Summary
Bleeding events have been observed among a subgroup of chronic lymphocytic leukaemia (CLL) patients treated with ibrutinib. We analysed data from two studies of single‐agent ibrutinib to better characterize bleeding events and pattern of anticoagulation and antiplatelet use. Among 327 ibrutinib‐treated patients, concomitant anticoagulation (11%) or antiplatelet use (34%) was common, but major bleeding was infrequent (2%). Bleeding events were primarily grade 1, and infrequently (1%) led to discontinuation. Among 175 patients receiving concomitant anticoagulant or antiplatelet agents, 5 had major bleeding events (3%). These events were typically observed in conjunction with other factors, such as coexisting medical conditions and/or concurrent medications.
Keywords: anticoagulation, bleeding, chronic lymphocytic leukaemia, haemorrhage, ibrutinib
Ibrutinib is a first‐in‐class, once‐daily inhibitor of Bruton tyrosine kinase (BTK) with single‐agent efficacy and a favourable benefit‐risk profile in patients with CLL. BTK and TEC are expressed in platelets and act downstream in glycoprotein (GP)VI signalling involved in collagen‐mediated platelet aggregation (Quek et al, 1998; Futatani et al, 2001; Atkinson et al, 2003; Liu et al, 2006). The two kinases probably possess redundant functions in collagen‐induced aggregation, however, BTK is thought to play a more dominant role than TEC (Atkinson et al, 2003). While patients with inherited BTK deficiency (X‐linked agammaglobulinaemia) do not demonstrate bleeding diathesis (Winkelstein et al, 2006), bleeding events have been reported in a subgroup of ibrutinib‐treated patients (Byrd et al, 2013, 2014, 2015; Wang et al, 2015). Patients with CLL are typically older and have medical comorbidities for which anticoagulation or antiplatelet agents are indicated. Given the increased bleeding risk associated with antiplatelet and/or anticoagulants, we assessed the pattern of use of these agents and characterized bleeding adverse events (AEs) observed in 2 multicentre studies of single‐agent ibrutinib in patients with CLL.
Methods
We conducted a secondary analysis of data from the phase 2 PCYC‐1102 (NCT01105247) study (Byrd et al, 2013) and interim analysis of the randomized phase 3 PCYC‐1112 (RESONATE; NCT01578707) study (Byrd et al, 2014) of ibrutinib versus ofatumumab to characterize concomitant anticoagulant and/or antiplatelet use and frequency of bleeding complications. Bleeding AEs were recorded/graded according to CTCAE v4.0. Major bleeding was defined as any grade ≥3 bleeding event or haemorrhage of any grade resulting in intraocular bleeding causing loss of vision, need for ≥2 units of red blood cell transfusion, hospitalization, prolonged hospitalization or any intracranial haemorrhage.
PCYC‐1102 enrolled treatment‐naïve CLL/small lymphocytic lymphoma (SLL) patients aged ≥65 years and previously treated patients to receive ibrutinib 420 or 840 mg/day (Byrd et al, 2013, 2015). PCYC‐1112 enrolled patients with CLL/SLL after ≥1 prior therapy; patients randomized to ibrutinib received 420 mg/day (Byrd et al, 2014). Details on haematology‐related eligibility criteria, protocol‐specified restrictions on concomitant warfarin use and caution against other agents are provided (Data S1).
