Abstract
Background
Comparative evidence for efficacy and safety of second‐generation cholinesterase inhibitors (ChEIs) is still sparse.
Objectives
The purpose of this research is to compare three ChEIs, donepezil, galantamine and rivastigmine, in patients with mild‐to‐moderate Alzheimer's disease (AD).
Methods
We conducted a systematic review for published articles and included randomised, double‐blind, placebo‐controlled trials and head‐to‐head randomised trials evaluating the efficacy and safety of ChEIs in patients with AD. We examined Alzheimer's Disease Assessment Scale, cognitive subscale (ADAS‐Cog), Neuropsychiatric Inventory (NPI), Clinician's Interview‐Based Impression of Change plus caregiver's input (CIBIC+) and Clinical Global Impression of Change (CGIC) as efficacy endpoints. Withdrawals due to adverse events and number of patients experiencing nausea, vomiting, diarrhoea and dizziness were examined as safety profiles. Network meta‐analyses were sequentially performed for efficacy and safety outcomes based on drug/dose treatment conditions.
Results
Among the 21 trials included, network meta‐analysis showed that all treatments were significantly more efficacious than placebo in cognition measured by ADAS‐Cog. All treatments except galantamine were significantly more efficacious than placebo in global change in CIBIC+ or CGIC. Across all conditions, no significant efficacy was observed in neuropsychiatric symptoms measured by NPI. Derived hierarchies in the efficacy of treatment conditions were variables across efficacy and safety.
Conclusions
Our analysis is the first attempt to incorporate available direct and indirect evidence. The results suggest that ChEIs should have significant efficacy for cognition and global change assessment, but the efficacy on neuropsychiatric symptoms is questionable in patients with mild‐to‐moderate AD.
Keywords: Alzheimer's disease, cholinesterase inhibitors, meta‐analysis
Introduction
Alzheimer's disease (AD) is the most common form of dementia. At present, there are no therapeutic interventions that halt or reverse disease progression, and the currently available medications, typically acetylcholinesterase inhibitors (ChEIs), are just palliative therapy for AD symptoms. Regarding patients with mild‐to‐moderate AD, the American Psychiatric Association recommends second generation ChEIs, donepezil, galantamine and rivastigmine for mild‐to‐moderate AD (Rabins et al., 2007). The National Institute for Health and Clinical Excellence guideline also recommends these three second‐generation ChEIs as options for managing mild‐to‐moderate AD (NICE, 2011).
To date, several reviews and meta‐analyses have been published summarising the efficacy and safety of second‐generation ChEIs for treatment of AD (Trinh et al., 2003; Birks, 2006; Hansen et al., 2008; Tan et al., 2014; Wang et al., 2014). However, comparative evidence is still sparse. Despite the slight variations in the mode of action of the three ChEIs, the Cochrane Dementia and Cognitive Improvement Group concluded that there is no evidence of any difference between them with respect to efficacy (Birks, 2006). On the other hand, a later meta‐analysis including adjusted indirect comparison revealed no significant differences between these drugs regarding cognition, but found the relative risk of global response to be better with donepezil and rivastigmine than galantamine and favoured donepezil over galantamine regarding neuropsychiatric symptoms (Hansen, et al., 2008). However, adjusted indirect comparisons did not combine results of direct and indirect comparisons. The network meta‐analysis (NMA) is a meta‐analysis in which multiple treatments are compared using both direct comparisons of interventions within randomised controlled trials and indirect comparisons across trials based on a common comparator (Salanti et al., 2008). The analysis is useful when investigators are interested in summarising results from more than two treatments and the hierarchy of these treatments. Although there are concerns about methodological issues of sample size, power, sources of bias and heterogeneity, for many comparisons, the network meta‐analysis may yield more reliable and definitive results than would a pairwise meta‐analysis (Mills et al., 2013).
We conducted a systematic review and Bayesian NMA of three ChEIs, donepezil, galantamine and rivastigmine, for the treatment of mild‐to‐moderate AD. Our intention was to provide comparative evidence and hierarchies for efficacy and safety between ChEIs in patients with mild‐to‐moderate AD.
Methods
Literature search
The initial literature search was conducted with using PubMed and EMBASE. Search keywords included: Alzheimer's disease, cholinesterase inhibitor, donepezil, galantamine and rivastigmine. The search covered English‐language reports published as full‐text articles before September 2014. Previously published systematic reviews, meta‐analyses and the Specialized Register of the Cochrane Dementia and Cognitive Improvement Group were also manually reviewed.
Inclusion/exclusion criteria and data extraction
We included results from randomised, double‐blinded, placebo‐controlled trials (RCTs) evaluating the efficacy of ChEIs in patients with mild‐to‐moderate AD with at least one of the following assessments: (1) Alzheimer's Disease Assessment Scale, cognitive subscale (ADAS‐Cog; Rosen and Mohs, 1984), (2) Neuropsychiatric Inventory (NPI; Cummings et al., 1994), (3) Clinician's Interview‐Based Impression of Change scale plus caregiver's input (CIBIC+; Knopman et al., 1994) and (4) Clinical Global Impression of Change (CGIC; Schneider et al., 1997). Results from randomised comparative trials, directly comparing one drug with another, were also included in the analysis. These “head‐to‐head” trials were not required to be double‐blinded in our inclusion criteria. Studies were excluded from the analysis if they examined patients with severe AD (defined as the Mini‐Mental State Examination < 10 or specifically indicated in the study design) or mild cognitive impairment. Studies that specifically examined high‐dose or low‐dose treatment were also excluded from the analysis.
Two authors (H. K., R. N.) independently reviewed identified articles during the initial literature search according to the above inclusion and exclusion criteria. The two reviewers discussed any discrepancies on article retrieval. When agreement between the two reviewers was not achieved, a third reviewer (T. O.) made the final decision. For each study included, patient characteristics, study design, drug name, dosage, sample size, efficacy outcomes and adverse event (AE) occurrences were extracted. In addition, the data were extracted from the results of the intent‐to‐treat population with last observation carried forward method, if it was available. The data extraction process was also independently conducted by two authors (H. K, R. N), and any discrepancy was discussed and resolved.
Outcome measures
The efficacy for cognition was evaluated by assessment of ADAS‐Cog and neuropsychiatric symptoms were assessed by NPI. The means and standard deviations of the change from baseline were extracted. The efficacy for clinical global change was evaluated by assessment of CIBIC+ and CGIC. Patients who was judged at least “minimal/slightly improved” (i.e. ≤3 points in CIBIC+ or CGIC) were counted. We also extracted the number of patients who withdrew from the study because of AEs. Finally, we extracted the numbers of patients with specific AEs: nausea, vomiting, diarrhoea and dizziness.
