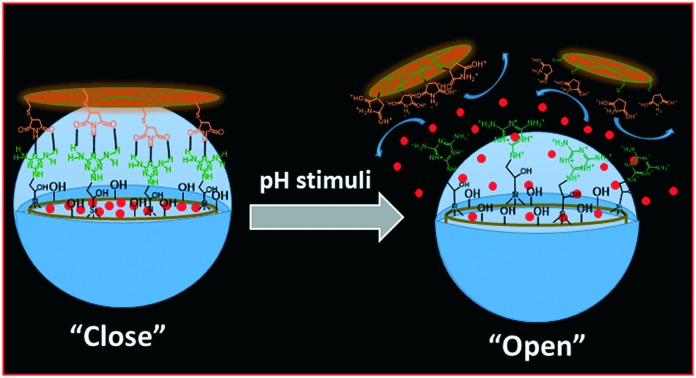
 Herein, we propose a “host–guest” complexation-based mesoporous silica drug carrier, MSNs@Mela@TTM, for pH-responsive drug delivery applications in cancer therapy.
Herein, we propose a “host–guest” complexation-based mesoporous silica drug carrier, MSNs@Mela@TTM, for pH-responsive drug delivery applications in cancer therapy.
Abstract
Mesoporous silica-based drug delivery carriers mostly require appropriate surface modifications to improve their drug delivery efficiency and to reduce their adverse side effects. In the present work, we have synthesised mesoporous silica nanoparticles and their surface was covered by using capping units such as tetrathio-maleimide (TTM) via a “host–guest” complexation mechanism for pH-responsive drug delivery applications. The surface-functionalised melamine (Mela) groups on the outer surface of the mesoporous silica nanoparticles act as “hosts” and the surface capped TTM units act as “guests” during the surface capping of the mesoporous silica nanoparticles via the “host–guest” complexation approach. After the encapsulation of cargoes into the mesopore channels, the melamine functional groups were covalently immobilised onto the outer surface of the cargo loaded MSNs and then the TTM units were introduced onto the outer surface of the silica nanoparticles as “gatekeepers” to obtain surface capped mesoporous silica (MSN@Mela@TTM/RhB) NPs to protect the loaded cargo molecules inside the mesopore channels and to prevent their premature leakage. The surface-capped TTM units controlled the drug release behavior with respect to the pH of the release medium. In this study, we used rhodamine B (RhB) as a model cargo to study the loading and pH-responsive release behavior of the MSN@Mela@TTM NPs. The encapsulated RhB molecules were retained inside the mesopore channels at physiological pH (pH 7.4) conditions while an enhanced release occurred at acidic pH (pH 5.0 and 4.0) conditions, respectively. Furthermore, the in vitro biocompatibility and the intracellular uptake efficiency of the synthesised MSNs@Mela@TTM NPs were examined by using the MDA-MB-231 cell line. The experimental results suggest that the MSNs@Mela@TTM nanoparticles are biocompatible and could be utilised for pH-stimuli responsive drug delivery applications.
1. Introduction
Intracellular delivery of drugs into the target sites in the body in a controlled manner is considered to be crucial and an important factor in cancer therapy.1 Various micro/nano- materials such as liposomes, nanomicelles, silica, and polymer nanoparticles have been developed for controlled drug delivery applications.2,3 Generally, an efficient drug carrier system should accommodate high amounts of drugs and release them into the target sites in a controlled manner without any considerable side effects.4 In the past two decades inorganic nanoparticles, specifically mesoporous silica nanoparticles, have attracted much attention in the field of drug delivery because of their excellent physicochemical characteristics such as high surface area, tunable pore size, pore volume, thermal and chemical stability, hydrophilicity, enriched surface silanol groups and easy surface modifications, biocompatibility, and biodegradability.5 Owing to their high surface area and mesoporosity, mesoporous silica nanoparticles show high drug-encapsulating capacity.6 Furthermore, the silica nanoparticles can be internalised into the cells via an endocytosis mechanism and therefore facilitate the release of loaded drugs selectively inside the cytoplasm.7
Among various stimuli that have been utilised as triggers, the pH stimuli are considered to be an ideal trigger for the selective release of anticancer drugs because the pH values of tumor tissues are slightly lower (6.5–7.2) than those of normal cells and blood (∼pH 7.4) and the pH values in lysosomes and liposomes are more acidic (pH 5.0–5.5).8 Therefore, the pH-stimuli can control site-specific drug release based on the pH value of the surrounding environment. The encapsulated drug molecules inside the mesopore channels are well protected by the incorporated capping molecules at physiological pH conditions and are released under acidic pH conditions by detachment of the capping molecules from the pore-mouth of the mesopores caused by the intracellular pH-stimuli. By this approach, target drug delivery can be achieved without considerable side effects on normal cells.9–12 Non-covalent interactions, specifically hydrogen bonding interactions, play a crucial role and have inspired many studies in host–guest chemistry and complexation phenomena.13 Over the past few decades, numerous attempts have been made to design a range of synthetic motifs that can form multiple hydrogen bonding complexation with different modes of interaction.14,15 Surface modification of the mesoporous silica nanoparticles with appropriate receptor functional groups which have an ability to form hydrogen bonds with suitable guest molecules, is considered to be attractive for various applications.16,17 Melamine, a well-known nitrogen-rich organic base that belongs to the family of heterocyclic organic molecules, can be applied as an artificial receptor to accommodate suitable guest molecules through complementary multipoint hydrogen bonding interactions.18 The complementary hydrogen bonding interactions are expected between melamine and maleimide units at physiological pH (pH 7.4) conditions and considerably negligible interactions are expected at acidic pH (pH < 7) conditions since the formation and dissociation of hydrogen bonds between melamine–maleimide groups are sensitive to pH variations.19
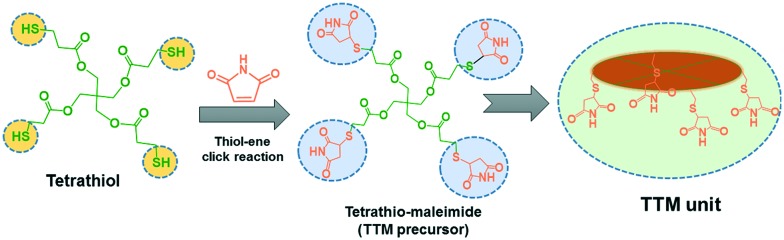
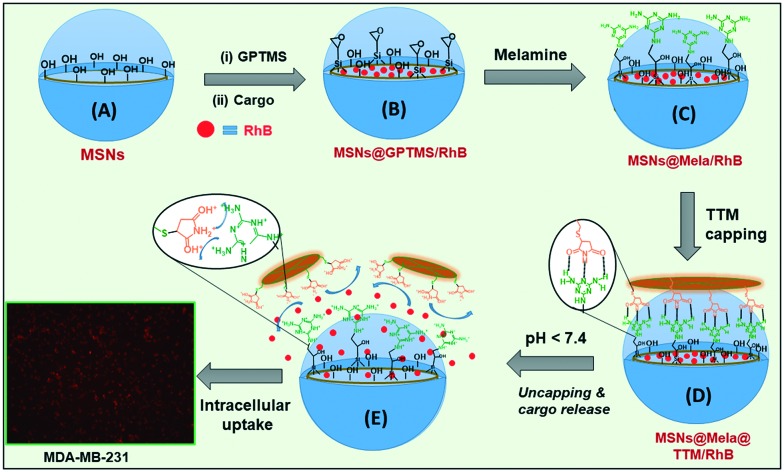
In the present work, we have synthesised surface capped “host–guest” complexation-based mesoporous silica (MSNs@Mela@TTM) nanoparticles for pH-stimuli responsive controlled drug delivery applications. The external surface of the mesoporous silica nanoparticles was functionalised with melamine derivatives which facilitate complementary multipoint hydrogen bonding interactions with the maleimide groups of the tetrathio-maleimide (TTM) capping units. The surface capped TTM units effectively protected the loaded cargo inside the mesopore channels and released them under acidic pH conditions. The synthesis of TTM units, surface functionalisation of melamine onto the MSNs, surface capping strategy, and pH-responsive release of the loaded cargo from the MSNs@Mela@TTM/RhB NPs are illustrated in Schemes 1 and 2.
