Abstract
Aims and objectives
To estimate the prevalence of difficult venous access in complex patients with multimorbidity and to identify associated risk factors.
Background
In highly complex patients, factors like ageing, the need for frequent use of irritant medication and multiple venous catheterisations to complete treatment could contribute to exhaustion of venous access.
Design
A cross‐sectional study was conducted.
Methods
‘Highly complex’ patients (n = 135) were recruited from March 2013–November 2013. The main study variable was the prevalence of difficult venous access, assessed using one of the following criteria: (1) a history of difficulties obtaining venous access based on more than two attempts to insert an intravenous line and (2) no visible or palpable veins. Other factors potentially associated with the risk of difficult access were also measured (age, gender and chronic illnesses). Univariate analysis was performed for each potential risk factor. Factors with p < 0·2 were then included in multivariable logistic regression analysis. Odds ratios were also calculated.
Results
The prevalence of difficult venous access was 59·3%. The univariate logistic regression analysis indicated that gender, a history of vascular access complications and osteoarticular disease were significantly associated with difficult venous access. The multivariable logistic regression showed that only gender was an independent risk factor and the odds ratios was 2·85.
Conclusions
The prevalence of difficult venous access is high in this population. Gender (female) is the only independent risk factor associated with this. Previous history of several attempts at catheter insertion is an important criterion in the assessment of difficult venous access.
Relevance to clinical practice
The prevalence of difficult venous access in complex patients is 59·3%. Significant risk factors include being female and a history of complications related to vascular access.
Keywords: comorbidity, fluid therapy, inpatients, peripheral catheterisation, vascular access devices
What does this paper contribute to the wider global clinical community?
The number of high complex patients needing intravenous therapy is increasing. To identify risk factors of poor venous access is essential to provide an optimal care.
The prevalence of difficult venous access in complex patients is 59·3%. Significant risk factors include being female and a history of complications related to vascular access.
It is defined a population whose venous capital is difficult to preserve. It is highlighted the need for a proactive and expert intervention of ITTs in this population.
Introduction
Highly complex patients are patients of advanced age, with comorbidities and severe functional limitations, and are considered of high risk in relation to mortality and healthcare use (Ollero Baturone et al. 2002, Jadad et al. 2010, Health, Social Services and Equality Ministry of Spain 2012).
In the Basque Country (Spain), the population of highly complex patients requiring case management are a mean of 75 years old and have a mean of 1·9 hospital admissions per year, higher values than the mean across Europe (Health, Social Services and Equality Ministry of Spain 2012). Overall, they represent 5% of patients but account for more than 40% of hospital bed days (Garcia‐Morillo et al. 2005) and 85% of the admissions of these patients are directly related to their multimorbidity (Garcia‐Morillo et al. 2005). The cost of care associated with these patients is sixfold higher than that for patients with a single chronic condition (Garcia‐Morillo et al. 2005).
When hospitalised, an increasing number of highly complex patients require intravenous therapy as a safe way to administer treatment (Ingram & Lavery 2005). The rate of complications in patients receiving intravenous therapy ranges between 10–25% (Hawes 2007, Gallieni et al. 2008). These complications include infiltration and extravasation with an incidence of 34% (Dougherty 2008), phlebitis with an incidence of 20% (Nassaji‐Zavareh & Ghorbani 2007) and others such as thrombophlebitis, pneumothorax, haemothorax, infections, thrombosis and catheter‐related bacteraemia (Royal College of Nursing IV Therapy Forum 2005). In the case of highly complex patients with multimorbidities, the situation is complicated by the process of ageing itself, as well as the need for frequent use of agents that are irritant to veins and for multiple venous catheters to complete treatments, which contribute to greater limitations and exhaustion of venous access (Hawes 2007).
Background
The difficulty of venous access is determined by whether there is a lack of visible and palpable veins together with a history of difficulty in placing venous catheters (Brannam et al. 2004, Jacobson & Winslow 2005, Hawes 2007, Lapostolle et al. 2007, Sebbane et al. 2013, Fields et al. 2014, Chiricolo et al. 2015). In highly complex patients, difficult venous access may lead to serious consequences at various different levels. Patient pain and overall suffering are increased by repeated attempts to obtain venous access, which in many cases lead to the insertion of catheters in inappropriate locations such as flexion areas (hand, wrist and antecubital fossa) or lower limbs, in turn increasing the risk of subsequent complications (Royal College of Nursing IV Therapy Forum 2005, Hawes 2007, Cicolini et al. 2009, Infusion Nurses Society 2011, Abolfotouh et al. 2014). Difficult access also leads to delays in the administration of medication, partial or total loss of the prescribed dose, and the need to place central venous lines, representing a greater risk for patients (Hawes 2007). It also causes an increase in the use of materials, nurse time (Hawes 2007), costs associated with complications and length of hospital stay.