Results
Median age was 68 years in PCYC‐1102 (N = 132) and 67 years in the PCYC‐1112 ibrutinib arm (N = 195). At the time of analysis, median follow‐up was 21·1 (range 0·7–29·0) and 9·6 months (range 0·3–16·6), respectively. Median platelet count at baseline was 105 (range, 2–310) × 109/l in PCYC‐1102 and 117 (range, 20–441) × 109/l in PCYC‐1112; 45% and 38% of patients, respectively, had platelet count <100 × 109/l and 12% and 11%, respectively, had platelet count <50 × 109/l. In both studies, we observed an increase in platelet count over time (Barrientos et al, 2014; Byrd et al, 2015). Concomitant anticoagulation or antiplatelet therapy was common; 35 patients (11%) received anticoagulants, 110 (34%) received antiplatelet agents and 30 (9%) received both (Table 1, Table SI). The most frequent anticoagulant was low‐molecular‐weight heparin (LMWH, 15%), albeit often limited in duration (<3 months in 33 of 48 patients). Eleven of 48 and 1 of 8 patients received therapeutic (versus only prophylactic) dosing of LMWH or other heparin, respectively. The most common antiplatelet agents included aspirin (21%) and non‐aspirin, non‐steroidal anti‐inflammatory drugs (NSAIDs; 28%).
Table 1.
Summary of grade >1 bleeding events by use of anticoagulation/antiplatelet agents with single‐agent ibrutinib
| Any ACc (n = 23) | Any APc (n = 67) | AC and APd (n = 13) | No AC or AP (n = 55) | Total (N = 132) | |
|---|---|---|---|---|---|
| Grade >1 bleeding events in PCYC‐1102 | |||||
| Median follow‐up (range), months | 22·1 (0·7–28·3) | 21·9 (1·2–29·0) | 22·1 (8·2–26·0) | 18·4 (0·9–27·3) | 21·1 (0·7–29·0) |
| Patients with any grade >1 bleeding events, n (%) | 4 (17) | 6 (9) | 1 (8) | 3 (6) | 12 (9) |
| Patients with major bleeding, n (%) | 2 (9) | 3 (4) | 1 (8) | 2 (4) | 6 (5) |
| Number of events | 4 | 7 | 1 | 6 | 16 |
| Events resolved,a n (%) | 4 (100) | 7 (100) | 1 (100) | 4 (67) | 14 (88) |
| Median event duration (range), daysa | – | – | – | – | 18 (3–169) |
| Median time to onset (range), daysb | 55 (21–361) | 193·5 (4–633) | 361 (361–361) | 12 (10–504) | 33 (4–633) |
| Any AC (n = 42) | Any AP (n = 73) | AC and AP (n = 17) | No AC or AP (n = 97) | Total (N = 195) | |
|---|---|---|---|---|---|
| Grade >1 bleeding events in PCYC‐1112 (RESONATE) ibrutinib arm | |||||
| Median follow‐up (range), months | 9·7 (0·3–14·2) | 10·0 (2·0–16·0) | 10·8 (2·0–14·2) | 9·3 (1·2–16·6) | 9·6 (0·3–16·6) |
| Patients with any grade >1 bleeding events, n (%) | 2 (5) | 2 (3) | 1 (6) | 5 (5) | 8 (4) |
| Patients with major bleeding, n (%) | 1 (2) | 1 (1) | 1 (6) | 1 (1) | 2 (1) |
| Number of events | 2 | 2 | 1 | 6 | 9 |
| Events resolved,a n (%) | 2 (100) | 2 (100) | 1 (100) | 6 (100) | 9 (100) |
| Median event duration (range), daysa | – | – | – | – | 11 (1–174) |
| Median time to onset (range), daysb | 276 (241–310) | 215 (120–310) | 310 (310–310) | 80 (18–102) | 111 (18–310) |
AC, anticoagulant; AP, antiplatelet agent.
For events ongoing at end of AE reporting period, earliest date of last dose date +30, study exit date was used.
Time from first dose of study treatment to onset of first grade >1 bleeding event.
Anticoagulant (with or without antiplatelet agents) or antiplatelet agent (with or without anticoagulation) given at any time for any duration during the study.
Both an anticoagulant and antiplatelet agent given at any time for any duration during the study.