Scheme 1. Synthesis of the tetrathio-maleimide (TTM) precursor.
Scheme 2. Schematic representation for the synthesis, cargo loading, melamine functionalization (A–C), and TTM capping onto the pore mouths (D) and pH-responsive uncapping of the TTM units (E) from the surface of the MSNs@Mela@TTM/RhB nanoparticles.
2. Experimental
2.1. Materials and reagents
Cetyltrimethylammonium bromide (CTAB), tetraethyl orthosilicate (TEOS), ammonium hydroxide (NH4OH, 28%), toluene, tetrahydrofuran (THF), (3-glycidoxypropyl)trimethoxysilane (GPTMS, 98%), melamine (99%), pentaerythritol tetrakis(3-mercaptopropionate) (tetrathiol, 95%), maleimide (99%) and rhodamine B (RhB) were purchased from Sigma Aldrich. All the reagents were used as received without further purification. A human breast cancer cell line (MDA-MB-231) was obtained from the Korean Cell Line Bank, South Korea.
2.2. Synthesis of tetrathio-maleimide (TTM) precursor
The tetrathio-maleimide (TTM) precursor was synthesised as follows. Approximately 0.43 g (4.4 mmol) of maleimide was dissolved in 50 mL of anhydrous ethanol. To this, 0.48 g (1.0 mmol) of tetrathiol was added and the obtained reaction mixture was stirred for 12 h at 45 °C. After the reaction was completed, the reaction mixture was concentrated under reduced pressure. The obtained precipitate was then purified with diethyl ether and dried under vacuum overnight at room temperature. The obtained product was labelled TTM (tetrathio-maleimide) (Scheme 1). Product: [(C–H)3(C–O–C O)(C O)2(CH–NH–S)]4, yield: ∼95%; 1H NMR (300 MHz, CDCl3): δ 1.3 (t, 18H, CH2O), δ 1.9 (t, 8H, C–H), δ 2.4 (t, 4H, NH).
2.3. Synthesis of mesoporous silica nanoparticles (MSNs)
Mesoporous silica nanoparticles were synthesised by following the reported procedure with slight modifications.20 Approximately 1.0 g of CTAB was dissolved in 480 mL distilled water and approximately 3.5 mL of aqueous 2.0 M NaOH solution was added to the CTAB solution. The obtained mixture was stirred at 80 °C for 30 min. To this, 5 mL of TEOS was added slowly under vigorous stirring and the obtained suspension mixture was further stirred for another 4 h at 80 °C. The resulting white precipitate was filtered, washed with deionised water and ethanol, and dried overnight at 70 °C (Scheme 2(A)). The as-synthesised samples were labelled MSNs@CTAB NPs.
2.4. Surface modification of GPTMS groups onto the as-synthesised MSN NPs
For surface modification of GPTMS groups, approximately 250 mg of the as-synthesised MSN samples were suspended in dry toluene (50 mL) and stirred. To this, approximately 10 mL of 0.5 M GPTMS was added slowly and the reaction suspension was stirred at 85 °C for 12 h under a N2 atmosphere. After the reaction, the resulting suspension was centrifuged, washed with toluene and dried at 60 °C. The GPTMS-modified sample was labelled MSNs@(CTAB)-GPTMS NPs (Scheme 2(B)). The occluded CTAB surfactant from the as-synthesised MSNs@(CTAB)-GPTMS NPs was then removed by a solvent extraction process by using an alcoholic solution of ammonium nitrate (30 mg NH4NO3 in 20 mL EtOH) at 60 °C. The extraction process was repeated three times to complete the removal of surfactant from the samples. The surfactant extracted mesoporous silica nanoparticles were labelled MSNs@GPTMS NPs (Scheme 2(B)).
2.5. RhB loading into the MSNs@GPTMS materials
In this study, we chose RhB as a model cargo for loading/release experiments. For RhB loading, approximately 0.25 g of the MSNs@GPTMS NPs were suspended in 5 mL of RhB solution (1 mg mL–1 in EtOH) at pH 7.0. The suspension was stirred for 12 h at room temperature. The RhB encapsulated samples were collected by centrifugation and the obtained particles were re-dispersed in 1 mL of water (pH 7.4) and immediately filtered out. The washing solution was combined with the supernatant and the final supernatant solution was used to determine the cargo loading efficiency by UV-vis spectroscopy at 550 nm. The encapsulation efficiency was determined from the initial and the final concentration of the RhB solution. The RhB encapsulation efficiency was determined using the following equation: Encapsulation efficiency (%) = [Mass of cargo in nanoparticles/Mass of cargo injected] × 100. From the above equations, the encapsulation efficiency of RhB into the MSNs@GPTMS NPs was determined to be approximately 65%. The RhB loaded samples were labelled MSNs@GPTMS@RhB NPs. We have used the hydrophobic solvent tetrahydrofuran (THF) to avoid or minimize the leakage of the encapsulated RhB from the MSNs@GPTMS/RhB nanoparticles during the surface modification and functionalization processes. During the surface modification processes, a considerably negligible amount of approximately ∼5% RhB leaked from the samples. The estimated RhB encapsulation efficiencies in MSNs@GPTMS/RhB NPs and MSNs@Mela@TTM/RhB NPs were determined to be ∼65% and ∼60%, respectively.
2.6. Melamine functionalisation onto the MSNs@GPTMS/RhB NP samples
Melamine functionalisation was performed by dispersing 200 mg of the MSNs@GPTMS/RhB samples in 60 mL THF and stirred at room temperature. To this, 5 mL (0.5 M) of melamine solution was added and the reaction suspension was stirred for 12 h. After 12 h of reaction, the obtained suspension was centrifuged, washed with THF to remove the unreacted melamine molecules and dried at 60 °C. The melamine-functionalised samples were labelled MSNs@Mela/RhB NPs (Scheme 2(C)).
2.7. Surface capping of TTM units onto the MSNs@Mela/RhB nanoparticles
The pore-mouth of the RhB loaded MSNs@Mela/RhB NPs were further capped by using TTM units. To perform this, 100 mg of the MSNs@Mela/RhB NPs were suspended in 50 mL THF. To this, 5 mL (0.5 M) of TTM precursor solution was added and the suspension mixture was stirred for 6 h at room temperature. During this process, the TTM units were capped onto the pore-mouth of the MSNs@Mela/RhB nanoparticles through a “host–guest” complexation process via multipoint hydrogen bonding interactions. After 6 h stirring, the TTM-capped MSNs@Mela/RhB samples were collected by centrifugation, washed with THF to remove the unattached TTM units and dried at 60 °C. The pore-mouth capped samples were labelled MSNs@Mela@TTM/RhB (Scheme 2(D)).
2.8. pH-stimuli responsive cargo release experiments
To examine the pH-stimuli responsive “uncapping” efficiency of the surface capped TTM units and the RhB release behaviour of the MSNs@Mela@TTM/RhB system, approximately 50 mg of the MSNs@Mela@TTM/RhB samples were placed in a dialysis membrane (MWCO 5 kDA) and added to 10 mL aqueous PBS buffer solution. The RhB release experiments were performed at different pH conditions (pH 7.4, 6.5, 5.0 and 4.0, respectively) for 24 h. The RhB release behaviour of all the samples was monitored for an initial 3 h at pH 7.4 and then the pH of the release medium was maintained at pH 7.4 or adjusted to pH 6.5, 5.0 and 4.0, respectively, using aqueous 0.1 M HCl solution. The RhB release process was monitored at predetermined time intervals using UV-vis spectrometry.
