 Polyhydroxyalkanoates are being explored exponentially for biomedical application. The collative reported information on polyhydroxyalkanoates may serve as a guide to attain novel biomaterials.
Polyhydroxyalkanoates are being explored exponentially for biomedical application. The collative reported information on polyhydroxyalkanoates may serve as a guide to attain novel biomaterials.
Abstract
Polyhydroxyalkanoates (PHAs) are biopolymers synthesized by bacteria under unbalanced growth conditions. These biopolymers are considered as potential biomaterials for future applications because they are biocompatible, biodegradable, and easy to produce and functionalize with strong mechanical strength. Currently, PHAs are being extensively innovated for biomedical applications due to their prerequisite properties. The wide range of biomedical applications includes drug delivery systems, implants, tissue engineering, scaffolds, artificial organ constructs, etc. In this article we review the utility of PHAs in various forms (bulk/nano) for biomedical applications so as to bring about the future vision for PHAs as biomaterials for the advancement of research and technology.
Introduction
Currently, biopolymers are more preferred because they are obtained from renewable resources and are eco-friendly. The renewable resources are plants, animals, fungi and microorganisms (mainly bacteria). The polymers obtained from plant and animal resources are polysaccharides (cellulose, chitosan, pectin), proteins (collagen, α-keratin), lipids (triglycerides, phospholipids), and polyesters (polylactides, cutin), which are being used widely for different applications.1 In addition to these, polymers obtained from microbes especially bacterial species are also being investigated because they are abundant and faster and easier to grow than polymers from plant or animal resources. The various polymers obtained from bacteria are exopolysaccharides, proteins, lipids and polyesters like polyhydroxyalkanoates (PHAs).
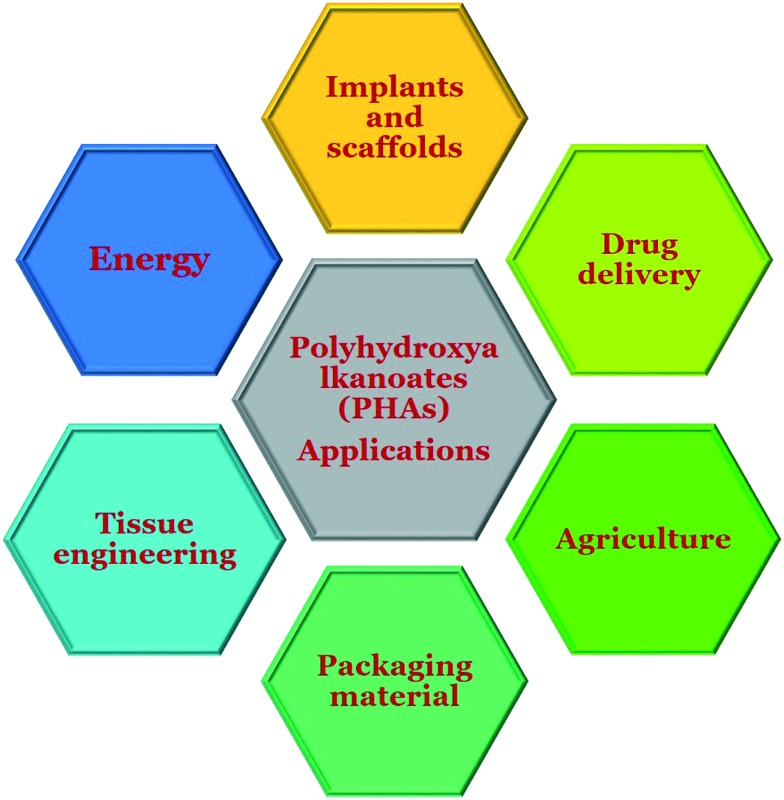
PHAs were observed for the first time by Beijerinck in 1888 as inclusion bodies inside bacteria. These granular inclusions were extracted and identified as poly(3-hydroxybutyrate) (PHB) by Lemoigne in 1927. This was the foundation stone in the history of PHAs.2 They are easy to produce and functionalize on a large scale with good mechanical strength.3 Though the lab production of PHAs is slightly expensive at present, the prerequisite properties of these polymers have driven them as biomaterials for biomedical applications. In addition, research on PHAs for other applications such as food packing, agriculture, energy, etc. is also being carried out. Fig. 1 shows the applications of PHAs in various fields.
Fig. 1. Various applications of polyhydroxyalkanoates.
In this review article, we summarize the information about the progress of the research, the technology of various PHAs and their origin and utility as biomaterials for biomedical applications.
PHAs and their bacterial sources
Polyhydroxyalkanoates are linear polyesters synthesized chemically by ring opening polymerization of β-lactones4 and naturally by both Gram-positive and Gram-negative bacteria under unfavourable growing conditions like physical or nutritional stress. They are well known for their biodegradability, biocompatibility and mechanical strength. In addition, it is easy and simple to culture bacteria in large quantities to isolate PHAs. Further, the desired structure and molecular weight of PHAs can be manipulated by changing the growth conditions.5,6 Various PHAs and their co-polymers that are isolated from different kinds of bacterial species are listed in Table 1. There are over 150 possible constituent monomers reported to date which are classified on the basis of the number of carbons in the monomer as short chain length (scl) PHAs (for example, PHB, poly(3-hydroxyvalerate) (PHV), poly(3-hydroxybutyrate-co-valerate) (PHBV)), medium chain length (mcl) PHAs (for example, polyhydroxyoctanoate (PHO), polyhydroxynonanoate (PHN), polyhydroxyhexanoate (PHHx), polyhydroxyheptanoate (PHHp)) and long chain length (lcl) PHAs.7 The methods to obtain various PHAs in the laboratory using various bacterial species are summarized in our earlier publication.8 Among these, PHB and PHBV are used most extensively for biomaterial investigations; hence, PHAs are commercially available. The various applications include biomedical applications such as drug delivery, development of scaffolds, implants, and biosensors,9 food coating, packing, agriculture, molded goods, non-woven fabrics, adhesives, textile, paper coating, automobiles, performance additives,10–14 energy materials, etc.15–27
Table 1. Different kinds of PHAs, bacterial resources and chemical structures.
| Polymer name | Bacterial resource | Chemical structure |
| Poly-3-hydroxybutyrate (P3HB) | Pseudomonas pseudomallei, Azotobacter chroococcum and Pseudomonas putida, P. oleovorans, Alcaligenes eutrophus, Zoogloea ramigera, Alcaligenes sp. A-04 Ralstonia eutropha |
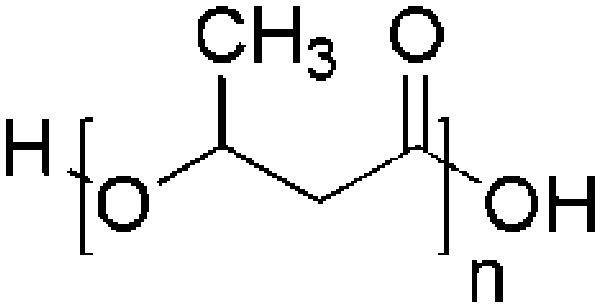
|
| Polyhydroxyvalerate (PHV) | P. oleovorans |
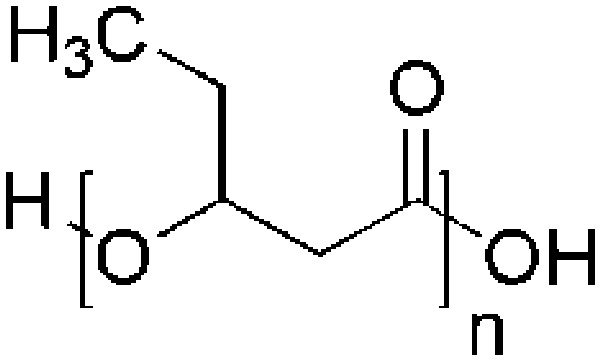
|
| Polyhydroxyhexanoate (PHHx) | P. putida KTHH03 |
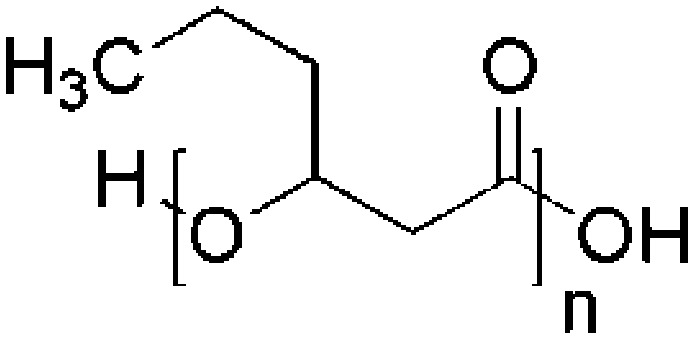
|
| Polyhydroxyheptanoate (PHHp) | P. putida KTHH03 |
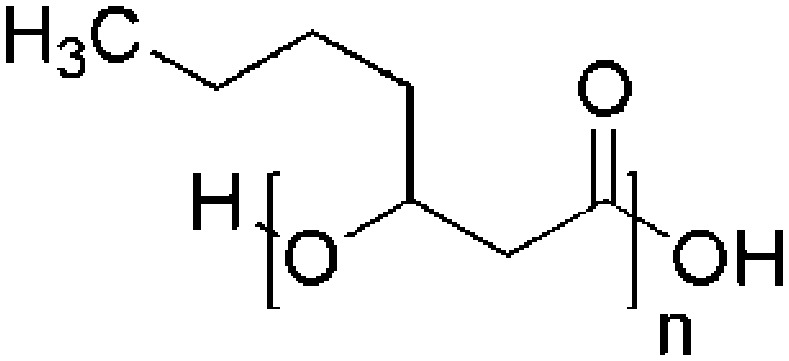
|
| Polyhydroxyoctanoate (PHO) | Streptomyces lividans 28 |
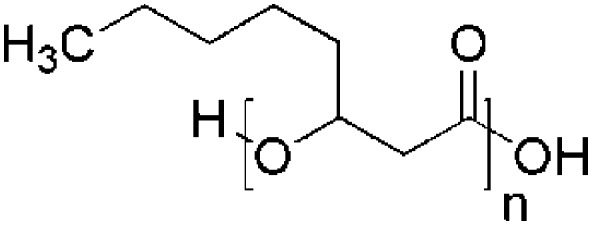
|
| Polyhydroxynonanoate (PHN) | Alcaligenes sp. |
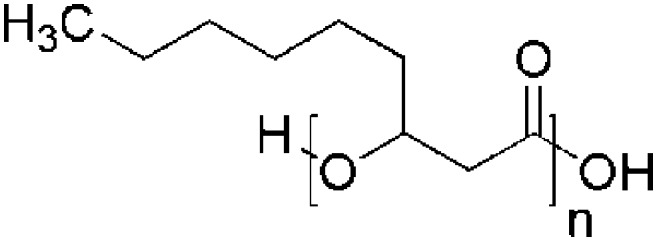
|
Biomedical applications
Generally, every living system develops some kind of response towards the inserted material (biomaterial), which may be favourable or unfavourable to the living system. The favourable response would be the acknowledgment of the biomaterial with improved bioactivity and the unfavourable response includes impediment of bioactivity around the material or other antagonistic organic signals to hinder the function of the biomaterial. Naturally obtained PHAs are stereo-specific (the carbons in the backbone are in R(–) configuration) with ester linkage, because of which they are easily degraded enzymatically and empower particular communications with biological entities enabling biocompatibility. Thus PHAs are being examined and being improved broadly for diverse biomedical applications.29,30,31 P(4HB) (TephaFLEX) was the first commercial product of PHAs that was approved by the FDA in 2007 as an absorbable suture. This indicated that PHAs have a bright prospect as a biomaterial in biomedical applications.32 It was found that P4HB can cross the blood brain barrier; therefore, PHAs and their copolymers are being examined to enhance their properties in order to empower their utility as biomaterials.30,33 Various methods such as epoxidation, carboxylation, chlorination, hydroxylation, and pyrolysis were used for modifying the properties of PHAs. The targeted properties were enhanced bioactivity, processability, compatibility, biodegradability or decreased crystallinity, hydrophobicity, and improved moldability and flexibility, etc.34 Accordingly the end applications include sutures, slings, stents, repair patches, cardiovascular patches, heart valves, orthopedic pins, adhesion barriers, tissue engineered cardiovascular devices, articular cartilage repair devices, nerve repair devices, tendon repair devices, guided tissue repair/regeneration devices, nerve guides, bone-marrow scaffolds, and wound dressings.27,35 The PHA based materials that are commercialized are Phantom Fiber™ as suture (Tornier Co.), MonoMax® as suture (Braun Surgical Co.), BioFiber™ as scaffold (P4HB polymer) (Tornier Co.), GalaFLEX as mesh (Galatea Corp.), and Tornier® as surgical mesh (Tornier Co.).36