The most common bleeding AEs were grade 1 petechiae and contusion in 13% and 20% (PCYC‐1102) and in 13% and 11% (PCYC‐1112), respectively (Fig 1A). These mild (grade 1) bleeding AEs did not lead to major complications. Prior history of any grade bleeding was the only factor predictive of mild bleeding in a multivariate analysis for risk factors that included anticoagulant/antiplatelet use, age, platelet count, bleeding history, fall risk, cytochrome P inhibitor use, and baseline hepatic and/or renal impairment. Major bleeding occurred in 5% on PCYC‐1102 [exposure‐adjusted incidence rate per 100 patient‐years (EAIR) 3·6], and in PCYC‐1112 occurred in 1% on ibrutinib (EAIR 1·4) versus 2% on ofatumumab (EAIR 4·4), with longer exposure on ibrutinib in the randomized study. No grade 4 or 5 bleeding AEs were reported. The sites of major bleeding AEs in ibrutinib‐treated patients included gastrointestinal (n = 4), central nervous system (n = 3), and postoperative excision site of a skin lesion (n = 1). Thus, major bleeding events were uncommon with ibrutinib (8 of 327, 2%) across the 2 studies (Table 1), and were typically observed in patients with various other factors (detailed in Table SII). These events were not associated with grade 4 thrombocytopenia, except in 1 patient with baseline platelet count of 2 × 109/l. Among the 175 patients who received any anticoagulant and/or antiplatelet therapy, 5 (3%) had major bleeding AEs; among the 152 patients who did not receive concomitant therapy, 3 (2%) had major bleeding AEs. All of these major bleeding AEs resolved. For the 5 of 8 patients with major bleeding who received concomitant anticoagulant and/or antiplatelet agents, 1 had received anticoagulation alone (LMWH), 2 had received antiplatelet therapy alone (1 aspirin, 1 NSAID), and 2 had received anticoagulation and antiplatelet therapy (1 warfarin and aspirin, 1 LMWH, other heparin and NSAID). Approximately 1 year following PCYC‐1112 interim analysis, major bleeding occurred in 2 additional patients on ibrutinib, including grade 3 spontaneous haematoma (psoas) and post‐traumatic grade 4 subdural haematoma. In both cases, bleeding events resolved and ibrutinib was reinitiated.
Figure 1.
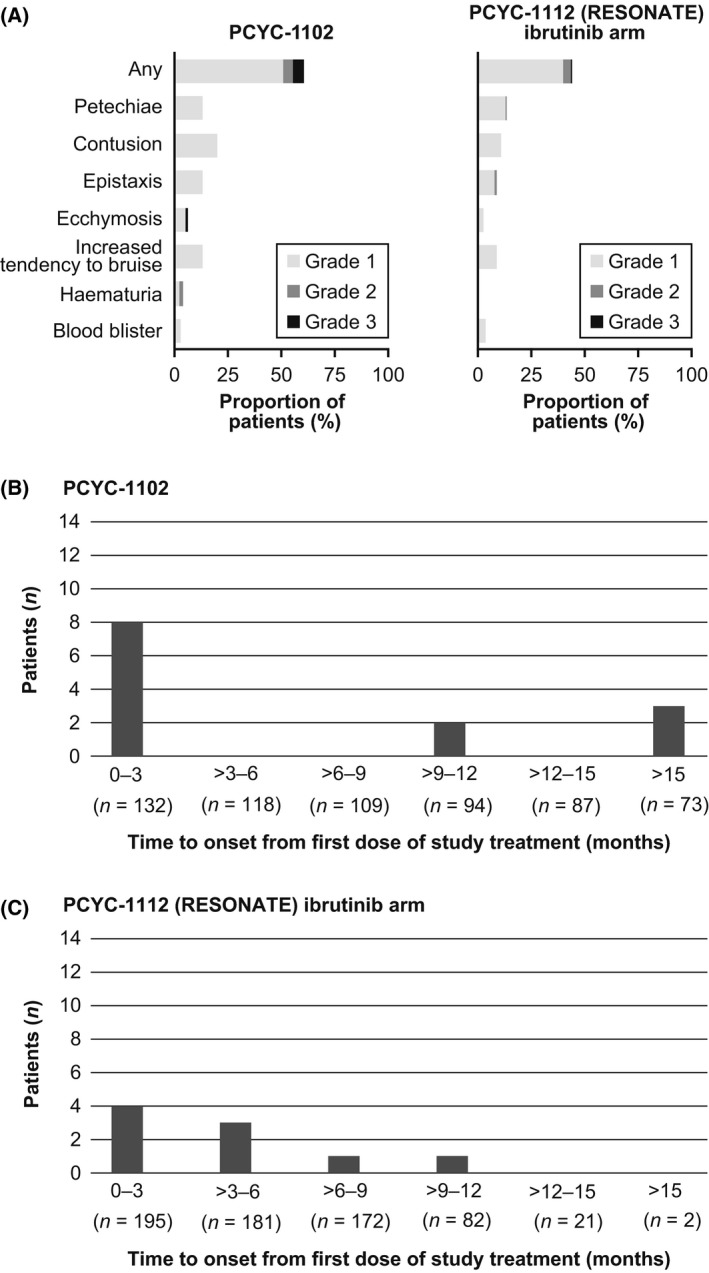