2.9. In vitro cytotoxicity analysis
For in vitro cytotoxicity analysis, we used doxorubicin (Dox) as the model anticancer drug. The in vitro cytotoxicity of the prepared MSNs@Mela NPs and MSNs@Mela@TTM NPs, with and without Dox loading was evaluated using MDA-MB-231 cells in a 3-(4,5′-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT) assay analysis. For MTT assay studies, the MDA-MB-231 cells were cultured in 96-well plates (1 × 104 cells per well) using 10% fetal bovine serum (FBS) supplemented Dulbecco's modified Eagle media (DMEM) at 37 °C in a humidified 5% CO2 atmosphere. The cultured cells were treated with the MSNs@Mela NP and MSNs@Mela@TTM/Dox NP samples, respectively, at various concentrations for 24 h. After 24 h incubation, the surface adhered and non-uptaken nanoparticles were removed by washing the cells using PBS buffer, then 20 μL of MTT reagent solution was added to each well, the formed formazan crystals were dissolved using dimethyl sulfoxide (DMSO) solvent, and then the optical density (OD) was determined at 570 nm.
Cell viability was determined using the following equation.
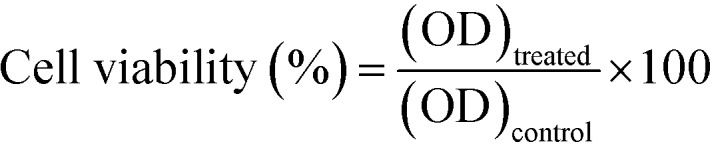 where ODtreated indicates the cells treated with the Dox-loaded MSNs@Mela@TTM/Dox samples and ODcontrol indicates the blank cells for 24 h incubation.
where ODtreated indicates the cells treated with the Dox-loaded MSNs@Mela@TTM/Dox samples and ODcontrol indicates the blank cells for 24 h incubation.
2.10. Intracellular uptake experiment
To monitor the intracellular uptake behaviour of the MSNs@Mela@TTM NPs nanoparticles were examined using MDA-MB-231 cells and monitored by fluorescence microscopy using RhB as a model cargo. For this experiment, MDA-MB-231 cells were treated with the MSNs@Mela@TTM/RhB nanoparticles with a concentration of 10 μg mL–1 and incubated for 6 h. After 6 h incubation, the cells were fixed with 4% paraformaldehyde for 15 min and then washed with PBS buffer and fluorescence images were taken by fluorescence microscopy.
2.11. Characterization
Low-angle X-ray diffraction (XRD) patterns of the samples were measured using an X'Pert-MPD system (Philips, Almelo, Netherlands) X-ray diffractometer with Cu Kα radiation. Fourier transform-infrared (FTIR) spectral analysis of the materials was performed on a Perkin-Elmer 1320 FTIR spectrometer using a KBr pelleting method. Field emission-transmission microscopy (FETEM) images were obtained by using a transmission electron microscope (JEOL JEM-2100F) at an accelerating voltage of 200 kV. Field-emission scanning electron micrographs (FESEM) were obtained using a field emission (FESEM JSM-6700, Japan) microscope. N2 adsorption–desorption measurements were carried out by using a Nova 4000e surface area and pore size analyzer at –196 °C. The samples were degassed at 120 °C for 4 h under vacuum prior to the measurements. Solid-state 29Si and 13C cross polarization magnetic angle spinning nuclear magnetic resonance (13C CP MAS NMR) spectral analyses were carried out using a 4 mm zirconia rotor spinning at 6 kHz (resonance frequency of 79.5 and 100 MHz for 29Si and 13C CP MAS NMR, respectively; 90° pulse width of 5 μs, contact time of 2 ms; recycle delay of 3 s). Thermogravimetric analysis of the samples was performed on a TGA 7, Pyris 1, Perkin Elmer thermal analyzer with a heating rate of 10 °C min–1 under an air atmosphere. The isoelectric point (IEP) was measured using an electrophoretic light scattering spectrophotometer (ELS-8000) by dispersing 15 mg of nanoparticles in 25 ml of distilled water followed by ultrasonication for 15 min.
3. Results and discussion
3.1. Structural and morphological characterisation
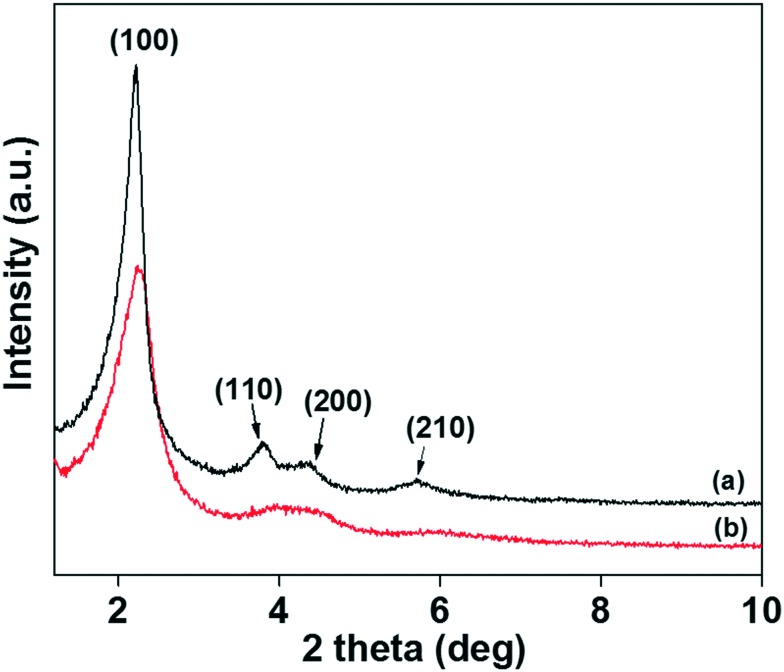
The XRD patterns of the MSNs@Mela NP and the surface capped MSNs@Mela@TTM materials without cargo loading are shown in Fig. 1(a and b). As shown in Fig. 1(a), the XRD pattern of the MSNs@Mela material shows a well-defined characteristic intense peak at 2θ = 2.25° and three weak reflection peaks at 2θ = 3.79°, 4.36° and 5.73° which were assigned to (100), (110), (200) and (210) respectively, indicating the formation of ordered hexagonal mesoporous materials. After TTM unit capping, the MSNs@Mela@TTM NP sample (Fig. 1(b)) shows all the characteristic peaks with slightly decreased peak intensities suggesting that the mesoporous structure is well-retained after surface modification processes.21 FTIR spectral analysis was performed to determine the presence of surface-modified organic functional groups on the MSNs.
Fig. 1. XRD patterns of the (a) MSNs@Mela NP and (b) MSNs@Mela@TTM materials.
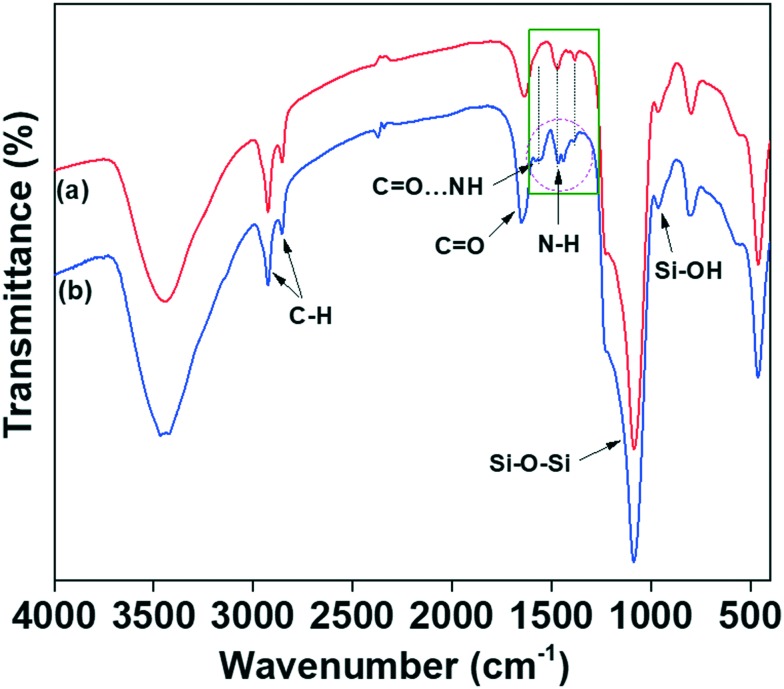
Fig. 2(a and b) show the FTIR spectra of the melamine-functionalised MSNs@Mela NPs without cargo loading and the TTM capped MSNs@Mela@TTM NP samples. As shown in Fig. 2(a), the MSNs@Mela NP sample shows the characteristic bands at 1089 cm–1 and 805 cm–1, which represent the asymmetric and symmetric stretching of Si–O–Si bridges. The peak at 965 cm–1 indicates the surface silanol Si–OH groups.22 Furthermore, the MSNs@Mela NPs shows the vibration bands at 2854 cm–1 and 2922 cm–1, which represent the C–H stretching of the propyl carbon chains.
