fluorobenzaldehyde (1 equiv., 384 mg, 3.1 mmol), tert-butyl isocyanide (1 equiv., 257 mg, 3.1 mmol) and TMSN3 (2 equiv., 357 mg, 3.1 mmol) for 13 h with 70% yield (0.70 mmol, 283 mg) as a white solid; m.p. = 188–191 °C; IR (KBr), νmax: 669, 754, 1215, 1530, 3021, 3368 cm–1; HPLC-PDA: tr = 15.61 min (% area = 99.40%); 1H NMR (400 MHz, CDCl3) δ: 7.69 (bs, 1H, HNH), 7.51–7.45 (m, 4H, HAr), 7.15–7.10 (m, 1H, HAr), 7.09–7.08 (t, J = 1.44 Hz, 3H, HAr), 5.67 (s, 1H, HCH), 4.11 (d, J = 14.88 Hz, 1H, HCH2), 3.70 (d, J = 13.96 Hz, 1H, HCH2), 3.12–3.06 (m, 1H, HCH2), 3.00–2.94 (m, 1H, HCH2), 280–2.77 (t, J = 5.64 Hz, 2H, HCH2), 1.71 (s, 9H, H3×CH3) ppm; 13C NMR (100 MHz, CDCl3): 154.27 (C-5), 136.06 (C–Ar), 131.56 (C–Ar), 131.47 (C–Ar), 131.37 (C–Ar), 131.33 (C–Ar), 127.22 (C–Ar), 121.36 (C–Ar), 119.40 (C–Ar), 117.89 (C–Ar), 115.79 (C–Ar), 115.54 (C–Ar), 110.71 (C–Ar), 108.07 (C–Ar), 63.10 (C–C(Me)3), 61.73 (C–CH), 48.15 (C–CH2), 46.88 (C–CH2), 30.22 (C–CH3), 21.49 (C–CH2) ppm; HRMS (EI) calcd. for [C23H25FN6 + H]+ 405.2203; found 405.2205.
2-((1-(tert-Butyl)-1H-tetrazol-5-yl)(4-chlorophenyl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (14c)30
According to GP, 14c was obtained by the reaction of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 344 mg, 2 mmol), 4-chlorobenzaldehyde (1 equiv., 281 mg, 2 mmol), tert-butyl isocyanide (1 equiv., 166 mg, 2 mmol), and TMSN3 (2 equiv., 461 mg, 2 mmol) in methanol (1.0 M, 2.5 mL) as solvent for 15 h with 66% yield (0.66 mmol, 277 mg) as a white solid; m.p. = 100–103 °C; IR (KBr), νmax: 670, 756, 1214, 1586, 3020, 3368 cm–1; HPLC-PDA: tr = 12.80 min (% area = 97.92%); 1H NMR (400 MHz, CDCl3) δ: 7.71 (bs, 1H, HNH), 7.68–7.45 (m, 2H, HAr), 7.44–7.43 (t, J = 2.04 Hz, 2H, HAr), 7.38–7.36 (m, 2H, HAr), 7.16–7.07 (m, 2H, HAr), 5.67 (s, 1H, HCH), 4.12 (d, J = 14.72 Hz, 1H, HCH2), 3.70 (d, J = 14.72 Hz, 1H, HCH2), 3.12–3.06 (m, 1H, HCH2), 3.00–2.94 (m, 1H, HCH2), 2.80–2.77 (t, J = 6.56 Hz, 2H, HCH2), 1.71 (s, 9H, H3×CH3) ppm; 13C NMR (100 MHz, CDCl3): 154.05 (C-5), 136.05 (C–Ar), 134.74 (C–Ar), 134.10 (C–Ar), 131.34 (C–Ar), 131.09 (C–Ar), 128.87 (C–Ar), 127.05 (C–Ar), 121.37 (C–Ar), 119.40 (C–Ar), 117.92 (C–Ar), 110.72 (C–Ar), 108.11 (C–Ar), 63.08 (C–C(Me)3), 61.70 (C–CH), 48.18 (C–CH2), 46.85 (C–CH2), 30.23 (C–CH3), 21.50 (C–CH2) ppm; HRMS (EI) calcd. for [C23H25ClN6 + H]+ 421.1907; found 421.1900.
2-((4-Bromophenyl)(1-(tert-butyl)-1H-tetrazol-5-yl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (14d)
According to GP, 14d was obtained by the reaction of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 344 mg, 2 mmol), 4-bromobenzaldehyde (1 equiv., 368 mg, 2 mmol), tert-butyl isocyanide (1 equiv., 166 mg, 2 mmol) and TMSN3 (2 equiv., 461 mg, 2 mmol) in methanol (1.0 M, 3 mL) as solvent for 16 h with 71% yield (0.71 mmol, 329 mg) as a white solid. m.p. = 193–196 °C; IR (KBr), νmax: 668, 754, 1214, 1580, 3015, 3369 cm–1; HPLC-PDA: tr = 23.09 min (% area = 95.00%); 1H NMR (400 MHz, CDCl3) δ: 7.64 (bs, 1H, HNH), 7.54–7.52 (m, 2H, HAr), 7.47 (d, J = 7.44 Hz, 1H, HAr), 7.39 (d, J = 8.52 Hz, 2H, HAr), 7.31 (d, J = 6.56 Hz, 1H, HAr), 7.16–7.12 (t, J = 7.12 Hz, 1H, HAr), 7.11–7.07 (t, J = 7.28 Hz, 1H, HAr), 5.66 (s, 1H, HCH), 4.12 (d, J = 13.80 Hz, 1H, HCH2), 3.70 (d, J = 14.88 Hz, 1H, HCH2), 3.12–3.07 (m, 1H, HCH2), 3.00–2.94 (m, 1H, HCH2), 2.80–2.77 (t, J = 5.68 Hz, 2H, HCH2), 1.71 (s, 9H, H3×CH3) ppm; 13C NMR (100 MHz, CDCl3): 153.87 (C-5), 136.02 (C–Ar), 134.64 (C–Ar), 131.83 (C–Ar), 131.38 (C–Ar), 130.89 (C–Ar), 128.87 (C–Ar), 127.09 (C–Ar), 122.90 (C–Ar), 121.42 (C–Ar), 119.40 (C–Ar), 117.92 (C–Ar), 110.77 (C–Ar), 108.15 (C–Ar), 63.22 (–C(Me)3), 62.14 (CH), 48.23 (CH2), 46.75 (CH2), 30.33 (3 × CH3), 21.50 (CH2) ppm; HRMS (EI) calcd. for [C23H25BrN6 + H]+ 465.1402; found 465.1403.