Frequency of bleeding events with single‐agent ibrutinib in patients with chronic lymphocytic leukaemia. (A) Frequency of bleeding adverse events (AEs) by severity grade for events occurring in at least 3% of patients treated on PCYC‐1102 (N = 132) and the PCYC‐1112 (RESONATE) ibrutinib arm (N = 195). The most common bleeding AEs were grade 1 petechiae and contusion in 13% and 20% (PCYC‐1102) and in 13% and 11% (RESONATE), respectively. No grade 4 or 5 bleeding events were reported. Frequency of grade >1 bleeding AEs by time to event onset for (B) PCYC‐1102 and (C) PCYC‐1112 (RESONATE) ibrutinib arm. The same patient could have experienced multiple grade >1 bleeding AEs, with different time to onset of events. [Correction added on 08 May 2017, after first online publication: Figure 1B, “(n=18)” was corrected to “(n=118)”].
Given the limited number of major haemorrhages, we evaluated all bleeding AEs grade >1 to understand the timing of clinically relevant bleeding events, grade >1 bleeding AEs occurred in 9% in PCYC‐1102, and 4% in the PCYC‐1112 ibrutinib arm (Table 1). Among these 25 grade >1 bleeding AEs, 13 events occurred in 12 patients (7%) treated with concomitant anticoagulation or antiplatelet agents (2 of 12 received both anticoagulant and antiplatelet agents) and 12 occurred in 8 patients (5%) who were not receiving these medications. In the PCYC‐1112 ofatumumab arm, grade >1 bleeding occurred in 5 patients (3%). The majority of these events on ibrutinib resolved, with a median event duration of 18 and 11 days on PCYC‐1102 and PCYC‐1112, respectively. Most of these bleeding AEs occurred early, during the first 3–6 months of therapy (Fig 1B, C). Bleeding AEs led to discontinuation of ibrutinib in 4 patients (1%) from the 2 studies.
Discussion
The spectrum of bleeding events commonly occurring during single‐agent ibrutinib therapy (largely petechiae, bruising) suggests a primary haemostatic defect, ie, inhibition of platelet‐mediated haemostasis. In patients with CLL, the disease itself has been associated with impairment in both ADP‐mediated and collagen‐mediated platelet aggregation (Lipsky et al, 2015). The role of BTK and other Tec family kinases in GPVI signalling has been known for over a decade (Quek et al, 1998; Futatani et al, 2001; Liu et al, 2006). Pharmacological inhibition of BTK by ibrutinib has been shown in vitro to similarly affect downstream signalling of GPVI and platelet adhesion on von Willebrand factor under arterial flow (Levade et al, 2014). Similarly, in vitro collagen‐mediated platelet aggregation of blood from ibrutinib‐treated patients was reduced, an effect that correlated with occurrence of bleeding AEs and was reversible upon drug cessation (Levade et al, 2014; Kamel et al, 2015). Given the short half‐life (4–6 h) of ibrutinib and lack of new BTK synthesis in platelets, platelet transfusion given after clearance of ibrutinib from the blood may correct haemostasis in cases of serious bleeding (Levade et al, 2014; Kamel et al, 2015).