Synthesis of results
We first analysed the descriptive data of the studies included and patient characteristics. A set of independent random‐effect meta‐analyses was then performed for each outcome. These traditional meta‐analyses assessed heterogeneities and publication biases across the studies prior to conducting NMA. The heterogeneities were evaluated using I 2 statistic, and the publication biases were evaluated using funnel plots. The meta‐analyses were performed using Metafor package (Viechtbauer, 2010) in R.
The NMA was performed on the efficacy in seven arms according to the treatment condition drugs and dose (dose‐based comparison: 5 mg donepezil versus 10 mg donepezil versus 16 mg galantamine versus 24 mg galantamine versus 6 and 12 mg rivastigmine versus 18 mg rivastigmine patch [9.5 cm2] versus placebo) and sequentially performed to four arms according to the treatment condition based on drugs (drug‐based comparison: donepezil versus galantamine versus rivastigmine versus placebo). The analyses were performed in the Bayesian hierarchical model framework with Markov chain Monte Carlo estimation using the GeMTC package (van Valkenhoef et al., 2012) in R. The number of tuning iterations was set at 1000 and the number of simulation iterations at 10 000. The model convergence was evaluated by visually inspecting the iteration plot and the potential scale reduction factor (Brooks and Gelman, 1997). Finally, tolerability was evaluated as five arms according to the drug (donepezil versus galantamine versus rivastigmine versus rivastigmine patch versus placebo). Because a meaningful heterogeneity was expected between rivastigmine and rivastigmine patch, we decided to analyse these two preparations as separate arms.
In the NMA, we summarised ADAS‐Cog as the relative empirical mean difference between treatment arms. To evaluate NPI‐10 and NPI‐12 simultaneously, we examined the standardised mean change (Hedges' adjusted g) from baseline for the NPI scores and again summarised as relative difference. For the dichotomous outcomes, including CIBIC+, CGI and safety data, we evaluated the proportion of these events summarised as the odds ratio between the treatment arms. For each summary statistic, a 95% credible interval (95% CrI) was computed. The hierarchy between drugs was estimated by the surface under the cumulative ranking (Salanti et al., 2014), which was computed by the ranking probabilities of the frequency table of iteration results.
Because we did not require included randomised comparative trials to be a double‐blinded, quality assessment for those studies was essential. We performed a set of sensitivity analyses to test the consistency of results.
Results
Literature search and description of studies
Figure 1 presents the summary of the literature search (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flowchart). A total of 911 potentially relevant articles were identified from initial database search, and 21 met our inclusion criteria, including 18 studies designed as double‐blind RCTs and 3 studies exclusively designed as randomised, head‐to‐head, drug comparisons. Ten studies investigated donepezil, six galantamine and nine rivastigmine. Twenty‐one studies assessed ADAS‐Cog, six NPI and fourteen CIBIC+ and/or CGIC.
Figure 1.
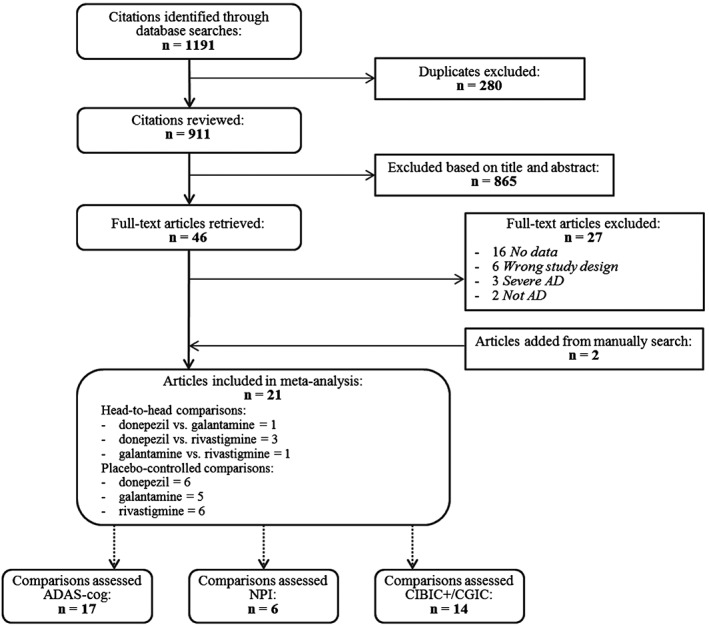
Literature review flowchart. AD, Alzheimer's disease; ADAS‐Cog, Alzheimer's Disease Assessment Scale, cognitive subscale; CGIC, Clinical Global Impression of Change; CIBIC+, Clinician's Interview‐based Impression of Change plus caregiver's input; NPI, Neuropsychiatric Inventory.
Table 1 presents the details of the 21 trials included. The total sample size was 9509. The studies were mainly conducted in North America and Europe. The mean study duration ranged from 12 to 104 weeks (average, 27.4). The characteristics of the study populations are summarised as follows: mean age at entry ranged from 69.4 to 78.4 years; 62.3% of participants were women. The mean Mini‐Mental State Examination score ranged from 15.1 to 21.5. The average scores of ADAS‐Cog and NPI at baseline ranged from 20.2 to 34.9 and from 10.3 to 35.2, respectively.
Table 1.