The current need to improve the management of difficult venous access, especially in this type of patient, has seen the emergence of intravenous therapy teams (ITTs) (Royer 2001, Hornsby et al. 2005, Kokotis 2005). These are mainly composed of nurses specialised in intravenous therapy and management of venous access and have been shown to improve outcomes in clinical practice (Mezey & Scholder 2003, Burns & Lamberth 2010, Centers for Disease Control and Prevention 2011), reducing the iatrogenic risks associated with intravenous therapy, and increasing patient safety and well‐being (Caballero 2006), as well as being more cost‐effective (Kokotis 2005).
As we have found no studies in the literature identifying the key factors that influence vascular deterioration of highly complex patients, we have designed the following study to estimate the prevalence of difficult venous access in highly complex hospitalised patients and identify associated risk factors.
Methods
Design
To address the objective, we designed a cross‐sectional study.
Participants
The target population included people who were classified as highly complex patients with multiple comorbidities (multimorbidity), based on the Kaiser Permanente pyramid.
The Kaiser pyramid model is a theoretical approach to stratify population according to their care needs. It was developed in the United States and has been extended to other countries.
In 2013, The Department of Health and Consumer Affairs of the Government of the Basque Country used this model to identify and stratify population with different health needs, from promotion and prevention to highly complex needs (Fig. 1).
Figure 1.
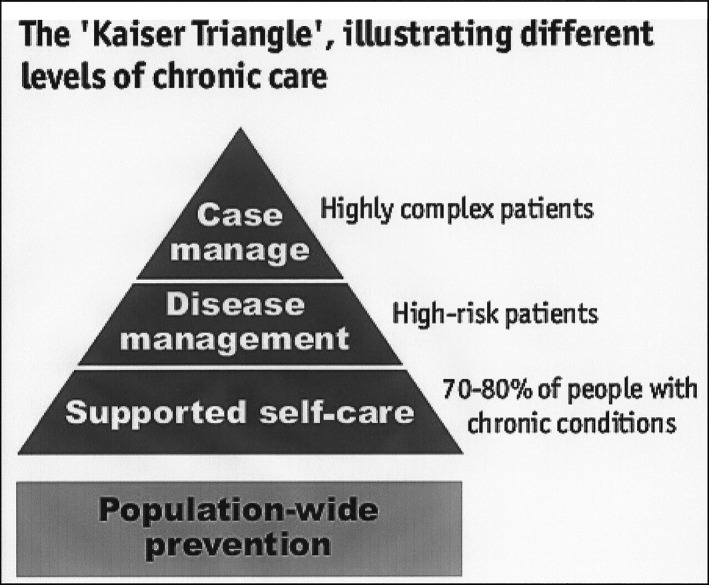
The Kaiser Triangle, illustrating different levels of chronic care. Source: NHS and University of Birmingham.
The study inclusion criteria were being admitted to hospital during the study period and being on the list of patients classified as highly complex.
The exclusion criteria were being in another level of the pyramid, unable to give informed consent form or treated previously by an ITT.
Candidate patients were identified by case management or liaison nurses of the participating hospitals, who were informed in writing whenever a patient classified as highly complex required hospital care. In turn, these nurses reported the hospital admission to the research team, and within 48 hours, a member of team in each participating centre visited the patient. After informing the patient about the study, informed consent was requested and we started to assess the study variables.
To detect a rate of 60% of difficult venous access, with an accuracy of 7% and an alpha error of 0·05, the sample size required was estimated to be 133 patients. Given the cross‐sectional nature of the study and that the difficulty of venous access was assessed after patient recruitment, we did not believe it to be necessary to consider potential losses.
Data collection
The main study variable was the presence of ‘difficult venous access’, based on whether patients met one or both of the following criteria: (1) a personal history of difficult venous access, based on patient report or a record of more than two attempts to insert an intravenous line, and (2) no visible or palpable veins appearing on either arm after placing a tourniquet.
The other variables were a series of factors that might predispose highly complex patients to difficult venous access. For this purpose, data were collected on a wide range of items (Table 1).
Table 1.
