Abstract
Purpose
The aim of this study was to investigate the value of biochemical profile of cyst fluid and diffusion‐weighted imaging (DWI) in differentiating hepatic hydatid cysts (HCs) from liver simple cysts.
Materials and methods
Forty‐six patients underwent MR imaging. Twenty‐nine patients had 29 hydatid cysts and 17 patients had liver simple cysts. Thirteen patients with hydatid cysts and seven patients with liver simple cysts were evaluated with cyst fluid biochemical analysis. The concentration of glucose, protein, calcium ion (Ca2+) electrolyte, macroscopic appearance, and parasitological sediment were evaluated in this study.
Results
In the respect of biochemical analysis cyst fluid, the concentration of glucose and calcium ion of HCs was significantly higher than that of the liver simple cysts. In the respect of DWI, in the b 1000 s/mm2 value in respect of mean application data center (ADC) values, there was a statistically significant difference between HCs group (the mean value was (2.50±0.79)×10−3 mm/s2) and liver simple cysts group (the mean value was (2.92±0.66)×10−3 mm/s2). However, no statistically significant results were obtained in the ADC measurements of b 500 s/mm2 between two groups.
Conclusion
The analysis of cyst fluid combined with the measurement of ADC values in the b 1000 s/mm2 value could be considered a promising parameter as an alternative to the differential diagnosis of hepatic hydatid cysts from liver simple cysts.
Keywords: cyst fluid, diffusion‐weighted imaging, hydatid cysts, liver simple cysts
1. Introduction
Hydatid disease is a parasitic disease caused by the larval stage of animal tapeworms, Echinococcus granulosus which produces unilocular cystic lesions. Hydatid disease is found in Mediterranean countries, the Middle East, Eastern Europe, Africa, Argentina, Chile, China, Australia, and New Zealand. The definitive and intermediate hosts for E. granulosus are dogs and sheep, respectively.1, 2, 3 The disease infected by E. granulosus most often spreads to the liver (50%‐70%) and less frequently the lung and in other parts of the body.4
Hydatid cysts are generally asymptomatic until expanding cysts gradually effect in the liver and elicit pressure symptoms. Cysts may grow for a period of 5‐20 years, and may be discovered incidentally on a routine ultrasound or CT examination. Expanding cysts present with abdominal pain or palpable mass in the right upper abdomen.2 HCs may compress the bile ducts and result in obstructive jaundice and cause fever, pruritus, eosinophilia, dissemination, or fatal anaphylaxis after a cyst ruptures into the peritoneum or biliary tract. Infection of the cyst can facilitate the development of liver abscesses and mechanic local complications, such as mass effect on bile ducts and vessels that can induce cholestasis, portal hypertension, and Budd‐Chiari syndrome.3 As a result, the diagnosis of HCs should be as fast as possible because of the relevant complications that may arise with disease progression. The diagnosis of HCs is usually made by positive serology and some characteristic radiologic features. Biochemical analysis within HCs also plays a definitive role in the metabolism, physiology and immunology of cystic echinococcosis.5 Radiologic appearance of HCs depends on the stage of maturity and ranges from completely liquid type to completely solid type.6 The diagnostic accuracy is high for cysts with characteristic features such as calcification, multivesicular appearance, and floating membrane.7 However, there are some diagnostic difficulties with unusual sites and appearances.8, 9 In addition, HCs of initial phase usually appear as a completely liquid cyst, and are therefore radiologically indistinguishable from liver simple cysts.6 There are a limited number of studies in literature which have researched the efficacy of biochemical profile of cyst fluid combined with DWI in HCs.
The aim of this study was to investigate the value of biochemical profile of cyst fluid and DWI in differentiating hepatic hydatid cysts from liver simple cysts.
2. Materials and Methods
2.1. Patients
This retrospective study included 46 consecutive patients (33 males, 13 females; age range 19‐56, mean age, 45±1.7) who underwent MR imaging from July 2012 to December 2013. The diagnosis of HCs was based on radiological imaging and serological tests [indirect haemagglutination tests (IHT), and enzyme‐linked immunosorbent assay (ELISA)] and pathology. It was considered to be positive when the titer exceeded a value of 1:32 for ITH and 1:200 for ELISA. Of these, 29 patients were diagnosed for HCs, 17 patients were diagnosed for liver simple cysts as serologically negative, without epidemic life history or significant change in the follow up period. Hydatid cyst and liver simple cyst fluids were obtained after surgical removal of cysts and ultrasound‐guided percutaneous needle biopsy from thirteen patients with HCs and seven patients with liver simple cysts. Informed consent was obtained from all the study participants.