Baseline characteristics of studies included
| Drug(s) | Study | Daily dose | Gender (Female, %) | Age (years) | Study duration (weeks) | Baseline MMSE | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|
| Cognition | Behaviour | Global | |||||||
| Donepezil | Rogers et al., 1998a | Placebo | 61 | 74 | 12 | 19.8 | ADAS‐Cog | — | CIBIC+ |
| 5 mg | 69 | 73.8 | 19.39 | ||||||
| 10 mg | 61 | 73.4 | 19.35 | ||||||
| Rogers et al., 1998b | Placebo | 61 | 72.6 | 24 | 19.4 | ADAS‐Cog | — | CIBIC+ | |
| 5 mg | 63 | 72.9 | 19.44 | ||||||
| 10 mg | 62 | 74.6 | 19.17 | ||||||
| Burns et al., 1999 | Placebo | 55 | 71 | 24 | 20 | — | — | CIBIC+ | |
| 5 mg | 61 | 72 | 20 | ||||||
| 10 mg | 57 | 72 | 20 | ||||||
| Homma et al., 2000 | Placebo | 66 | 69.4 | 24 | 16.6 | ADAS‐Cog | — | CGIC | |
| 5 mg | 68 | 70.1 | 17.8 | ||||||
| Frölich et al., 2011 | Placebo | 55 | 73.5 | 12 | — | ADAS‐Cog | |||
| 10 mg | 66 | 73.9 | — | ||||||
| Maher‐Edwards et al., 2011 | Placebo | 70 | 71.6 | 24 | 18.3 | ADAS‐Cog | — | CIBIC+ | |
| 10 mg | 63 | 71.1 | 19.2 | ||||||
| Haig et al., 2014 | Placebo | 62 | 70.3 | 12 | 18 | ADAS‐Cog | NPI | — | |
| 10 mg | 60 | 70.5 | 17.9 | ||||||
| Galantamine | Raskind et al., 2000 | Placebo | 62 | 75.3 | 24 | 19.2 | ADAS‐Cog | — | CIBIC+ |
| 24 mg | 66 | 75.9 | 19.5 | ||||||
| Tariot et al., 2000 | Placebo | 62 | 77.1 | 21 | 17.7 | ADAS‐Cog | NPI | — | |
| 16 mg | 62 | 76.3 | 17.8 | ||||||
| 24 mg | 67 | 77.7 | 17.7 | ||||||
| Wilcock et al., 2000 | Placebo | 61 | 72.7 | 24 | 19.3 | ADAS‐Cog | — | CIBIC+ | |
| 24 mg | 63 | 71.9 | 19.5 | ||||||
| Wilkinson and Murray 2001 | Placebo | 59 | 74.2 | 12 | 18.7 | ADAS‐Cog | — | CGIC | |
| 18 mg | 56 | 72.7 | 18.8 | ||||||
| 24 mg | 59 | 72.9 | 18.2 | ||||||
| Brodaty et al., 2005 | Placebo | 64 | 76.3 | 26 | 18.08 | ADAS‐Cog | NPI | CIBIC+ | |
| 16–24 mg | 64 | 76.5 | 17.8 | ||||||
| Rivastigmine | Corey‐Bloom et al., 1998 | Placebo | 58 | 74.8 | 26 | 20 | ADAS‐Cog | — | CIBIC+ |
| 6–12 mg | 68 | 73.8 | 19.62 | ||||||
| Forette et al., 1999 | Placebo | ‐ | 72.5 | 18 | 19.2 | ADAS‐Cog | — | CIBIC+ | |
| 6–12 mg | ‐ | 70.7 | 19.6 | ||||||
| Rösler et al., 1999 | Placebo | ‐ | — | 26 | — | ADAS‐Cog | — | CIBIC+ | |
| 6–12 mg | 59 | 72 | 19.9 | ||||||
| Feldman and Lane 2007 | Placebo | 60 | 71.7 | 26 | 18.8 | ADAS‐Cog | — | — | |
| 2–12 mg | 59 | 71.2 | 18.4 | ||||||
| Winblad et al., 2007 | Placebo | 67 | 73.9 | 24 | 16.4 | ADAS‐Cog | NPI | CGIC | |
| 3–12 mg | 66 | 72.8 | 16.4 | ||||||
| (P) 18 mg | 68 | 73.6 | 16.7 | ||||||
| Nakamura et al., 2011 | Placebo | 68 | 74.5 | 24 | 16.6 | ADAS‐Cog | — | CIBIC+ | |
| (P) 18 mg | 68 | 75.1 | 16.5 | ||||||
| Donepezil(D) versus Galantamine(G) versus Rivastigmine(R) | Cumbo and Ligori 2014 | (D) 10 mg | 55 | 78.4 | 52 | 16.3 | — | NPI | — |
| (G) 24 mg | 52 | 78.2 | 16.4 | ||||||
| (R) 12 mg | 54 | 78.3 | 16.2 | ||||||
| Donepezil versus Rivastigmine | Bullock et al., 2005 | (D) 5–10 mg | 69 | 75.8 | 104 | 15.13 | — | NPI | — |
| (R) 3–12 mg | 69 | 75.9 | 15.15 | ||||||
| Wilkinson et al., 2002 | (D) 5–10 mg | 54 | 74 | 12 | 21.5 | ADAS‐Cog | — | — | |
| (R) 3–12 mg | 64 | 74.9 | 20.7 | ||||||
ADAS‐cog, Alzheimer's Disease Assessment Scale, cognitive subscale; CIBIC+, Clinician's Interview‐Based Impression of Change with caregiver's input; CGIC, Clinical Global Impression of Change; (D), donepezil; (G), galantamine; MMSE, Mini‐Mental State Examination; NPI, Neuropsychiatric Inventory; (P), rivastigmine patch; (R), rivastigmine.
Table 2 summarises all I 2 values from preliminary meta‐analysis performed for each efficacy measure of each drug–placebo arm. Cognition and clinical global change gave a hint of heterogeneity among included trials (I 2 = 44.19% and 52.72%, respectively). For each drug–placebo arm, Rivastigmine‐placebo arm in cognition and clinical global change gave a hint of heterogeneity (I 2 = 63.79% and 51.30%, respectively). All funnel plots (data not shown) showed a fairly symmetric distribution, indicating no hint of publication bias.
Table 2.
I 2 values for heterogeneity analysis
| ADAS‐cog | NPI | CIBIC+/CGIC | |
|---|---|---|---|
| Overall | 44.19 | <0.00 | 52.72 |
| Donepezil | 30.47 | na* | <0.00 |
| Galantamine | <0.00 | <0.00 | <0.00 |
| Rivastigmine | 63.79 | <0.00 | 51.3 |
ADAS‐cog, Alzheimer's Disease Assessment Scale, cognitive subscale; CIBIC+, Clinician's Interview‐Based Impression of Change with caregiver's input; CGIC, Clinical Global Impression of Change.
Heterogeneity test was not conducted, because only a single NPI‐Placebo arm evaluated NPI.
Efficacy
Cognition
Across all NMAs, models were well converged (potential scale reduction factor = 1.00–1.03). Of the 21 studies included, 17 reported sufficient data on the efficacy for cognition, 7 for donepezil, 5 for galantamine and 6 for rivastigmine. Because a sign of heterogeneity was observed, a post‐hoc analysis was performed to further investigate the potential source of the heterogeneity. A linear regression analysis revealed that there was a significant time trend between the efficacy for cognition and the publication year in the placebo group (p = 0.017), but not in the treatment group (p = 0.606) (Figure 2A).
Figure 2.