2-((1-(tert-Butyl)-1H-tetrazol-5-yl)(3-nitrophenyl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (14e)
According to GP, 14e was obtained by the reaction of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 361 mg, 2.1 mmol), 3-nitrobenzaldehyde (1 equiv., 317 mg, 2.1 mmol), tert-butyl isocyanide (1 equiv., 174 mg, 2.1 mmol) and TMSN3 (2 equiv., 484 mg, 2.1 mmol) in methanol (1.0 M, 3 mL) as solvent for 18 h with 69% yield (0.69 mmol, 298 mg) as a yellow solid; m.p. = 95–98 °C; IR (KBr), νmax: 669, 755, 1215, 1520, 3012, 3368 cm–1; HPLC-PDA: tr = 18.02 min (% area = 95.40%); 1H NMR (400 MHz, CDCl3) δ: 8.41 (t, J = 1.40 Hz, 1H, HAr), 8.26 (d, J = 8.48 Hz, 1H, HAr), 8.01 (d, J = 7.60 Hz, 1H, HAr), 7.71 (s, 1H, HNH), 7.63–7.59 (t, J = 8.00 Hz, 1H, HAr), 7.47 (d, J = 8.00 Hz, 1H, HAr), 7.30 (d, J = 4.44 Hz, 1H, HAr), 7.16–7.13 (t, J = 7.12 Hz, 1H, HAr), 7.11–7.08 (t, J = 7.60 Hz, 1H, HAr), 5.79 (s, 1H, HCH), 4.06 (d, J = 14.52 Hz, 1H, HCH2), 3.75 (d, J = 14.36 Hz, 1H, HCH2), 3.13–3.07 (m, 1H, HCH2), 3.00–2.94 (m, 1H, HCH2), 2.82–2.79 (m, 2H, HCH2), 1.77 (s, 9H, H3×CH3) ppm; 13C NMR (100 MHz, CDCl3): 153.04 (C-5), 148.30 (C–Ar), 137.50 (C–Ar), 136.07 (C–Ar), 135.87 (C–Ar), 130.75 (C–Ar), 129.66 (C–Ar), 127.05 (C–Ar), 124.58 (C–Ar), 123.74 (C–Ar), 121.67 (C–Ar), 119.55 (C–Ar), 117.97 (C–Ar), 110.77 (C–Ar), 108.21 (C–Ar), 63.18 (–C(Me)3), 62.09 (CH), 48.14 (CH2), 46.75 (CH2), 30.28 (3 × CH3), 21.65 (CH2) ppm; HRMS (EI) calcd. for [C23H25N7O2 + H]+ 432.2148; found 432.2141.
2-((1-(tert-Butyl)-1H-tetrazol-5-yl)(4-nitrophenyl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (14f)
According to GP, 14f was synthesized by the reaction of solution of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 361 mg, 2.1 mmol), 4-nitrobenzaldehyde (1 equiv., 317 mg, 2.1 mmol), tert-butyl isocyanide (1 equiv., 174 mg, 2.1 mmol) and TMSN3 (2 equiv., 484 mg, 2.1 mmol) in methanol (1.0 M, 2 mL) as solvent for 20 h with 72% yield (0.72 mmol, 310 mg) as a yellow solid; m.p. = 178–181 °C; IR (KBr), νmax: 669, 758, 1215, 1584, 3019, 3369 cm–1; HPLC-PDA: tr = 18.06 min (% area = 99.17%); 1H NMR (400 MHz, CDCl3) δ: 8.27 (d, J = 8.92 Hz, 2H, HAr), 7.75 (d, J = 8.92 Hz, 2H, HAr), 7.65 (bs, 1H, HNH), 7.47 (d, J = 7.64 Hz, 1H, HAr), 7.30 (s, 1H, HAr), 7.17–7.08 (m, 2H, HAr), 5.81 (s, 1H, HCH), 4.12 (d, J = 14.04 Hz, 1H, HCH2), 3.72 (d, J = 14.68 Hz, 1H, HCH2), 3.15–3.09 (m, 1H, HCH2), 3.00–2.95 (m, 1H, HCH2), 2.82–2.79 (t, J = 6.36 Hz, 2H, HCH2), 1.75 (s, 9H, H3×CH3) ppm; 13C NMR (100 MHz, CDCl3): 153.09 (C-5), 148.06 (C–Ar), 142.73 (C–Ar), 136.12 (C–Ar), 130.70 (C–Ar), 126.95 (C–Ar), 123.69 (C–Ar), 121.62 (C–Ar), 119.45 (C–Ar), 117.97 (C–Ar), 110.67 (C–Ar), 108.11 (C–Ar), 63.03 (–C(Me)3), 62.04 (CH), 48.23 (CH2), 46.85 (CH2), 30.31 (3 × CH3), 21.63 (CH2) ppm; HRMS (EI) calcd. for [C23H25N7O2 + H]+ 432.2148; found 432.2149.
4-((1-(tert-Butyl)-1H-tetrazol-5-yl)(1,3,4,9-tetrahydro-2H-pyrido[3,4-b]indol-2-yl)methyl)benzonitrile (14g)
According to GP, 14g was synthesized by the reaction of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 258 mg, 1.5 mmol), 4-cyanobenzaldehyde (1 equiv., 196 mg, 1.5 mmol), tert-butyl isocyanide (1 equiv., 124 mg, 1.5 mmol) and TMSN3 (2 equiv., 346 mg, 1.5 mmol) in methanol (1.0 M, 1.5 mL) as solvent for 21 h with 78% yield (0.78 mmol, 321 mg) as a white solid; m.p. = 75–78 °C; IR (KBr), νmax: 669, 758, 1215, 1580, 3022, 3369 cm–1; HPLC-PDA: tr = 19.00 min (% area = 96.60%); 1H NMR (400 MHz, CDCl3) δ: 7.74 (bs, 1H, HNH), 7.71–7.65 (m, 4H, HAr), 7.47 (d, J = 7.96 Hz, 1H, HAr), 7.29 (d, J = 7.80 Hz, 1H, HAr), 7.16–7.12 (t, J = 7.08 Hz, 1H, HAr), 7.11–7.07 (t, J = 7.64 Hz, 1H, HAr), 5.75 (s, 1H, HCH), 4.09 (d, J = 14.16 Hz, 1H, HCH2), 3.69 (d, J = 14.20 Hz, 1H, HCH2), 3.12–3.07 (m, 1H, HCH2), 2.98–2.92 (m, 1H, HCH2), 2.80–2.77 (t, J = 6.04 Hz, 2H, HCH2), 1.73 (s, 9H, H3×CH3) ppm; 13C NMR (100 MHz, CDCl3): 153.23 (C-5), 140.86 (C–Ar), 136.07 (C–Ar), 132.37 (C–Ar), 130.89 (C–Ar), 130.45 (C–Ar), 127.05 (C–Ar), 121.57 (C–Ar), 119.55 (C–Ar), 118.22 (C–Ar), 117.92 (C–Ar), 112.79 (C–Ar), 110.82 (C–Ar), 108.11 (C–Ar), 63.28 (–C(Me)3), 61.99 (CH), 48.23 (CH2), 46.71 (CH2), 30.33 (3 × CH3), 21.60 (CH2) ppm; HRMS (EI) calcd. for [C24H25N7 + H]+ 412.2250; found 412.2253.
2-((1-(tert-Butyl)-1H-tetrazol-5-yl)(4-methoxyphenyl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (14h)
According to GP, 14g was synthesized by the reaction of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 258 mg, 1.5 mmol), 4-methoxybenzaldehyde (1 equiv., 204 mg, 1.5 mmol), tert-butyl isocyanide (1 equiv., 124 mg, 1.5 mmol) and TMSN3 (2 equiv., 346 mg, 1.5 mmol) in methanol (1.0 M, 3 mL) as solvent for 24 h with 75% yield (0.75 mmol, 312 mg) as a white solid; m.p. = 108–111 °C; IR (KBr), νmax: 669, 754, 1215, 2400, 3018, 3367 cm–1; HPLC-PDA: tr = 12.61 min (% area = 96.34%); 1H NMR (400 MHz, CDCl3) δ: 7.70 (bs, 1H, HNH), 7.47–7.44 (m, 1H, HAr), 7.41–7.36 (m, 3H, HAr), 7.14–7.12 (m, 1H, HAr), 7.11–7.06 (m, 1H, HAr), 6.91 (d, J = 8.48 Hz, 2H, HAr), 5.24 (s, 1H, HCH), 4.11 (d, J = 14.68 Hz, 1H, HCH2), 3.82 (s, 3H, H–OCH3), 3.71 (d, J = 14.68 Hz, 1H, HCH2), 3.12–3.06 (m, 1H, HCH2), 3.01–2.96 (m, 1H, HCH2), 2.80–2.76 (m, 2H, HCH2), 1.69 (s, 9H, H3×CH3) ppm; 13C NMR (100 MHz, CDCl3): 154.72 (C-5), 139.82 (C–Ar), 136.09 (C–Ar), 131.69 (C–Ar), 131.01 (C–Ar), 127.49 (C–Ar), 121.23 (C–Ar), 119.23 (C–Ar), 117.84 (C–Ar), 114.01 (C–Ar), 110.75 (C–Ar), 109.13 (C–Ar), 108.05 (C–Ar), 63.20 (–C(Me)3), 61.57 (CH), 55.30 (–OCH3), 48.21 (CH2), 46.91 (CH2), 30.18 (3 × CH3), 21.40 (CH2) ppm; HRMS (EI) calcd. for [C24H28N6O + H]+ 417.2403; found 417.2417.