PHAs are functionally modified by epoxidation, carboxylation, chlorination, hydroxylation, and pyrolysis to increase the bioactivity, processability, compatibility, and biodegradability or to decrease crystallinity and hydrophobicity, and enhance moldability and flexibility, etc.37 For example; the review by Zibiao Li et al. highlighted the latest advancement in the engineered systems of PHA-based water soluble polymers, functionalized PHAs with polar groups, and the block/graft copolymerization of PHAs with hydrophilic components in various polymeric designs to manipulate the properties.38 He also explains in another review about the importance of water soluble PHAs in therapeutic applications.39 It is well documented in both reviews that chemically modified water soluble PHAs have a significant effect on materials building and showed incredible quality, providing satisfactory smart biomaterials. These water soluble PHAs are used in new aspects of controlled drug release, cancer therapy, DNA/siRNA delivery and tissue engineering.39 Further, the biocompatibility of PHB-based biomaterials is enhanced by PEGylation, which have great potential as biological scaffolds supporting nerve repair.40 In the following sub-sections we detailed the investigations reported on various PHAs as biomaterials for drug delivery, implants, implant coatings, tissue engineering, biosensors and scaffolds.
Drug delivery systems
Conventional drug delivery systems comprise tablets, injections, ointments, liquid medicines and syrups. These methods of drug delivery deal with several side effects along with problems like overdose in one stroke, including the need of booster doses, inappropriate release, non-specific intake by non-targeted cells, generalized circulation all over the body, etc. The problems associated with conventional drug administration have given an approach to new ideas and methods, i.e. controlled release technology. The drug delivery system is a smart application to deliver drugs in a controlled fashion with therapeutically optimal rate and dose regimen in order to minimize side effects and toxicity due to drug overdose. Liposomes, proliposomes, cyclodextrins, gels, microspheres, pro-drugs, etc. are being used as drug delivery systems. However, the stratified advancement in modern drug delivery started with the use of polymers as carriers. Polymers can provide controlled release of bioactive agents with constant drug dosing over a period of time. As a result, polymers are being studied extensively as drug delivery systems. Basically any drug delivery system consists of three basic components: (a) a polymeric excipient as carrier, (b) a targeting moiety, and (c) a biologically active agent. Some systems might have extra components added for precise targeting or for targeting mobile cells. These modern drug delivery systems are superior to conventional drug assimilation considering several aspects like the minimum amount of drug required to work efficiently, drug delivery to treat the desired cells thereby increasing the therapeutic benefits, reducing side effects and protecting healthy cells and tissues. This method of treatment is effective against a wide range of diseases and disorders like leprosy, conjunctivitis, ulcers, urinary tract infections, prion diseases and cancer. The drug delivery route could be oral or intravenous for which different polymers are being used as excipients.
Natural and synthetic polymers are approved for different administrative routes using polymers such as cellulose derivatives, PEG, polyacrylic acid, PLGA (poly(lactide-co-glycolic acid)), PCL, PLA and proteins. The degradability of such polymers adds to their utility in tissue engineering or as controlled drug release vehicles.41 Though several synthetic and natural polymers are being studied, natural polymers have gained importance because they are renewable and easy to produce and can be fabricated with good mechanical and thermal properties. There are several biocompatible and biodegradable polymers that are in practice; however there is always a need for new polymers with improved properties for various applications. In this way, PHAs of microbial origin were identified and are being studied profusely. Though they are being exploited in a wide range of applications, apparently they are investigated more as drug delivery systems. From the early 1990s, PHAs have been studied for drug delivery as they degrade by surface erosion.32 Their biodegradability, biocompatibility and non-toxic nature has enabled PHAs to circulate in the system for prolonged periods; therefore they were used for a wide payload spectrum of therapeutic agents.42 Accordingly, loading of bioactive agents in matrices of PHAs, starting from antibiotics, anti-inflammatory agents, anticancer agents, anesthetics, hormones, steroids, vaccines, proteins, and nucleic acids (DNA, RNA) to other biological macromolecules, has been carried out for more than three decades.4,43,44 For drug release one of the important aspects to be considered is degradation; which was well studied for PHB/PEG based poly(ester urethane)s by X. J. Loh et al.45 The thermo-gels of these copolymers were used as injectables for targeting tumours for long term sustained release to make improvements in chemotherapy and minimise side effects.46 In another paper he showed the gel hydrolysis of copolymer (PHB–PEG–PPG) and the protein release.47 Also, multi-arm PHB based tri-block copolymers were reported for preparing thermo-responsive hydrogels. Here poly(N-isopropyl acrylamide) (PNIPAM) and polypropylene methacrylate (PPGMA) blocks were involved in the response to room temperature and body temperature. This strategy was used for delivering the drug. The toxicity of the co-polymers was checked against CCD-112CoN human fibroblast cell lines.48
A new composite material was prepared by solvent casting with PHBV and natural rubber. The material was developed for drug delivery using flurbiprofen as a model drug, where the drug release rate was influenced by variation in the natural rubber content.49 The hydrophilicity was enhanced by modifying pendent groups of hydrophobic polyhydroxyoctanoate (PHO) with carboxylic acids. The drug release profile of this polymer recorded an increase in doxorubicin release and cell proliferation studies also showed better cell proliferation.50 In addition to drugs, PHAs were also studied for releasing nucleic acids and proteins; for example; cationic PHA was synthesized to obtain poly(hydroxyoctanoate)-co-(hydroxy-11-(bis(2-hydroxyethyl)-amino)-10-hydroxyundecanoate) to immobilize plasmid DNA and its delivery was monitored,51 and inactive extracellular PHA depolymerase and mcl PHA poly[(3-hydroxyoctanoate)-co-(3-hydroxyhexanoate)] were used for immobilization and delivery of proteins.52 High performance PHAs were studied as transdermal drug delivery systems (TDDS), which are composed of monomers like 3-hydroxyhexanoic acid and 3-hydroxyoctanoic acid. In the presence of dendrimers these polymers facilitated crystallization and better dissolution of various drugs like ketoprofen, clonidine, and tamsulosin which were used for observing the release studies.53,54 P(3-HB-co-3-HV) and P(3HB-co-4-HB) rods were fabricated with drug and used for treating implant-related osteomyelitis in a rat model. Apart from bulk materials, micro-materials