2.2. Biochemical analysis
21G×200 mm PTC needle (Hakko Company) were used in the extraction for both hydatid cyst and simple liver cyst fluid specimen. Fluid of 13 HCs and seven simple cysts was thoroughly shaken up, respectively, before being sent to the biochemistry lab. The content of Na+, Cl−, Ca2+, blood urea nitrogen (BUN), total protein (TP), albumin (ALB), globulin (GLB), white globulin ratio, alkaline phosphatase (ALP), pH value, glucose (Glu) were evaluated in this study.
2.3. Magnetic Resonance Imaging (MRI)
From 2012 to 2013, all patients underwent MR examination with 1.5 T scanner (GE Healthcare, Milwaukee, WI, USA) using an eight‐element phased‐array body coil. All patients were examined initially with the routine MRI protocol. The imaging sequences included axial T1‐weighted, breathing gating gradient echo sequence and a single stimulate spin echo sequence. T1WI spin echo sequence, (125/4.8 mseconds; matrix size, 320×224;),T2WI quick spin echo sequence control technology and the breath gate, TR 4286 mseconds, TE 87 mseconds, slice thickness 8 mm, gap, 2 mm; Scanning field 360‐460 mm, matrix size, 256×256, DWI with single‐shot echo‐planar imaging (EPI). Specific scanning parameters: TR 2000‐3000 mseconds, TE 50‐70 mseconds, Echo spacing (ES) 0.8 seconds, matrix size 128×256, bandwidth 2080 Hz/pixel, slice thickness 8 mm, gap, 3 mm, view field 350 mm, single signal collection. DWI imaging were acquired using the following b values: 0, 500, and 1000 s/mm2. Application data center (ADC) maps were reconstructed from these images.
2.4. Quantitative analysis
All measurements were performed on an advanced workstation (AW4.2; GE Healthcare). Images were obtained with values of b 500, 1000 s/mm2. An ADC map of the images was automatically generated and the ADC values were measured on this map for all patients. The circular region of interest (ROI) used for quantitative measurements of ADC values of HCs was placed centrally, and the size of the ROI was kept as large as possible, covering at least two‐thirds of the cyst, yet avoiding interference from the surrounding liver tissue and major blood vessels. The mean ADC values of cysts and liver were determined on images with values of b 500, 1000 s/mm2. The average of three measurements was recorded as the final signal intensity of ADC. Signal intensity of the cysts, cyst‐to‐liver signal intensity ratio, ADC of the cysts, and cyst‐to‐liver ADC ratio were calculated.
2.5. Statistical analysis
Data were expressed as mean±standard deviation. Statistical analysis was performed by the Kolmogorov‐Smirnov test when equal variance of some parameters were assumed, Mann‐Whitney U test when equal variance of some parameters were not assumed when appropriate, Spearman analysis. Analyses were performed with the use of SPSS version 18.0 (SPSS, Chicago, IL, USA). P<.05 was considered to be statistically significant.
3. Results
3.1. Cyst fluid biochemical analysis
The result of biochemical analyses of the cyst fluids of HCs and liver simple cysts are shown in Table 1. Hydatid cyst fluids contained significantly more glucose and Ca2+ (P<.05) than liver simple cysts. However, there was no significant difference in all the other chemical parameters between HCs and liver simple cysts (Figure 1).
Table 1.