2-((1-(tert-Butyl)-1H-tetrazol-5-yl)(3,4-dimethoxyphenyl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (14i)
According to GP, 14i was obtained by the reaction of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 206 mg, 1.2 mmol), 3,4-dimethoxybenzaldehyde (1 equiv., 199 mg, 1.2 mmol), tert-butyl isocyanide (1 equiv., 100 mg, 1.2 mmol) and TMSN3 (2 equiv., 276 mg, 1.2 mmol) in methanol (1.0 M, 1 mL) as solvent for 24 h with 70% yield (0.70 mmol, 312) as a white solid; m.p. = 107–110 °C; IR (KBr), νmax: 669, 758, 1216, 1578, 3021, 3368 cm–1; HPLC-PDA: tr = 12.85 min (% area = 98.82%); 1H NMR (400 MHz, CDCl3) δ: 7.65 (s, 1H, HNH), 7.48 (d, J = 7.80 Hz, 1H, HAr), 7.30 (d, J = 7.80 Hz, 1H, HAr), 7.20 (d, J = 1.60 Hz, 1H, HAr), 7.16–7.12 (t, J = 7.08 Hz, 1H, HAr), 7.11–7.07 (t, J = 7.28 Hz, 1H, HAr), 6.83 (s, 2H, HAr), 5.60 (s, 1H, HCH), 4.09 (d, J = 15.24 Hz, 1H, HCH2), 3.90 (s, 3H, H–OCH3), 3.88 (s, 3H, H–OCH3), 3.72 (d, J = 13.84 Hz, 1H, HCH2), 3.13–3.07 (m, 1H, HCH2), 3.03–2.98 (m, 1H, HCH2), 2.81–2.78 (t, J = 5.32 Hz, 2H, HCH2), 1.70 (s, 9H, H3×CH3) ppm; 13C NMR (100 MHz, CDCl3): 154.77 (C-5), 149.34 (C–Ar), 136.02 (C–Ar), 131.58 (C–Ar), 128.18 (C–Ar), 127.15 (C–Ar), 122.16 (C–Ar), 121.37 (C–Ar), 119.35 (C–Ar), 117.87 (C–Ar), 112.50 (C–Ar), 110.72 (C–Ar), 110.48 (C–Ar), 108.16 (C–Ar), 63.47 (–C(Me)3), 61.60 (CH), 56.13 (–OCH3), 55.88 (OCH3), 48.14 (CH2), 47.10 (CH2), 30.18 (3 × CH3), 21.31 (CH2) ppm; HRMS (EI) calcd. for [C25H30N6O2 + H]+ 447.2508; found 447.2518.
2-((1-(tert-Butyl)-1H-tetrazol-5-yl)(3,4,5-trimethoxyphenyl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (14j)
According to GP, 14j was obtained by the reaction of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 86 mg, 0.5 mmol), 3,4,5-trimethoxybenzaldehyde (1 equiv., 98 mg, 0.5 mmol), tert-butyl isocyanide (1 equiv., 41 mg, 0.5 mmol) and TMSN3 (2 equiv., 115 mg, 0.5 mmol) in methanol (1.0 M, 1.5 mL) as solvent for 18 h with 82% yield (0.82 mmol, 390 mg) as a white solid; m.p. = 143–146 °C; IR (KBr), νmax: 669, 754, 1214, 1520, 3020, 3369 cm–1; HPLC-PDA: tr = 9.52 min (% area = 98.43%); 1H NMR (400 MHz, CDCl3) δ: 7.80 (bs, 1H, HNH), 7.48 (d, J = 7.24 Hz, 1H, HAr), 7.29 (d, J = 7.24 Hz, 1H, HAr), 7.13–7.07 (m, 2H, HAr), 6.71 (s, 2H, HAr), 5.57 (s, 1H, HCH), 4.08 (d, J = 13.40 Hz, 1H, HCH2), 3.87 (s, 3H, H–OCH3), 3.83 (s, 6H, H2×–OCH3), 3.73 (d, J = 14.68 Hz, 1H, HCH2), 3.13–3.07 (m, 1H, HCH2), 3.04–2.98 (m, 1H, HCH2), 2.82–2.79 (t, J = 10.80 Hz, 2H, HCH2), 1.72 (s, 9H, H3×CH3) ppm; 13C NMR (100 MHz, CDCl3): 154.60 (C-5), 153.30 (C–Ar), 138.17 (C–Ar), 136.07 (C–Ar), 131.49 (C–Ar), 131.29 (C–Ar), 127.17 (C–Ar), 121.34 (C–Ar), 119.32 (C–Ar), 117.86 (C–Ar), 110.77 (C–Ar), 108.02 (C–Ar), 106.94 (C–Ar), 106.79 (C–Ar), 63.72 (–C(Me)3), 61.60 (CH), 60.86 (–OCH3), 56.32 (2 × –OCH3), 48.23 (CH2), 47.15 (CH2), 30.28 (3 × CH3), 21.26 (CH2) ppm; HRMS (EI) calcd. for [C26H32N6O3 + H]+ 477.2614; found 477.2612.
2-((1-(tert-Butyl)-1H-tetrazol-5-yl)(4-isopropylphenyl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (14k)
According to GP, 14k was obtained by the reaction of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 120 mg, 0.7 mmol), 4-isopropylbenzaldehyde (1 equiv., 104 mg, 0.7 mmol), tert-butyl isocyanide (1 equiv., 58 mg, 0.7 mmol) and TMSN3 (2 equiv., 161 mg, 0.7 mmol) in methanol (1.0 M, 1 mL) as solvent for 12 h with 72% yield (0.72 mmol, 308 mg) as a white solid; m.p. = 105–108 °C; IR (KBr), νmax: 668, 756, 1216, 1589, 3022, 3368 cm–1; HPLC-PDA: tr = 12.78 min (% area = 96.60%); 1H NMR (400 MHz, CDCl3) δ: 7.67 (s, 1H, HNH), 7.46 (d, J = 7.88 Hz, 1H, HAr), 7.42 (d, J = 7.84 Hz, 2H, HAr), 7.27–7.26 (m, 1H, HAr), 7.24–7.22 (m, 2H, HAr), 7.14–7.10 (t, J = 7.36 Hz, 1H, HAr), 7.102–7.06 (t, J = 7.76 Hz, 1H, HAr), 5.64 (s, 1H, HCH), 4.12 (d, J = 13.84 Hz, 1H, HCH2), 3.72 (d, J = 14.04 Hz, 1H, HCH2), 3.12–3.07 (m, 1H, HCH2), 3.02–2.97 (m, 1H, HCH2), 2.94–2.89 (m, 1H, H–C(Me)2), 2.81–2.77 (m, 2H, HCH2), 1.70 (s, 9H, H3×CH3), 1.33 (s, 6H, H2×CH3) ppm; 13C NMR (100 MHz, CDCl3): 154.58 (C-5), 149.38 (C–Ar), 136.06 (C–Ar), 135.62 (C–Ar), 132.61 (C–Ar), 131.68 (C–Ar), 130.32 (C–Ar), 129.76 (C–Ar), 127.22 (C–Ar), 126.67 (C–Ar), 121.25 (C–Ar), 119.27 (C–Ar), 117.86 (C–Ar), 110.69 (C–Ar), 108.12 (C–Ar), 63.57 (–C(Me)3), 61.56 (CH), 48.34 (CH2), 46.82 (CH2), 33.77 (–C(Me)2), 30.22 (3 × CH3), 23.86 (2 × CH3), 21.46 (CH2) ppm; HRMS (EI) calcd. for [C26H32N6 + H]+ 429.2767; found 429.2768.