were also designed using PHAs. For instance, microspheres of PHB were used for controlled release of activators or inhibitors of enzymes or for sustained delivery to tissues of blood vessels. Microspheres of PHB and PHBV copolymers were prepared by a solvent evaporation method and studied for sustained release of progesterone.55 Microcapsules of PHBV encapsulating the enzyme catalase were used as delivery systems to treat cancer. This therapy is known as anti-metastatic therapy, where encapsulation of catalase using a polymer coating protects the inner sensitive material from the external conditions and is also responsible for delivering drugs to target sites in the active state.56 In another study, photodynamic therapy (PDT) was developed whereby PHBV and PCL microspheres were used for biodegradable drug delivery systems.57 PHBV micro-particles with gellan gum hydrogels were prepared in the form of injectables by a double emulsion solvent evaporation method. Here the purpose is to localize delivery and for long term retention of both hydrophilic and hydrophobic active agents loaded in micro-particles.58 In the case of antibiotic delivery to the infected site, PHA drug delivery systems were proved to provide and maintain adequate concentrations and were very effective against the treatment of resistant infections.59,60 PHB matrices as implants to release hormone in a sustained manner were studied and proved effective.61 Similarly, the PHA matrix worked as a depot to control the release of hormone in a better way when it was coated with cyanoacrylate.62 An implantable tablet of PHB and calcium acetate–glycerol adduct with an active agent or bio-regulating peptide was studied for release in buffer along with an adjuvant, proposing a newer approach of administration of complex combinations of bioactive materials in a single stroke.63 Biodegradation and compatibility studies on implants were carried out both in vivo and in vitro to prove the suitability of PHAs as implantable drug delivery systems.64
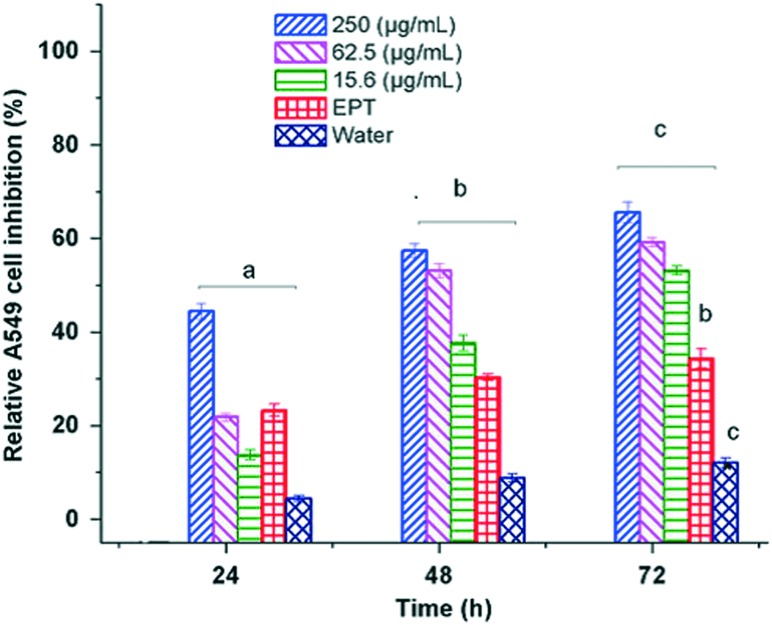
Currently different forms of therapeutic delivery carriers of PHA based nano-vehicles like nanoparticles, micelles, liposomes and vesicles are being studied experimentally, which proved their efficiency in terms of increased bioavailability of drug and better encapsulation of diverse compounds. Polymeric nanoparticles with a targeting moiety are extensively used for targeted drug delivery to treat cancers. It is very crucial that drugs target cancerous cells in the human body in order to make them site specific and reduce side effects. The nanoparticles are reported in a wide variety of sizes and shapes, which show active penetration via all possible pathways like active and passive targeting, specific cellular or subcellular trafficking, and facial control of cargo release. Many research groups are involved in the development of polyhydroxyalkanoates as nanoparticles by sophisticated engineering and encapsulation of anticancer agents to precisely target tumour sites.65 The nano-delivery system can take several shapes such as nanoparticles, micelles, dendrimers, liposomes, nanohybrids, nanosheets, nanoplexes, and nanotubes or horns or rods with bioactive agents. PHBV nanoparticles were loaded with the anticancer drug ellipticine and studied in vitro for drug release using cancer cell lines. The rate of inhibition of cancer cells was twice greater when treated with ellipticine loaded nanoparticles than ellipticine alone.66 The cytotoxicity study by MTT assay using human non-small lung carcinoma cells A549 showed effective activity of the released drug by the nanocarriers (Fig. 2).66 With advanced technologies in molecular biology and genetic engineering, easily detectable nanoparticles of PHB are produced for targeted drug delivery.67 For example, the PHA synthase gene in genetically modified E. coli is linked with green fluorescent protein (GFP), which aids ease of detection, and single chain variable fragment (A33scFv) antibody, which is involved in targeting the expected cell or protein. The translation of the gene yields a protein chain which gives nanoparticles after single step click chemistry. These amphiphilic micelles with a hydrophobic core are loaded with anticancer drug and antibody fragment at the periphery, which works precisely for colon cancer.68
Fig. 2. In vitro cytotoxicity tests of ellipticine loaded PHBV-12 nanoparticles at different concentrations for A549 cell line [(a–c) Student–Newman–Keuls test (P < 0.05)] (reprinted with permission from Masood et al., 2013, J. Mater. Sci.: Mater. Med.).
Implants and implant coatings
Generally implant materials are designed using polymers, metals and ceramics to replace or repair the damaged portion of the body tissue or organ. Ideally the material used as an implant should be mechanically strong and sustain the dynamic conditions of the body. Though there are several biomaterials used in practice as implants, biomaterials with improved properties are under investigation. As described in the above sections, PHAs are at par with polyethylene in their mechanical strength and hence are being investigated extensively. Moreover, slow hydrolytic and non-enzymatic degradation of PHAs in the human body by surface erosion makes the crystalline PHAs more suitable and therefore are investigated for implant applications like nerve repair and bone implants and as supporting material for bone implants.69 PHAs are melt processable thermoplastics and hence can be molded in desirable shapes. Besides these properties, hydrophobicity is a limiting factor for PHAs to be used for implant application because extensive blood contact may lead to encapsulation and thrombi formation as reported in some cases. Thus, to enhance compatibility and to reduce hydrophobicity, PHAs are being blended or copolymerized with other hydrophilic polymers like poly(ethylene glycol) (PEG). In doing so, certain characteristics of PHAs were modified like reduction of crystallinity and increased rate of hydrolysis.70
PHAs are mostly used as hard implants like cartilage, ligaments and meniscus. As reported with modifications of PHAs in flexibility and elasticity, they may also be suitable for soft tissue implants like blood vessels, intestines, nerves, etc.71 Procedures for 3D structured prototypes or implants were developed using several techniques like fast prototyping with selective sintering of embedded laser using PHAs.72 P4HB monofilament mesh is used to form 3D resorbable implants to relieve temporarily deformed host tissue or anatomical shapes like hernia.73 Breast implants of P4HB and its copolymers are developed with sufficient mechanical strength to support a reconstructed breast; they degrade gradually, allowing breast tissue parenchymal cells to grow without losing support. These self-reinforced implants are able to deform temporarily and can resume their 3D shape.74 For the necessary mechanical strength and elasticity balance, block co-polymers of different PHB and PEG ratios were manipulated to attain tunable properties for various biomedical applications, where Young's modulus and stress and strain at break can be adjusted according to the length of PHB and PEG chains in the respective blocks.75 A 3D model of an implant of a composite of PHB and PHBV loaded with wollastonite was analyzed for the free energy of the interface–surface interaction for applications in bone formation and deposition of drugs.76 PHBV is considered as a bio-absorbable polymeric material (BPM), which is being utilized as an implant construct for the occlusions in aneurysms. The BPM in the form of hybrid bioactive coils is immobilized with growth factors in order to accelerate histopathological transformation of an unorganized clot into fibrous connective tissue.77 There are many such examples where PHAs are used as implants in animal models successfully. The review by Q. Wu et al., elaborated such examples like artificial esophagus, vascular patches, nerve conduit, artificial cartilage etc.42
