Abstract
Objective
The aim of this study was to evaluate the angiogenicity of a combination of BM‐EPCs and BM‐MSCs in vitro in the presence of SP and its working mechanism.
Methods
BM‐MSCs and BM‐EPCs were cocultured with or without SP. ELISA and RT‐PCR were performed to detect angiogenic factors such as VEGF and PDGF‐BB. N‐cadherin was detected by Western blot analysis. The tubular network‐forming ability was evaluated by a Matrigel tube‐forming assay.
Results
BM‐EPCs coculture with BM‐MSCs strongly stimulated the recruitment of BM‐MSCs onto the BM‐EPC‐generated endothelial tubular network. Upon SP treatment, endothelial branching point, tubule length, and tubular recruitment of BM‐MSCs were further increased and stabilized. The coculture of BM‐EPCs and BM‐MSCs synergistically stimulated expression of VEGF, VEGF receptor, N‐cadherin, and PDGF‐BB, all of which were further enhanced by SP treatment. Blockade of PDGF‐BB by its functional blocking antibodies markedly reduced the BM‐MSC incorporation into the endothelial tubules. SP‐pretreated BM‐MSCs were preferentially incorporated into the preformed BM‐EPC tubular network.
Conclusions
BM‐EPCs along with SP promote the pericyte‐like coverage of BM‐MSCs on endothelial tubules possibly through the induction of PDGF‐BB.
Keywords: EPC, MSC, PDGF‐BB, pericytes, substance P
Abbreviations
- Ab
antibody
- BBB
blood‐brain barrier
- BM‐EPC
bone marrow‐derived endothelial precursor cells
- BM‐MSC
bone marrow‐derived mesenchymal stem cells
- CFU
colony‐forming units
- CNS
central nervous system
- ELISA
enzyme‐linked immunosorbent assay
- NK‐1R
neurokinin‐1 receptor
- PDGF
platelet‐derived growth factor
- RP
receptor antagonist
- SP
substance P
- VEGF
vascular endothelial growth factor
- α‐SMA
α‐smooth muscle actin
1. INTRODUCTION
Brain injury, such as in the case of ischemic stroke, is accompanied by BBB damage, leading to increased vascular permeability, edema, and inflammatory cells infiltration, leading to a cascade of inflammatory secondary tissue damage.1 Many stem cell therapies using either MSCs or EPCs have been attempted in animal stroke models as well as in clinical trials to restore a functional BBB and interrupt damage in the early stages of the stroke, but no successful outcome has been reported.2 Considering that the BBB is mainly composed of inner tubular endothelial cells, outward‐encircling pericytes, and astrocyte end feet, more than one cell type may be required as reparative stem or precursor cells for the repair of the BBB following a stroke.
In the BBB of the CNS, the inner endothelial tube is tightly encircled by pericytes, which share a basement membrane with endothelial cells and form direct synaptic‐like peg‐socket focal contacts between two cell types through N‐cadherin and connexins.3, 4 Thereby, pericytes maintain endothelial tight junctions and regulate the capillary diameter and cerebrovascular flow under physiological conditions. Notably, close cooperation between brain endothelial cells and pericytes in the BBB has been reported in platelet‐derived growth factor (PDGF)‐B or PDGFR beta null mice, which results in a complete loss of pericytes, causing the rupture of CNS microvessels, micro‐aneurysms, and embryonic lethality.5, 6 Furthermore, after brain injury, pericytes seem to be more vulnerable than any other cell type in the BBB.6, 7, 8, 9 A reparative stem cell therapy targeting the replacement and recruitment of both pericytes and endothelial cells is in high demand.
Bone marrow is a reservoir of autologous stem cells such as MSCs, EPCs, and hematopoietic stem cells, which can be harvested from bone marrow aspirates and peripheral blood by the specific stem cell mobilizer.10 EPCs have been utilized for neovascularization after transplantation post‐CNS insult and myocardial infarction.11, 12 BM‐MSCs have been used in the treatment of a variety of tissue injuries and diseases because of their capacity to regulate severe immune and inflammatory responses by secreting protective trophic factors.13, 14 It is also expected that BM‐MSCs may play a similar role as pericytes in the blood vessel, based on their similar marker expression patterns and phenotype.15, 16 However, dual cell therapy with both BM‐EPCs and BM‐MSCs aimed toward tight blood vessel formation and investigation into their cooperated roles has not been widely studied.