2-((1-Cyclohexyl-1H-tetrazol-5-yl)(4-fluorophenyl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (14l)
According to GP, 14l was obtained by the reaction of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 86 mg, 0.5 mmol), 4-fluorobenzaldehyde (1 equiv., 62 mg, 0.5 mmol), cyclohexyl isocyanide (1 equiv., 55 mg, 0.5 mmol) and TMSN3 (2 equiv., 115 mg, 0.5 mmol) in methanol (1.0 M, 1.2 mL) as solvent for 22 h with 68% yield (0.68 mmol, 293 mg) as a yellow solid; m.p. = 123–126 °C; IR (KBr), νmax: 668, 753, 1215, 2400, 3019, 3369 cm–1; HPLC-PDA: tr = 28.47 min (% area = 96.42%); 1H NMR (400 MHZ, CDCl3) δ: 7.77 (bs, 1H, HNH), 7.54–7.50 (m, 3H, HAr), 7.32 (d, J = 7.88 Hz, 1H, HAr), 7.19–7.15 (t, J = 7.64 Hz, 1H, HAr), 7.12–7.08 (t, J = 8.28 Hz, 3H, HAr), 5.38 (s, 1H, HCH), 4.57–4.50 (m, 1H, Hcy), 3.95 (d, J = 14.68 Hz, 1H, HCH2), 3.65 (d, J = 14.68 Hz, 1H, HCH2), 3.08–3.01 (m, 1H, HCH2), 2.92–2.88 (m, 1H, HCH2), 2.86–2.82 (m, 2H, HCH2), 2.02–1.98 (m, 1H, Hcy), 1.92–1.89 (t, J = 8.92 Hz, 4H, Hcy) 1.87–1.77 (m, 1H, Hcy), 1.59 (d, J = 12.76 Hz, 1H, Hcy), 1.34–1.30 (m, 3H, Hcy) ppm; 13C NMR (100 MHz, CDCl3): 153.40 (C-5), 136.11 (C–Ar), 131.58 (C–Ar), 130.84 (C–Ar), 130.33 (C–Ar), 130.25 (C–Ar), 126.97 (C–Ar), 121.59 (C–Ar), 119.50 (C–Ar), 117.96 (C–Ar), 116.04 (C–Ar), 115.83 (C–Ar), 110.89 (C–Ar), 108.02 (C–Ar), 62.84 (CH), 58.25 (C–Cy), 48.98 (CH2), 48.21 (CH2), 32.88 (C–Cy), 32.84 (C–Cy), 25.39 (C–Cy), 25.23 (C–Cy), 24.73 (C–Cy), 21.04 (CH2) ppm; HRMS (EI) calcd. for [C25H27FN6 + H]+ 431.2359; found 431.2351.
2-((4-Chlorophenyl)(1-cyclohexyl-1H-tetrazol-5-yl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (14m)
According to GP, 14m was obtained by the reaction of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 206 mg, 1.2 mmol), 4-chlorobenzaldehyde (1 equiv., 125 mg, 1.2 mmol), cyclohexyl isocyanide (1 equiv., 131 mg, 1.2 mmol) and TMSN3 (2 equiv., 276 mg, 1.2 mmol) in methanol (1.0 M, 1.5 mL) as solvent for 22 h with 74% yield (0.74 mmol, 330 mg) as a white solid; m.p. = 187–190 °C; IR (KBr), νmax: 669, 757, 1215, 1584, 3019, 3368 cm–1; HPLC-PDA: tr = 23.41 min (% area = 97.36%); 1H NMR (400 MHz, CDCl3) δ: 7.80 (bs, 1H, HNH), 7.50–7.47(m, 3H, HAr), 7.39 (d, J = 8.52 Hz, 2H, HAr), 7.32 (d, J = 8.00 Hz, 1H, HAr), 7.18–7.15 (t, J = 7.44 Hz, 1H, HAr), 7.14–7.10 (t, J = 7.44 Hz, 1H, HAr), 5.38 (s, 1H, HCH), 4.57–4.49 (m, 1H, Hcy), 3.95 (d, J = 14.56 Hz, 1H, HCH2), 3.65 (d, J = 14.36 Hz, 1H, HCH2), 3.07–3.01 (m, 1H, HCH2), 2.92–2.88 (m, 1H, HCH2), 2.86–2.81 (m, 2H, HCH2), 2.01–1.95 (m, 1H, Hcy), 1.92–1.87 (m, 3H, Hcy) 1.84–1.75 (t, J = 23.60 Hz, 2H, Hcy), 1.61 (d, J = 11.88 Hz, 1H, Hcy), 1.34–1.25 (m, 3H, Hcy) ppm; 13C NMR (100 MHz, CDCl3): 153.16 (C-5), 136.12 (C–Ar), 134.72 (C–Ar), 134.24 (C–Ar), 130.73 (C–Ar), 129.92 (C–Ar), 129.17 (C–Ar), 126.95 (C–Ar), 121.63 (C–Ar), 119.51 (C–Ar), 117.96 (C–Ar), 110.92 (C–Ar), 107.96 (C–Ar), 62.89 (CH), 58.30 (C–Cy), 48.99 (CH2), 48.19 (CH2), 32.92 (C–Cy), 32.86 (C–Cy), 25.38 (C–Cy), 25.22 (C–Cy), 24.73 (C–Cy), 21.02 (CH2) ppm; HRMS (EI) calcd. for [C25H27ClN6 + H]+ 447.2064; found 447.2076.