The implantable devices are coated with a layer of different PHAs, like P4HB and its copolymers, e.g., on a cochlear implant, for drug elusion and also to impart lubrication to the device for the ease of insertion of the electrodes.78 The stainless steel prosthetic implant or stent is spray coated with PHBV and shikonin, a derivative of naphthazarin, to prevent thrombosis and restenosis.79 The titanium implant is flame-spray coated with a combination of PMMA and PHBV and checked for thickness, roughness, adhesion, wettability, and in vitro biocompatibility.80 Different PHAs and their derivatives are studied for their applications as implant materials by in vitro and in vivo methods. Certain examples like implants of PHB and PHBHHx were tested and proved suitable with chondrocytes and exhibited adequate deposition of calcium and phosphorus, similar to the natural hydroxyapatite material. Therefore, its effective role in physiological functioning of the cartilage regeneration was substantiated for tissue engineering.81
Nanocomposites have a huge potential in biomedical applications and are progressing towards taking care of a variety of diverse applications for improving the quality of life. Different nano-forms of PHA and its derivatives are studied by in vitro and in vivo methods. The review by P. Dwivedi et al. summarizes the challenges involved in implanting biomedical devices and prostheses leading to nosocomial infections, more severely with the multi-drug resistant superbug, which had directed current efforts to develop self-sterilizing nano-composite biomaterials.82 Hence electrospun nanocomposites of PHBV with silk fibroin and nano-hydroxyapatite (HA) are being developed for bone tissue engineering.83 The nanofiber matrices composed of PHB and PHBV are subjected to cell adhesion studies against different cell lines like epithelial cells, fibroblasts and hepatocytes. These cells showed a high adhesion and proliferation rate in vitro, signifying a suitable nano-sized material for growth and proliferation and thus more favourable for application. In addition to these studies PHBV substrate was tested for compatibility with retinal pigment epithelial cells in order to use it for sub-retinal transplantation and to replace diseased or damaged retinal pigment epithelium.84
Scaffolds and tissue engineering
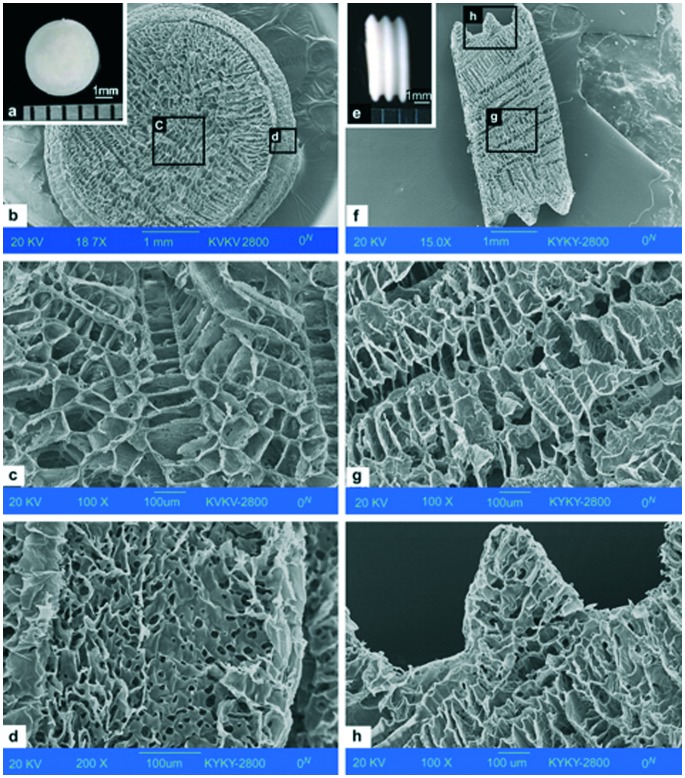
Scaffolds are biomaterials used as supports to repair or regenerate tissue. Scaffolds may be prepared from natural or synthetic polymers. The scaffolds used in the human body are biocompatible and may be biodegradable. Though several polymers are being used as scaffolds, there is always a need for new polymers with better properties. Hence, PHAs were studied as scaffolds owing to their good mechanical properties. A porous scaffold made of PHBHHx is shown in Fig. 3.85
Fig. 3. Appearance of a three-dimensional PHBHHx scaffold. (a) Surface of the scaffold, scale bar: 1 mm. (b) Cross-section of the scaffold by SEM, scale bar: 1 mm. (c) Porous structure in the middle of the cross section by SEM, scale bar: 100 μm. (d) Edge of the scaffold by SEM, scale bar: 100 μm. (e) Side view of the scaffold, scale bar: 1 mm. (f) Radial section of the scaffold by SEM, scale bar: 1 mm. (g) Porous structure in the middle of the radial section by SEM, scale bar: 100 μm. (h) Protuberant part from the side of the scaffold by SEM, scale bar: 100 μm (reprinted with permission from Wang et al., 2008, Biomaterials).
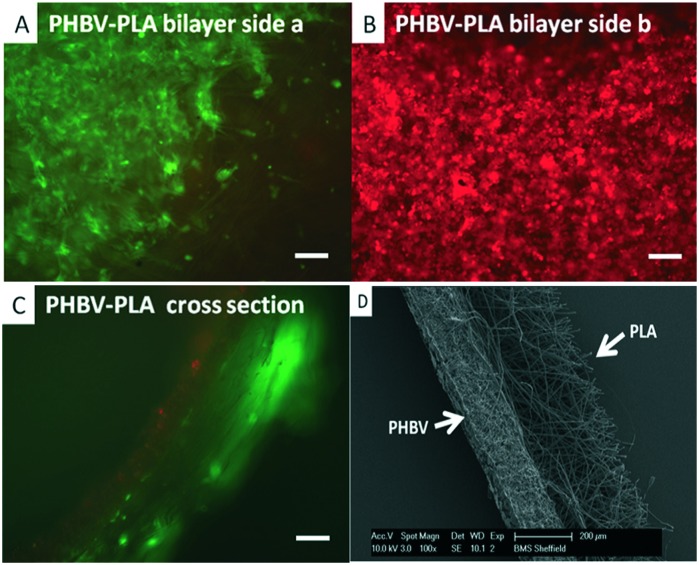
However, as studied, PHAs are hard and brittle in nature. To make them soft and elastic, composites of PHAs with inorganic materials like bioglass, hydroxyapatite (HA), clay, etc. were studied extensively, where they showed good mechanical properties and increased hydrophilicity and degradation rate.86 To elaborate this, composites of PHB or PHBV with HA were studied for bone implant application. These implants exhibited a compressive strength of 62 MPa which is equal to the bone strength of humans. A series of 3D implants and filling materials were made using P3HB and P3HB/HA composites and tested in vivo. These implants showed noticeable osteoplastic properties with normal reparative osteogenesis.87 Now they are suggested mostly for biomedical applications worldwide. Scaffolds of combinations of PHBV with a variety of materials like collagen, calcium phosphate and also some porous composites with sol–gel bioactive glass were studied in vivo mostly for bone tissue engineering.84 Porous scaffolds of PHB were also developed with natural corals to grow osteoblast cells which showed high cell viability. Also polymers like PHB, PHBHHx, PHBV and grafted PHAs with collagen, gelatin, chitosan, acrylic acid, etc. were studied for their potential as bone implants and cell attachments.88 Scaffolds of PHB and gelatin were studied for proliferating adrenocortical cells and found suitable for tissue engineering applications. PHBV is the only polymer which was studied more in bulk as well as in the nanoform to understand its suitability for scaffolds. As expected, the nanoform was proved more suitable to grow rabbit derived chondrocytes than films. Its nano size caused an increase in its surface area which in turn is responsible for better attachment of cells to scaffolds.84 Copolymers with a repeating unit of one scl and one mcl in the PHA backbone were tested for tissue engineering and the properties were found similar to those of petroleum derived polymers.89 It is known that porous scaffolds are more favorable for cell proliferation. The method in which scaffolds are made more porous by salt incorporation and leaching was tested with PHBV polymer, where the resultant scaffolds degraded faster. The degradation of this polymer was further enhanced with the incorporation of chitosan and sodium alginate.90 Polymer devices and 3D implants of PHB/PHV have been proven to be biocompatible in vitro against fibroblasts, hepatocytes, endothelial cells, and osteoblasts and also in vivo in animals for both short and long durations. The results suggested that PHAs are safe for various purposes like scaffolds for functioning cells, monofilament suture fibers for stitching wounds in surgery, and implants for reparative osteogenesis and bone defects in oral surgery.91 Other bi- and trilayer nanofibrous scaffolds co-cultured with fibroblast and mesenchymal progenitor cells (hESMPs) showed cell adhesion and proliferation using fluorescent dyes (Fig. 4).92
Fig. 4. Co-culture of Celltracker™ labelled fibroblasts (green) and hESMPs (red) on bilayer membranes of PHBV–PLA. After 7 days (A) fibroblasts (green) are confined to the PLA face with no sign of hESMPs (red). (B) On the opposite face of PHBV, hESMPs are grown without growth of fibroblast. (C) A cross section of a PHBV–PLA membrane shows each cell type on its respective side. (D) Scanning electron micrographs (SEMs) of an electrospun scaffold (cross section) of PHBV–PLA. The PHBV region on the left side is dense while the PLA region has a more open structure (reprinted with permission from Bye et al., 2013, Biomaterials Science).