In this study, we first evaluated whether EPCs and MSCs derived from the bone marrow can concertedly work in the reconstruction of a pericyte‐covered vascular network, using a Matrigel tubular network assay. Because we are targeting the BBB, which is regarded as the most pericyte‐dense area in the body with an endothelial‐pericyte ratio between 1:1 and 3:1, in this study, BM‐EPCs and BM‐MSCs were applied at a ratio of 2:1 by considering of the physiological condition as well as the capability of BM‐MSCs on immune modulation.3 We also evaluated whether the SP/NK‐1R signaling pathway, known to be expressed and to activate cell proliferation and migration in both cell types,17, 18 is involved in the pericyte coverage of the endothelia. Finally, the important regulators of the endothelial tubular network as well as pericyte‐like coverage of the endothelia were investigated. Using this in vitro coculture system, the feasibility of a dual stem cell therapy in BBB reconstruction after transplantation following ischemic stroke can be pretested.
2. MATERIALS AND METHODS
2.1. Primary culture and identification of rat BM‐EPCs and BM‐MSCs
BM‐EPCs and BM‐MSCs were obtained from the femurs of adult LEW/Crlj rats (Orientbio, Sungnam, Korea), as described previously, with modifications.19 Briefly, the single cells were washed several times and cultured in MSC growth medium (Lonza Inc., Walkersville, MD, USA) for BM‐MSC culture, or in endothelial growth medium‐2MV (Lonza Inc.) on a dish precoated with 1 μg/mL rat fibronectin (Sigma‐Aldrich, St. Louis, MO, USA) for BM‐EPC culture. After 24 hours of culture at 37°C in a humidified atmosphere of 5% CO₂, the unattached cells were removed. The medium was changed every 3 days, and the cells were subcultured until they reached 80% confluency, by seeding at a density of 3 × 105 cells per 100 mm2 on culture dishes. For MSC and EPC identification, marker expression and CFUs were assessed as described previously.10 Briefly, MSCs were identified by CD29+, CD106+, NK‐1R+, α‐SMA+, CD45−, and CD34− markers using FACS analysis and immunofluorescence staining, and EPCs were identified by CD34+, CD31+, UEA lectin binding+, and α‐SMA− markers. Approximately 90% of the cells satisfied these specific marker expression profiles. Cells from passage 2 to passage 4 were used in this study (Figures S1 and S2).
2.2. Matrigel tube formation
A Matrigel tube formation assay was performed for the in vitro angiogenesis assessment.20 A 48‐well plate was treated with 150 μL of Matrigel (BD Biosciences, San Jose, CA, USA) for 30 minutes at 37°C. For optimal simulation of the cell proportions in the BBB in vivo, BM‐EPCs and BM‐MSCs were plated at a ratio of 2:1 with a density of 2 × 10⁴ and 1 × 10⁴ cells per well, respectively. To track the behavior of the inoculated cells, BM‐EPCs and BM‐MSCs were labeled with PKH26 red and PKH26 green (Sigma‐Aldrich), respectively. To analyze the effect of SP and PDGF‐BB, cells were pretreated with SP (Merck Millipore, Darmstadt, Germany) or PDGF‐BB functional blocking Ab (Abcam, Cambridge, MA, USA) at concentrations of 100 nmol/L and 20 μg/mL for 3 hours before Matrigel assay or concurrently treated at the time of coculture. The cells and Matrigel were fixed with 3.7% formaldehyde, and the tube length, bifurcation points, and numbers of the tube‐incorporated BM‐MSCs were measured under a stereomicroscope (Olympus, Tokyo, Japan) or fluorescence microscope (Leica, Wetzlar, Germany). All the experiments were performed as triplicate independent experiments, and the statistics were analyzed using NIH image J software.21
2.3. Enzyme‐linked immunosorbent assay (ELISA)
The culture media were collected from equal cell quantities of BM‐MSCs, BM‐EPCs, or BM‐EPC/BM‐MSC cocultures at a ratio of 2:1. VEGF and PDGF‐BB levels were measured using VEGF and PDGF‐BB ELISA kits (R&D system, Inc., Minneapolis, MN, USA). Data from three independent experiments were statistically analyzed.