Potential risk factors for difficult venous access
| Age and gender |
| Number of hospital admissions in the year before the study |
| Level of independence (Barthel Index) |
| Chronic illnesses: number and diagnoses |
| History of treatment with anticoagulants, immunosuppressants, corticoids or chemotherapy |
| Previous major surgery |
| Limited limb function |
| Peripheral venous disease |
| Toxic habits |
| History of multiple attempts to place catheters for intravenous therapy |
| History of complications associated with vascular access: phlebitis, thrombosis, haematomas, infiltrations and extravasations, among others |
| Body mass index |
The Barthel Index was used to measure the ‘level of independence’ (see Fig. 2). We considered scores <20 as total dependence, 20–40 as severe dependence, 45–55 moderate dependence and 60 or more as low dependence.
Figure 2.

Barthel Index to measure the ‘level of independence’.
For the factor ‘number of chronic diseases’, we considered a cut‐off point (median).
We considered some potentially aggressive intravenous treatments such as anticoagulants, immunosuppressants, corticoids and chemotherapy.
The factor ‘limited limb function’ refers to limitations due to hemiplegia, breast surgery or burns.
The ‘toxic habits’ factor refers to the previous history of parenteral drug habit.
To measure the item ‘History of multiple attempts to place catheters for intravenous therapy’, we established three ranges: <10, between 10–20 and more than 20 punctures in the year before the study. For the analysis, we estimate that patients who have received more than 10 punctures have suffered damage to the venous access, considering this a risk factor.
Regarding body mass index (BMI), 30 was established as the cut‐off point for severe obesity, which was hypothesised as having greater difficulty for venous access.
All the data were recorded in field notebooks. Prior to this, we trained all members of the research team in the collection of data on the study variables. Subsequently, the data were entered into a dedicated Microsoft Access database for the statistical analysis.
The study was carried out between 1 March–21 November 2013 in OSI Araba University Hospital in the city of Vitoria‐Gasteiz (500 beds) and Alto Deba Hospital in the municipality of Arrasate‐Mondragón (95 beds), both public hospitals in the network of the Basque Health Service.
Ethical approval
All patients gave written informed consent prior to their inclusion in the study. They were told about the potential benefits and risks associated with their participation. The anonymity of participants was safeguarded in accordance with the Spanish Organic Law 15/1999 of 13 December, on the Protection of Personal Data, and the principles of the Declaration of Helsinki. The study was approved by the Ethics Committee of the OSI Araba University Hospital (reference number 2013‐007) and was included on the hospital insurance policy.
Data analysis
For the descriptive analysis, frequencies and percentages were calculated for qualitative variables. For the continuous variables, means and standard deviations or medians, interquartile range, minimum and maximum were calculated depending of the normality of the variable.
Means were compared using the Student's t‐test, and medians were compared using the nonparametric Mann–Whitney U‐test.
Binary logistic regression was used for the univariate analysis, to explore the association between each of the risk factors and difficult venous access. Means were compared using the nonparametric Mann–Whitney U‐test, given that the data did not meet the normality assumptions. All factors with a p < 0·2 in this univariate analysis were included in a multivariable logistic regression model. Odds ratios were calculated to describe the probability of having difficult venous access. The level of significance was set at 0·05 for all the tests. Data were analysed using the SPSS® version 21.0 (International Business Machines Corporation (IBM), Armonk, New York, USA).
Results
We recruited 135 patients, of whom more than half (65·2%) were women.
The general characteristics of the sample are the following ones:
The mean age was 78·4 years (SD 9·4), the youngest patient was 49 years old, and the oldest one was 99.
The median of hospitalisations in the year before the study was 1 (IQR 0–2) with a maximum number of 11 hospitalisations. In addition, length of hospital stay had a median of six days (IQR 4–9), the minimum was 1 and the maximum 39.
Regarding catheterisation, venous access was considered difficult in 59·3% of the sample (95% CI 50·8–67·9), in all cases on the basis that they had a personal history of more than two attempts to insert an intravenous line (though some patients also recalled past difficulties obtaining venous access and/or lacked visible or palpable veins).
In relation to risk factors, the median Barthel Index of the total sample was 90 points (IQR 60–100) and the minimum and maximum were 0 and 100, respectively.
The median of chronic diseases was 3 (IQR 3–4) with minimum of 1 and maximum of 6. According to this result, this median was established as the cut‐off point for univariate analysis. The most common were cardiovascular diseases and respiratory conditions, diagnosed in 94·8 and 67·4% of participants, respectively. Other chronic illnesses included diabetes (48·9%), osteoarticular disease (28·9%) and obesity (13·3%).