Si-Wu Peng et al. studied the cell proliferation for films of various PHAs like PHB, PHBV, P3HB4HB, PHBHHx and PHBVHHx using rat osteoblast cells. In their study, degradation products and leached out fractions were checked against well-known cancer induction genes like c-Fos, Ki63 and p53. The results confirmed the suitability of PHAs as biomaterials, disproving their tumor inducing ability at gene level.93 Another cell proliferation study with oligomers of PHAs like oligo-3HB, 3-HHx and oligo-3H-co-4-HB was carried out in which oligo-3HB and 3HHx showed good cell viability in the experiment.94 In another study, a triblock co-polymer, poly(N-isopropylacrylamide)-block-poly[(R)-3-hydroxybutyrate]-block-poly(N-isopropylacrylamide) (PNIPAAm-PHB-PNIPAAm), was designed and used as a thermo-responsive substrate for cell culturing by coating it with gelatin. This substrate helps detaching cultured cells by cooling at 4 °C for 20 min where trypsinisation was not needed.95 Also gels of multi block poly(ester urethane)s consisting of segments of PHB, PEG and PPG were prepared for enhancing cell attachment as compared to the commercially available PEG–PPG–PEG triblock copolymer, which was proposed for applications in tissue engineering and scaffolds.96
Polymeric scaffolds for cardiac regeneration were made using PLGA, PHBV and poly(dioxanone). These single layered, porous scaffolds showed biocompatibility with cardiac stem cells. Sutures of PHB and PHBV were compared with silk and catgut sutures, where PHA sutures exhibited the necessary strength during recovery from muscle-fascial cuts. Adverse effects like inflammation necrosis, calcification, and malignization of the cicatrices at the site of implantation were not observed in the case of PHA as filament which is in contrast to the filaments of PLGA and poly(dioxane). Even the macrophage response towards both PHB and PHBV filaments was normal throughout the implantation period.97 Elastomeric mcl PHA films were implanted in rats subcutaneously and showed no rejection symptoms like necrosis, abscess or tumourigenesis even after 6 weeks of implantation.98 PHBV scaffolds were checked for the chondrogenic differentiation ability of human adipose derived stem cells (hASCs) in vivo on a mouse model. This study proves the support of the PHBV scaffold for the production of neocartilage in a heterotopic site during implantation for 16 weeks.99 It is well known that the peripheral nervous system shows poor recovery by Wallerian degeneration in small nerve gaps, which improved with the aid of electrospun nanofibers of PHA scaffolds loaded with stem cells on the traumatic injury of a rat model.100
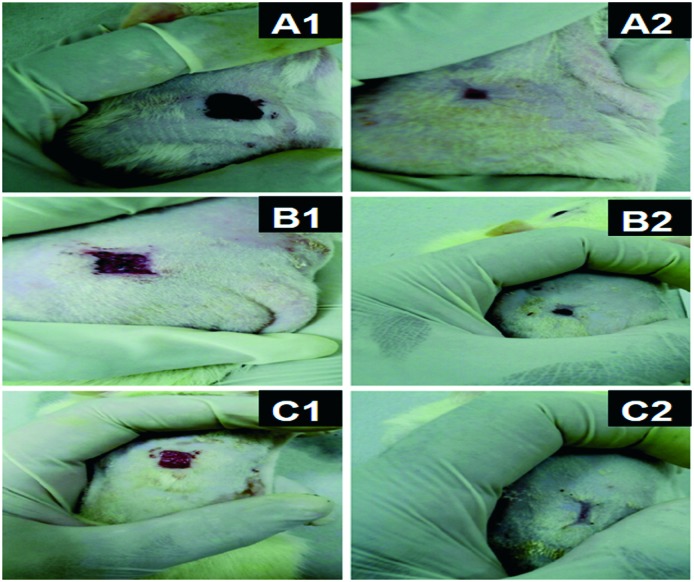
Advanced wound healing materials should support damaged skin by covering and provide properties such as non-adherence to wound, maintaining moisture balance, non-toxicity to tissue, enabling gaseous exchange at the site of the target area, hemostasis, and maintaining aseptic conditions to avoid infections.101 Studies in this direction were done using biodegradable PHAs as implants for wound healing. For example, a polymeric film of PHA and guar gum loaded with curcumin was studied for its application in wound healing, which showed good antibacterial properties.102 PHBV fibers were also studied and proved to be better than the application of direct medicinal ointment on the wound. Fig. 5 demonstrates the wound healing property of such fibers.103 PEGylated PHB-based biomaterials have great potential as biological scaffolds supporting nerve repair.40
Fig. 5. Microscopic image of the wound contracture over time for (A1) 1 week and (A2) 2 weeks using PHBV fibers, (B1) 1 week and (B2) 2 weeks using R-Spondin 1 and (C1) 1 week and (C2) 2 weeks using PHBV fibers loaded with R-Spondin 1 (reprinted with permission from Kuppan et al., 2011, Biomacromolecules).
As a ready reference, Table 2 lists many such applications of different PHAs in different areas.
Table 2. Different PHAs, copolymers of PHAs and their applications.
| Sr. no. | Various modified PHAs and their copolymers | Applications of PHAs |
| 1. | PHB conjugated with PHA synthase enzyme | Drug delivery for colon cancer therapy |
| 2. | Copolymers of scl and mcl PHAs or unsaturated mcl PHAs | For tissue engineering application and transdermal drug delivery |
| 3. | Tuned valerate content in PHBV | As a drug carrier for cancer therapy |
| 4. | Enzyme mediated microporous PHAs | For scaffolds in biomedical applications |
| 5. | Lipase catalyzed mcl poly(1′-O-3-hydroxyacyl sucrose) | For biomedical and industrial applications |
| 6. | PHBV with natural rubber | Delivery of model drug flurbiprofen |
| 7. | Artificial PHO-co-HHx | For targeted binding of proteins using extracellular mcl-PHA depolymerase52 |
| 8. | Cationic PHO-co-(hydroxy-11-(bis(2-hydroxyethyl)-amino)-10-hydroxyundecanoate) | Delivery of plasmid DNA51 |
| 9. | PHO with pendent groups of modified carboxylic acid (PHO75COOH25) | Helps in cell adhesion for 3D engineering, drug release and cell proliferation studies50 |
| 10. | Blend of PHB and PHBHHx | Rabbit articular cartilage chondrocyte adhesion studies104 |
| 11. | PHA and starburst polyamidoamine dendrimer | For transdermal drug delivery54 |
| 12. | High quality PHA-co-terpolyesters | For biomedical and pharmaceutical applications105 |
| 13. | PHBV–PHO diblock co-polymers with P4HB | For drug delivery and drug elution for cochlear implants78,106 |
| 14. | Co-poly(3-hydroxy-8-phenyloctanoate-3-hydroxy-6-phenylhexanoate) and co-poly(3-hydroxy-7-phenylheptanoate-3-hydroxy-5-phenylvalerate) | As a biodegradable polymer and for biomedical applications107 |
| 15. | Microcomposites of clay with PHB | For pharmaceutical, cosmetic and industrial applications108 |
| 16. | PHA nanocapsules,109 PHA conjugated nanocontainers110 and nanoparticles of PHBHHx111 | For controlled drug release in drug delivery systems |
| 17. | Antibiotic releasing PHB, PHBV, P3HB–3HV and P3HB–4HV implants | For the treatment of implant related osteomyelitis112,113 |
| 18. | Gels of PHB-co-PHH and P3HB-co-4HB | As anti-adhesion gels for preventing postoperative adhesion114 |
| 19. | PHAs obtained from Pseudomonas sp. | For drug release application115,116 |
| 20. | Nanoparticles of PHBHHx | For implant coating and tissue regeneration in 3D hydrogel/stem cell model117 |
| 21. | Graft polymers of PHBHV with HEMA and MAA | Micro-patterned surfaces suitable for biomedical applications118 |
| 22. | Nanofiber mats, films of PHA | For controlled local drug delivery system against periodontitis119 |
| 23. | PHBHHx films and scaffolds with good mechanical properties | For the growth and proliferation of chondrocytes81 |
| 24. | PHB, P4HB and nanofibers of PHBV | For gastrointestinal patches and sutures120 and nerve regeneration devices for axon regeneration121 or artificial nerve graft122 |
| 25. | Magnetic nanospheres of PHB/PHBV and iron oxide | For targeted cancer therapy123,124 |
| 26. | Poly(3-hydroxybutyrate-co-3-hydroxy-10-undecanoate) (PHBU) scaffolds | For soft tissue replacement125 |
| 27. | Microparticles of PHOHHX-co-PEG | For drug delivery126 |
| 28. | PHAs and their composites with ceramics | For ligament and tendon scaffolds,127 wound management and maxillofacial treatment128 |
| 29. | Microcapsules of PHB | Encapsulation and slow release of l-leuprolide acetate129 |
| 30. | Electrospun nanofiber mats of PHBV, PLA and polyglycerol sebacate (PGS) blends | For ventricular regeneration130 |
| 31. | Nanoparticles of copolymers of PHB and PHBHHx and PCL | For intracellular drug release of rhodamine B isothiocyanate (RBITC)131 |
| 32. | Nano-containers of PHB, PHBV blends with PEG | Slow and sustained release of the drug thymoquinone132 |
| 33. | Macrocapsules derived with PHBHO–PEG and derivatives of folic acid | Loaded with drugs like Adriamycin, epiadriamycin, taxol or camptothecin for antitumor therapy133 |
| 34. | Composite of PHB with hydroxyapatite (HA) and PHBV with LA and apatite | For bone implant134 and tissue engineering135 |
| 35. | PHAs as solid substrate | For denitrification of waste water136 |
| 36. | Micro/nano-sized drug carriers of PHBV | For skin penetrating drug delivery137 |
| 37. | Drug encapsulated PHBV nanofibers | For controlled drug delivery with lower drug crystallinity138 |
Conclusions
In this review copolymers of PHAs were well studied and found more suitable as biomaterials for various biomedical applications; as a result, a few products based on PHAs are in use. Further, it is inferred that the prerequisite properties such as biocompatibility, biodegradability, and easy production and modification either chemically or physically have enabled investigators to develop new PHAs with improved properties so as to expand their utility. Herein the collective information on development of biomaterials for various biomedical applications would provide a ready reference for those working in related areas to design new materials for new applications. Accordingly in the near future, we may anticipate some breakthrough results in almost all research fields based on co-polymers of PHAs.
Conflict of interest
The authors declare no competing interests.
Acknowledgments
The authors acknowledge the CSC0302 and CSC0134, CSIR, New Delhi, AcSIR and CSIR-NCL for the financial support and facilities.
Biographies

Bhagyashri S. Thorat Gadgil
Bhagyashri S. Thorat Gadgil completed her M.Sc. in Biotechnology from Shivaji University, India. Currently she is pursuing her PhD in polymer sciences and engineering, which is interdisciplinary. Her research interest is to develop new blends and composites of biopolymers and study their applications in different fields. She has been working on blends of polyhydroxyalkanoates for the last four years as a part of her PhD work.

Naresh Killi
Naresh Killi obtained his M.Sc. (2011) from Andhra University, India. Currently he is pursuing his PhD at CSIR-National Chemical Laboratory, Pune, India. His research interest is mainly focussed on design and synthesis of biodegradable/functional polymers as materials for biomedical applications.