2.4. RT‐PCR analysis
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. The template cDNAs were synthesized using a Prime‐script RT‐PCR kit (Takara Bio. Inc., Shiga, Japan) with 1 μg of total RNA and the following primer sequences for RT‐PCR: rat VEGF (sense: TGT ACA AGT GCC AGC TAA GGA A; antisense: CAC ACG TAG TTT GCT GGA CAA G), rat VEGFR (sense: CCT TAG ATA GCC CGG AAC GC; antisense: GCC ACA CTC AGT CAC CAA CA, USA), and rat β‐actin (sense: CTT CTG CAT CCT GTC AGC GAT GC; antisense: AGA AGA GCT ATGA GCT GCC TGA CG).
2.5. Western blotting
The cell lysates were prepared using a Cell Lysis Kit (Cell Signaling, Danvers, Massachusetts, USA) according to the manufacturer's instructions, subjected to separation by 10% SDS‐PAGE, and transferred to nitrocellulose membranes. The blots were blocked with 5% skim milk in PBS. Western blots were then probed with antibodies recognizing N‐cadherin (1:300; Abcam). Signals were detected using a Chemilluminator (Vilber Lourmat, Marne, France).
3. RESULTS
3.1. BM‐EPCs exhibit inherent endothelial tube‐forming capacity on the Matrigel but BM‐MSCs do not
BM‐EPCs and BM‐MSCs are expected to be candidate stem and precursor cells for dual cell therapeutics for the treatment of various types of vascular damage. To test this in vitro, BM‐EPCs or BM‐MSCs were plated on Matrigel, and their tubular formations were examined (Figure 1). BM‐MSCs did not form a tubular network after 3‐hour incubation (Figure 1A) and rather formed pellet‐like structures after longer incubation times (data not shown). However, BM‐EPCs showed a rather elongated morphology at 1 hour on the Matrigel plate, tubule‐like connections at 1.5 hours, and a mature tubular network at 3 hours (Figure 1A).
Figure 1.
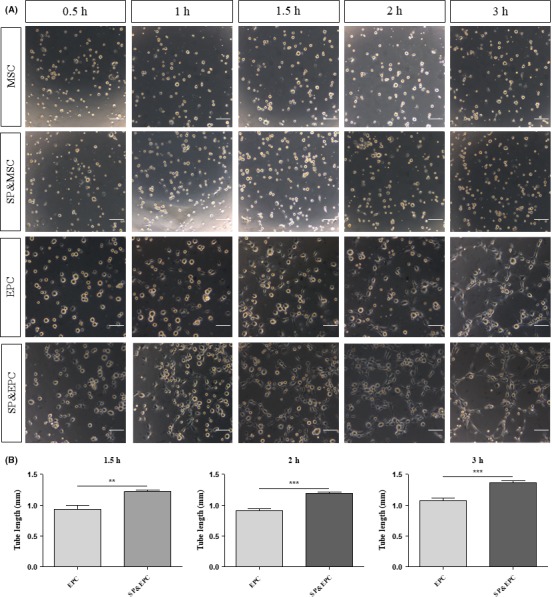
Tubular network‐forming capacity of BM‐MSCs and BM‐EPCs with or without SP treatment. (A) BM‐MSCs or BM‐EPCs were seeded at 2 × 104 cells/well on Matrigel‐coated wells in a 48‐well plate with or without 100 nmol/L SP. Phase‐contrast images were taken at indicated time points under a stereomicroscope. Scale bar = 50 μm. (B) The quantification of tube length in the vascular tube structure was measured using Image J. Data are presented as mean ± SEM (**P < .005, ***P < .0005, student's t test)
Both BM‐EPCs and BM‐MSCs express SP receptor NK‐1R,22 and SP is known to mobilize BM‐MSCs from the bone marrow into the blood and aid in their recruitment to the injured tissue.19 We explored whether SP treatment can affect the tube‐forming capacity of the MSCs and/or EPCs. The SP treatment did not affect MSC behavior but resulted in an approximately 27% increase in EPC tube‐forming capacity (Figure 1A,B).