Among ibrutinib‐treated patients who received any anticoagulant or antiplatelet agent, major bleeding was reported in 9% and 4% in PCYC‐1102 (median follow‐up 22 months) and 2% and 1% in PCYC‐1112 (median follow‐up 10 months), respectively. For comparison, 18% of CLL patients in the Surveillance, Epidemiology, and End Results (SEER) ‐Medicare cancer registry had at least one major haemorrhage, corresponding to an EAIR of 6 (Gifkins et al, 2015). Moreover, the incidence of major haemorrhage was reported to be more than 8 times higher among patients with CLL than that of the age‐ and gender‐matched general population (Gifkins et al, 2015). In our analysis, there were no major bleeding AEs in patients receiving newer direct oral anticoagulants, however, the number of patients treated with these agents (n = 4) was too small to sufficiently assess bleeding risk with these agents. Further studies, one ongoing (Farooqui et al, 2015), are needed to determine the safety of concomitant anticoagulant use with ibrutinib.
Precautions on concomitant use of anticoagulants and antiplatelet agents, along with adherence to perioperative ibrutinib‐withholding guidelines, as applied in the PCYC‐1112 study and reflected in the ibrutinib label, resulted in a small number of major bleeding complications. Exclusion criteria applied in this study may limit the global application of results to the general CLL population. Patients with haematological malignancies, including CLL, are probably at increased risk for major bleeding because of the disease itself, comorbid conditions, concomitant medications or clinically significant thrombocytopenia. Thus, care should be exercised when initiating ibrutinib therapy in patients on anticoagulants who are at increased risk at baseline for experiencing major haemorrhagic events. The data presented here do not alter the risk‐benefit balance supporting ibrutinib treatment for CLL, but suggest that both patients and their treating physicians should be appropriately informed regarding the potential risk for bleeding.
Authorship contributions
JAJ designed the analysis, analysed and interpreted data, and wrote the manuscript; PH, SC, CT, RRF, PMB, SJS, TJK, IWF, UJ, JAB, JCB and SMO provided patients, collected data, and critically reviewed the manuscript; MC, JM and DFJ designed the analysis, analysed and interpreted data, and critically reviewed the manuscript. All authors approved the manuscript for submission.
Disclosure of conflicts of interest
JAJ: consultancy and research funding from Pharmacyclics, AbbVie, Janssen; PH: honoraria, consultancy and research funding from Roche, GSK, Janssen, Gilead, AbbVie, honoraria and research funding from Novartis, Pharmacyclics, research funding from Celgene; SC: consultancy for Janssen, Pharmacyclics, research funding from AbbVie, Pharmacyclics; CT: honoraria, consultancy and research funding from Janssen; RRF: honoraria, consultancy and speakers bureau for Pharmacyclics; PMB: consultancy for Pharmacyclics, AbbVie, research funding from Pharmacyclics; SJS: research funding from Pharmacyclics; TJK: consultancy for AbbVie, Genentech, Gilead, research funding from AbbVie, Genentech, Pharmacyclics; IWF: research funding from Janssen and Pharmacyclics; UJ: honoraria, consultancy and travel expenses for Janssen; JAB: consultancy for Janssen, Portola, research funding from Pharmacyclics, Gilead, travel expenses for Roche, Janssen; MC, employment with Pharmacyclics LLC, an AbbVie Company, stock ownership with AbbVie, Johnson & Johnson; JN: employment with Pharmacyclics LLC, an AbbVie Company, stock ownership with Amgen, AbbVie; DFJ, employment with Pharmacyclics LLC, an AbbVie Company, stock ownership with AbbVie; JCB: no relevant conflicts of interest to disclose; SOB: honoraria, consultancy and research funding from Pharmacyclics, honoraria and consultancy for Janssen.