GVN Rathna
GVN Rathna, PhD (Chemistry), is working as a Senior Scientist at CSIR-National Chemical Laboratory, Pune, India. She is working on several projects such as hydrogels, biomaterials, nanotechnology, solar energy and pest control. She was a postdoctoral fellow at the University of Wisconsin, Madison, USA. She was awarded the National Science Council award by Taiwan. She has published more than 20 research papers, a couple of book chapters, and US patents in the field of biomaterials. She is recognised as an established scientist by the Royal Society of UK, 2017. Currently she is working on the development of new biomaterials for advanced drug delivery systems and coatings.
References
- Quirino R. L., Garrison T. F., Kessler M. R. Green Chem. 2014;16:1700–1715. [Google Scholar]
- Khanna S., Srivastava A. K. Process Biochem. 2005;40:607–619. [Google Scholar]
- Liu H., Jiang N., Mao H., Wang Z. Gongcheng Suliao Yingyong. 2014;42:131–134. [Google Scholar]
- Li Z. B., Loh X. J. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2017;9(3) doi: 10.1002/wnan.1429. [DOI] [PubMed] [Google Scholar]
- Tomizawa S., Hyakutake M., Saito Y., Agus J., Mizuno K., Abe H., Tsuge T. Biomacromolecules. 2011;12:2660–2666. doi: 10.1021/bm2004687. [DOI] [PubMed] [Google Scholar]
- Tsuge T. Polym. J. 2016;48:1051–1057. [Google Scholar]
- Akaraonye E., Keshavarz T., Roy I. J. Chem. Technol. Biotechnol. 2010;85:732–743. [Google Scholar]
- Rathna G. V. N. and Ghosh S., Bacterial Polymers: Resources, Synthesis and Applications, In Biopolymers, John Wiley & Sons, Inc., 2011, pp. 291–316. [Google Scholar]
- Guo M., Stuckey D. C., Murphy R. J. Green Chem. 2013;15:706–717. [Google Scholar]
- Freier T., Biopolyesters in Tissue Engineering Applications, In Polymers for Regenerative Medicine, ed. C. Werner, Springer, Berlin Heidelberg, 2006, pp. 1–61. [Google Scholar]
- Kumar M. N. R. React. Funct. Polym. 2000;46:1–27. [Google Scholar]
- Subbiah T., Bhat G., Tock R., Parameswaran S., Ramkumar S. J. Appl. Polym. Sci. 2005;96:557–569. [Google Scholar]
- Berger J., Reist M., Mayer J. M., Felt O., Peppas N. A., Gurny R. Eur. J. Pharm. Biopharm. 2004;57:19–34. doi: 10.1016/s0939-6411(03)00161-9. [DOI] [PubMed] [Google Scholar]
- Bugnicourt E., Cinelli P., Lazzeri A., Alvarez V. eXPRESS Polym. Lett. 2014;8:791–808. [Google Scholar]
- Kulprecha S., Phonprapai C. and Chanchaichaovivat A., Characterization and production of poly–β–hydroxybutyric acid, A biodegradable thermoplastic from Alcaligenes sp. A-04, Elsevier, 1995, pp. 1377–1384. [Google Scholar]
- Fletcher M., Lessmann J. M., Loeb G. I. Biofouling. 1991;4:129–140. [Google Scholar]
- Kenis P. R. Appl. Microbiol. 1968;16:1253. doi: 10.1128/am.16.8.1253-1254.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik R., Kanari B., Upadhyay S. World J. Microbiol. Biotechnol. 2000;16:407–414. [Google Scholar]
- Jensen-Spaulding A., Cabral K., Shuler M. L., Lion L. W. Water Res. 2004;38:2231–2240. doi: 10.1016/j.watres.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Cambon-Bonavita M. A., Raguenes G., Jean J., Vincent P., Guezennec J. J. Appl. Microbiol. 2002;93:310–315. doi: 10.1046/j.1365-2672.2002.01689.x. [DOI] [PubMed] [Google Scholar]
- Kent J. L., Osborn M. J. Biochemistry. 1968;7:4396–4408. doi: 10.1021/bi00852a036. [DOI] [PubMed] [Google Scholar]
- Kienle-Burgdorf A. Schweiz. Lab.-Z. 1995;52:306–315. [Google Scholar]
- Yousef N. E. New Egypt. J. Microbiol. 2006;13:222–236. [Google Scholar]
- Dohse D. M., Lion L. W. Environ. Sci. Technol. 1994;28:541–548. doi: 10.1021/es00053a003. [DOI] [PubMed] [Google Scholar]
- Horowitz D. M., Sanders J. K. M. J. Am. Chem. Soc. 1994;116:2695–2702. [Google Scholar]
- Reusch R. N. Biochemistry. 2000;65:280–295. [PubMed] [Google Scholar]
- Rinaudo M. Prog. Polym. Sci. 2006;31:603–632. [Google Scholar]
- Tripathi G., Mahishi L., Ramachander T. and Rawal S., Process for the production of polyhydroxyoctanoate by streptomyces lividans, US 20020090687 A1, 2002.
- Zinn M., Witholt B., Egli T. Adv. Drug Delivery Rev. 2001;53:5–21. doi: 10.1016/s0169-409x(01)00218-6. [DOI] [PubMed] [Google Scholar]
- Visakh P. M., Polyhydroxyalkanoates (PHAs), their Blends, Composites and Nanocomposites: State of the Art, New Challenges and Opportunities, In Polyhydroxyalkanoate (PHA) based Blends, Composites and Nanocomposites, The Royal Society of Chemistry, 2015, ch. 1, pp. 1–17. [Google Scholar]
- Hazer D. B., Kılıçay E., Hazer B. Mater. Sci. Eng., C. 2012;32:637–647. [Google Scholar]
- Shrivastav A., Kim H.-Y., Kim Y.-R., BioMed Res. Int., 2013. , 581684 , , 581613 pp. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazer D. B., Kilicay E., Hazer B. Mater. Sci. Eng., C. 2012;32:637–647. [Google Scholar]
- Wiesbrock F., Ebner C., Stelzer F., Weinberg A. and Kuehn K.-D., Hybrid polymers for medical applications, Chemical Indexing Equivalent to 158:77765 (EP), WO2012174580A1, Technische Universitaet Graz, Austria, Medical University of Graz, AT&S Austria Technologie & Systemtechnik AG, Heraeus Medical GmbH, 2012, p. 35.
- Schmidt D., Stock U. A., Hoerstrup S. P. Philos. Trans. R. Soc., B. 2007;362:1505–1512. doi: 10.1098/rstb.2007.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manavitehrani I., Fathi A., Badr H., Daly S., Negahi Shirazi A., Dehghani F. Polymer. 2016;8:20. doi: 10.3390/polym8010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai D., Loh X. J. ACS Sustainable Chem. Eng. 2014;2:106–119. [Google Scholar]
- Li Z. B., Yang J., Loh X. J. NPG Asia Mater. 2016;8:e265. doi: 10.1038/am.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. B., Loh X. J. Chem. Soc. Rev. 2015;44:2865–2879. doi: 10.1039/c5cs00089k. [DOI] [PubMed] [Google Scholar]
- Chan R. T. H., Russell R. A., Marcal H., Lee T. H., Holden P. J., Foster L. J. R. Biomacromolecules. 2014;15:339–349. doi: 10.1021/bm401572a. [DOI] [PubMed] [Google Scholar]
- Lu S., Shen X., Zhou C., Duan C., Zhang H. Gongcheng Suliao Yingyong. 2014;42:109–113. [Google Scholar]
- Wu Q., Wang Y., Chen G. Q. Artif. Cells, Blood Substitutes, Biotechnol. 2009;37:1–12. doi: 10.1080/10731190802664429. [DOI] [PubMed] [Google Scholar]
- Nobes G. A. R., Marchessault R. H., Maysinger D. Drug Delivery. 1998;5:167–177. doi: 10.3109/10717549809052032. [DOI] [PubMed] [Google Scholar]
- Kalhapure R. S., Suleman N., Mocktar C., Seedat N., Govender T. J. Pharm. Sci. 2015;104:872–905. doi: 10.1002/jps.24298. [DOI] [PubMed] [Google Scholar]
- Loh X. J., Tan K. K., Li X., Li J. Biomaterials. 2006;27:1841–1850. doi: 10.1016/j.biomaterials.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Wu Y. L., Wang H., Qiu Y. K., Liow S. S., Li Z. B., Loh X. J. Adv. Healthcare Mater. 2016;5:2679–2685. doi: 10.1002/adhm.201600723. [DOI] [PubMed] [Google Scholar]
- Loh X. J., Goh S. H., Li J. Biomaterials. 2007;28:4113–4123. doi: 10.1016/j.biomaterials.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Barouti G., Liow S. S., Dou Q. Q., Ye H. Y., Orione C., Guillaume S. M., Loh X. J. Chem. – Eur. J. 2016;22:10501–10512. doi: 10.1002/chem.201601404. [DOI] [PubMed] [Google Scholar]
- Coelho J. F. J., Gois J. R., Fonseca A. C., Gil M. H. J. Appl. Polym. Sci. 2010;116:718–726. [Google Scholar]
- Renard E., Timbart L., Vergnol G., Langlois V. J. Appl. Polym. Sci. 2010;117:1888–1896. [Google Scholar]
- Sparks J., Scholz C. Biomacromolecules. 2009;10:1715–1719. doi: 10.1021/bm900372x. [DOI] [PubMed] [Google Scholar]
- Ihssen J., Magnani D., Thony-Meyer L., Ren Q. Biomacromolecules. 2009;10:1854–1864. doi: 10.1021/bm9002859. [DOI] [PubMed] [Google Scholar]
- Wang Z., Itoh Y., Hosaka Y., Kobayashi I., Nakano Y., Maeda I., Umeda F., Yamakawa J., Nishimine M., Suenobu T., Fukuzumi S., Kawase M., Yagi K. J. Biosci. Bioeng. 2003;96:537–540. doi: 10.1016/S1389-1723(04)70146-2. [DOI] [PubMed] [Google Scholar]
- Wang Z., Itoh Y., Hosaka Y., Kobayashi I., Nakano Y., Maeda I., Umeda F., Yamakawa J., Kawase M., Yagi K. J. Biosci. Bioeng. 2003;95:541–543. doi: 10.1016/s1389-1723(03)80059-2. [DOI] [PubMed] [Google Scholar]
- Gangrade N., Price J. C. J. Microencapsulation. 1991;8:185–202. doi: 10.3109/02652049109071487. [DOI] [PubMed] [Google Scholar]
- Campos E., Branquinho J., Carreira A. S., Carvalho A., Coimbra P., Ferreira P., Gil M. H. Eur. Polym. J. 2013;49:2005–2021. [Google Scholar]
- Simioni A. R., Vaccari C., Re M. I., Tedesco A. C. J. Mater. Sci. 2008;43:580–584. [Google Scholar]
- Pacheco D. P., Amaral M. H., Reis R. L., Marques A. P., Correlo V. M. Int. J. Pharm. 2015;478:398–408. doi: 10.1016/j.ijpharm.2014.11.036. [DOI] [PubMed] [Google Scholar]
- Gursel I., Yagmurlu F., Korkusuz F., Hasirci V. J. Microencapsulation. 2002;19:153–164. doi: 10.1080/02652040110065413. [DOI] [PubMed] [Google Scholar]
- Gould P. L., Holland S. J., Tighe B. J. Int. J. Pharm. 1987;38:231–237. [Google Scholar]
- Koenig W., Seidel H. R. and Sandow J. K., Sustained-release peptide hormone pharmaceuticals, EP133988A2, Hoechst A.-G., Fed. Rep. Ger., 1985, p. 15.