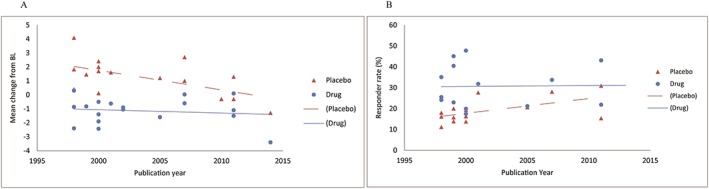
Association between efficacy ((A) ADAS‐Cog; (B) CIBIC+/CGIC) and publication year. ADAS‐Cog, Alzheimer's Disease Assessment Scale, cognitive subscale; CGIC, Clinical Global Impression of Change; CIBIC+, Clinician's Interview‐Based Impression of Change plus caregiver's input.
Figure 3A presents a network comparing 17 eligible studies as a dose‐based comparison. The analysis revealed that all drugs and dosages were associated with a significantly greater improvement than placebo (Figure 4A), and 24 mg galantamine showed a significantly greater improvement than rivastigmine patch (Figure 5A). The derived hierarchy across dosages was 24 mg galantamine > 15 mg galantamine > 12 mg rivastigmine > 5 mg donepezil > 10 mg donepezil > rivastigmine patch. NMA for a drug‐based comparison revealed that all drugs had a significantly greater improvement than placebo (Figure 4B), and the derived hierarchy was galantamine > rivastigmine > donepezil (Figure 5B).
Figure 3.
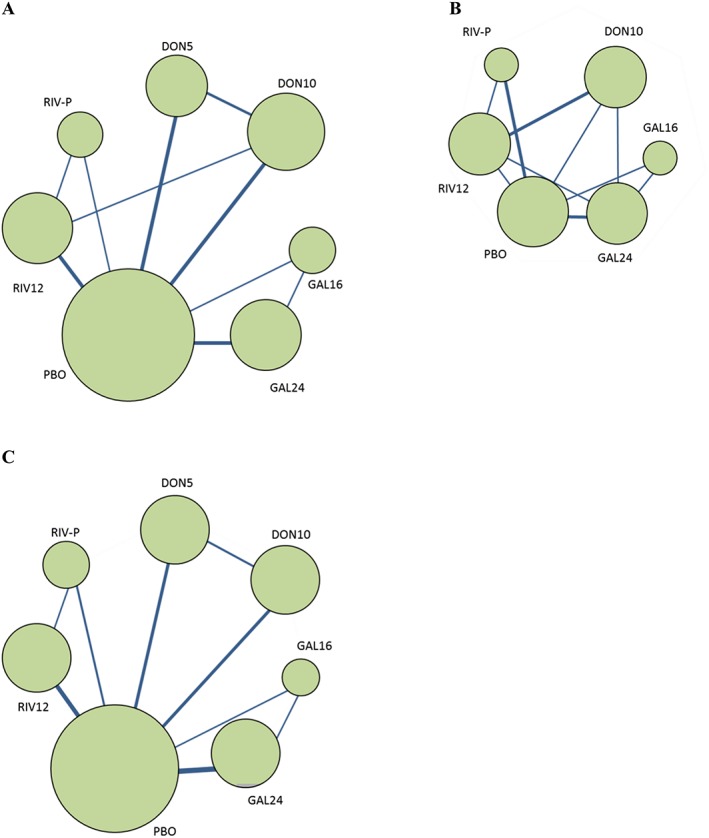
Network diagram of three efficacy measures ((A) ADAS‐Cog; (B) NPI; (C) CIBIC+/CGIC). The sizes of the nodes indicate the number of trials that evaluated the treatments. The nodes are linked by a line when the treatments were directly comparable, the thickness of which indicates the number of trials. ADAS‐Cog, Alzheimer's Disease Assessment Scale, cognitive subscale; CGIC, Clinical Global Impression of Change; CIBIC+, Clinician's Interview‐Based Impression of Change plus caregiver's input; DON5, 5 mg donepezil; DON10, 10 mg donepezil; GAL16, 16 mg galantamine; GAL24, 24 mg galantamine; NPI, Neuropsychiatric Inventory; PBO, placebo; RIV12, 12 mg rivastigmine;. RIV‐P, rivastigmine patch (9.5 cm2).
Figure 4.
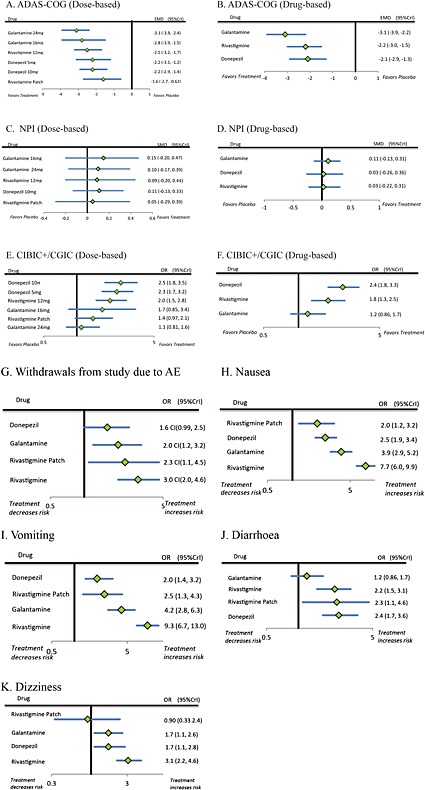
Forest plots of the efficacy and safety of ChEIs ((A) ADAS‐Cog [dose‐based]; (B) ADAS‐Cog [drug‐based]; (C) NPI [dose‐based]; (D) NPI [drug‐based]; (E) CIBIC+/CGIC [dose‐based]; (F) CIBIC+/CGIC [drug‐based]; (G) Withdrawals from study due to AE; (H) Nausea; (I) Vomiting; (J) Diarrhoea; (K) Dizziness) among patients with mild to moderate AD. ADAS‐Cog, Alzheimer's Disease Assessment Scale, cognitive subscale; AE, adverse event; CGIC, Clinical Global Impression of Change; ChEIs, cholinesterase inhibitors; CIBIC+, Clinician's Interview‐Based Impression of Change plus caregiver's input; CrI, credible interval; EMD, empirical mean difference of change on total ADAS‐Cog score between placebo and treatment; NPI, Neuropsychiatric Inventory; OR, odds ratio; SMD, standardized mean difference.
Figure 5.