Fig. 2. FTIR spectra of (a) MSNs@Mela NP and (b) MSNs@Mela@TTM materials.
The vibration peak at 1565 cm–1 represents the N–C stretching and the characteristic peak at 1469 cm–1 for the C–C bands, indicating the presence of melamine groups in the MSNs@Mela NPs.23 After TTM unit capping, the MSNs@Mela@TTM NP sample (Fig. 2(b)) spectra show that the amine part of the melamine groups had a noticeable peak shift (approximately 7 cm–1) from 1439 cm–1 to 1434 cm–1 and a reduced peak intensity at 1466 cm–1, which signified the interaction of surface capped TTM units with melamine groups by hydrogen bonding interactions.24 In addition, the MSNs@Mela@TTM NPs' spectra showed the presence of N–H and C–O groups of the maleimide functionalities which clearly revealed that the TTM units can successfully be capped onto the surface of the MSNs@Mela NPs through hydrogen bonding interactions.19
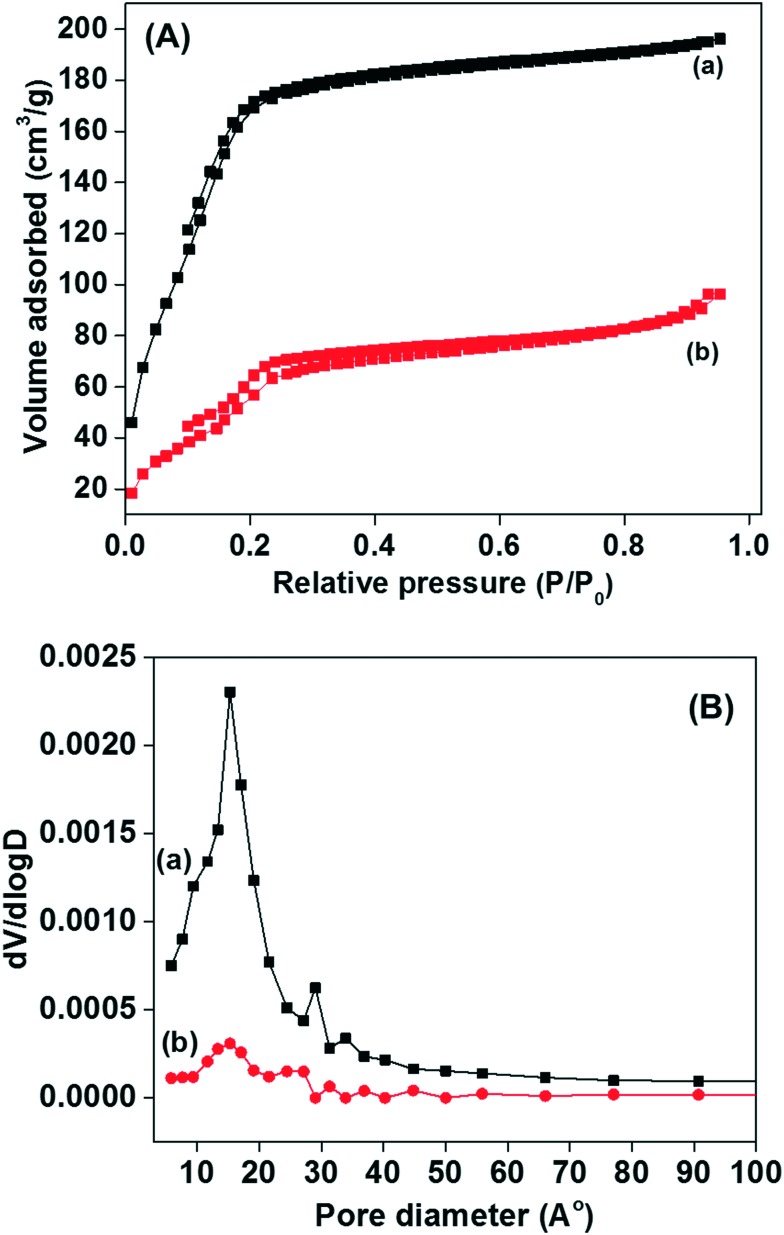
Nitrogen adsorption–desorption measurements were performed to determine the physicochemical properties of the synthesised materials. Fig. 3(A and B) display the N2 adsorption–desorption isotherm and pore size distribution curves of the MSNs@Mela NPs without cargo loading and the RhB loaded and TTM unit capped MSNs@Mela@TTM/RhB materials. As displayed in Fig. 3A(a and b), both samples exhibited a type-IV-like isotherm with an H1 hysteresis loop. However, the isotherm curves indicate the presence of intrinsic mesoporosity which may be due to the presence of irregular mesopores. The surface area, pore size and pore volume of the prepared MSNs@Mela NPs are 889 m2 g–1, ∼2.3 nm, and 0.48 cm3 g–1, respectively. In contrast, after RhB loading and TTM capping, the MSNs@Mela@TTM/RhB sample showed a reduced surface area (108 m2 g–1) and wide pore size distribution (Fig. 3B(b)). The reduced surface area and wide pore size distribution observed for the MSNs@Mela@TTM/RhB sample might be due to the pore filling effect of the encapsulated RhB cargo molecules inside the mesopore channels.
Fig. 3. (A) N2 adsorption–desorption isotherms and (B) pore size distribution curves of the (a) MSNs@Mela NP and (b) MSNs@Mela@TTM/RhB materials.
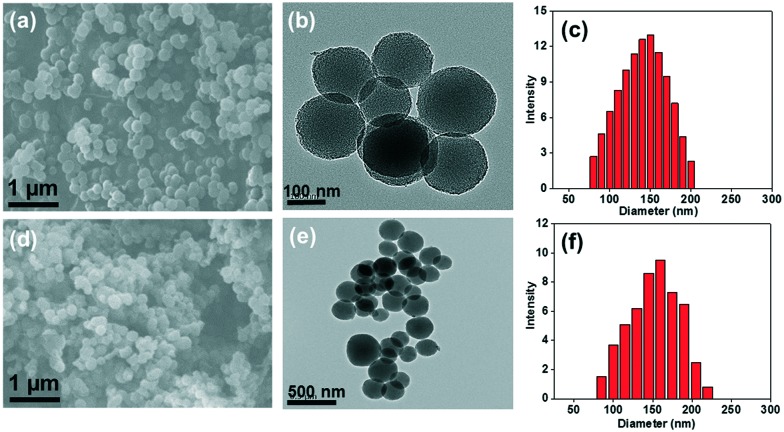
The morphology and mesostructural arrangements of the MSNs@Mela NP and MSNs@Mela@TTM/RhB samples were examined through SEM and TEM analysis (Fig. 4). As shown in the SEM image (Fig. 4(a)), the MSNs@Mela material shows the formation of spherical particles. On the other hand, the SEM image of the MSNs@Mela@TTM/RhB nanoparticles (Fig. 4(d)) shows spherical particles with slight aggregation which might be due to the surface modification and TTM group functionalisation. Fig. 4(b and e) shows the TEM images of the MSNs@Mela NPs and MSNs@Mela@TTM/RhB NPs revealing the presence of mesopores in the synthesised nanoparticles. Dynamic light scattering (DLS) analysis of the MSNs@Mela NPs shows (Fig. 4(c)) that the average particle size lay between 120–150 nm. In contrast, the MSNs@Mela@TTM/RhB NPs (Fig. 4(f)) has an average particle size that lies between 130–160 nm. A slight increase in the particle size occurred due to the surface modification and TTM groups functionalisation processes.