Regarding previous treatments, 77% of participants had been on anticoagulants and 54·1% corticosteroids. The prevalence of other factors was as follows: 12·6% for previous major surgery, 14·8% for limited limb function, 36·3% for peripheral venous disease and 14·8% for toxic habits. The year prior to the start of the study, 44·5% of the patients had undergone more than 10 attempts to obtain venous access, 43% had developed haematoma associated with needle punctures, 11·9% had developed phlebitis, and 11·9% had had infiltration.
The characteristics of the sample in relation to difficult and not difficult venous access can be seen in Tables 2 and 3.
Table 2.
Univariate logistic regression analysis for difficult venous access risk factors
| Variable category | Difficult venous access | |||
|---|---|---|---|---|
| N (%) | p | Odds ratio | 95% CI | |
| Gender n (%) | ||||
| Female (n = 47) | 35 (74·5) | 0·010 | 2·79 | 1·28–6·07 |
| Male (n = 88) | 45 (51·1) | |||
| Chronic illnesses (≥4 conditions) n (%) | ||||
| Yes (n = 50) | 32 (64·0) | 0·391 | 1·37 | 0·67–2·81 |
| No (n = 85) | 48 (56·5) | |||
| Cardiovascular disease n (%) | ||||
| Yes (n = 128) | 78 (60·9) | 0·112 | 3·90 | 0·73–20·88 |
| No (n = 7) | 2 (28·6) | |||
| Chronic respiratory disease n (%) | ||||
| Yes (n = 91) | 51 (56·0) | 0·276 | 0·66 | 0·32–1·39 |
| No (n = 44) | 29 (65·9) | |||
| Chronic kidney failure n (%) | ||||
| Yes (n = 27) | 16 (59·3) | 0·999 | 1·00 | 0·42–2·36 |
| No (n = 108) | 64 (59·3) | |||
| Diabetes n (%) | ||||
| Yes (n = 66) | 41 (62·1) | 0·508 | 1·26 | 0·63–2·51 |
| No (n = 69) | 39 (56·5) | |||
| Obesity (body mass index >30 kg/m2) n (%) | ||||
| ≥30 (n = 39) | 28 (71·8) | 0·062 | 2·15 | 0·96–4·82 |
| <30 (n = 96) | 52 (54·2) | |||
| Osteoarticular disease n (%) | ||||
| Yes (n = 39) | 29 (74·4) | 0·025 | 2·56 | 1·12–5·83 |
| No (n = 96) | 51 (53·1) | |||
| History of treatment with: anticoagulants, immunosuppressants, corticoids or chemotherapy n (%) | ||||
| Yes (n = 119) | 72 (60·5) | 0·424 | 1·53 | 0·54–4·36 |
| No (n = 16) | 8 (50·0) | |||
| Previous major surgery n (%) | ||||
| Yes (n = 17) | 13 (76·5) | 0·132 | 2·47 | 0·76–8·04 |
| No (n = 118) | 67 (56·8) | |||
| Limited limb function (any limb) n (%) | ||||
| Yes (n = 20) | 14 (70·0) | 0·294 | 1·73 | 0·62–4·83 |
| No (n = 115) | 66 (57·4) | |||
| Peripheral venous disease n (%) | ||||
| Yes (n = 49) | 30 (61·2) | 0·726 | 1·14 | 0·56–2·33 |
| No (n = 86) | 50 (58·1) | |||
| Toxic habits n (%) | ||||
| Yes (n = 20) | 11 (55·0) | 0·675 | 0·82 | 0·31–2·12 |
| No (n = 115) | 69 (60·0) | |||
| History of >10 venipuncture attempts n (%) | ||||
| Yes (n = 60) | 38 (63·3) | 0·441 | 1·32 | 0·66–2·65 |
| No (n = 74) | 42 (56·8) | |||
| History of vascular access‐related complications n (%) | ||||
| Yes (n = 64) | 44 (68·8) | 0·034 | 2·14 | 1·06–4·33 |
| No (n = 71) | 36 (50·7) | |||
| Haematomas n (%) | ||||
| Yes (n = 58) | 39 (67·2) | 0·103 | 1·80 | 0·888–3·658 |
| No (n = 77) | 41 (53·2) | |||
| Cancer n (%) | ||||
| Yes (n = 25) | 11 (44·0) | 0·090 | 2·14 | 0·89–5·15 |
| No (n = 110) | 69 (62·7) | |||
Table 3.