2-((2-Chlorophenyl)(1-cyclohexyl-1H-tetrazol-5-yl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (14n)
According to GP, 14n was obtained by the reaction of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 124 mg, 1.3 mmol), 2-chlorobenzaldehyde (1 equiv., 182 mg, 1.3 mmol), cyclohexyl isocyanide (1 equiv., 142 mg, 1.3 mmol) and TMSN3 (2 equiv., 300 mg, 1.3 mmol) in methanol (1.0 M, 1.5 mL) as solvent for 15 h with 82% yield (0.82 mmol, 366 mg) as a white solid; m.p. = 188–191 °C; IR (KBr), νmax: 669, 759, 1213, 2413, 3020, 3369 cm–1; HPLC-PDA: tr = 30.29 min (% area = 96.54%); 1H NMR (400 MHz, CDCl3) δ: 7.91 (d, J = 7.72 Hz, 1H, HAr), 7.81 (bs, 1H, HNH), 7.49 (d, J = 7.44 Hz, 1H, HAr), 7.45 (d, J = 7.64 Hz, 1H, HAr), 7.38–7.33 (m, 1H, HAr), 7.31–7.29 (m, 2H, HAr), 7.17–7.13 (t, J = 7.60 Hz, 1H, HAr), 7.12–7.08 (t, J = 7.60 Hz, 1H, HAr), 5.84 (s, 1H, HCH), 4.43–4.36 (m, 1H, Hcy), 3.86–3.77 (m, 2H, HCH2), 3.12–3.06 (m, 1H, HCH2), 2.98–2.92 (m, 1H, HCH2), 2.88–2.80 (m, 2H, HCH2), 2.02–1.95 (m, 1H, Hcy), 1.90–1.80 (m, 4H, Hcy) 1.72–1.69 (t, J = 6.72 Hz, 1H, Hcy), 1.61 (d, J = 4.44 Hz, 1H, Hcy), 1.33–1.28 (m, 3H, Hcy) ppm; 13C NMR (100 MHz, CDCl3): 153.26 (C-5), 136.11 (C–Ar), 134.02 (C–Ar), 133.54 (C–Ar), 131.14 (C–Ar), 130.95 (C–Ar), 130.00 (C–Ar), 129.93 (C–Ar), 127.59 (C–Ar), 127.06 (C–Ar), 121.48 (C–Ar), 119.39 (C–Ar), 117.95 (C–Ar), 110.84 (C–Ar), 108.16 (C–Ar), 58.56 (CH), 58.24 (C–Cy), 48.77 (CH2), 48.17 (CH2), 33.00 (C–Cy), 32.88 (C–Cy), 25.31 (C–Cy), 24.75 (C–Cy), 21.14 (CH2) ppm; HRMS (EI) calcd. for [C25H27ClN6 + H]+ 447.2064; found 447.2097.
2-((4-Bromophenyl)(1-cyclohexyl-1H-tetrazol-5-yl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (14o)
According to GP, 14o was obtained by the reaction of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 344 mg, 2 mmol), 4-bromobenzaldehyde (1 equiv., 368 mg, 2 mmol), cyclohexyl isocyanide (1 equiv., 218 mg, 2 mmol) and TMSN3 (2 equiv., 461 mg, 2 mmol) in methanol (1.0 M, 2 mL) as solvent for 16 h with 80% yield (0.80 mmol, 392 mg) as a yellow solid; m.p. = 99–102 °C; IR (KBr), νmax: 669, 757, 1215, 2400, 3019, 3366 cm–1; HPLC-PDA: tr = 24.23 min (% area = 98.49%); 1H NMR (400 MHz, CDCl3) δ: 7.73 (bs, 1H, HNH), 7.55 (d, J = 8.40 Hz, 2H, HAr), 7.50 (d, J = 7.56 Hz, 1H, HAr), 7.44–7.40 (m, 2H, HAr), 7.32 (d, J = 7.68 Hz, 1H, HAr), 7.19–7.10 (m, 2H, HAr), 5.36 (s, 1H, HCH), 4.58–4.50 (m, 1H, Hcy), 3.95 (d, J = 14.88 Hz, 1H, HCH2), 3.64 (d, J = 14.48 Hz, 1H, HCH2), 3.08–3.00 (m, 1H, HCH2), 2.92–2.88 (m, 1H, HCH2), 2.87–2.82 (m, 2H, HCH2), 2.03–1.99 (m, 1H, Hcy), 1.97–1.87 (m, 3H, Hcy) 1.81 (d, J = 15.32 Hz, 1H, Hcy), 1.72 (bs, 1H, Hcy), 1.32–1.29 (m, 4H, Hcy) ppm; 13C NMR (100 MHz, CDCl3): 153.07 (C-5), 137.46 (C–Ar), 136.11 (C–Ar), 134.85 (C–Ar), 134.72 (C–Ar), 132.12 (C–Ar), 130.22 (C–Ar), 126.97 (C–Ar), 122.81 (C–Ar), 121.64 (C–Ar), 119.54 (C–Ar), 117.97 (C–Ar), 110.89 (C–Ar), 109.18 (C–Ar), 108.03 (C–Ar), 62.98 (CH), 58.30 (C–Cy), 48.99 (CH2), 48.20 (CH2), 32.88 (C–Cy), 25.24 (C–Cy), 24.73 (C–Cy), 21.05 (CH2) ppm; HRMS (EI) calcd. for [C25H27BrN6 + H]+ 491.1559; found 491.1592.
2-((1-Cyclohexyl-1H-tetrazol-5-yl)(4-nitrophenyl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (14p)
According to GP, 14p was obtained by the reaction of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 138 mg, 0.8 mmol), 4-nitrobenzaldehyde (1 equiv., 121 mg, 0.8 mmol), cyclohexyl isocyanide (1 equiv., 87 mg, 0.8 mmol) and TMSN3 (2 equiv., 184 mg, 0.8 mmol) in methanol (1.0 M, 1.5 mL) as solvent for 18 h with 83% yield (0.83 mmol, 379 mg) as a white solid; m.p. = 106–109 °C; IR (KBr), νmax: 669, 754, 1215, 1584, 3022, 3369 cm–1; HPLC-PDA: tr = 21.65 min (% area = 95.07%); 1H NMR (400 MHz, CDCl3) δ: 8.29 (d, J = 8.92 Hz, 2H, HAr), 7.79 (d, J = 9.12 Hz, 3H, HAr, HNH), 7.51 (d, J = 7.44 Hz, 1H, HAr), 7.32 (d, J = 8.08 Hz, 1H, HAr), 7.18–7.11 (m, 1H, HAr), 5.24 (s, 1H, HCH), 4.59–4.51 (m, 1H, Hcy), 3.93 (d, J = 14.48 Hz, 1H, HCH2), 3.68 (d, J = 14.24 Hz, 1H, HCH2), 3.09–3.00 (m, 1H, HCH2), 2.95–2.91 (m, 1H, HCH2), 2.89–2.84 (m, 2H, HCH2), 2.05–1.99 (m, 1H, Hcy), 1.95–1.89 (m, 2H, Hcy), 1.87–1.85 (t, J = 2.96 Hz, 3H, Hcy), 1.75 (d, J = 6.80 Hz, 1H, Hcy), 1.37–1.29 (m, 3H, Hcy) ppm; 13C NMR (100 MHz, CDCl3): 152.26 (C-5), 148.01 (C–Ar), 142.76 (C–Ar), 137.50 (C–Ar), 136.12 (C–Ar), 129.64 (C–Ar), 126.87 (C–Ar), 124.04 (C–Ar), 121.80 (C–Ar), 120.13 (C–Ar), 118.00 (C–Ar), 110.91 (C–Ar), 109.12 (C–Ar), 107.99 (C–Ar), 62.72 (CH), 58.50 (C–Cy), 48.97 (CH2), 48.01 (CH2), 32.92 (C–Cy), 25.24 (C–Cy), 24.67 (C–Cy), 21.11 (CH2) ppm; HRMS (EI) calcd. for [C25H27N7O2 + H]+ 458.2304; found 458.2309.