Supporting information
Data S1. Methods.
Table SI. Summary of concomitant anticoagulants and/or antiplatelet therapy in PCYC‐1102 and PCYC‐1112 (RESONATE) ibrutinib arm.
Table SII. Summary of baseline characteristics and major bleeding events in patients treated with ibrutinib.
Acknowledgements
We thank the patients who participated in these studies, their supportive family members, and the investigators and clinical research staff from the study centers. This study was sponsored by Pharmacyclics LLC, an AbbVie Company. Medical writing assistance in the preparation of this manuscript was provided by Maoko Naganuma, MSc, CMPP, and was funded by Pharmacyclics LLC, an AbbVie Company.
The copyright line for this article was changed on 27 July 2018 after original online publication
References
- Atkinson, B.T. , Ellmeier, W. & Watson, S.P. (2003) Tec regulates platelet activation by GPVI in the absence of Btk. Blood, 102, 3592–3599. [DOI] [PubMed] [Google Scholar]
- Barrientos, J.C. , O'Brien, S. , Brown, J.R. , Kay, N.E. , Reddy, N.M. , Coutre, S. , Tam, C. , Mulligan, S. , Jaeger, U. , Devereux, S. , Pocock, C. , Robak, T. , Schuster, S.J. , Schuh, A. , Gill, D. , Bloor, A. , Dearden, C. , Moreno, C. , Cull, G. , Hamblin, M. , Jones, J.A. , Kierschniak, T. , Eckert, K. , Suzuki, S. , Hsu, E. , James, D.F. , Byrd, J.C. & Hillmen, P. (2014) Hematologic and immunologic function and patient well‐being for the phase III RESONATE(TM) study of ibrutinib vs ofatumumab in relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Blood, 124(21): 4696. [Google Scholar]
- Byrd, J.C. , O'Brien, S. & James, D.F. (2013) Ibrutinib in relapsed chronic lymphocytic leukemia. New England Journal of Medicine, 369, 1278–1279. [DOI] [PubMed] [Google Scholar]
- Byrd, J.C. , Brown, J.R. , O'Brien, S. , Barrientos, J.C. , Kay, N.E. , Reddy, N.M. , Coutre, S. , Tam, C.S. , Mulligan, S.P. , Jaeger, U. , Devereux, S. , Barr, P.M. , Furman, R.R. , Kipps, T.J. , Cymbalista, F. , Pocock, C. , Thornton, P. , Caligaris‐Cappio, F. , Robak, T. , Delgado, J. , Schuster, S.J. , Montillo, M. , Schuh, A. , de Vos, S. , Gill, D. , Bloor, A. , Dearden, C. , Moreno, C. , Jones, J.J. , Chu, A.D. , Fardis, M. , McGreivy, J. , Clow, F. , James, D.F. , Hillmen, P. & Investigators, R. (2014) Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. New England Journal of Medicine, 371, 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd, J.C. , Furman, R.R. , Coutre, S.E. , Burger, J.A. , Blum, K.A. , Coleman, M. , Wierda, W.G. , Jones, J.A. , Zhao, W. , Heerema, N.A. , Johnson, A.J. , Shaw, Y. , Bilotti, E. , Zhou, C. , James, D.F. & O'Brien, S. (2015) Three‐year follow‐up of treatment‐naive and previously treated patients with CLL and SLL receiving single‐agent ibrutinib. Blood, 125, 2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui, M. , Valdez, J. , Soto, S. , Bray, A. , Tian, X. & Wiestner, A. (2015) Atrial fibrillation in CLL/SLL patients on ibrutinib. Blood, 126(23): 2933. [Google Scholar]