Fig. 4. SEM, TEM images and the corresponding average particle size distributions of the MSNs@Mela NP (a–c) and MSNs@Mela@TTM/RhB (d–f) materials, respectively.
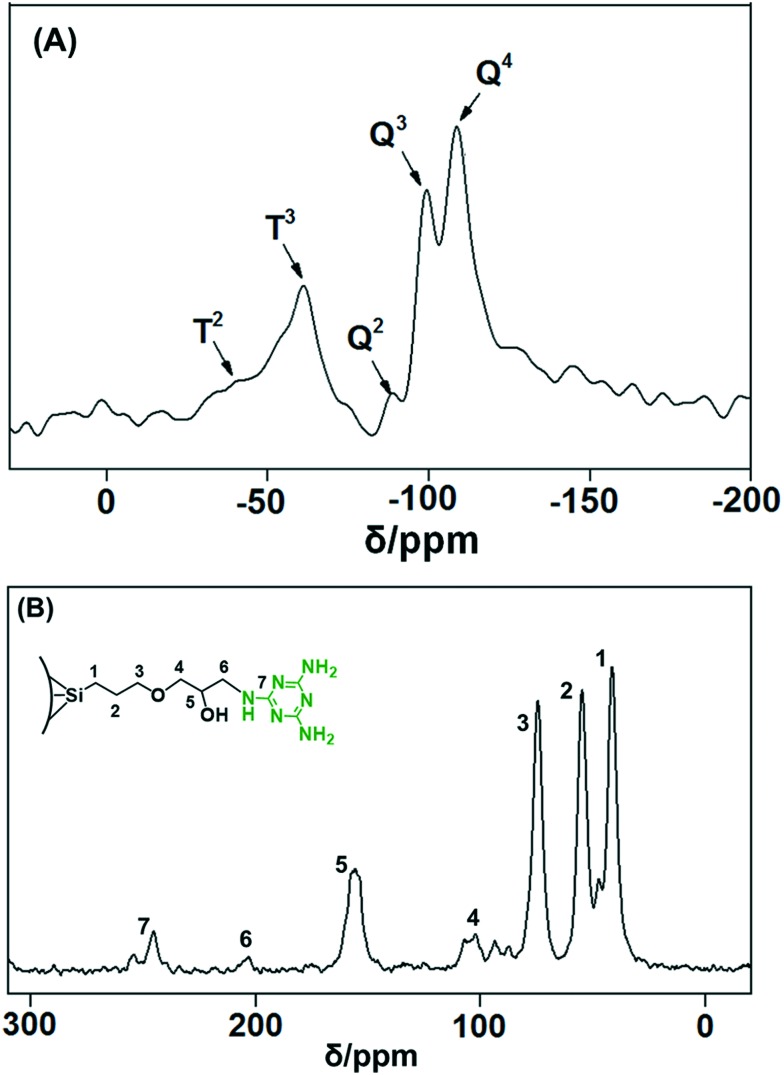
The melamine group-functionalised MSNs@Mela NPs were analysed by solid-state 29Si and 13C CP MAS NMR spectral analyses. Fig. 5(A) shows the 29Si CP MAS NMR spectrum for the MSNs@Mela NPs without cargo loading. As observed in Fig. 5(A), the spectrum shows both Q and T signals, which implies that the MSNs@Mela NPs are composed of a condensed silica matrix and covalently attached organosilane functional moieties. The Q peaks, specifically, the –109.2 ppm (Q4), –99.3 ppm (Q3) and –89.5 ppm (Q2) signals, confirm the formation of a silica network. In addition, the T signals appearing at –58.6 ppm (T3) and –47.6 ppm (T2) indicate the covalently attached melamine functionalities present in the silica network.25Fig. 5(B) shows the 13C CP MAS NMR spectrum of the MSNs@Mela NPs without cargo loading. The 13C CP MAS NMR spectrum in Fig. 5(B) shows three resonance signals; the carbon resonance signals at 38 ppm, 52 ppm, and 85 ppm are assigned to the carbon atoms of –Si–CH2–, the middle –CH2– and the last –CH2– group of the propyl carbon chains (–Si–CH2–CH2–CH2–), respectively.26 Furthermore, the additional resonance peaks at 102 and 156 ppm, indicative of –C–N– and O–H groups, respectively, indicate the carbon atoms of epoxy groups and melamine groups. The resonance peaks at 205 and 254 ppm represent the N–C–N and C N groups, which confirm the presence of the functionalised melamine groups on the MSNs@Mela NPs.27
Fig. 5. Solid-state (A) 29Si CP and (B) 13C CP MAS NMR spectra of the MSNs@Mela NPs.
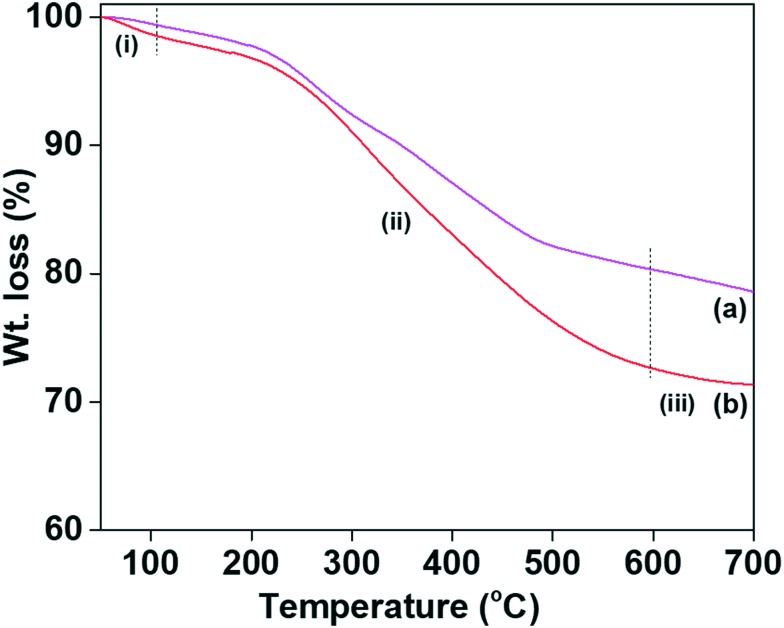
TG analysis was performed to determine the quantity of the immobilised melamine groups on the MSNs@Mela NPs and TTM unit capped MSNs@Mela@TTM NPs without cargo loading which is shown in Fig. 6(a and b). As shown in Fig. 6(a and b), both the samples showed an initial weight loss of approximately ∼1.2 wt% at 100 °C due to the evaporation of physisorbed solvent or moisture. The MSNs@Mela NP and MSNs@Mela@TTM NP samples showed gradual weight losses of approximately 17.8 wt% and 24.5 wt% in the temperature region 101–650 °C, corresponding to the decomposition of the functionalised melamine groups and the surface capped TTM units, respectively.28 A weight loss of approximately ∼25 wt% occurred due to the collective decomposition of organic moieties which evidenced that a considerable amount of organic functional groups was incorporated with the MSNs@Mela@TTM materials.
Fig. 6. TG analysis of the (a) MSNs@Mela NP and (b) MSNs@Mela@TTM materials.