4-((1-Cyclohexyl-1H-tetrazol-5-yl)(1,3,4,9-tetrahydro-2H-pyrido[3,4-b]indol-2-yl)methyl)benzonitrile (14q)
According to GP, 14q was obtained by the reaction of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 215 mg, 1.25 mmol), 4-cyanobenzaldehyde (1 equiv., 164 mg, 1.25 mmol), cyclohexyl isocyanide (1 equiv., 136 mg, 1.25 mmol) and TMSN3 (2 equiv., 288 mg, 1.25 mmol) in methanol (1.0 M, 2 mL) as solvent for 18 h with 85% yield (0.85 mmol, 372 mg) as a white solid; m.p. = 90–93 °C; IR (KBr), νmax: 668, 758, 1216, 1580, 3022, 3368 cm–1; HPLC-PDA: tr = 23.52 min (% area = 96.76%); 1H NMR (400 MHz, CDCl3) δ: 7.76 (bs, 1H, HNH), 7.71 (s, 4H, HAr), 7.50 (d, J = 7.44 Hz, 1H, HAr), 7.32 (d, J = 7.80 Hz, 1H, HAr), 7.19–7.11 (m, 2H, HAr), 5.24 (s, 1H, HCH), 4.55–4.50 (m, 1H, Hcy), 3.92 (d, J = 14.52 Hz, 1H, HCH2), 3.66 (d, J = 14.88 Hz, 1H, HCH2), 3.05–2.99 (m, 1H, HCH2), 2.93–2.89 (m, 1H, HCH2), 2.87–2.83 (m, 2H, HCH2), 2.30 (d, J = 12.76 Hz, 1H, Hcy), 2.03–1.99 (m, 1H, Hcy), 1.96 (d, J = 5.32 Hz, 1H, Hcy), 1.85–1.82 (t, J = 3.16 Hz, 2H, Hcy), 1.74 (d, J = 9.24 Hz, 2H, Hcy) 1.34–1.29 (m, 3H, Hcy) ppm; 13C NMR (100 MHz, CDCl3): 152.39 (C-5), 141.06 (C–Ar), 137.48 (C–Ar), 136.12 (C–Ar), 132.66 (C–Ar), 130.41 (C–Ar), 129.41 (C–Ar), 126.87 (C–Ar), 121.74 (C–Ar), 119.59 (C–Ar), 117.98 (C–Ar), 112.73 (C–Ar), 110.93 (C–Ar), 109.14 (C–Ar), 107.92 (C–Ar), 62.93 (CH), 58.45 (C–Cy), 48.98 (CH2), 48.06 (CH2), 32.89 (C–Cy), 31.58 (C–Cy), 25.21 (C–Cy), 24.68 (C–Cy), 22.65 (CH2) ppm; HRMS (EI) calcd. for [C26H27N7 + H]+ 438.2406; found 438.2409.
2-((1-Cyclohexyl-1H-tetrazol-5-yl)(4-methoxyphenyl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (14r)
According to GP, 14r was obtained by the reaction of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 430 mg, 2.5 mmol), 4-methoxybenzaldehyde (1 equiv., 340 mg, 2.5 mmol), cyclohexyl isocyanide (1 equiv., 273 mg, 2.5 mmol) and TMSN3 (2 equiv., 576 mg, 2.5 mmol) in methanol (1.0 M, 3 mL) as solvent with 74% yield (0.74 mmol, 327 mg) as a white solid; m.p. = 94–97 °C; IR (KBr), νmax: 669, 752, 1284, 1584, 3021, 3369 cm–1; HPLC-PDA: tr = 12.41 min (% area = 95.49%); 1H NMR (400 MHz, CDCl3) δ: 7.74 (s, 1H, HNH), 7.50 (d, J = 7.88 Hz, 1H, HAr), 7.44–7.40 (m, 2H, HAr), 7.32 (d, J = 8.08 Hz, 1H, HAr), 7.18–7.10 (m, 1H, HAr), 6.93 (d, J = 8.28 Hz, 2H, HAr), 5.24 (s, 1H, HCH), 4.58–4.51 (m, 1H, Hcy), 3.97 (d, J = 14.92 Hz, 1H, HCH2), 3.82 (s, 3H, H–OCH3), 3.65 (d, J = 14.88 Hz, 1H, HCH2), 3.09–3.03 (m, 1H, HCH2), 2.92–2.88 (m, 1H, HCH2), 2.85–2.82 (m, 2H, HCH2), 2.30 (d, J = 12.12 Hz, 1H, Hcy), 1.99–1.95 (m, 1H, Hcy), 1.88–1.84 (m, 3H, Hcy), 1.74–1.70 (m, 2H, Hcy), 1.31–1.28 (m, 3H, Hcy) ppm; 13C NMR (100 MHz, CDCl3): 153.88 (C-5), 140.58 (C–Ar), 137.42 (C–Ar), 136.11 (C–Ar), 132.47 (C–Ar), 131.15 (C–Ar), 129.75 (C–Ar), 127.72 (C–Ar), 127.06 (C–Ar), 121.48 (C–Ar), 119.43 (C–Ar), 117.93 (C–Ar), 114.29 (C–Ar), 110.87 (C–Ar), 109.20 (C–Ar), 108.07 (C–Ar), 63.13 (CH), 58.11 (C–Cy), 49.01 (CH2), 48.32 (CH2), 32.83 (C–Cy), 25.41 (C–Cy), 24.78 (C–Cy), 21.04 (CH2) ppm; HRMS (EI) calcd. for [C26H30N6O + H]+ 443.2559; found 443.2545.
2-((1-Cyclohexyl-1H-tetrazol-5-yl)(3,4-dimethoxyphenyl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (14s)
According to GP, 14s was obtained by the reaction of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 34 mg, 0.2 mmol), 3,4-dimethoxybenzaldehyde (1 equiv., 33 mg, 0.2 mmol), cyclohexyl isocyanide (1 equiv., 22 mg, 0.2 mmol) and TMSN3 (2 equiv., 46 mg, 0.2 mmol) in methanol (1.0 M, 0.5 mL) as solvent for 20 h with 82% yield (0.82 mmol, 387 mg) as a white solid; m.p. = 219–222 °C; IR (KBr), νmax: 669, 757, 1215, 1584, 3020, 3368 cm–1; HPLC-PDA: tr = 12.67 min (% area = 98.40%); 1H NMR (400 MHz, CDCl3) δ: 7.77 (s, 1H, HNH), 7.51 (d, J = 7.44 Hz, 1H, HAr), 7.32 (d, J = 7.44 Hz, 1H, HAr), 7.18–7.15 (t, J = 7.08 Hz, 1H, HAr), 7.14–7.10 (t, J = 7.60 Hz, 1H, HAr), 7.07 (d, J = 1.92 Hz, 1H, HAr), 7.01 (d, J = 7.96 Hz, 1H, HAr), 6.86 (d, J = 8.12 Hz, 1H, HAr), 5.28 (s, 1H, HCH), 4.58–4.52 (t, J = 11.56 Hz, 1H, Hcy), 3.96 (d, J = 14.92 Hz, 1H, HCH2), 3.89 (s, 3H, H–OCH3), 3.86 (s, 3H, H–OCH3), 3.68 (d, J = 14.92 Hz, 1H, HCH2), 3.10–3.04 (m, 1H, HCH2), 2.95–2.90 (m, 1H, HCH2), 2.85–2.82 (t, J = 5.64 Hz, 2H, HCH2), 1.98–1.86 (m, 4H, Hcy), 1.72–1.65 (m, 3H, Hcy), 1.32–1.27 (t, J = 10.12 Hz, 3H, Hcy) ppm; 13C NMR (100 MHz, CDCl3): 153.86 (C-5), 149.51 (C–Ar), 149.29 (C–Ar), 136.09 (C–Ar), 131.06 (C–Ar), 128.44 (C–Ar), 127.03 (C–Ar), 121.54 (C–Ar), 120.79 (C–Ar), 119.47 (C–Ar), 117.94 (C–Ar), 111.27 (C–Ar), 111.05 (C–Ar), 110.89 (C–Ar), 108.04 (C–Ar), 63.26 (CH), 58.13 (C–Cy), 49.01 (CH2), 48.42 (CH2), 32.83 (C–Cy), 25.42 (C–Cy), 25.32 (C–Cy), 24.76 (C–Cy), 20.85 (CH2) ppm; HRMS (EI) calcd. for [C27H32N6O2 + H]+ 473.2665; found 473.2661.