Hydatid cysts and simple cyst fluid analysis (±s)
| Detection index | Hydatid cyst (n=13) | Simple cyst (n=7) | U‐value | P‐value |
|---|---|---|---|---|
| Osmolality | 270.155±35.08 | 279.463±16.83 | 0.656 | .520 |
| Total protein | 1.046±1.91 | 1.857±1.31 | 0.984 | .338 |
| Albumin | 0.762±1.82 | 1.386±1.20 | 0.810 | .428 |
| Globulin | 0.300±0.29 | 0.528±0.53 | 1.251 | .227 |
| Albumin | 2.100±4.40 | 3.043±3.85 | 0.464 | .649 |
| Urea/nitrogen | 7.317±2.58 | 5.134±2.96 | −1.718 | .103 |
| Glucose | 2.842±2.03 | 0.140±0.24 | −3.459 | .003 |
| Total cholesterol | 0.149±0.44 | 0.090±0.12 | 0.343 | .736 |
| Na+ | 137.445±16.96 | 145.556±5.74 | 1.213 | .242 |
| Cl− | 99.117±21.77 | 125.629±4.27 | 3.151 | .006 |
| Ca2+ | 3.932±1.41 | 0.73±0.49 | −5.900 | .000 |
| Inorganic phosphorus | 0.136±0.25 | 0.053±0.022 | −0.911 | .374 |
| Amylase | 1.85±2.51 | 2.00±2.24 | 0.135 | .894 |
| Proportion | 1.0039±0.0015 | 1.0045±0.0010 | 0.829 | .418 |
| PH | 7.613±0.21 | 7.864±0.47 | 1.625 | .123 |
The mean value of liver glucose concentration was higher than non‐parasitic cyst fluid's, P=.03. The Ca2+ concentration of Hydatid cyst fluid was higher than non‐parasitic cyst fluid, P<.001. The concentration of protein and other components were not statistically different.
Figure 1.

(A) Pictures of specimen. 50 mL water, 5%, 10%, 50% glucose solution were shown from left to right, respectively. (B) ADC map and ADC value measurement (b value 1000 s/mm2). (C) b value 1000 s/mm2, low signal of water, highest signal of 50% glucose solution. (D) Signal intensity measurement. (E) b value 500 s/mm2, highest signal of water. (F) b value 0 s/mm2, highest signal of 50% glucose solution
3.2. ADC value measurement
In the b 1000 s/mm2 value in respect of mean ADC values, there was a statistically significant difference between HCS group (the mean value was (2.50±0.79)×10−3 mm/s2) and liver simple cysts group (the mean value was (2.92±0.66)×10−3 mm/s2). However, no statistically significant results were obtained in the ADC measurements of b 500 s/mm2 between two groups (Table 2; Figures 2 and 3).
Table 2.
ADC value (×10−3 mm/s2) and signal strength measurement rate of hydatid cysts under different b value ()
| Classification | b value 500 s/mm2 | b value 1000 s/mm2 | ||||
|---|---|---|---|---|---|---|
| ADC value of lesions | ADC value of liver | Signal strength ratio | ADC value of lesions | ADC value of liver | Signal strength ratio | |
| Hydatid cyst | 3.095 | 1.409 | 1.1517 | 2.501 | 1.363 | 0.86293 |
| 0.670 | 0.563 | 0.5339 | 0.789 | 0.421 | 0.5515 | |
| Simple cyst | 3.313 | 1.429 | 0.94693 | 2.916 | 1.293 | 0.5086 |
| 0.467 | 0.517 | 0.3967 | 0.658 | 0.382 | 0.2372 | |
| U‐value | 242.5 | 293.5 | 246.0 | 192.0 | 287.5 | 186.0 |
| P‐value | .173 | .686 | .195 | .021 | .605 | .015 |
b value 1000 s/mm2, ADC value of hydatid cysts were 0.00250±0.00079 mm/s2. The ADC value of simple liver cysts were 0.00292±0.000658 mm/s2. The ADC value of simple cysts were significantly higher than the value of hydatid cysts, The difference between the two groups was significant (P=.021). b value 500 s/mm2, the ADC value and signal intensity ratio between the two groups were not statistically different (Figures 2 and 3).
Figure 2.