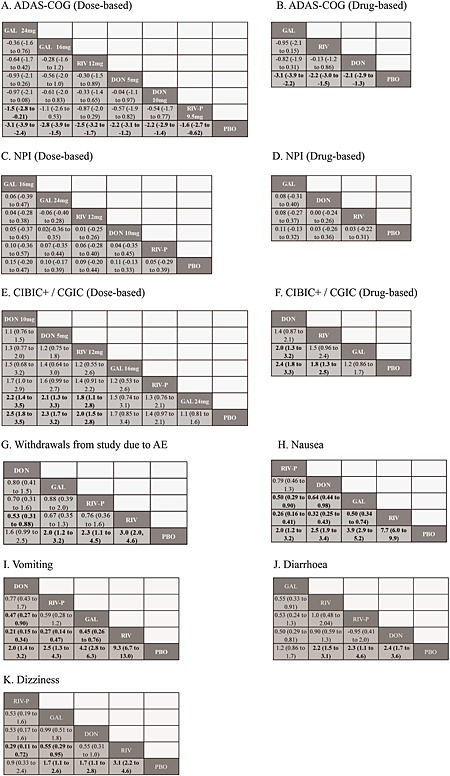
League tables of efficacy and safety of ChEIs ((A) ADAS‐Cog [dose‐based]; (B) ADAS‐Cog [drug‐based]; (C) NPI [dose‐based]; (D) NPI [drug‐based]; (E) CIBIC+/CGIC [dose‐based]; (F) CIBIC+/CGIC [drug‐based]; (G) Withdrawals from study due to AE; (H) Nausea; (I) Vomiting; (J) Diarrhoea; (K) Dizziness). Drugs are ordered based on effect size ranking derived from the network meta‐analyses. Placebo was listed at the bottom as a comparison. For tolerability, the odds ratio with 95% CrI lower than 1.0 indicates that column‐defining treatment decreases the risk of adverse event comparing with the row‐defining treatment. ADAS‐Cog, Alzheimer's Disease Assessment Scale, cognitive subscale; AE, adverse event; CGIC, Clinical Global Impression of Change; ChEIs, cholinesterase inhibitors; CIBIC+, Clinician's Interview‐Based Impression of Change plus caregiver's input; DON, donepezill; GAL, galantamine; NPI, Neuropsychiatric Inventory; PBO, placebo; RIV, rivastigmine; RIV‐P, rivastigmine patch.
Neuropsychiatric symptoms
Six studies reported sufficient data for the efficacy of neuropsychiatric symptoms, three for donepezil, three for galantamine and three for rivastigmine. Preliminary meta‐analysis did not indicate a significant heterogeneity (I 2 < 0.01%) across through the extracted data for neuropsychiatric symptoms. The funnel plot showed a fairly symmetric distribution, indicating no hint of publication bias.
Figure 3B presents a network comparing six eligible studies as a dose‐based comparison. The analysis revealed that none of the drugs and dosages had a significantly greater improvement than placebo (Figure 4C), and the derived hierarchy across dosages was 16 mg galantamine > 24 mg galantamine > 12 mg rivastigmine > 10 mg donepezil > rivastigmine patch (Figure 5C). NMA for a drug‐based analysis revealed that none of drugs had a significantly greater improvement than placebo (Figure 4D). The derived hierarchy was galantamine > donepezil > rivastigmine (Figure 5D).
Clinical global change
Fourteen studies reported sufficient data on the efficacy for clinical global change; five for donepezil, four for galantamine and five for rivastigmine. Because a sign of heterogeneity was observed, the same analysis performed in the analysis of cognition was applied. The responder rate was arc‐sin transformed to normalise the distribution. A significant time trend between the efficacy and the publication year in the placebo group (p = 0.044) was observed, but not in the treatment group (p = 0.950) (Figure 2B).
Figure 3C presents a network comparing 14 eligible studies as a dose‐based comparison. The analysis revealed that 5 mg donepezil, 10 mg donepezil and 12 mg rivastigmine had a significantly greater improvement than placebo and 24 mg galantamine (Figure 4E). The derived hierarchy was 10 mg donepezil > 5 mg donepezil > 12 mg rivastigmine > 16 mg galantamine > rivastigmine patch > 24 mg galantamine (Figure 5E). NMA for a drug‐based analysis revealed that all drugs except galantamine had a significantly greater improvement than placebo (Figure 4F), and the derived hierarchy was donepezil > rivastigmine > galantamine (Figure 5F).
Tolerability
Withdrawals from study due to adverse events
Nineteen studies provided data on withdrawals from study due to AEs. NMA revealed that all drugs except donepezil had a significantly higher risk of AEs than placebo (Figure 4G). The risk of AEs with donepezil was also significantly less than with rivastigmine (Figure 5G). The derived hierarchy across drugs was donepezil > galantamine > rivastigmine patch > rivastigmine.
Nausea
Twenty‐one studies provided data on nausea. NMA revealed that all drugs had a significantly higher risk than placebo. The risk associated with rivastigmine patch was significantly less than with galantamine (Figure 4H). The risk associated with donepezil was also significantly less than with galantamine and rivastigmine. The risk associated with galantamine was also significantly less than with rivastigmine. The derived hierarchy across drugs was rivastigmine patch > donepezil > galantamine > rivastigmine (Figure 5H).
Vomiting
Nineteen studies provided data on vomiting. NMA revealed that all drugs had a significantly higher risk than placebo. The risk for donepezil was significantly less than for galantamine and rivastigmine. The risk for rivastigmine patch was significantly less than for rivastigmine. The risk for galantamine was significantly less than for rivastigmine (Figure 4I). The derived hierarchy across drugs was donepezil > rivastigmine patch > galantamine > rivastigmine (Figure 5I).
Diarrhoea
Fourteen studies provided data on diarrhoea. NMA revealed that all drugs except galantamine had a significantly higher risk than placebo (Figure 4J). The risk associated with galantamine was significantly less than with rivastigmine and donepezil (Figure 5J). The derived hierarchy across drugs was galantamine > rivastigmine > rivastigmine patch > donepezil.
Dizziness
Fourteen studies provided data on dizziness. NMA revealed that all drugs except rivastigmine patch had a significantly higher risk than placebo (Figure 4K). The risk for rivastigmine patch was significantly less than for rivastigmine (Figure 5K). The risk for galantamine was also significantly less than for rivastigmine. The derived hierarchy across drugs was rivastigmine patch > galantamine > donepezil > rivastigmine.