Comparison of continuous variables between both groups
| Variable | DVA | Not DVA | p |
|---|---|---|---|
| Number of hospitalisations in the previous year | 1 (0–2) | 1 (0–2) | 0·219b |
| Barthel Index | 85 (55–100) | 90 (70–100) | 0·155b |
| Age | 79·1 (9·0) | 77·9 (9·6) | 0·487a |
Mann–Whitney U‐test.
Student's t‐test.
The univariate analysis of the association between these potential risk factors and difficult venous access indicated that gender (p = 0·01), a history of complications related to intravenous therapy (p = 0·034) and osteoarticular disease (p = 0·025) were statistically significant. Among the other variables, only obesity was found to be close to significance (Tables 2 and 3).
The following factors were included in the multivariable logistic regression analysis: cardiovascular disease (p = 0·112), cancer (p = 0·09), previous major surgery (p = 0·132), level of independence (p = 0·172), the presence of haematomas (p = 0·103), and obesity (p = 0·062), as well as the aforementioned factors found to be statistically significant in the univariate analysis. The multivariable analysis showed that only gender (p = 0·008) was a significant independent risk factor. The odds ratio for this factor was 2·85 (95% CI 1·31–6·25).
Discussion
In the literature, we have not found any studies similar to ours in a sample of highly complex patients. However, in other populations, prospective studies have been carried out assessing the difficulty of venous access by counting the number of attempts to insert an intravenous line and these have identified factors that may have an influence on success (Brannam et al. 2004, Jacobson & Winslow 2005, Lapostolle et al. 2007, Bensghir et al. 2012, Sebbane et al. 2013, Fields et al. 2014, Chiricolo et al. 2015). Some of these studies assessed the difficulty of venous access based on a physical examination and characteristics of the veins (Jacobson & Winslow 2005, Sebbane et al. 2013), while others considered venipuncture attempts prior to the current hospitalisation and the presence of chronic conditions (Fields et al. 2014, Chiricolo et al. 2015). The results of our study show a high prevalence of difficult venous access in highly complex patients, notably higher than the rates observed in studies focused on other population groups (Lapostolle et al. 2007, Fields et al. 2014). In classifying our patients as having difficult access, the factor with the greatest weight was a history of more than two attempts to insert an intravenous line, the presence of visible or palpable veins being less important.
Our findings indicate that when venous access is required in patients, although a physical examination is essential, we should also consider patient history of catheterisation to predict the difficulty of venous access. In fact, Fields et al. (2014) noted that, although it may not be easy to assess this history, it may be a better predictive factor than the condition of the patient at the time.
Regarding associated factors, being female was found to be significantly associated with difficult venous access. Among the women, there was a greater prevalence of difficult access and a threefold higher risk of difficult access than in the men. Some previous research has linked being female to higher rates of phlebitis (Tagalakis et al. 2002, Forni et al. 2010, Dychter et al. 2012) and fewer successful attempts to place intravenous devices in peripheral blood vessels (Jacobson & Winslow 2005). Further, women tend to be more affected by osteoarticular disease, and in our study, this disease was found to be significantly associated with the risk of difficult venous access.
On the other hand, age was not statistically significant. This is consistent with the results of the Jacobson and the Bensghir study, which assessed variables associated with intravenous catheter insertion failure in peripheral veins (Jacobson & Winslow 2005, Bensghir et al. 2012). However, age may be clinically relevant, given that older individuals have undergone anatomical changes typical of ageing and tend to have a weaker vascular system (Wengström & Margulies 2008, Dychter et al. 2012). Various authors have clearly advocated a proactive effort to avoid damage to vessels in this group of patients (Kokotis 2005, Dychter et al. 2012).
Chronic illnesses do not seem to be associated with the difficulty of venous access, except in the case of osteoarticular disease. In contrast, some studies assessing the difficulty of inserting intravenous devices in a peripheral vein have considered chronicity to be a potential risk factor for difficult venous access, but without specifying the number or type of conditions (Brannam et al. 2004, Kokotis 2005, Costantino et al. 2010). Chronicity has also been associated with a greater risk of phlebitis (Tagalakis et al. 2002). Further, the Lapostolle study found a significant association with diabetes, but this condition was evaluated together with other factors such as chemotherapy and recurrent hospital admission (Lapostolle et al. 2007), making it difficult to assess which of them was directly related to the difficulty of venous access. Fields et al. (2014) indicated that diabetes may be associated with difficult access due to the frequent medical attention these patients require or morphological changes in their veins.