3.2. BM‐MSCs in coculture with BM‐EPCs stimulate recruitment to the tubular network formed by EPCs, which is further enhanced by SP treatment
Only BM‐EPCs showed an inherent capacity to reform a tubular network on Matrigel. However, the coculture of BM‐MSCs and BM‐EPCs may stimulate the incorporation of BM‐MSCs onto the outer surface of endothelial tubules as pericyte‐like cells. As this occurs in vitro, a similar outcome may be expected after cell transplantation to the vascular injury. When BM‐MSCs and BM‐EPCs were cocultured on Matrigel after the specific fluorescence labeling, PKH‐red‐labeled BM‐MSCs were no longer randomly distributed but were recruited to the PKH‐green‐labeled endothelial tubular network (Figure 2A,B). Upon SP cotreatment, branching points increased by 46% (Figure 2D), the tubule length of the endothelial tubular network increased by 30.6% (Figure 2E), and tubular recruitment of BM‐MSCs increased by 39% (Figure 2C‐F), which also resulted in a more elongated morphology of cells lining the surface of the tubules (Figure 2B). However, this tubular coverage with BM‐MSCs under the coculture decomposed following longer incubation up to 18 hours (Figure 2G). In contrast, in the SP‐treated coculture, the tubular coverage with BM‐MSCs was well maintained and more tightly incorporated for a longer period. Thus, SP treatment may stabilize and reinforce BM‐MSC incorporation into the endothelial tubular network.
Figure 2.
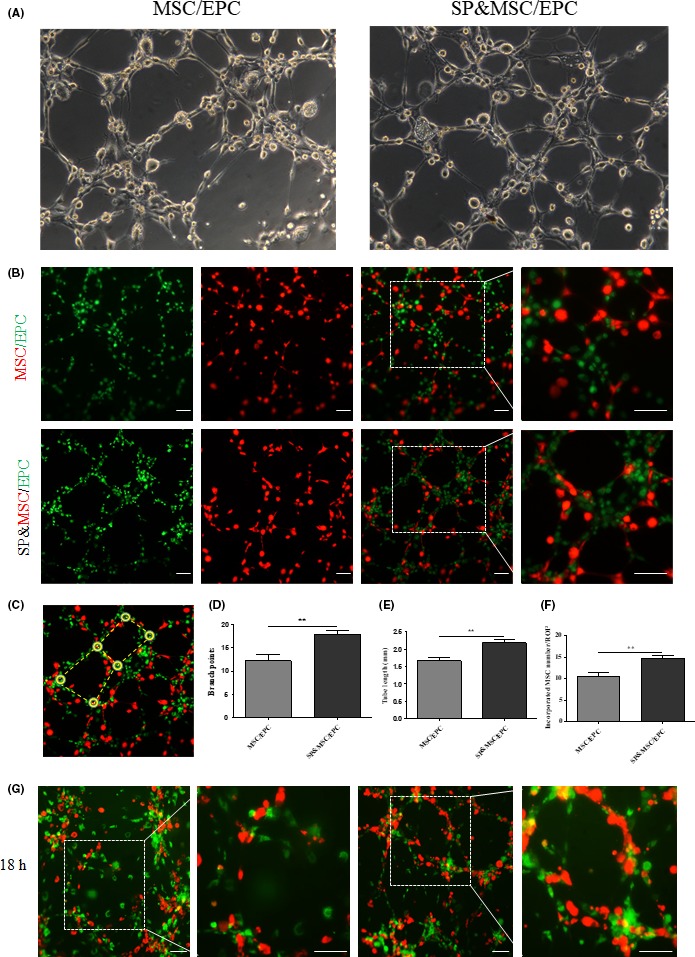
Effect of BM‐MSC and BM‐EPC coculture with or without SP treatment on the pericyte‐like incorporation and tubular network. (A) BM‐EPCs and BM‐MSCs at a density of 3 × 104 cells/well at a ratio of 2:1 were plated for the coculture on Matrigel. At 3 h, a tubular structure was clearly observed by stereomicroscope. The coculture resulted in most of the BM‐MSCs being incorporated into the tubular network. SP treatment resulted in a longer and better organized tube. (B‐G) The cells were labeled with PKH‐red for BM‐MSCs and PKH‐green for BM‐EPCs to discriminate between the two cell types. The tubular structure was imaged by a fluorescence microscope at 3 h (B‐F) and 18 h (G) postseeding. (C) The solid round circle and dash indicate branch points and tube length, respectively, for quantification analysis. The branch points (D), tube length (E), and tubular‐associated PKH‐red BM‐MSCs (F) are presented as mean ± SEM (**P < .005, student's t test). Scale bar = 50 μm
3.3. BM‐MSCs and BM‐EPCs in coculture synergistically stimulate expression of VEGF, VEGF receptor, PDGF‐BB, and N‐cadherin
BM‐MSC and BM‐EPC cocultures with SP exhibited enhanced tubular network formation as well as pericyte‐like coverage of the tubules in Matrigel. To investigate the underlying mechanism of this synergistic effect, the expression of VEGF, VEGF receptor, PDGF‐BB, and N‐cadherin, all of which are important for angiogenesis and pericyte coverage on the endothelia,5 was examined. Importantly, the coculture itself exhibited increased VEGF secretion in the medium, which was further enhanced by SP treatment (Figure 3A). This synergistic VEGF upregulation was sustained up to 3 days in the coculture, based on RT‐PCR analysis (Figure 3B‐D). Furthermore, VEGF receptor expression was also increased in the coculture (Figure 3B,D).