- Fraser H. M., Sandow J., Seidel H. R., Lunn S. F. Acta Endocrinol. 1989;121:841–848. doi: 10.1530/acta.0.1210841. [DOI] [PubMed] [Google Scholar]
- Humke R., Seidel H. R. and Von Rechenberg W., Controlled-release parenteral implants containing poly(hydroxybutyric acid) and calcium acetate-glycerol adducts and bioregulating peptides, Hoechst A.-G., Fed. Rep. Ger., 1988, p. 6.
- Korsatko W. and Wabnegg B., Sustained-release tablets, Chemie Linz A.-G., Austria, Lentia G.m.b.H., 1984, p. 21. [Google Scholar]
- Masood F. Mater. Sci. Eng., C. 2016;60:569–578. doi: 10.1016/j.msec.2015.11.067. [DOI] [PubMed] [Google Scholar]
- Masood F., Chen P., Yasin T., Hasan F., Ahmad B., Hameed A. J. Mater. Sci.: Mater. Med. 2013;24:1927–1937. doi: 10.1007/s10856-013-4946-x. [DOI] [PubMed] [Google Scholar]
- Masani M. Y. A., Parveez G. K. A., Izawati A. M. D., Lan C. P., Akmar A. S. N. Plasmid. 2009;62:191–200. doi: 10.1016/j.plasmid.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Kwon H.-S., Jung S.-G., Kim H.-Y., Parker S. A., Batt C. A., Kim Y.-R. J. Mater. Chem. B. 2014;2:3965–3971. doi: 10.1039/c4tb00304g. [DOI] [PubMed] [Google Scholar]
- Masood F. Mater. Sci. Eng., C. 2016;60:569–578. doi: 10.1016/j.msec.2015.11.067. [DOI] [PubMed] [Google Scholar]
- Scholz C. ACS Symp. Ser. 2000;764:328–334. [Google Scholar]
- Li Y., Thouas G. A., Chen Q.-Z. RSC Adv. 2012;2:8229–8242. [Google Scholar]
- De Oliveira M. F. and Da Silva J. V. L., Procedure for obtaining the three-dimensional structure of prototypes and or the implants made of biodegradable raw materials, by means of a technique of fast prototyping with technology of selective sintering of embedded laser; prototype and or biodegradable implant and resulting biodegradable waste by-products, Centro de Tecnologia da Informacco Renato Archer, Brazil, 2016, p. 21. [Google Scholar]
- Rizk S., Fosco A., Ganatra A., Martin D. P. and Williams S. F., Three-dimensional resorbable implants comprising poly-4-hydroxybutyrate copolymers for tissue reinforcement and hernia repair, WO2015167807A1, Tepha, Inc., USA, 2015, p. 33.
- Felix F., Fosco A., Martin D. P., Moses A., Van Natta B., Rizk S. and Williams S. F., Absorbable implants for plastic surgery, WO2015006737A1, Tepha, Inc., USA, 2015, p. 59.
- Li X., Loh X. J., Wang K., He C. B., Li J. Biomacromolecules. 2005;6:2740–2747. doi: 10.1021/bm050234g. [DOI] [PubMed] [Google Scholar]
- Volova T. G., Shishatskaja E. I., Mironov P. V., Goreva A. V. Perspekt. Mater. 2009:43–50. [Google Scholar]
- Murayama Y. and Vinuela F., Bioabsorbable polymeric implants and a method of using the same to create occlusions, Cont.-in-part of U.S. Ser. No. 406, 306, US8388643B2, University of California, USA, 2013, p. 21.
- Lenarz T., Schmitz K.-P., Behrend D., Sternberg K., Williams S. and Martin D., Drug eluting cochlear implants, WO2012064526A1, Tepha, Inc., USA, 2012, p. 28.
- Sevastianov V., Coating compositions for implants especially stents containing biocompatible polymers and shikonin, WO2004009148A1, Ls Medcap GmbH, Germany, 2004, p. 49.
- Chebbi A., Podporska J., Stokes J. DVS-Ber. 2011;276:a67. [Google Scholar]
- Zhao K., Deng Y., Chun Chen J., Chen G.-Q. Biomaterials. 2003;24:1041–1045. doi: 10.1016/s0142-9612(02)00426-x. [DOI] [PubMed] [Google Scholar]
- Dwivedi P., Narvi S. S., Tewari R. P. J. Appl. Biomater. Funct. Mater. 2013;11:129–142. doi: 10.5301/JABFM.2013.11544. [DOI] [PubMed] [Google Scholar]
- Paşcu E. I., Stokes J., McGuinness G. B. Mater. Sci. Eng., C. 2013;33:4905–4916. doi: 10.1016/j.msec.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Chen G.-Q., Wu Q. Biomaterials. 2005;26:6565–6578. doi: 10.1016/j.biomaterials.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Wang Y., Bian Y.-Z., Wu Q., Chen G.-Q. Biomaterials. 2008;29:2858–2868. doi: 10.1016/j.biomaterials.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Misra S. K., Valappil S. P., Roy I., Boccaccini A. R. Biomacromolecules. 2006;7:2249–2258. doi: 10.1021/bm060317c. [DOI] [PubMed] [Google Scholar]
- Shishatskaya E. I., Kamendov I. V., Starosvetsky S. I., Vinnik Y. S., Markelova N. N., Shageev A. A., Khorzhevsky V. A., Peryanova O. V., Shumilova A. A. Artif. Cells, Nanomed., Biotechnol. 2014;42:344–355. doi: 10.3109/21691401.2013.816312. [DOI] [PubMed] [Google Scholar]
- Bradley M., Khan F., Oreffo R. O. C. and Tare R. S., Biocompatible polymer blends for cell attachments, WO2010023463A2, University Court of the University of Edinburgh, UK, 2010, p. 58.