Several studies have analysed obesity in other populations (Brannam et al. 2004, Hawes 2007, Costantino et al. 2010, Dargin et al. 2010, Bensghir et al. 2012, Dychter et al. 2012, Sebbane et al. 2013, Fields et al. 2014), with similar results to ours; namely, this variable was not found to be significantly associated with difficult access (Jacobson & Winslow 2005, Lapostolle et al. 2007, Fields et al. 2014). However, its clinical relevance should be assessed given that the associated increase in subcutaneous tissue makes it more difficult to find veins in the arms (Fields et al. 2014, Frank 2016).
A history of treatment with anticoagulants or corticosteroids is relevant in the patient anamnesis as a real or potential indicator of multiple venipuncture procedures, given that patients treated with these drugs often require long and repeated intravenous therapy and hence multiple insertions of intravenous catheters (Fields et al. 2014).
The number of previous placements of intravenous devices has been considered a risk factor as well as used in defining difficult venous access in other studies (Hawes 2007, Lapostolle et al. 2007, Fields et al. 2014, Chiricolo et al. 2015). Unlike in these previous studies, however, we have quantified the number of intravenous placements retrospectively, finding a high percentage of patients had undergone multiple venipunctures. Therefore, we consider it essential to include this variable in the assessment of the patient. Likewise, it is essential to collect data on the history of complications related to vascular access, as we demonstrate in this study. According to the literature, the most common complications are infiltration and phlebitis (Tagalakis et al. 2002, Nassaji‐Zavareh & Ghorbani 2007, Dougherty 2008, Dychter et al. 2012, Frank 2016), and perhaps, the presence of haematomas should be added to this list, given the high prevalence of this factor in our study.
Limitations
One of the limitations of the study was that the number of venous punctures was quantified by interviewing the patient, with the potential for memory bias. Although we reviewed patient medical records to minimise this bias, we found that the number of attempts to place a catheter is not usually recorded. Another factor that may have had an impact on this issue was that we did not assess the experience/skills of the nurses carrying out these procedures. Fields underlines that it is difficult to collect data assessing the experience of the health professional involved retrospectively, but it should nevertheless be taken into account (Fields et al. 2014). Success in placing intravenous devices has been measured prospectively in other studies (Hornsby et al. 2005, Jacobson & Winslow 2005), with nurses with greater experience requiring fewer attempts (Jacobson & Winslow 2005). Accordingly, future research should address this issue to provide further evidence.
The sampling was not random and a major disadvantage of this is seasonal variability if the recruitment period is short. In our study, this was addressed by recruiting over a relatively long period, including months with higher and lower rates of hospital admission of complex patients. Lastly, another potential source of bias is interviewer bias, this being minimised in our study by training researchers and the use of the same data collection tools.
Conclusion
To conclude, we found a high prevalence of difficult venous access, especially among women, and it was generally related to a history of more than two attempts to insert an intravenous line, rather than to veins not being visible or palpable. The risk of difficult venous access was associated with being female, osteoarticular disease and a history of complications related to vascular access.
A strength of this study was that it assesses the difficulty of venous access proactively, combining physical examination and an analysis of the patient′s history regarding vascular access, and considers a wide range of variables. It is also pioneering in that it considers difficult vascular access specifically in complex patients.
The results may have implications for clinical practice, as they describe a population group in which it is difficult to preserve venous access. They highlight the need for proactive intervention by ITTs in this population, to ensure the adequate placement of intravenous devices and minimise the number of venipunctures.
Further research is required focusing on what are the most important risk factors for difficult venous access and thereby extending the findings of our study. In addition, similar analysis could be performed in groups at other levels of the Kaiser pyramid. On the other hand, it would be interesting to explore the impact of ITTs on the management of venous access devices in complex patients.
Relevance to clinical practice
The number of highly complex patients needing intravenous therapy is increasing. Identifying risk factors for poor venous access is essential to providing optimal care.
The prevalence of difficult venous access in complex patients is 59·3%. Significant risk factors include being female and a history of complications related to vascular access.
A population whose venous access is difficult to preserve is defined, and the need for a proactive and expert intervention of ITTs in this population is highlighted.
Funding
This study was fully funded by the Department of Health and Consumer Affairs of the Government of the Basque Country through a 2012 health research grant (reference: 2012111020).
Contributions
All authors have contributed similarly to the preparation of this manuscript.
Conflict of interest
The authors have no conflicts of interest to declare in relation to this work.
Acknowledgements
We would like to thank all the patients who participated in this study, the Department of Health and Consumer′s affairs of the Government of the Basque Country and the Basque Health Service (Osakidetza).