Figure 3.
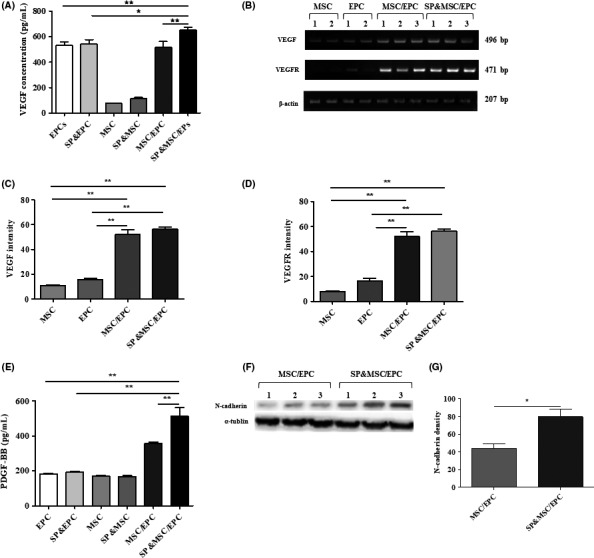
Analysis of VEGF, VEGF receptor, PDGF‐BB, and N‐cadherin. (A) ELISA of VEGF. The cells were plated at a density of 3 × 105 cells/well for individual cell culture but were plated at a ratio of 2:1 in the coculture. Cells were treated with 100 nmol/L SP at the time of cell plating. VEGF was measured from the culture at day 1. Data represent the mean ± SEM (*P < .05, **P < .005, student's t test) (B) RT‐PCR analysis of VEGF and VEGFR at day 3 culture. The quantitative analysis of VEGF (C) and VEGFR (D) expressions are presented as the mean ± SEM (*P < .05, **P < .005, student's t test). (E) ELISA of PDGF‐BB at day 1 culture. PDGF‐BB expression is presented as the mean ± SEM (**P < .005, student's t test) (F,G) Western blot analysis of N‐cadherin expression at day 3 coculture with or without SP treatment. The intensity of α‐tubulin was used as an internal control. Densitometry of N‐cadherin band is presented as the mean ± SEM (*P < .05, student's t test)
PDGF‐BB is one of the important factors involved in pericyte recruitment and attachment on the endothelia, possibly through stimulation of N‐cadherin‐mediated homophilic interactions. PDGF‐BB secretion was also increased in the coculture and was further stimulated by SP treatment (Figure 3E). Similarly, N‐cadherin expression in the coculture was also increased by 81.5% following SP treatment (Figure 3F,G). Thus, enhanced expression of VEGF and/or PDGF‐BB in the coculture and their further increase by SP treatment may be responsible for the synergistic effect of the BM‐EPCs and BM‐MSCs on the vascular tubular network.
3.4. PDGF‐BB blocking reduces BM‐MSC coverage of the endothelial tubules
To further confirm the role of PDGF‐BB in the pericyte‐like cell recruitment of BM‐MSCs on the endothelia, PDGF‐BB expression was examined in the coculture on Matrigel (Figure 4A). The coculture strongly induced PDGF‐BB secretion at 3 hours after cell plating, which was further stimulated by the SP treatment. However, NK‐1 receptor blocker reduced but did not eliminate the expression of PDGF‐BB under coculture conditions, suggesting that SP‐NK‐1R may not be the only signaling pathway involved. To test the importance of PDGF‐BB in the BM‐MSC recruitment and tubular network, functional blocking antibodies against PDGF‐BB were applied in the coculture on Matrigel (Figure 4B,C). Blocking PDGF‐BB significantly reduced the vascular tubular network as well as the endothelia‐recruited BM‐MSCs by 59.03 ± 1.718% and 54.7 ± 4.39%, respectively, in the presence and absence of SP (Figure 4C).