- Tappel R. C., Pan W. Y., Bergey N. S., Wang Q., Patterson I. L., Ozumba O. A., Matsumoto K., Taguchi S., Nomura C. T. ACS Sustainable Chem. Eng. 2014;2:1879–1887. [Google Scholar]
- Ansari N. F., Amirul A. A. Appl. Biochem. Biotechnol. 2013;170:690–709. doi: 10.1007/s12010-013-0216-0. [DOI] [PubMed] [Google Scholar]
- Shishatskaya E. I. Macromol. Symp. 2008;269:65–81. [Google Scholar]
- Bye F. J., Bissoli J., Black L., Bullock A. J., Puwanun S., Moharamzadeh K., Reilly G. C., Ryan A. J., MacNeil S. Biomater. Sci. 2013;1:942–951. doi: 10.1039/c3bm60074b. [DOI] [PubMed] [Google Scholar]
- Peng S.-W., Guo X.-Y., Shang G.-G., Li J., Xu X.-Y., You M.-L., Li P., Chen G.-Q. Biomaterials. 2011;32:2546–2555. doi: 10.1016/j.biomaterials.2010.12.051. [DOI] [PubMed] [Google Scholar]
- Yang X.-D., Zou X.-H., Dai Z.-W., Luo R.-C., Wei C.-J., Chen G.-Q. J. Biomater. Sci., Polym. Ed. 2009;20:1729–1746. doi: 10.1163/156856208X386291. [DOI] [PubMed] [Google Scholar]
- Loh X. J., Gong J. S., Sakuragi M., Kitajima T., Liu M. Z., Li J., Ito Y. Macromol. Biosci. 2009;9:1069–1079. doi: 10.1002/mabi.200900081. [DOI] [PubMed] [Google Scholar]
- Loh X. J., Goh S. H., Li J. J. Phys. Chem. B. 2009;113:11822–11830. doi: 10.1021/jp903984r. [DOI] [PubMed] [Google Scholar]
- Volova T., Shishatskaya E., Sevastianov V., Efremov S., Mogilnaya O. Biochem. Eng. J. 2003;16:125–133. [Google Scholar]
- Hazer D. B., Hazer B., Kaymaz F. Biomed. Mater. 2009;4:035011. doi: 10.1088/1748-6041/4/3/035011. [DOI] [PubMed] [Google Scholar]
- Liu J., Zhao B., Zhang Y., Lin Y., Hu P., Ye C. J. Biomed. Mater. Res., Part A. 2010;94:603–610. doi: 10.1002/jbm.a.32730. [DOI] [PubMed] [Google Scholar]
- Biazar E. Int. J. Polym. Mater. Polym. Biomater. 2014;63:898–908. [Google Scholar]
- Sharma J., Lizu M., Stewart M., Zygula K., Lu Y., Chauhan R., Yan X., Guo Z., Wujcik E. K., Wei S. Polymer. 2015;7:186–219. [Google Scholar]
- Pramanik N., Mitra T., Khamrai M., Bhattacharyya A., Mukhopadhyay P., Gnanamani A., Basu R. K., Kundu P. P. RSC Adv. 2015;5:63489–63501. [Google Scholar]
- Kuppan P., Vasanthan K. S., Sundaramurthi D., Krishnan U. M., Sethuraman S. Biomacromolecules. 2011;12:3156–3165. doi: 10.1021/bm200618w. [DOI] [PubMed] [Google Scholar]
- Zheng Z., Bei F.-F., Tian H.-L., Chen G.-Q. Biomaterials. 2005;26:3537–3548. doi: 10.1016/j.biomaterials.2004.09.041. [DOI] [PubMed] [Google Scholar]
- Koller M., Hesse P., Bona R., Kutschera C., Atlic A., Braunegg G. Macromol. Symp. 2007;253:33–39. doi: 10.1002/mabi.200600211. [DOI] [PubMed] [Google Scholar]
- Vergnol G., Sow H., Renard E., Haroun F., Langlois V. React. Funct. Polym. 2012;72:260–267. [Google Scholar]
- Abraham G. A., Gallardo A., San Roman J., Olivera E. R., Jodra R., García B., Miñambres B., García J. L., Luengo J. M. Biomacromolecules. 2001;2:562–567. doi: 10.1021/bm010018h. [DOI] [PubMed] [Google Scholar]
- da Silva-Valenzuela M. D., Wang S. H., Wiebeck H. and Valenzuela-Diaz F. R., Nanocomposite Microcapsules from Powders of Polyhydroxybutyrate (PHB) and Smectite Clays, In Advanced Powder Technology Vii, ed. L. Salgado and F. Ambrozio, 2010, pp. 794–798. [Google Scholar]
- Yu P. H. F., Chan A. H. Y. and Ho K. P., Preparation of polyhydroxyalkanoate (PHA) nanocapsules as protein carriers, EP2756842A1, Nano and Advanced Materials Institute Limited, Peop. Rep. China, 2014, p. 14.
- Yoon S. C., Choi M. H. and Shah M., Amphiphilic polyhydroxyalkanoate-mPEG copolymer nanocontainer for drug delivery, Chemical Indexing Equivalent to 155:442191 (KR), WO2012124838A1, Industry-Academic Cooperation Foundation Gyeongsang National University, S. Korea, 2012, p. 26.
- Lu X.-Y., Wu D.-C., Li Z.-J., Chen G.-Q., Prog. Mol. Biol. Transl. Sci., 2011, 104 , 299 –323 , , 293 plates . [DOI] [PubMed] [Google Scholar]
- Gürsel I., Korkusuz F., Türesin F., Gürdal Alaeddinoǧlu N., Hasırcı V. Biomaterials. 2000;22:73–80. doi: 10.1016/s0142-9612(00)00170-8. [DOI] [PubMed] [Google Scholar]
- Türesin F., Gürsel I., Hasirci V. J. Biomater. Sci., Polym. Ed. 2001;12:195–207. doi: 10.1163/156856201750180924. [DOI] [PubMed] [Google Scholar]
- Chen G. and Dai Z., Anti-adhesion gel containing polyhydroxyalkanoates (PHA) and hydrophilic organic solvent, and application thereof, CN101618045A, Shantou University, Peop. Rep. China, 2010, p. 28.
- Kabilan S., Ayyasamy M., Jayavel S., Paramasamy G., Int. J. Microbiol., 2012. , 317828 , , 317810 pp. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai R., Keshavarz T., Roether J. A., Boccaccini A. R., Roy I. Mater. Sci. Eng., R. 2011;72:29–47. [Google Scholar]
- Dong C.-L., Webb W. R., Peng Q., Tang J. Z., Forsyth N. R., Chen G.-Q., El Haj A. J. J. Biomed. Mater. Res., Part A. 2015;103:282–288. doi: 10.1002/jbm.a.35149. [DOI] [PubMed] [Google Scholar]
- Versace D.-L., Dubot P., Cenedese P., Lalevee J., Soppera O., Malval J.-P., Renard E., Langlois V. Green Chem. 2012;14:788–798. [Google Scholar]
- Joshi D., Garg T., Goyal A. K., Rath G. Drug Delivery. 2015:1–15. doi: 10.2174/1567201812666141205131331. [DOI] [PubMed] [Google Scholar]
- Lobler M., Sass M., Kunze C., Schmitz K.-P., Hopt U. T. Biomaterials. 2001;23:577–583. doi: 10.1016/s0142-9612(01)00144-2. [DOI] [PubMed] [Google Scholar]
- Terenghi G., Mohanna P.-N. and Martin D. P., Nerve regeneration devices comprising polyhydroxyalkanoate with improved axonal regeneration rates and methods of use, WO2005020825A1, Tepha, Inc., USA, 2005, p. 18.
- Biazar E., Keshel S. H. Cell Commun. Adhes. 2013;20:41–49. doi: 10.3109/15419061.2013.774378. [DOI] [PubMed] [Google Scholar]
- Erdal E., Kavaz D., Sam M., Demirbilek M., Demirbilek M. E., Saglam N., Denkbas E. B. J. Biomed. Nanotechnol. 2012;8:800–808. [PubMed] [Google Scholar]
- Vilos C., Gutierrez M., Escobar R. A., Morales F., Denardin J. C., Velasquez L., Altbir D. Electron. J. Biotechnol. 2013;16(5):8. [Google Scholar]
- Levine A. C., Sparano A., Twigg F. F., Numata K., Nomura C. T. ACS Biomater. Sci. Eng. 2015;1:567–576. doi: 10.1021/acsbiomaterials.5b00052. [DOI] [PubMed] [Google Scholar]
- Babinot J., Guigner J.-M., Renard E., Langlois V. J. Colloid Interface Sci. 2012;375:88–93. doi: 10.1016/j.jcis.2012.02.042. [DOI] [PubMed] [Google Scholar]
- Rathbone S., Furrer P., Luebben J., Zinn M., Cartmell S. J. Biomed. Mater. Res., Part A. 2010;93:1391–1403. doi: 10.1002/jbm.a.32641. [DOI] [PubMed] [Google Scholar]
- Keshavarz T., Roy I. Curr. Opin. Microbiol. 2010;13:321–326. doi: 10.1016/j.mib.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Ajellal N., Thomas C. M., Aubry T., Grohens Y., Carpentier J.-F. New J. Chem. 2011;35:876–880. [Google Scholar]
- Kenar H., Kose G. T., Hasirci V. J. Mater. Sci.: Mater. Med. 2010;21:989–997. doi: 10.1007/s10856-009-3917-8. [DOI] [PubMed] [Google Scholar]
- Xiong Y.-C., Yao Y.-C., Zhan X.-Y., Chen G.-Q. J. Biomater. Sci., Polym. Ed. 2010;21:127–140. doi: 10.1163/156856209X410283. [DOI] [PubMed] [Google Scholar]
- Shah M., Naseer M. I., Choi M. H., Kim M. O., Yoon S. C. Int. J. Pharm. 2010;400:165–175. doi: 10.1016/j.ijpharm.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Zhao L., Zhang C., Lv L., Dong Y., Chen Z. and Zhang X., Poly(hydroxyalkanoate) targeting carrier and antitumor agent-loaded nanoparticle, and preparation method thereof, CN101513530A, Shanxi University, Peop. Rep. China, 2009, p. 7.
- Doyle C., Tanner E. T., Bonfield W. Biomaterials. 1991;12:841–847. doi: 10.1016/0142-9612(91)90072-i. [DOI] [PubMed] [Google Scholar]
- Saska S., Christovam L. M., Beatrice C. G., Lucas A. A., Neto P. I., Pereira F. D., Oliveria M. F., Pereira V. A., Capote T. O., Ribeiro S. L. Tissue Eng., Part A. 2015;21:S119–S119. [Google Scholar]
- Hiraishi A., Khan S. T. Appl. Microbiol. Biotechnol. 2003;61:103–109. doi: 10.1007/s00253-002-1198-y. [DOI] [PubMed] [Google Scholar]
- Eke G., Kuzmina A. M., Goreva A. V., Shishatskaya E. I., Hasirci N., Hasirci V. J. Mater. Sci.: Mater. Med. 2014;25:1471–1481. doi: 10.1007/s10856-014-5169-5. [DOI] [PubMed] [Google Scholar]
- Souza M. A., Sakamoto K. Y., Mattoso L. H. C. J. Nanomater. 2014:129035. [Google Scholar]