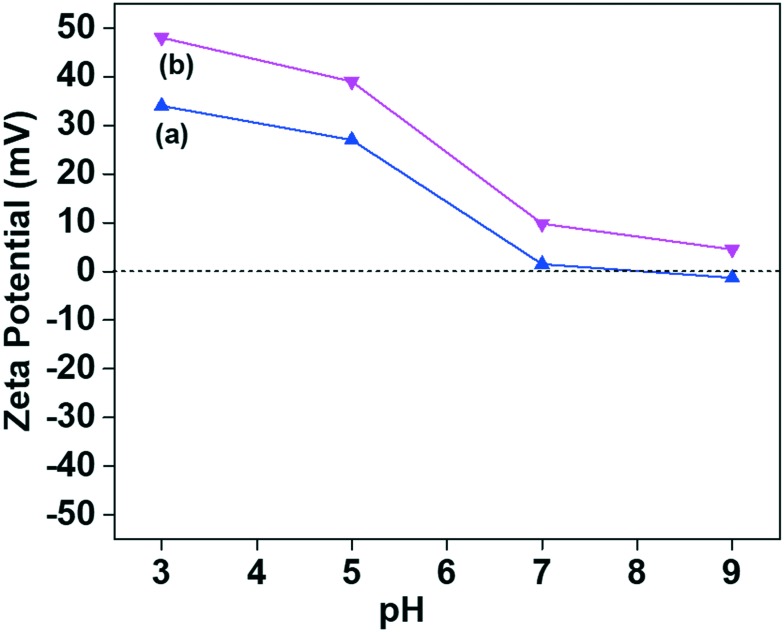
Fig. 7 shows the pH-dependent zeta potential values of the MSNS@Mela NP and MSNs@Mela@TTM samples without cargo loading which were examined in phosphate buffer saline (PBS) solution at different pH conditions (pH 3, 5, 7, and 9). After melamine group functionalisation and TTM capping, the MSNs@Mela NP and MSNs@Mela@TTM NP samples showed higher positive charge values. The maximum positive charge value observed at pH 3 was +34.2 and +48.5, respectively. Upon further increasing the pH value to 7 and 9, the values are reduced to +1.3 and –1.6 for the MSNs@Mela NPs and +9.8 and +4.5 for the MSNs@Mela@TTM NPs, respectively (Fig. 7(a and b)), which supports the presence of functionalised melamine groups and the surface capped TTM units on the MSNs@Mela NP and MSNs@Mela@TTM NP samples.29
Fig. 7. Zeta-potential measurements of the (a) MSNs@Mela NP and (b) MSNs@Mela@TTM materials.
3.2. pH-stimuli responsive cargo release from the MSNs@Mela@TTM/RhB samples
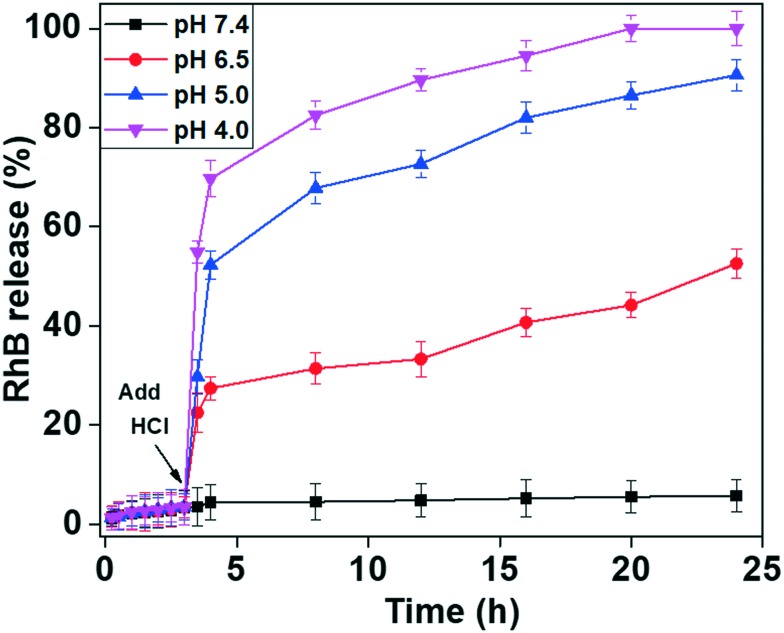
The capping molecule TTM units were introduced onto the MSNs@Mela/RhB NP system to prevent the premature leakage of loaded RhB molecules from the MSNs@Mela/RhB nanoparticles and to demonstrate the pH-stimuli triggered release efficiency of the cargo molecules from the MSNs@Mela@TTM/RhB system (Scheme 2(D)). As illustrated in Scheme 2(D), the maleimide part of the TTM units can interact with the functionalised melamine groups onto the MSNs@Mela/RhB NPs through multipoint hydrogen bonding interactions.30 Thus, the surface-capped TTM units can efficiently protect the encapsulated cargo molecules inside the mesopore channels of the MSNs@Mela@TTM/RhB materials in a physiological pH (pH 7.4) environment. We performed RhB release experiments at different pH conditions (pH 7.4, 6.5, 5.0, and 4.0) to examine the pH-stimuli-triggered RhB cargo release behaviour of the MSNs@Mela@TTM/RhB NPs system. The RhB release behaviour of the MSNs@Mela@TTM/RhB samples was monitored by UV-vis spectrometry at 550 nm. As observed in Fig. 8, the MSNs@Mela@TTM/RhB system showed considerably negligible amounts of released RhB at pH 7.4 after a 24 h release period. This evidenced that the capping molecule TTM can effectively protect the encapsulated cargo molecules inside the mesopore channels and supported that the TTM units may be utilised as an efficient capping molecule. To examine the pH responsiveness of the TTM capping units, the pH of the release medium were adjusted to different pH conditions (pH 6.5, 5.0, and 4.0) after 3 h by using 0.1 M HCl solution. The RhB release behaviour was monitored by UV-vis spectrometry at different time intervals. As can be seen in Fig. 8, RhB release did not occur considerably during the initial 3 h at pH 7.4 prior to adding HCl solution. In contrast, after adding HCl to make the release medium acidic (pH 6.5, 5.0 and 4.0, respectively) an enhanced RhB release was observed with respect to the pH of the release medium. The enhanced cargo release at acidic pH conditions might be due to the pH-stimuli-induced uncapping of the TTM units suggesting the pH-stimuli responsive uncapping behaviour of the TTM units.
Fig. 8. pH-stimuli responsive RhB release efficiency from the RhB loaded, surface-capped MSNs@Mela@TTM/RhB materials at different pH conditions (pH 7.4, 6.5, 5.0, and 4.0), respectively. The arrow indicates the addition of HCl solution to the release medium after 3 h.
The RhB release was significantly enhanced at higher acidic pH (pH 5.0 and 4.0) as compared to the mildly acidic conditions (pH 6.5) (Fig. 8). A considerably negligible amount (∼7%) of released RhB was observed at pH 7.4 at 24 h. In contrast, approximately 37.8%, 65.9%, and 83.9% released RhB were observed at an initial 8 h and approximately 45.8%, 89.5%, and 100% were observed at pH 6.5, 5.0, and 4.0, respectively after a 24 h release period. From Fig. 8, it was observed that considerably less amounts of RhB was released at pH 7.4 and 6.5 conditions, which revealed that the uncapping of the TTM units from the pore-mouth of the MSNs@Mela@TTM/RhB system was not fully activated at physiological (pH 7.4) and mildly acidic pH (pH 6.5) conditions. This is because the hydrogen bonding interactions between the melamine functional groups and the maleimide parts of the TTM units are stronger and therefore the TTM capping units properly and partially closed the pore channels of the MSNs@Mela@TTM/RhB system. On the other hand, strong protonation occurs in melamine functionalities and maleimide groups at reduced pH conditions (pH 5.0 and 4.0), which induced the dissociation of the hydrogen bonding interactions. Due to the strong protonation and electrostatic repulsive force, the surface-capped TTM units were detached completely from the pore-mouth of the MSNs@Mela@TTM@RhB system, and therefore the loaded RhB molecules diffused out freely from the mesopore channels of the MSNs@Mela@TTM@RhB system (Scheme 1(E)). The cargo release experiments clearly evidenced that the TTM units can act as efficient “gatekeepers” and thus the proposed nanovalve-based MSNs@Mela@TTM/RhB system could be utilised for pH-stimuli responsive drug delivery applications in cancer therapy.