2-((1-Cyclohexyl-1H-tetrazol-5-yl)(3,4,5-trimethoxyphenyl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (14t)
According to GP, 14t was obtained by the reaction of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 103 mg, 0.6 mmol), 3,4,5-trimethoxybenzaldehyde (1 equiv., 118 mg, 0.6 mmol), cyclohexyl isocyanide (1 equiv., 65 mg, 0.6 mmol) and TMSN3 (2 equiv., 138 mg, 0.6 mmol) in methanol (1.0 M, 1.5 mL) as solvent for 8 h with 85% yield (0.85 mmol, 427 mg) as a white solid; m.p. = 193–196 °C; IR (KBr), νmax: 669, 757, 1214, 1585, 3020, 3368 cm–1; HPLC-PDA: tr = 13.05 min (% area = 97.52%); 1H NMR (400 MHz, CDCl3) δ: 7.80 (bs, 1H, HNH), 7.52 (d, J = 7.60 Hz, 1H, HAr), 7.33 (d, J = 7.64 Hz, 1H, HAr), 7.19–7.16 (t, J = 7.08 Hz, 1H, HAr), 7.15–7.11 (t, J = 7.44 Hz, 1H, HAr), 6.74 (s, 2H, HAr), 5.30 (s, 1H, HCH), 4.66–4.58 (m, 1H, Hcy), 3.98 (d, J = 14.72 Hz, 1H, HCH2), 3.85 (s, 3H, H–OCH3), 3.84 (s, 6H, H2×–OCH3), 3.66–3.50 (t, J = 14.72 Hz, 1H, HCH2), 3.11–3.06 (m, 1H, HCH2), 2.96–2.91 (m, 1H, HCH2), 2.87–2.84 (m, 2H, HCH2), 2.03–1.97 (m, 1H, Hcy), 1.92–1.85 (m, 3H, Hcy) 1.78–1.65 (m, 3H, Hcy), 1.33–1.25 (m, 3H, Hcy) ppm; 13C NMR (100 MHz, CDCl3): 153.64 (C-5), 138.12 (C–Ar), 136.11 (C–Ar), 131.75 (C–Ar), 130.97 (C–Ar), 126.97 (C–Ar), 121.60 (C–Ar), 119.50 (C–Ar), 117.93 (C–Ar), 110.93 (C–Ar), 107.94 (C–Ar), 105.32 (C–Ar), 63.74 (CH), 60.90 (–OMe), 58.26 (C–Cy), 56.28 (2 × –OMe), 49.22 (CH2), 48.62 (CH2), 32.90 (C–Cy), 32.83 (C–Cy), 25.48 (C–Cy), 25.38 (C–Cy), 24.75 (C–Cy), 20.86 (CH2) ppm; HRMS (EI) calcd. for [C28H34N603 + H]+ 503.2771; found 503.2771.
4-((1-Cyclohexyl-1H-tetrazol-5-yl)(1,3,4,9-tetrahydro-2H-pyrido[3,4-b]indol-2-yl)methyl)phenol (14u)
According to GP, 14u was obtained by the reaction of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1 equiv., 86 mg, 0.5 mmol), 4-hydroxybenzaldehyde (1 equiv., 61 mg, 0.5 mmol), cyclohexyl isocyanide (1 equiv., 54 mg, 0.5 mmol) and TMSN3 (2 equiv., 115 mg, 0.5 mmol) in methanol (1.0 M, 1 mL) as solvent for 12 h with 65% yield (0.65 mmol, 278 mg) as a white solid; m.p. = 102–105 °C; IR (KBr), νmax: 669, 756, 1215, 1524, 3020, 3370 cm–1; HPLC-PDA: tr = 12.72 min (% area = 99.50%); 1H NMR (400 MHz, CDCl3) δ: 7.81 (bs, 1H, HNH), 7.49 (d, J = 7.64 Hz, 1H, HAr), 7.34–7.29 (m, 3H, HAr), 7.17–7.139 (t, J = 6.56 Hz, 1H, HAr), 7.13–7.09 (t, J = 7.48 Hz, 1H, HAr), 6.86–6.83 (m, 2H, HAr), 5.26 (s, 1H, HCH), 4.58–4.51 (m, 1H, Hcy), 3.93 (d, J = 15.32 Hz, 1H, HCH2), 3.61 (d, J = 15.12 Hz, 1H, HCH2), 3.01–2.96 (m, 1H, HCH2), 2.92–2.87 (m, 1H, HCH2), 2.84–2.80 (m, 2H, HCH2), 1.96–1.90 (m, 2H, Hcy), 1.87–1.83 (m, 3H, Hcy), 1.73–1.64 (m, 2H, Hcy), 1.30–1.26 (m, 3H, Hcy) ppm; 13C NMR (100 MHz, CDCl3): 154.31 (C-5), 136.14 (C–Ar), 131.11 (C–Ar), 129.88 (C–Ar), 126.97 (C–Ar), 126.66 (C–Ar), 121.44 (C–Ar), 119.35 (C–Ar), 117.89 (C–Ar), 116.19 (C–Ar), 111.02 (C–Ar), 109.21 (C–Ar), 107.75 (C–Ar), 63.24 (CH), 58.32 (C–Cy), 49.16 (CH2), 48.12 (CH2), 32.80 (C–Cy), 25.32 (C–Cy), 24.74 (C–Cy), 20.87 (CH2) ppm; HRMS (EI) calcd. for [C25H28N6O + H]+ 429.2403; found 429.2496.
Conclusions
In conclusion, a series of tetrahydro β-carboline tetrazole hybrids were synthesised in good yield by the efficient, easy and one-step Ugi multicomponent reaction and identified as potent antileishmanial agents. The biological data revealed that most of the synthesized derivatives of tetrahydro β-carboline tetrazoles have exhibited moderate to potent in vitro antileishmanial activity with a good selectivity index compared to the standard drugs miltefosine and SSG. Compound 14t showed highest activity against intracellular amastigotes in vitro. This compound has also displayed a promising in vivo potency in the L. donovani/golden hamster model. In the pharmacokinetic study, it indicates high volume of distribution and systemic clearance. Therefore, these β-carboline analogues are found to be good candidates for a new lead optimization in the field of antileishmanial chemotherapy.
Conflict of interest
The authors declare no competing interests.
Supplementary Material
Acknowledgments
We are grateful to the Council of Scientific and Industrial Research (to P. P., A. K. P. and P. S. C.) and the University Grand Commission, New Delhi (to D. S. C.) for the financial support in the form of a fellowship. We are thankful to the S. A. I. F. Division of CDRI, Lucknow for providing the spectroscopic data (CDRI communication no. is 9461).
Footnotes
†Electronic supplementary information (ESI) available: 1H and 13C NMR data are available. See DOI: 10.1039/c7md00125h
References
- Sharma U., Singh S. J. Vector Borne Dis. 2008;45:255–272. [PubMed] [Google Scholar]
- Hussain H., Al-Harrasi A., Al-Rawahi A., Green I. R., Gibbons S. Chem. Rev. 2014;114:10369–10428. doi: 10.1021/cr400552x. [DOI] [PubMed] [Google Scholar]
- Chappuis F., Sundar S., Hailu A., Ghalib H., Rijal S., Peeling R. W., Alvar J., Boelaert M., Chattopadhyay A., Jafurulla M. Nat. Rev. Microbiol. Biochem. Biophys. Res. Commun. 2007;2011;5416:873–882. 7–12. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- WHO Report, http://www.who.int/mediacentre/factsheets/fs375/en/ (accessed 27.09.16).