[The copyright line for this article was changed on 26 July 2018 after original online publication]
References
- Abolfotouh MA, Salam M, Bani‐Mustafa A, White D & Balkhy HH (2014) Prospective study of incidence and predictors of peripheral intravenous catheter‐induced complications. Therapeutics and Clinical Risk Management 10, 993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensghir M, Chkoura K, Mounir K, Drissi M, Elwali A, Ahtil R, Meziane M, Alaoui H, Elmoqadem A, Lahlou J, Hatim A, Azendour H & Drissi Kamili N (2012) Peripheral intravenous access in the operating room: characteristics and predictors of difficulty. French Annals of Anesthesia and Reanimation 31, 600–604. [DOI] [PubMed] [Google Scholar]
- Brannam L, Blaivas M, Lyon M & Flake M (2004) Emergency nurses’ utilization of ultrasound guidance for placement of peripheral intravenous lines in difficult‐access patients. Academic Emergency Medicine 11, 1361–1363. [DOI] [PubMed] [Google Scholar]
- Burns T & Lamberth B (2010) Facility wide benefits of radiology vascular access teams. Radiology Management 32, 28–32; quiz 33‐4. [PubMed] [Google Scholar]
- Caballero C (2006) Propuesta de formación de equipo de terapia intravenosa (Proposal to establish an Intravenous Therapy Team). Revista Rol de Enfermería (Nursing Rol) 29, 34–38. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2011) 2011 Guidelines for the Prevention of Intravascular Catheter‐Related Infections. Available at: http://www.cdc.gov/hicpac/BSI/BSI-guidelines-2011.html (accessed 15 May 2014).
- Chiricolo G, Balk A, Raio C, Wen W, Mihailos A & Ayala S (2015) Higher success rates and satisfaction in difficult venous access patients with a guide wire–associated peripheral venous catheter. The American Journal of Emergency Medicine 33, 1742–1744. [DOI] [PubMed] [Google Scholar]
- Cicolini G, Bonghi AP, Di Labio L & Di Mascio R (2009) Position of peripheral venous cannulae and the incidence of thrombophlebitis: an observational study. Journal of Advanced Nursing 65, 268–1273. [DOI] [PubMed] [Google Scholar]
- Costantino TG, Kirtz JF & Satz WA (2010) Ultrasound‐guided peripheral venous access vs. the external jugular vein as the initial approach to the patient with difficult vascular access. The Journal of Emergency Medicine 39, 462–467. [DOI] [PubMed] [Google Scholar]
- Dargin JM, Rebholz CM, Lowenstein RA, Mitchell PM & Feldman JA (2010) Ultrasonography‐guided peripheral intravenous catheter survival in ED patients with difficult access. The American Journal of Emergency Medicine 28, 1–7. [DOI] [PubMed] [Google Scholar]
- Dougherty L (2008) IV therapy: recognizing the differences between infiltration and extravasation. The British Journal of Nursing 17, 896, 898–901. [DOI] [PubMed] [Google Scholar]
- Dychter SS, Gold DA, Carson D & Haller M (2012) Intravenous therapy: a review of complications and economic considerations of peripheral access. Journal of Infusion Nursing 35, 84–91. [DOI] [PubMed] [Google Scholar]
- Fields JM, Piela NE, Au AK & Ku BS (2014) Risk factors associated with difficult venous access in adult ED patients. The American Journal of Emergency Medicine 32, 1179–1182. [DOI] [PubMed] [Google Scholar]
- Forni C, Loro L, Tremosini M, Trofa C, D'Alessandro F, Sabbatini T, Kapron M, Genco R, Schiavone M, Borri C, Bombino C, Notarnicola T, Amodeo A, Boschi R, Capezzali D, Mosci D & Mini S (2010) Cohort study of peripheral catheter related complications and identification of predictive factors in a population of orthopedic patients. Assistenza Infermieristica e Ricerca: AIR (Nursing assistance and search) 29, 166–173. [PubMed] [Google Scholar]
- Frank RL (2016) Peripheral Venous Access in Adults. Available at: http://www.uptodate.com/contents/peripheral-venous-access-in-adults?source=search_result&search=peripheral+intravenous+catheter&selectedTitle=1~30 (accessed 8 October 2016).