3.3. Cytotoxicity study
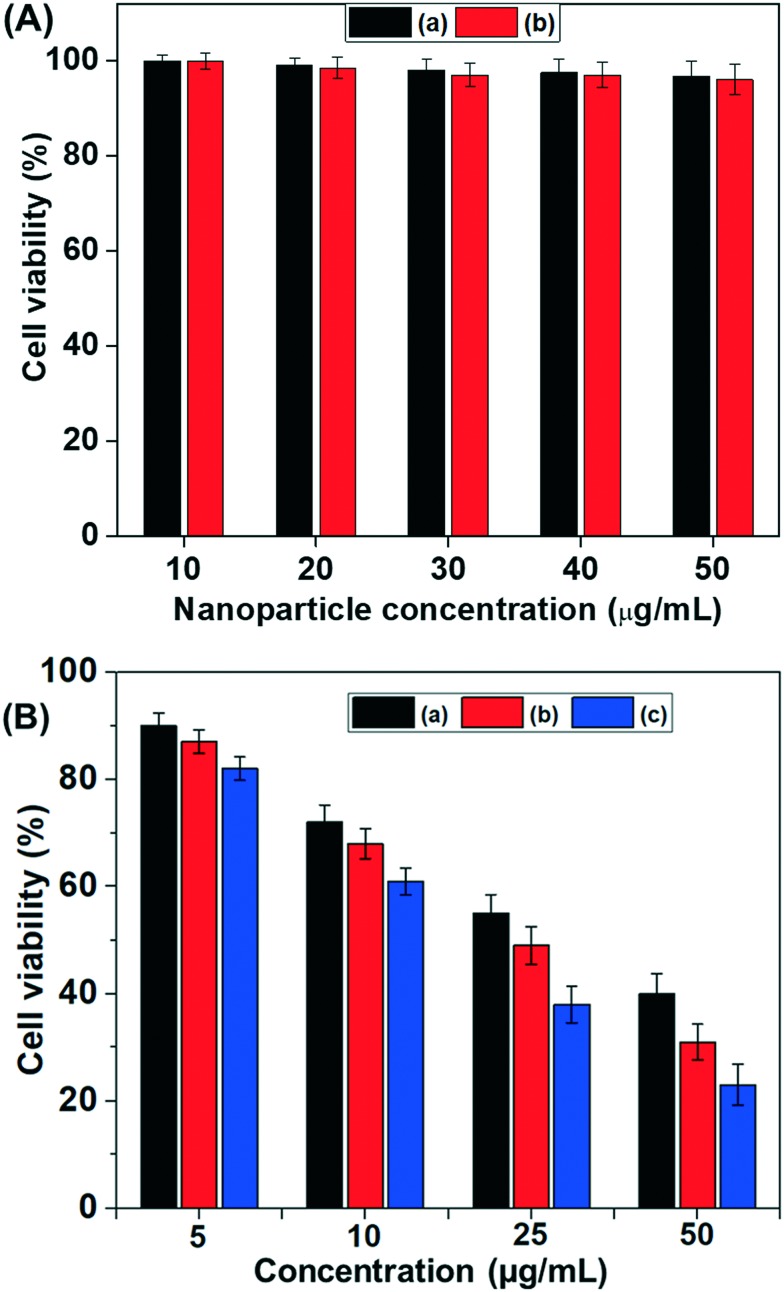
To evaluate the in vitro cytocompatibility of the MSNs@Mela NPs and MSNs@Mela@TTM NPs without cargo loading they were tested against MDA-MB-231 cells by MTT assay analysis with different sample concentrations (10–50 μg mL–1). As shown in Fig. 9(A), the MSNs@Mela NPs and the MSNs@Mela@TTM NPs showed approximately >95% cell viability at the tested concentrations after 24 h incubation, which indicates that the synthesised MSNs@Mela@TTM NP system is biocompatible (Fig. 9(A)). In addition, to determine the in vitro cytotoxicity effects, we used doxorubicin (Dox) as a model anticancer drug. We examine the cytotoxicity effect of different concentrations (5, 10, 25 and 50 μg mL–1) of free Dox and the same concentration of Dox loaded MSNs@Mela/Dox NPs and MSNs@Mela@TTM/Dox NPs against MDA-MB-231 cells (Fig. 9(B)). As shown Fig. 9(B), all the samples showed dose-dependent toxicity to MDA-MB-231 cells. Specifically, free Dox showed approximately ∼57% cytotoxicity to MDA-MB-231 cells when treated with 50 μg mL–1 free Dox. On the other hand, the Dox (50 μg mL–1) loaded MSNs@Mela/Dox NP and MSNs@Mela@TTM/Dox samples showed cytotoxicity of about 67% and ∼78% to MDA-MB-231 cells after a 24 h incubation period. It is noted that the TTM unit capped MSNs@Mela@TTM/Dox sample showed considerably higher cytotoxicity to MDA-MB-231 cells as compared to free Dox and MSNs@Mela/Dox NPs at the same concentrations (Fig. 9(B)). This might be due to the slow release of Dox molecules from the mesopore channels which could be controlled by the surface capped TTM gatekeeper units under mildly acidic pH conditions. In addition, the free Dox molecules can be internalised into the cells by a passive diffusion pathway whereas the Dox loaded nanoparticles can be taken up by cells via an endocytosis mechanism. Thus, the Dox loaded MSNs@Mela/Dox NPs and MSNs@Mela@TTM/Dox NPs can release more amounts of drugs into the cell cytoplasm and therefore show a greater cytotoxic effect as compared to the cells treated with free Dox.
Fig. 9. (A) In vitro cytotoxicity of the (a) MSNs@Mela NPs; (b) MSNs@Mela@TTM NPs without cargo loading were respectively treated at different concentrations against MDA-MB-231 cells. (B) In vitro cytotoxicity of (a) free Dox treated at different (5, 10, 25 and 50 μg mL–1) concentrations, and (b and c) the same concentrations of Dox loaded MSNs@Mela/Dox and MSNs@Mela@TTM/Dox nanoparticles, respectively treated at different (5, 10, 25 and 50 μg mL–1) concentrations.
3.4. Cellular uptake study
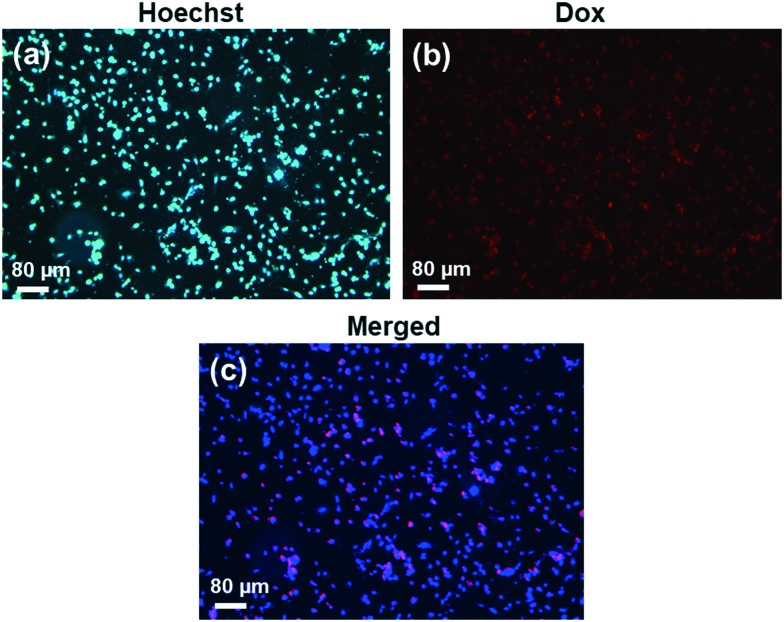
The intracellular uptake behaviour of the Dox loaded MSNs@Mela@TTM/Dox nanoparticles was evaluated on MDA-MB-231 cells by fluorescence microscopy analysis. For this experiment, approximately 10 μg mL–1 of the Dox loaded MSNs@Mela@TTM/Dox samples was treated with the MDA-MB-231 cells and the cells were incubated for 6 h. Then, the sample-treated cells were washed with PBS buffer and observed under a fluorescence microscope to determine the presence of internalised MSNs@Mela@TTM/Dox nanoparticles inside the MDA-MB-231 cells. As observed in Fig. 10(a), the nucleus stained control cell image showed that no red fluorescence in the cytoplasm was observed. On the other hand, the cells treated with the MSNs@Mela@TTM/Dox samples show red fluorescence (Fig. 10(b)), which evidenced that the nanoparticles were internalised into the MBA-MD-231 cells. Moreover, the merged cell image (Fig. 10(c)) showed red fluorescence signals in the cytoplasm which implies that the MSNs@Mela@TTM/Dox nanoparticles can be effectively taken up by the MDA-MB-231 cells in a short incubation period.