Discussion
The present analysis demonstrated the modest beneficial effects of all ChEIs on cognition, but not on neuropsychiatric symptoms compared with placebo in patients with mild‐to‐moderate AD. Beneficial effects on clinical global change were found for donepezil and rivastigmine, but not for galantamine compared with placebo. Regarding hierarchies of efficacy for cognition, 24 mg galantamine showed superior efficacy to rivastigmine patch. In clinical global change, both 5 and 10 mg of donepezil and 12 mg rivastigmine showed superior efficacy to 24 mg galantamine. To our knowledge, this is the first NMA of ChEIs treatment in patients with AD in which compared using both direct and indirect comparisons of interventions across trials simultaneously. This should strengthen the findings from our analysis over previous meta‐analyses (Trinh et al., 2003; Birks, 2006; Hansen et al., 2008; Lockhart et al., 2011;Tan et al., 2014; Wang et al., 2014). The study quality of head‐to‐head trials might be questionable, because two out of three head‐to‐head trials were not double‐blinded. However, sensitivity analysis without these two trials demonstrated essentially the same results (data not shown).
Previous pair‐wise meta‐analyses and adjusted indirect comparisons revealed the modest efficacy of ChEIs on cognition, but there has been no evidence of any differences between them (Hansen et al., 2008). The present analysis also demonstrated essentially the same result. A slight difference from previous analyses is that 24 mg galantamine treatment was superior to rivastigmine patch with respect to efficacy for cognition. However, the number of trials of rivastigmine patch was limited, and this might affect the present results. Taken together, ChEIs have modest but robust efficacy on cognition; however, hierarchies of efficacy for cognition among them are still unclear. Although AD is a progressive disease and long‐term efficacy on cognition is clinically important, meta‐analyses, including this one, mainly selected placebo‐controlled RCTs demonstrating relatively short‐term efficacy on cognition. Because a long‐term placebo‐controlled study is ethically questionable, high‐quality long‐term head‐to‐head trials are essential to clarify comparative differences between ChEIs.
Previous meta‐analyses have consistently demonstrated that ChEIs have beneficial effects on neuropsychiatric symptoms (Trinh et al., 2003; Hansen et al., 2008; Lockhart et al., 2011; Tan et al., 2014; Wang et al., 2014). However, differences in efficacy between each ChEI were inconsistent. Furthermore, some systematic reviews suggested that pharmacological therapies are not particularly effective for management of neuropsychiatric symptoms of dementia (Sink et al., 2005, Rodda et al., 2009). Unlike previous meta‐analyses, the present analysis demonstrated that none of the currently available ChEIs had beneficial effects on neuropsychiatric symptoms compared with placebo in patients with mild‐to‐moderate AD. We excluded trials enrolling patients with severe AD. Conversely, recent meta‐analyses include severe AD (Hansen et al., 2008; Di Santo et al., 2013; Tan et al., 2014, Wang et al., 2014). Although a previous meta‐analysis Di Santo et al. (2013) documented no relationship between severity of dementia and efficacy of ChEIs on neuropsychiatric symptoms, patients with AD deteriorate progressively and may present with varying degrees of severity of disease, which may influence the results from pooled data. Indeed, a recent study demonstrated that NPI total score significantly increased with dementia stage in patients with AD (Hashimoto et al., 2015). Not only severity but also features of neuropsychiatric symptoms might be influenced by dementia stage. Another recent study demonstrated that mood symptoms would be developed in the early course of the disease; on the other hand, psychotic symptoms and agitation would characterise the middle‐to‐late stages of dementia in patients with AD (Lopez et al., 2003). These data suggest that patients with AD having neuropsychiatric symptoms would have varied clinical manifestations when severity of dementia and feature of symptoms are not optimally controlled. Such heterogeneity might contribute to incongruent results from both each trial and pooled analyses for pharmacotherapy. To verify efficacy of ChEIs on neuropsychiatric symptoms, further placebo‐controlled studies targeting individual psychiatric symptoms in targeting subjects in specific should be needed.
Regarding clinical global change, donepezil showed superior efficacy to galantamine. We speculate that differences in the year of study conduct might be associated with the hierarchy of clinical global change. Trials of donepezil and oral rivastigmine were conducted earlier than those of galantamine and rivastigmine patch. There was a significantly positive correlation between when the study was carried out and responder rates of clinical global change assigned to placebo. Namely, placebo effects on global clinical change increased over time. Meanwhile, no significant correlation between when the study was carried out and responder rate of clinical global change assigned to study drugs was noted. Therefore, increasing placebo effects may contribute in decreasing drug‐placebo differences in galantamine and rivastigmine patch studies. Increasing placebo effects over time were found in antipsychotic and antidepressant trials (Walsh et al., 2002; Rutherford et al., 2014). We assume that similar phenomena could occur in trials of ChEIs. Increasing placebo effects over time were observed not only for global clinical change but also for cognition. We believe that increasing placebo effects over time should be taken into account in the interpretation of the results of meta‐analyses. Further analysis is needed to clarify factors related to increasing placebo effects over time in anti‐dementia drug trials. Such effects should be also taken in to consideration forward anti‐dementia trials.
In the present analysis, we evaluated nausea, vomiting, diarrhoea, dizziness and withdrawals due to AEs as tolerability profiles of each ChEI, because these AEs are the most common ones in ChEIs trials. A previous meta‐analysis documented the frequency with which these events were reported, generally lowest for donepezil and highest for rivastigmine (Hansen 2008). It also documented that the relative risk of withdrawing for any reason or because of AEs was similar for donepezil compared with placebo, but the relative risk was statistically significantly greater for galantamine and rivastigmine compared with placebo. Regarding withdrawal due to AEs, our analysis demonstrated essentially the same result; all drugs, except donepezil, had a significantly higher risk than placebo. Other safety profiles such as nausea, vomiting and dizziness were concordant with safety profiles documented in the previous analysis. A difference from the previous analysis was that diarrhoea was less frequent with galantamine than other ChEIs. In this analysis, we separately analysed oral rivastigmine and rivastigmine patch; rivastigmine patch showed superior tolerability. A study (Winblad et al., 2007) of oral versus rivastigmine patch also demonstrated the superior tolerability of the patch. Differences in tolerability of rivastigmine could be explained by differences in formulation. Mortality was generally reported, and most of them have been identified as not related with drugs. The data also distributed fairly even across all drugs and placebo arms (data not shown).