- Gallieni M, Pittiruti M & Biffi R (2008) Vascular access in oncology patients. CA: A Cancer Journal for Clinicians 58, 323–346. [DOI] [PubMed] [Google Scholar]
- Garcia‐Morillo JS, Bernabeu‐Wittel M, Ollero‐Baturone M, Aguilar‐Guisad M, Ramirez‐Duque N, Gonzalez de la Puente MA, Limpo P, Romero‐Carmona S & Cuello‐Contreras JA (2005) Incidence and clinical features of patients with comorbidity attended in internal medicine areas. Clinical Medicine 125, 5–9. [DOI] [PubMed] [Google Scholar]
- Hawes ML (2007) A proactive approach to combating venous depletion in the hospital setting. Journal of Infusion Nursing 30, 33–44. [DOI] [PubMed] [Google Scholar]
- Health, Social Services and Equality Ministry of Spain (2012) Estrategia para el abordaje de la cronicidad en el sistema nacional de salud (Strategy for approach of chronicity in the national health system). Health, Social Services and Equality Ministry of Spain, Madrid, Spain. [Google Scholar]
- Hornsby S, Matter K, Beets B, Casey S & Kokotis K (2005) Cost losses associated with the “PICC, stick, and run team” concept”. Journal of Infusion Nursing 28, 45–53. [DOI] [PubMed] [Google Scholar]
- Infusion Nurses Society (2011) Infusion Nursing Standards of Practice (2011). Journal of Infusion Nursing 34(1S), S1–S109. [DOI] [PubMed] [Google Scholar]
- Ingram P & Lavery I (2005) Peripheral intravenous therapy: key risks and implications for practice. Nursing Standard 19, 55–64; quiz 66. [DOI] [PubMed] [Google Scholar]
- Jacobson AF & Winslow EH (2005) Variables influencing intravenous catheter insertion difficulty and failure: an analysis of 339 intravenous catheter insertions. Heart & Lung: The Journal of Acute and Critical Care 34, 345–359. [DOI] [PubMed] [Google Scholar]
- Jadad A, Cabrera A, Martos F, Smith R & Lyons R (2010) When People Live with Multiple Chronic Diseases: A Collaborative Approach to an Emerging Global Challenge. Andalusian School of Public Health, Granada. [Google Scholar]
- Kokotis K (2005) Cost containment and infusion services. Journal of Infusion Nursing 28, S22–S32. [DOI] [PubMed] [Google Scholar]
- Lapostolle F, Catineau J, Garrigue B, Monmarteau V, Houssaye T, Vecci I, Tréoux V, Crocheton N & Adnet F (2007) Prospective evaluation of peripheral venous access difficulty in emergency care. Intensive Care Medicine 33, 1452–1457. [DOI] [PubMed] [Google Scholar]
- Mezey M & Scholder J (2003) Editorial. Journal of Infusion Nursing 26, 125–126. [DOI] [PubMed] [Google Scholar]
- Nassaji‐Zavareh M & Ghorbani R (2007) Peripheral intravenous catheter‐related phlebitis and related risk factors. Singapore Medical Journal 48, 733–736. [PubMed] [Google Scholar]
- Ollero Baturone M, Álvarez Tello M, Barón Franco B, Bernabéu Wittel M, Codina Lanaspa A, Fernández Moyano A, Garrido Porras E, Ortiz Camúñez MA, de Rojas García Paso J & Romero Alonso A (2002) Atención al paciente pluripatológico: proceso asistencial integrado (Highly complex patient care: integrated care process).
- Royal College of Nursing IV Therapy Forum (2005) Standards for Infusion Therapy. Available at: http://www.rcn.org.uk/_data/assets/pdf_file/0005/78593/002179_pdf (accessed 15 May 2014).
- Royer T (2001) Nurse‐driven interventional technology: a cost and benefit perspective. Journal of Infusion Nursing 24, 326–331. [DOI] [PubMed] [Google Scholar]
- Sebbane M, Claret P, Lefebvre S, Mercier G, Rubenovitch J, Jreige R, Eledjam J & de La Coussaye J (2013) Predicting peripheral venous access difficulty in the emergency department using body mass index and a clinical evaluation of venous accessibility. The Journal of Emergency Medicine 44, 299–305. [DOI] [PubMed] [Google Scholar]
- Tagalakis V, Kahn SR, Libman M & Blostein M (2002) The epidemiology of peripheral vein infusion thrombophlebitis: a critical review. The American Journal of Medicine 113, 146–151. [DOI] [PubMed] [Google Scholar]
- Wengström Y & Margulies A (2008) European oncology nursing society extravasation guidelines. European Journal of Oncology Nursing 12, 357–361. [DOI] [PubMed] [Google Scholar]