Fig. 10. Fluorescence microscopy images of MDA-MB-231 cells incubated with (a) control and (b and c) Dox loaded and TTM-capped MSNs@Mela@TTM/Dox NPs incubated for 6 h.
4. Conclusions
In the present study, we synthesised a “host–guest” complexation-based mesoporous silica MSNs@Mela@TTM nanovalve-based drug carrier system for pH-stimuli responsive controlled drug delivery applications. We used RhB as a model cargo to demonstrate the pH-stimuli responsive release efficiency of the MSNs@Mela@TTM/RhB nanocarrier system and the cargo release behaviour which were examined at different pH conditions (pH 7.4, 6.5, 5.0, and 4.0), respectively. The experimental results evidenced that the MSNs@Mela@TTM NP system exhibited an excellent pH-stimuli responsive-controlled release behaviour. The detachment of the surface-capped TTM units is mainly dependent on the dissociation of the hydrogen bonding interactions induced by the acidic pH environment and electrostatic repulsion. Furthermore, the obtained results evidenced the prominent role of the surface capped TTM groups which can effectively control the drug release behaviour with respect to the pH of the release medium. In addition, the MTT assay analysis and intracellular uptake experiments against MDA-MB-231 cells support that the synthesised MSNs@Mela@TTM material is biocompatible and can be readily taken up by MDA-MB-231 breast cancer cells. Therefore, the MSN@Mela@TTM NP system could be utilised for pH-stimuli responsive drug delivery applications in cancer therapy. The proposed strategy is an easy and alternative approach to design “host–guest” complexation-based mesoporous silica nanovalve-based drug carrier systems for pH-stimuli responsive controlled drug delivery applications in cancer therapy.
5. Conflict of interests
The authors declare no competing interests.
Acknowledgments
This research was supported by a grant from the Marine Biotechnology Program (20150220) and funded by the Ministry of Oceans and Fisheries, Republic of Korea.
References
- Minelli C., Lowe S. B., Stevens M. M. Small. 2010;6:2336. doi: 10.1002/smll.201000523. [DOI] [PubMed] [Google Scholar]
- Alemdaroglu F. E., Alemdaroglu N. C., Landduth P., Herrmann A. Adv. Mater. 2008;20:899. [Google Scholar]
- Farrell D., Alper J., Ptak K., Panaro N. J., Grodzinski P., Barker A. D. ACS Nano. 2010;4:589. doi: 10.1021/nn100073g. [DOI] [PubMed] [Google Scholar]
- Bae Y. H., Park K. J. Controlled Release. 2011;153:198. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet-Regi M., Balas F., Arcos D. Angew. Chem. 2007;119:7692. doi: 10.1002/anie.200604488. [DOI] [PubMed] [Google Scholar]
- Lu F., Wu S. H., Hung Y., Mou C. Y. Small. 2009;5:1408. doi: 10.1002/smll.200900005. [DOI] [PubMed] [Google Scholar]
- Liu J., Hartono S. B., Jin Y. G., Li Z., Lu G. Q., Qiao S. Z. J. Mater. Chem. 2010;20:4595. [Google Scholar]
- Schmid S. L., Fuchs R., Male P., Mellman I. Cell. 1988;52:73. doi: 10.1016/0092-8674(88)90532-6. [DOI] [PubMed] [Google Scholar]
- Chen T., Yang N., Fu J. Chem. Commun. 2013;49:6555. doi: 10.1039/c3cc43221a. [DOI] [PubMed] [Google Scholar]
- Wang T., Wang M., Ding C., Fu J. Chem. Commun. 2014;50:12469. doi: 10.1039/c4cc05677a. [DOI] [PubMed] [Google Scholar]
- Wang M., Chen T., Ding C., Fu J. Chem. Commun. 2014;50:5068. doi: 10.1039/c4cc01442a. [DOI] [PubMed] [Google Scholar]
- Wang M., Gong G., Feng G., Wang T., Ding C., Zhou B., Jiang W., Fu J. ACS Appl. Mater. Interfaces. 2016;8:23289. doi: 10.1021/acsami.6b07603. [DOI] [PubMed] [Google Scholar]
- Schneider H. J. Angew. Chem. 1991;103:1419. [Google Scholar]
- Ogoshi H., Hatakeyama H., Kotani J., Kawashima A., Kuroda Y. J. Am. Chem. Soc. 1991;113:8181. [Google Scholar]
- Sivakova S., Rowan S. J. Chem. Soc. Rev. 2005;34:9. doi: 10.1039/b304608g. [DOI] [PubMed] [Google Scholar]
- Sarkar K., Dhara K., Nandi M., Roy P., Bhaumik A., Banerjee P. Adv. Funct. Mater. 2009;19:223. [Google Scholar]
- Cho E. J., Kang J. K., Jung J. W. Mater. Lett. 2007;61:5157. [Google Scholar]
- Sigal-Batikoff I., Konovalov O., Singh A., Berman A. Langmuir. 2010;26:16424. doi: 10.1021/la102166k. [DOI] [PubMed] [Google Scholar]
- Fertier L., Tháron C., Carcel C., Trens P., Man M. W. C. Chem. Mater. 2011;23:2100. [Google Scholar]
- Radu D. R., Lia C. Y., Jeftinija K., Rowe E. W., Jeftinija S., Lin V. J. Am. Chem. Soc. 2004;126:13216. doi: 10.1021/ja046275m. [DOI] [PubMed] [Google Scholar]
- Shin H. J., Ko C. H., Ryoo R. J. Mater. Chem. 2001;11:260. [Google Scholar]
- Chang J. S., Kong Z. L., Hwang D. F., Chang K. L. B. Chem. Mater. 2006;18:702. [Google Scholar]
- Fu J., Zhu Y., Zhao Y. J. Mater. Chem. B. 2014;2:3538. doi: 10.1039/c4tb00387j. [DOI] [PubMed] [Google Scholar]
- Moorthy M. S., Seo D. J., Song H. J., Park S. S., Ha C. S. J. Mater. Chem. A. 2013;1:12485. [Google Scholar]
- Moorthy M. S., Kim M. J., Bae J. H., Park S. S., Saravanan N., Kim S. H., Ha C. S. Eur. J. Inorg. Chem. 2013;2013:3028. [Google Scholar]
- Moorthy M. S., Park J. H., Bae J. H., Kim S. H., Ha C. S. J. Mater. Chem. B. 2014;2:6487. doi: 10.1039/c4tb00808a. [DOI] [PubMed] [Google Scholar]
- Whitenal W., Asefa T., Ozin G. A. Adv. Funct. Mater. 2005;15:1696. [Google Scholar]
- Chen S., You B., Zhou S., Wu L. J. Appl. Polym. Sci. 2009;112:3634. [Google Scholar]
- Lee C. H., Lo L. W., Mou C. Y., Yang C. S. Adv. Funct. Mater. 2008;18:3283. [Google Scholar]
- Herder M., Pätzel M., Grubert L., Hecht S. Chem. Commun. 2011;47:460. doi: 10.1039/c0cc02339f. [DOI] [PubMed] [Google Scholar]