Other limitations that were not discussed previously could restrict the study speculations. First, we examined the effect of publication year as a possible factor of heterogeneity of efficacy, but there might be other factors of heterogeneity that could lead to inconsistency in the NMA, such as the study duration and the quality of the study. While all but one of the studies included were short term, study duration was still a variable and might be a factor to consider. Second, the result on neuropsychiatric symptoms was derived based on relatively small number of trials. This might affect the wider intervals of estimated effect size. Third, our results for tolerability were only based on the studies that reported at least one of the efficacy outcomes, and thus, additional studies reporting safety information, but not eligible for inclusion in our analysis, may exist. Finally, some of our trials used flexible drug doses. Except for the rivastigmine trials, we attempted to estimate and categorise the dosages used in each trial with the information provided in their reports. Despite some limitations, we believe our results are the first attempt to quantitatively synthesise the hierarchy of the efficacy and tolerability of ChEI treatment for mild‐to‐moderate AD. Overall, the results indicate benefits in cognition and clinical global change but the efficacy on neuropsychiatric symptoms is questionable in patients with mild‐to‐moderate AD. Our results might suggest a possible perspective for anti‐dementia drug trials, such as increasing placebo effects over time and heterogeneity of neuropsychiatric symptoms in AD.
Transparency
Contributors
T. O. designed the study. HK designed the analysis. H. K., T. O. and R. N. literature searched and abstracted the data. H. K. and R. N. performed the statistical analysis. All authors interpreted the data and drafted the manuscript. All authors reviewed and approved the manuscript publication.
Competing interests
All authors have disclosed that they are full‐time employees of Janssen Pharmaceutical K.K. of Johnson & Johnson in Japan.
Declaration of funding
This study was funded by Janssen Pharmaceutical K.K. of Johnson & Johnson in Japan.
Financial declaration
All authors have disclosed that they are full‐time employees of Janssen Pharmaceutical K.K. of Johnson & Johnson in Japan.
Ethics approval
This study does not require ethics approval and consent form each participant, because (1) the present meta‐analysis does not directly involve human participants as stated in the MRC guidance and (2) the studies used in the present have previously satisfied regulatory requirements and been peer reviewed.
Previous presentation
This study has been presented at the 12th International Conference on Alzheimer's & Parkinson's diseases, March 19, 2015, Nice, France.
Key points.
Comparative evidence for efficacy and safety of cholinesterase inhibitors (ChEIs) in patients with Alzheimer's disease (AD) is still sparse.
We conducted the network meta‐analysis (NMA) to provide comparative evidences and hierarchies for efficacy between three cholinesterase inhibitors (ChEIs) in patients with mild‐to‐moderate Alzheimer's disease (AD).
The NMA showed significant, relatively similar, efficacy on cognition in mild‐to‐moderate AD patients, and all treatments except galantamine were significantly more efficacious than placebo in global change. However, efficacy on neuropsychiatric symptoms in patients with mild‐to‐moderate AD is questionable.
The safety profiles of ChEIs were similar across three drugs.
Kobayashi, H. , Ohnishi, T. , Nakagawa, R. , and Yoshizawa, K. (2016) The comparative efficacy and safety of cholinesterase inhibitors in patients with mild‐to‐moderate Alzheimer's disease: a Bayesian network meta‐analysis. Int J Geriatr Psychiatry, 31: 892–904. doi: 10.1002/gps.4405.
References
- Birks J. 2006. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev 25 CD005593: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodaty H, Corey‐Bloom J, Potocnik FC, et al. 2005. Galantamine prolonged‐release formulation in the treatment of mild to moderate Alzheimer's disease. Dement Geriatr Cogn Disord 20: 120–132. [DOI] [PubMed] [Google Scholar]
- Brooks SP, Gelman A. 1997. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat 7: 434–455. [Google Scholar]
- Bullock R, Touchon J, Bergman H, et al. 2005. Rivastigmine and donepezil treatment in moderate to moderately‐severe Alzheimer's disease over a 2‐year period. Curr Med Res Opin 21: 1317–1327. [DOI] [PubMed] [Google Scholar]
- Burns A, Rossor M, Hecker J, et al. 1999. The effects of donepezil in Alzheimer's disease—results from a multinational trial. Dement Geriatr Cogn Disord 10: 237–244. [DOI] [PubMed] [Google Scholar]
- Corey‐Bloom J, Anand R, Veach J. 1998. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer's disease. Int J Geriatr Psychopharmacol 1: 55–65. [Google Scholar]
- Cumbo E, Ligori LD. 2014. Differential effects of current specific treatments on behavioral and psychological symptoms in patients with Alzheimer's disease: a 12‐month, randomized, open‐label trial. J Alzheimers Dis 39: 477–485. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, et al. 1994. The Neuropsychiatric Inventory comprehensive assessment of psychopathology in dementia. Neurology 44: 2308–2314. [DOI] [PubMed] [Google Scholar]
- Di Santo SG, Prinelli F, Adorni F, Caltagirone C, Musicco M. 2013. A meta‐analysis of the efficacy of donepezil, rivastigmine, galantamine, and memantine in relation to severity of Alzheimer's disease. J Alzheimers Dis 35: 349–361. [DOI] [PubMed] [Google Scholar]
- Feldman HH, Lane R. 2007. Rivastigmine: a placebo controlled trial of twice daily and three times daily regimens in patients with Alzheimer's disease. J Neurol Neurosurg Psychiatry 78: 1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forette F, Anand R, Gharabawi G. 1999. A phase II study in patients with Alzheimer's disease to assess the preliminary efficacy and maximum tolerated dose of rivastigmine (Exelon). Eur J Neurol 6: 423–429. [DOI] [PubMed] [Google Scholar]
- Frölich L, Ashwood T, Nilsson J, et al. 2011. Effects of AZD3480 on cognition in patients with mild‐to‐moderate Alzheimer's disease: a phase IIb dose‐finding study. J Alzheimers Dis 24: 363–374. [DOI] [PubMed] [Google Scholar]
- Haig GM, Pritchett Y, Meier A, et al. 2014. A randomized study of H3 antagonist ABT‐288 in mild‐to‐moderate Alzheimer's dementia. J Alzheimers Dis 42: 959–971. [DOI] [PubMed] [Google Scholar]