Figure 4.
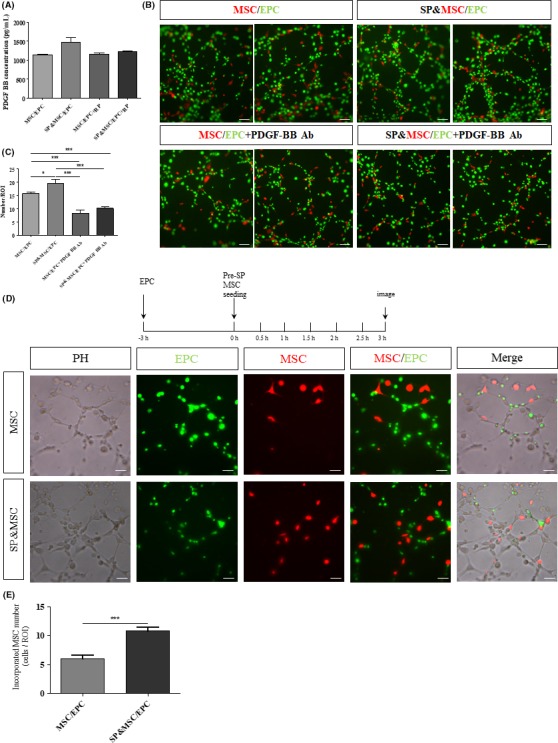
Inhibition of SP‐mediated PDGF‐BB induction by its receptor blockade and inhibition of tubular incorporation of BM‐MSCs by PDGF‐BB functional blocking antibodies. (A) ELISA analysis of PDGF‐BB in the coculture on Matrigel at 3 h. SP‐stimulated PDGF‐BB secretion was blocked by NK‐1 RP. Data are presented as the mean ± SEM. (B,C) The cocultures of PKH‐red‐labeled BM‐MSCs and PKH‐green‐labeled BM‐EPCs were treated with SP and/or PDGF‐BB functional blocking Ab. At 3 h, the tubular network and tubular incorporation of BM‐MSCs were strongly diminished. The quantification analyses are presented as the mean ± SEM (*P < .05, ***P < .0005, student's t test). (D,E) Preferential tubular incorporation of SP‐pretreated BM‐MSCs. PKH‐green‐labeled BM‐EPCs were seeded on Matrigel at a density of 2 × 104 at 3 h in advance to reconstruct an endothelial tubular network. To test the effect of SP on BM‐MSC tubular incorporation capacity, PKH‐red‐labeled BM‐MSCs at a density of 2 × 103 cells/well with or without SP pretreatment for 3 h were plated. The quantitative analysis of tubular‐incorporated BM‐MSCs is presented as the mean ± SEM (***P < .0005, student's t test). Scale bar = 50 μm
Under ischemic conditions, such as a stroke, pericytes have been shown to be more vulnerable than endothelial cells. Early loss of pericytes leads to hyperpermeability, which results in tissue edema and inflammatory cell infiltration.23 To test whether BM‐MSCs can be incorporated into the preformed vessels lacking in pericytes, PKH‐green‐labeled EPCs alone were seeded on Matrigel to form a tubular network 3 hours in advance, and PKH‐red‐labeled MSCs with or without SP pretreatment were inoculated to examine their endothelial recruitment (Figure 4D,E). Recruitment of BM‐MSCs on the preformed endothelia tubule was significantly enhanced by SP pretreatment of the BM‐MSCs (by approximately 60%), and the cell morphology was much more elongated along the tubular network.
4. DISCUSSION
Stroke is a common type of vascular occlusion or hemorrhage rupture in the brain. Currently, early resolution of the blood clots within 6 hours after a stroke is the only clinically approved treatment, and a very small portion of patients qualify for such treatment.24 Despite decades of research on numerous animals and clinical trials, the outcomes of stem cell therapies have not reached clinical expectations.19 Severe tissue‐destructive inflammatory microenvironments and a very low rate of homing and survival of the transplanted cells have been suggested as reasons contributing to their therapeutic inefficiency.19 However, using a proper combination of multiple reparative stem cells instead of just one specific cell type may be a promising method for BBB repair. In the present study, we have demonstrated in vitro that a combination of bone marrow‐derived EPCs and MSCs can be used to reconstruct the vascular network de novo synergistically by acting as endothelial cells and pericyte‐like cells, respectively. This effect can be further enhanced by SP cotreatment. Furthermore, SP‐treated BM‐MSCs were preferentially incorporated into preexisting BM‐EPC tubular structures, which mimic a capillary with pericyte loss in a micro‐aneurysm and ischemic brain injury.