- Bogdon C., Gessner A., Solbach W., Rollinghoff M. Curr. Opin. Immunol. 1996;8:517–525. doi: 10.1016/s0952-7915(96)80040-9. [DOI] [PubMed] [Google Scholar]
- Liu D., Uzonna J. E., Santos J. L., Andrade A. A., Dias A. A., Bonjardim C. A., Reis L. F., Teixeira S. M., Horta M. F. Front. Cell. Infect. Microbiol. J. Interferon Cytokine Res. 2012;2006;226:83. 682–688. [Google Scholar]
- Murray H. W., Berman J. D., Davies C. R., Saravia N. G. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- Maltezou H. C., Guerin P. J., Olliaro P., Sundar S., Boelaert M., Croft S. L., Desjeux P., Wasunna M. K., Bryceson A. D. J. Biomed. Biotechnol. Lancet Infect. Dis. 2010;2002;20102:617521. 494–501. [Google Scholar]
- Wasan K. M., Wasan E. K., Gershkovich P., Zhu X., Tidwell R. R., Werbovetz K. A., Clement J. G., Thornton S. J. J. Infect. Dis. 2009;200:357–360. doi: 10.1086/600105. [DOI] [PubMed] [Google Scholar]
- Guidelines on Use of Oral Drug Miltefosine for the Treatment of Kala-azar in the Endemic States on a Pilot Basis, Government of India, Available at: http://www.nvbdcp.gov.in/Doc/Guidelines%20on%20miltefosine. pdf (accessed 27.09.16).
- Ashok P., Lathiya H., Murugesan S. Eur. J. Med. Chem. 2015;97:928–936. doi: 10.1016/j.ejmech.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Di Giorgio C., Delmas F., Ollivier E., Elias R., Balansard G., Timon-David P. Exp. Parasitol. 2004;106:67–74. doi: 10.1016/j.exppara.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Costa E. V., Pinheiro M. L., Xavier C. M., Silva J. R., Amaral A. C., Souza A. D., Barison A., Campos F. R., Ferreira A. G., Machado G. M., Leon L. L. J. Nat. Prod. 2006;69:292–294. doi: 10.1021/np050422s. [DOI] [PubMed] [Google Scholar]
- Kam T. S., Sim K. M., Koyano T., Komiyama K. Phytochemistry. 1999;50:75–79. [Google Scholar]
- Martinez-Perez J. A., Iyengar S., Shannon H. E., Bleakman D., Alt A., Clawson D. K., Arnold B. M., Bell M. G., Bleisch T. J., Castano A. M., Del Prado M., Dominguez E., Escribano A. M., Filla S. A., Ho K. H., Hudziak K. J., Jones C. K., Mateo A., Mathes B. M., Mattiuz E. L., Ogden A. M. L., Simmons R. M. A., Stack D. R., Stratford R. E., Winter M. A., Wu Z., Ornstein P. L. Bioorg. Med. Chem. Lett. 2013;23:6463–6466. doi: 10.1016/j.bmcl.2013.09.045. [DOI] [PubMed] [Google Scholar]
- Faria J. V., Santos M. S. D., Bernardino A. M. R., Becker K. M., Machado G. M. C., Rodrigues R. F., Cavalheiro M. M. C., Leonor L. L. Bioorg. Med. Chem. Lett. 2013;23:6310–6312. doi: 10.1016/j.bmcl.2013.09.062. [DOI] [PubMed] [Google Scholar]
- Karabanovich G., Roh J., Smutny T., Nemecek J., Vicherek P., Stolarikova J., Vejsova M., Dufkova I., Vavrova K., Pavek P., Klimesova V., Hrabalek A. Eur. J. Med. Chem. 2014;82:324–340. doi: 10.1016/j.ejmech.2014.05.069. [DOI] [PubMed] [Google Scholar]
- Upadhayaya R. S., Jain S., Sinha N., Kishore N., Chandra R., Arora S. K. Eur. J. Med. Chem. 2004;39:579–592. doi: 10.1016/j.ejmech.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Newman D. J., Noren-Muller A., Reis-Correa I. J., Prinz H., Rosenbaum C., Saxena K., Schwalbe H. J., Vestweber D., Cagna G., Schunk S., Schwarz O., Schiewe H., Waldmann H. J. Med. Chem. Proc. Natl. Acad. Sci. U. S. A. 2008;2006;51103:2589–2599. 10606–10611. [Google Scholar]
- Kumar A., Katiyar S. B., Gupta S., Chauhan P. M. S. Eur. J. Med. Chem. 2006;41:106–113. doi: 10.1016/j.ejmech.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Kumar R., Khan S., Verma A., Srivastava S., Viswakarma P., Gupta S., Meena S., Singh N., Sarkar J., Chauhan P. M. S. Eur. J. Med. Chem. 2010;45:3274–3280. doi: 10.1016/j.ejmech.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Chauhan S. S., Gupta L., Mittal M., Vishwakarma P., Gupta S., Chauhan P. M. S. Bioorg. Med. Chem. Lett. 2010;20:6191–6194. doi: 10.1016/j.bmcl.2010.08.119. [DOI] [PubMed] [Google Scholar]
- Chauhan S. S., Pandey S., Shivahare R., Ramalingam K., Krishna S., Vishwakarma P., Siddiqi M. I., Gupta S., Goyal N., Chauhan P. M. S. MedChemComm. 2015;6:351–356. [Google Scholar]
- Sharma M., Chauhan K., Shivahare R., Vishwakarma P., Suthar M. K., Sharma A., Gupta S., Saxena J. K., Lal J., Chandra P., Kumar B., Chauhan P. M. S. J. Med. Chem. 2013;56:4374–4392. doi: 10.1021/jm400053v. [DOI] [PubMed] [Google Scholar]
- Ashok P., Chander S., Tejeria A., Garcia-Calvo L., Balana-Fouce R., Murugesan S. Eur. J. Med. Chem. 2016;123:814–821. doi: 10.1016/j.ejmech.2016.08.014. [DOI] [PubMed] [Google Scholar]
- Gohil V. M., Brahmbhatt K. G., Loiseau P. M., Bhutani K. K. Bioorg. Med. Chem. Lett. 2012;22:3905–3907. doi: 10.1016/j.bmcl.2012.04.115. [DOI] [PubMed] [Google Scholar]
- Snyder H. R., Hansch C. H., Katz L., Parmerter S. M., Spaeth E. C., Snyder H. R., Hansch C. H., Katz L., Parmerter S. M., Spaeth E. C. J. Am. Chem. Soc. 1948;70:219–221. doi: 10.1021/ja01181a063. [DOI] [PubMed] [Google Scholar]
- Malik M. Y., Jaiswal S., Sharma A., Shukla M., Lal J. Drug Metab. Rev. 2016;48:281–327. doi: 10.3109/03602532.2016.1157600. [DOI] [PubMed] [Google Scholar]
- Sangshetti J. N., Kalam Khan F. A., Kulkarni A. A., Patil R. H., Pachpinde M. A., Lohar K. S., Shinde D. B. Bioorg. Med. Chem. Lett. 2016;26:829–835. doi: 10.1016/j.bmcl.2015.12.085. [DOI] [PubMed] [Google Scholar]
- Cardenas-Galindo L. E., Islas-Jacome A., Alvarez-Rodriguez N. V., El Kaim L., Gamez-Montano R. Synthesis. 2014;46:49–56. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.