- Hansen RA, Gartlehner G, Webb AP, et al. 2008. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer's disease: a systematic review and meta‐analysis. Clin Interv Aging 3: 211–225. [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Yatabe Y, Ishikawa T, et al. 2015. Relationship between dementia severity and behavioral and psychological symptoms of dementia in dementia with Lewy bodies and Alzheimer's disease patients. Dement Geriatr Cogn Disord Extra 5: 244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma A, Takeda M, Imai Y, et al. 2000. Clinical efficacy and safety of donepezil on cognitive and global function in patients with Alzheimer's disease: a 24‐week, multicentre, double‐blind, placebo‐controlled study in Japan. Dement Geriatr Cogn Disord 11: 299–313. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Knapp MJ, Gracon SI, et al. 1994. The Clinician Interview‐Based Impression (CIBI): A clinician's global change rating scale in Alzheimer's disease. Neurology 44: 2315–2321. [DOI] [PubMed] [Google Scholar]
- Lockhart IA, Orme ME, Mitchell SA. 2011. The efficacy of licensed‐indication use of donepezil and memantine monotherapies for treating behavioural and psychological symptoms of dementia in patients with Alzheimer's disease: systematic review and meta‐analysis. Dement Geriatr Cogn Dis Extra 1: 212–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Sweet RA, et al. 2003. Psychiatric symptoms vary with the severity of dementia in probable Alzheimer's disease. J Neuropsychiatry Clin Neurosci 15: 346–353. [DOI] [PubMed] [Google Scholar]
- Maher‐Edwards G, Dixon R, Hunter J, et al. 2011. SB‐742457 and donepezil in Alzheimer's disease: a randomized, placebo‐controlled study. Int J Geriatr Psychiatry 26: 536–544. [DOI] [PubMed] [Google Scholar]
- Mills EJ, Thorlund K, Ioannidis JP. 2013. Demystifying trial networks and network meta‐analysis. BMJ 346: f2914. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Imai Y, Shigeta M, et al. 2011. A 24‐week, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy, safety and tolerability of the rivastigmine patch in Japanese patients with Alzheimer's disease. Dementd Geriatr Cogn Dis Extra 1: 163–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Clinical Excellence (NICE) . 2011. Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer's disease. NICE Technology Appraisal Guidance [TA217]
- Rabins PV, Blacker D, Rovner BW, et al. 2007. American Psychiatric Association practice guideline for the treatment of patients with Alzheimer's disease and other dementias. Second edition. Am J Psychiatry 164: 5–56. [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Wessel T, et al. 2000. Galantamine in AD: A 6‐month randomized, placebo‐controlled trial with a 6‐month extension. Neurology 54: 2261–2268. [DOI] [PubMed] [Google Scholar]
- Rodda J, Morgan S, Walker Z. 2009. Are cholinesterase inhibitors effective in the management of the behavioral and psychological symptoms of dementia in Alzheimer's disease? A systematic review of randomized, placebo‐controlled, trials of donepezil, rivastigmine and galantamine. Int Psychogeriatr 21: 813–824. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Farlow MR, Doody RS, et al. 1998a. A 24‐week, double‐blind, placebo‐controlled trial of donepezil in patients with Alzheimer's disease. Neurology 50: 136–145. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Doody RS, Mohs RC, et al. 1998b. Donepezil improves cognition and global function in Alzheimer's disease: a 15‐week, double‐blind, placebo‐controlled study. Arch Intern Med 158: 1021–1031. [DOI] [PubMed] [Google Scholar]
- Rosen WG, Mohs RC, Davis KL. 1984. A new rating scale for Alzheimer's disease. Am J Psychiatry 141: 1356–1364. [DOI] [PubMed] [Google Scholar]
- Rösler M, Anand R, Cicin‐Sain A, et al. 1999. Efficacy and safety of rivastigmine in patients with Alzheimer's disease: international randomised controlled trial. BMJ 318: 633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford BR, Pott E, Tandler JM, et al. 2014. Placebo response in antipsychotic clinical trials: a meta‐analysis. JAMA Psychiatry 71: 1409–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanti G, Ades AE, Ioannidis JP. 2014. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. J Clin Epidemiol 64: 163–171. [DOI] [PubMed] [Google Scholar]
- Salanti G, Higgins JP, Ades AE, et al. 2008. Evaluation of networks of randomized trials. Stat Methods Med Res 17: 279–301. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Olin JT, Doody RS, et al. 1997. Validity and reliability of the Alzheimer's Disease Cooperative Study—Clinical Global Impression of Change. Alzheimer Dis Assoc Disord 11(Suppl 2): S22–S32. [DOI] [PubMed] [Google Scholar]
- Sink KM, Holden KF, Yaffe K. 2005. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 293(5): 596–608. [DOI] [PubMed] [Google Scholar]
- Tan C‐C, Yua J‐T, Wang H‐F, et al. 2014. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer's disease: a systematic review and meta‐analysis. J Alzheimers Dis 41: 615–631. [DOI] [PubMed] [Google Scholar]
- Tariot PN, Solomon PR, Morris JC, et al. 2000. A 5‐month, randomized, placebo‐controlled trial of galantamine in AD. Neurology2000 54: 2269–2276. [DOI] [PubMed] [Google Scholar]
- Trinh NH, Hoblyn J, Mohanty S, et al. 2003. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer's disease: a meta‐analysis. JAMA 289: 210–216. [DOI] [PubMed] [Google Scholar]
- van Valkenhoef G, Lu G, de Brock B, et al. 2012. Automating network meta‐analysis. Res Synth Meth 3: 285–299. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. 2010. Conducting meta‐analyses in R with the metaphor package. J Stat Softw 36: 1–48. [Google Scholar]
- Walsh BT, Seidman SN, Sysko R, et al. 2002. Placebo response in studies of major depression: variable, substantial, and growing. JAMA 287: 1840–1847. [DOI] [PubMed] [Google Scholar]
- Wang J, Yu JT, Wang HF, et al. 2014. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer's disease: a systematic review and meta‐analysis. J Neurol Neurosurg Psychiatry 86: 101–109. [DOI] [PubMed] [Google Scholar]
- Wilcock GK, Lilienfeld S, Gaens E. 2000. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer's disease: multicentre randomised controlled trial. BMJ 321: 1445–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D, Murray J. 2001. Galantamine: a randomized, double‐blind, dose comparison in patients with Alzheimer's disease. Int J Geriatr Psychiatry 16: 852–857. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Passmore AP, Bullock R, et al. 2002. A multinational, randomised, 12‐week, comparative study of donepezil and rivastigmine in patients with mild to moderate Alzheimer's disease. Int J Clin Pract 56: 441–446. [PubMed] [Google Scholar]
- Winblad B, Cummings J, Andreasen N, et al. 2007. A six‐month double‐blind, randomized, placebo‐controlled study of a transdermal patch in Alzheimer's disease–rivastigmine patch versus capsule. Int J Geriatr Psychiatry 22: 456–467. [DOI] [PubMed] [Google Scholar]