- Futatani, T. , Watanabe, C. , Baba, Y. , Tsukada, S. & Ochs, H.D. (2001) Bruton's tyrosine kinase is present in normal platelets and its absence identifies patients with X‐linked agammaglobulinaemia and carrier females. British Journal of Haematology, 114, 141–149. [DOI] [PubMed] [Google Scholar]
- Gifkins, D.M. , Matcho, A. , Yang, H. , Xu, Y. , Gooden, M.A. & Wildgust, M. (2015) Incidence of major hemorrhage among CLL and MCL patients compared to the general elderly population: an analysis of the US SEER‐Medicare linked database. Blood, 126(23): 3268. [Google Scholar]
- Kamel, S. , Horton, L. , Ysebaert, L. , Levade, M. , Burbury, K. , Tan, S. , Cole‐Sinclair, M. , Reynolds, J. , Filshie, R. , Schischka, S. , Khot, A. , Sandhu, S. , Keating, M.J. , Nandurkar, H. & Tam, C.S. (2015) Ibrutinib inhibits collagen‐mediated but not ADP‐mediated platelet aggregation. Leukemia, 29, 783–787. [DOI] [PubMed] [Google Scholar]
- Levade, M. , David, E. , Garcia, C. , Laurent, P.A. , Cadot, S. , Michallet, A.S. , Bordet, J.C. , Tam, C. , Sie, P. , Ysebaert, L. & Payrastre, B. (2014) Ibrutinib treatment affects collagen and von Willebrand factor‐dependent platelet functions. Blood, 124, 3991–3995. [DOI] [PubMed] [Google Scholar]
- Lipsky, A.H. , Farooqui, M.Z. , Tian, X. , Martyr, S. , Cullinane, A.M. , Nghiem, K. , Sun, C. , Valdez, J. , Niemann, C.U. , Herman, S.E. , Saba, N. , Soto, S. , Marti, G. , Uzel, G. , Holland, S.M. , Lozier, J.N. & Wiestner, A. (2015) Incidence and risk factors of bleeding‐related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica, 100, 1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Fitzgerald, M.E. , Berndt, M.C. , Jackson, C.W. & Gartner, T.K. (2006) Bruton tyrosine kinase is essential for botrocetin/VWF‐induced signaling and GPIb‐dependent thrombus formation in vivo. Blood, 108, 2596–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quek, L.S. , Bolen, J. & Watson, S.P. (1998) A role for Bruton's tyrosine kinase (Btk) in platelet activation by collagen. Current Biology, 8, 1137–1140. [DOI] [PubMed] [Google Scholar]
- Wang, M.L. , Blum, K.A. , Martin, P. , Goy, A. , Auer, R. , Kahl, B.S. , Jurczak, W. , Advani, R.H. , Romaguera, J.E. , Williams, M.E. , Barrientos, J.C. , Chmielowska, E. , Radford, J. , Stilgenbauer, S. , Dreyling, M. , Jedrzejczak, W.W. , Johnson, P. , Spurgeon, S.E. , Zhang, L. , Baher, L. , Cheng, M. , Lee, D. , Beaupre, D.M. & Rule, S. (2015) Long‐term follow‐up of MCL patients treated with single‐agent ibrutinib: updated safety and efficacy results. Blood, 126, 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelstein, J.A. , Marino, M.C. , Lederman, H.M. , Jones, S.M. , Sullivan, K. , Burks, A.W. , Conley, M.E. , Cunningham‐Rundles, C. & Ochs, H.D. (2006) X‐linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine (Baltimore), 85, 193–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Methods.
Table SI. Summary of concomitant anticoagulants and/or antiplatelet therapy in PCYC‐1102 and PCYC‐1112 (RESONATE) ibrutinib arm.
Table SII. Summary of baseline characteristics and major bleeding events in patients treated with ibrutinib.