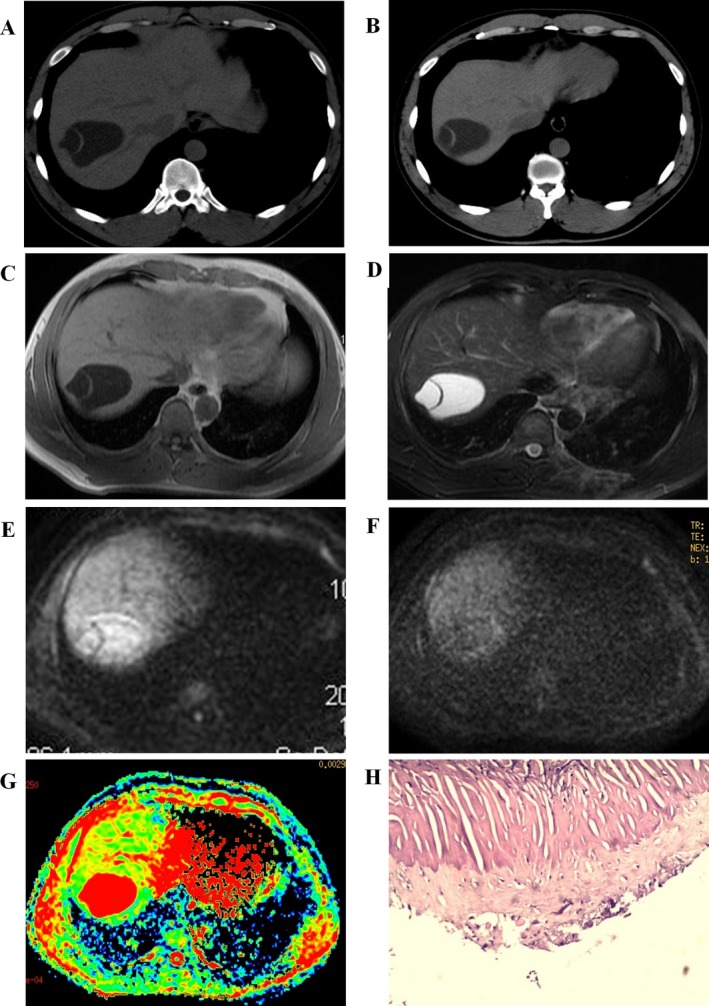
Liver upper right lobe hydatid cysts. (A, B) CT images, low‐density cystic lesion in the upper right lobe of liver, non‐wall structure, with floating band between the calcification. (C, D) T1WI T2WI images, low signal indicates ribbon‐like calcification. (E, F, G) b value of 500/1000 ADC images, high signal lesions, ribbon‐like calcification with low signal. (H) Pathological picture of a hydatid cyst
Figure 3.

Liver right lobe simple cyst. (A) CT scan shows right lobe cystic mass of liver, non‐wall structure. (B) MRI T2 coronal. (C) b value 1000 s/mm2 (D) ADC pseudo‐color pictures, ADC value was lower than the adjacent liver parenchyma
4. Discussion
At biochemical analysis, there is usually eosinophilia, and a serologic test is positive in 25% of HCs patients.10 The biochemical analysis of hydatid cyst fluid can also provide valuable information on the diagnosis of HCs.5 Shaafie et al. have demonstrated significantly higher concentration of uric acid in human hydatid fluids compared with other cyst fluids in the natural intermediate hosts (sheep, goats, cattle, camel).
In this study, the concentration of glucose and Ca2+ was found to be significantly higher in hydatid cyst fluid compared with liver simple cyst. We could indicate that the hydatid cyst fluid contained more compositions such as high level glucose and Ca2+.
Imaging methods such as ultrasound (US), computed tomography (CT), and magnetic resonance (MR) are essential in diagnosis of hydathid disease.1, 2, 11, 12 The imaging characteristics of HCs have been established and include sharply defined cystic lesions with a thick wall.13, 14, 15 They sometimes have septa, daughter cysts, and/or calcifications, a solid component is rarely seen. US is the first choice in radiological imaging for the diagnosis of HCs.13
CT offers equivalent information with that derived by US for diagnosis of HCs. It gives better information about the location of the cyst in the liver, the daughter and exogenous cyst. However, imaging features of HCs may sometimes overlap with those of liver simple cyst. For instance, both simple cysts and complete liquid type HCs display high signal intensity on T2WI and low signal intensity on T1WI, with well‐defined margins. Recent reports have suggested that DWI can be helpful in the characterization of cystic lesions in brain, liver, ovary, and uterus, to differentiate arachnoid cysts with epidermoid cysts, liver simple cysts with HCs and liver abscesses, ovarian cysts with endometrial cystic neoplasms.16, 17, 18, 19, 20 In these studies, the differences in ADCs of cysts were attributed to the difference in cellular density. However, to our knowledge, the role of biochemical analysis of hydatid cyst fluid combined with DWI in the differential diagnosis of HCs and liver simple cysts has not been reported previously.