The characteristics defining EPCs based on origin and markers expression have been debated for years.19 It was previously claimed that EPCs originate in the peripheral blood rather than the bone marrow.25 This study has revealed that BM‐EPCs are able to form an endothelial tubular network within 3 hours autonomously, which is the function of reparative EPCs in vitro. On the other hand, BM‐MSCs themselves remain in a pellet‐like morphology even after a long incubation. However, in cooperation with BM‐EPCs, BM‐MSCs can be incorporated into the EPC‐made endothelia, and this synergy can be further enhanced by SP cotreatment. A possible mechanism underlying the concerted cooperation between BM‐EPCs and BM‐MSCs in the vascular network formation could be the synergistic induction of VEGF and its receptor, PDGF‐BB, and N‐cadherin under coculture conditions.
PDGF‐BB has been reported to play a critical role in the pericyte recruitment process, which leads to vessel maturation and stabilization in the developmental phase as well as tissue repair in adults.5 Our data in this study clearly support that both BM‐EPCs and BM‐MSCs synergistically act on the PDGF‐BB induction as early as 3 hours in coculture and may then induce N‐cadherin expression, which is later engaged in homotypic intercellular association between two cells. This initial event may also reinforce the tight junction formation between endothelial cells as well as further coverage of pericytes along the basal membrane of the endothelial cells. This is supported by the finding that the functional blockade of PDGF‐BB led to nearly complete inhibition of recruitment of BM‐MSCs to the endothelial tubules and partial disruption of the endothelial tube structure of BM‐EPCs. Accordingly, PDGF‐BB seems to be critical for the maintenance of both the endothelial tube itself and pericyte‐like encircling of BM‐MSCs on the endothelia.
It was reported by our group that neuropeptide SP works as a stem cell mobilizer for BM‐MSCs and then promotes tissue repair by recruiting mobilized cells to the injured tissue.10 In addition, SP has also been reported to modulate the immune environment by increasing the anti‐inflammatory M2 type macrophage after spinal cord injury, which leads to less apoptosis of neighboring cells and better functional recovery.26, 27 Taken together with the role of SP in the suppression of the injury‐mediated inflammatory response and as a BM‐MSC mobilizer, our finding that SP stimulated PDGF‐BB induction and more stable interaction of BM‐MSCs on the endothelial network provides a rationale to develop SP as a supplementary adjuvant to improve the therapeutic outcomes of dual stem cell therapies by enhancing angiogenesis and pericyte recruitment to make mature, tight BBB vessels in ischemic vascular damage repair.
5. PERSPECTIVE
This study proposes the rationale and working mechanism of dual cell therapy with BM‐EPCs and BM‐MSCs in conjunction with SP supplementation for the repair of the BBB following ischemic stroke in vivo, possibly by stimulating secretion of the angiogenic factors VEGF and PDGF‐BB and N‐cadherin expression, culminating in pericyte‐like coverage of BM‐MSCs on BM‐EPC‐regenerated endothelial tubules.
Supporting information
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Ministry of Science, ICT & Future Planning (2012M3A9C6050499, 2016M3A9B4917320).
Zhang M, Ahn W, Kim S, Hong HS, Quan C, Son Y. Endothelial precursor cells stimulate pericyte‐like coverage of bone marrow‐derived mesenchymal stem cells through platelet‐derived growth factor‐BB induction, which is enhanced by substance P. Microcirculation. 2017;24:e12394 10.1111/micc.12394
[The copyright line for this article was changed on August 2, 2018, after original online publication]
Contributor Information
Chengshi Quan, Email: quancs@jlu.edu.cn.
Youngsook Son, Email: ysson@khu.ac.kr.