In our study, in the b 1000 s/mm2 value in respect of mean ADC values, there was a statistically significant difference between HCs group (the mean value was (2.50±0.79)×10−3 mm/s2) and liver simple cysts group (the mean value was (2.92±0.66)×10−3 mm/s2). This agrees well with the previous reports that the average ADC values of HCs type I (consists of a pure fluid collection, that is, a non‐complicated unilocular or monovesicular cyst) was significantly lower than of the liver simple cysts group. As the liver simple cysts have low viscosity, which results in the high ADC value, while the concentration of glucose and Ca2+ in HCs was significantly higher than that in liver simple cysts, thus the diffusion of water molecules of HCs is restricted which results in decreased ADC values. This is partly parallel to the previous reports.2, 17, 20, 21, 22 However, no statistically significant results were obtained in the ADC measurements of b 500 s/mm2 between two groups. We speculated that the low b values are sensitive to perfusion, which result in falsely elevated parenchymal organ and less ADC values.23
However, there are some limitations of our study. The most significant limitation of this study is the smaller patients' number evaluated with cyst fluid biochemical analysis than evaluated with DWI. Studies with more patients would result in a higher significance level, although our results suggest some significant trends. A large number of HCs and liver simple cysts patients included in the cyst fluid biochemical analysis would provide more information. Another important limitation is the lack of one to one comparison between the chemical analysis of cyst fluid and the measurement of ADC values of HCs and liver simple cysts. Future studies on larger series comparing between the chemical analysis of cyst fluid and the measurement of ADC values in the b 1000 s/mm2 value of HCs and liver simple cysts are needed.
In conclusion, the combination of cyst fluid analysis with the measurement of ADC values in the b 1000 s/mm2 value could be considered a promising parameter as an alternative to the differential diagnosis of hepatic hydatid cysts from liver simple cysts.
Shanshan W, Hui L, Yan L, et al. The study of biochemical profile of cyst fluid and diffusion‐weighted magnetic resonance imaging in differentiating hepatic hydatid cysts from liver simple cysts. J Clin Lab Anal. 2018;32:e22192 10.1002/jcla.22192
[The copyright line for this article was changed on 25 July 2018 after original online publication.]
References
- 1. Czermak BV, Akhan O, Hiemetzberger R, et al. Echinococcosis of the liver. Abdom Imaging. 2008;33:133‐143. [DOI] [PubMed] [Google Scholar]
- 2. Pedrosa I, Saiz A, Arrazola J, Ferreiros J, Pedrosa CS. Hydatid disease: radiologic and pathologic features and complications. Radiographics. 2000;20:795‐817. [DOI] [PubMed] [Google Scholar]
- 3. Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nunnari G, Pinzone MR, Gruttadauria S, et al. Hepatic echinococcosis: clinical and therapeutic aspects. World J Gastroenterol. 2012;18:1448‐1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shaafie IA, Khan AH, Rambabu K. Biochemical profiles of hydatid cyst fluids of Echinococcus granulosus of human and animal origin in Libya. J Helminthol. 1999;73:255‐258. [DOI] [PubMed] [Google Scholar]
- 6. Caremani M, Lapini L, Caremani D, Occhini U. Sonographic diagnosis of hydatidosis: the sign of the cyst wall. Eur J Ultrasound. 2003;16:217‐223. [DOI] [PubMed] [Google Scholar]