REFERENCES
- 1. Abdul‐Muneer PM, Chandra N, Haorah J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol Neurobiol. 2015;51:966‐979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glover LE, Tajiri N, Weinbren NL, et al. A step‐up approach for cell therapy in stroke: translational hurdles of bone marrow‐derived stem cells. Transl Stroke Res. 2012;3:90‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193‐215. [DOI] [PubMed] [Google Scholar]
- 4. Hirschi KK, Rohovsky SA. D’Amore PA. PDGF, TGF‐b, and Heterotypic Cell–Cell interactions Mediate Endothelial Cell–induced Recruitment of 10T1/2 Cells and Their Differentiation to a Smooth Muscle Fate. J Cell Biol. 1998;141:805‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398‐1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood‐brain barrier. Nature. 2010;468:557‐561. [DOI] [PubMed] [Google Scholar]
- 10. Hong HS, Lee J, Lee E, et al. A new role of substance P as an injury‐inducible messenger for mobilization of CD29(+) stromal‐like cells. Nat Med. 2009;15:425‐435. [DOI] [PubMed] [Google Scholar]
- 11. Zhao YH, Yuan B, Chen J, et al. Endothelial progenitor cells: therapeutic perspective for ischemic stroke. CNS Neurosci Ther. 2013;19:67‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hong SJ, Kihlken J, Choi SC, March KL, Lim DS. Intramyocardial transplantation of human adipose‐derived stromal cell and endothelial progenitor cell mixture was not superior to individual cell type transplantation in improving left ventricular function in rats with myocardial infarction. Int J Cardiol. 2013;164:205‐211. [DOI] [PubMed] [Google Scholar]
- 13. Brooke G, Cook M, Blair C, et al. Therapeutic applications of mesenchymal stromal cells. Semin Cell Dev Biol. 2007;18:846‐858. [DOI] [PubMed] [Google Scholar]
- 14. Dharmasaroja P. Bone marrow‐derived mesenchymal stem cells for the treatment of ischemic stroke. J Clin Neurosci. 2009;16:12‐20. [DOI] [PubMed] [Google Scholar]
- 15. Paul G, Ozen I, Christophersen NS, et al. The adult human brain harbors multipotent perivascular mesenchymal stem cells. PLoS ONE. 2012;7:e35577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301‐313. [DOI] [PubMed] [Google Scholar]
- 17. Jin Y, Hong HS, Son Y. Substance P enhances mesenchymal stem cells‐mediated immune modulation. Cytokine. 2015;71:145‐153. [DOI] [PubMed] [Google Scholar]
- 18. Amadesi S, Reni C, Katare R, et al. Role for substance p‐based nociceptive signaling in progenitor cell activation and angiogenesis during ischemia in mice and in human subjects. Circulation. 2012;125:1774‐1786, S1771–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tang YH, Ma YY, Zhang ZJ, Wang YT, Yang GY. Opportunities and challenges: stem cell‐based therapy for the treatment of ischemic stroke. CNS Neurosci Ther. 2015;21:337‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arnaoutova I, George J, Kleinman HK, Benton G. The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis. 2009;12:267‐274. [DOI] [PubMed] [Google Scholar]
- 21. Rasband WS. Image J. US National Institutes of Health, Bethesda, Maryland, USA. htpp://rsb.info.nih.gov/ij/. 1997‐2017. Accessed March 2, 2013. [Google Scholar]
- 22. Kang HS, Trzaska KA, Corcoran K, Chang VT, Rameshwar P. Neurokinin receptors: relevance to the emerging immune system. Arch Immunol Ther Exp. 2004;52:338‐347. [PubMed] [Google Scholar]
- 23. ElAli A, Theriault P, Rivest S. The role of pericytes in neurovascular unit remodeling in brain disorders. Int J Mol Sci. 2014;15:6453‐6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chapman SN, Mehndiratta P, Johansen MC, McMurry TL, Johnston KC, Southerland AM. Current perspectives on the use of intravenous recombinant tissue plasminogen activator (tPA) for treatment of acute ischemic stroke. Vasc Health Risk Manag. 2014;10:75‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen L, Huang H, Xi H, et al. A prospective randomized double‐blind clinical trial using a combination of olfactory ensheathing cells and Schwann cells for the treatment of chronic complete spinal cord injuries. Cell Transplant. 2014;23(Suppl 1):S35‐S44. [DOI] [PubMed] [Google Scholar]
- 26. Jiang MH, Chung E, Chi GF, et al. Substance P induces M2‐type macrophages after spinal cord injury. NeuroReport. 2012;23:786‐792. [DOI] [PubMed] [Google Scholar]
- 27. Jiang MH, Lim JE, Chi GF, et al. reduces apoptotic cell death possibly by modulating the immune response at the early stage after spinal cord injury. NeuroReport. 2013;24:846‐851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