- 7. WHO Informal Working Group . International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop. 2003;85:253‐261. [DOI] [PubMed] [Google Scholar]
- 8. Ilica AT, Kocaoglu M, Zeybek N, et al. Extrahepatic abdominal hydatid disease caused by Echinococcus granulosus: imaging findings. AJR Am J Roentgenol. 2007;189:337‐343. [DOI] [PubMed] [Google Scholar]
- 9. Andronikou S, Welman CJ, Kader E. Classic and unusual appearances of hydatid disease in children. Pediatr Radiol. 2002;32:817‐828. [DOI] [PubMed] [Google Scholar]
- 10. Mortele KJ, Ros PR. Cystic focal liver lesions in the adult: differential CT and MR imaging features. Radiographics. 2001;21:895‐910. [DOI] [PubMed] [Google Scholar]
- 11. Rozanes I, Guven K, Acunas B, Emre A. Cystic echinococcal liver disease: new insights into an old disease and an algorithm for therapy planning. Cardiovasc Intervent Radiol. 2007;30:1112‐1116. [DOI] [PubMed] [Google Scholar]
- 12. Filippou D, Tselepis D, Filippou G, Papadopoulos V. Advances in liver echinococcosis: diagnosis and treatment. Clin Gastroenterol Hepatol. 2007;5:152‐159. [DOI] [PubMed] [Google Scholar]
- 13. Gharbi HA, Hassine W, Brauner MW, Dupuch K. Ultrasound examination of the hydatic liver. Radiology. 1981;139:459‐463. [DOI] [PubMed] [Google Scholar]
- 14. Haliloglu M, Saatci I, Akhan O, Ozmen MN, Besim A. Spectrum of imaging findings in pediatric hydatid disease. AJR Am J Roentgenol. 1997;169:1627‐1631. [DOI] [PubMed] [Google Scholar]
- 15. Tuzun M, Hekimoglu B. Pictorial essay. Various locations of cystic and alveolar hydatid disease: CT appearances. J Comput Assist Tomogr. 2001;25:81‐87. [DOI] [PubMed] [Google Scholar]
- 16. Chan JH, Tsui EY, Luk SH, et al. Diffusion‐weighted MR imaging of the liver: distinguishing hepatic abscess from cystic or necrotic tumor. Abdom Imaging. 2001;26:161‐165. [DOI] [PubMed] [Google Scholar]
- 17. Inan N, Arslan A, Akansel G, et al. Diffusion‐weighted imaging in the differential diagnosis of simple and hydatid cysts of the liver. AJR Am J Roentgenol. 2007;189:1031‐1036. [DOI] [PubMed] [Google Scholar]
- 18. Nakayama T, Yoshimitsu K, Irie H, et al. Diffusion‐weighted echo‐planar MR imaging and ADC mapping in the differential diagnosis of ovarian cystic masses: usefulness of detecting keratinoid substances in mature cystic teratomas. J Magn Reson Imaging. 2005;22:271‐278. [DOI] [PubMed] [Google Scholar]
- 19. Cece H, Gündogan M, Karakas Ö. The role of diffusion‐weighted magnetic resonance imaging in the classification of hepatic hydatid cysts. Eur J Radiol. 2013;82:90‐94. [DOI] [PubMed] [Google Scholar]
- 20. Oruc E, Yildirim N, Topal NB, Kilicturgay S, Akgoz S, Savci G. The role of diffusion‐weighted MRI in the classification of liver hydatid cysts and differentiation of simple cysts and abscesses from hydatid cysts. Diagn Interv Radiol. 2010;16:279‐287. [DOI] [PubMed] [Google Scholar]
- 21. Sonmez G, Sivrioglu AK, Mutlu H, et al. Is it possible to differentiate between hydatid and simple cysts in the liver by means of diffusion‐weighted magnetic resonance imaging? Clin Imaging. 2012;36:41‐45. [DOI] [PubMed] [Google Scholar]
- 22. Volders WK, Gelin G, Stessens RC. Best cases from the AFIP. Hydatid cyst of the kidney: radiologic‐pathologic correlation. Radiographics. 2001;21:Spec No:S255‐60. [DOI] [PubMed] [Google Scholar]
- 23. Yamada I, Aung W, Himeno Y, Nakagawa T, Shibuya H. Diffusion coefficients in abdominal organs and hepatic lesions: evaluation with intravoxel incoherent motion echo‐planar MR imaging. Radiology. 1999;210:617‐623. [DOI] [PubMed] [Google Scholar]


