Abstract
Background
Quantification of burden of chronic spontaneous urticaria (CSU) vs. psoriasis (PsO) is limited.
Objective
To evaluate the burden associated with CSU vs. PsO of all severities (overall PsO), mild and moderate/severe PsO.
Methods
This retrospective cross‐sectional analysis compared data from adult patients with chronic urticaria (CU), used as a proxy for CSU, and PsO from the National Health and Wellness Survey in France, Germany, Italy, Spain and the United Kingdom. Outcomes included mental and physical component summary scores (MCS and PCS) calculated from the Short Form (SF)‐36v2 or SF‐12v2, SF‐6D health utility scores, self‐reported psychological complaints (anxiety, depression and sleep difficulties), work productivity and activity impairment, and self‐reported healthcare resource utilization. Bivariate and multivariate analyses for each outcome and comparative groups were conducted.
Results
This analysis included 769 CU and 7857 PsO (26.9% moderate/severe) patients. Following adjustment for covariates, CU patients showed a greater health‐related quality of life (HRQoL) impairment vs. overall PsO (MCS: −2.4, PCS: −1.6, SF‐6D: −0.03; all P < 0.001). CU patients showed a higher risk of anxiety, depression and sleep difficulties [odds ratio (OR): 1.63, 1.34 and 1.56, respectively; all P < 0.01] and greater healthcare resource use vs. overall PsO. The overall activity impairment was significantly greater in CU patients than in overall PsO patients (P = 0.001), while the impact on work was not significantly different. The results vs. moderate/severe PsO group showed no significant differences on all outcomes.
Conclusion
Burden of illness in CU is higher than PsO of all severities but similar to that observed in moderate/severe PsO. Both diseases have a similar negative impact on work productivity.
Introduction
Dermatological diseases are the fourth leading cause of global non‐fatal burden, expressed as years lost due to disability, and the 18th leading cause of global health burden, expressed as disability‐adjusted life years (DALYs).1 Although non‐fatal, dermatological diseases have an impact on the health‐related quality of life (HRQoL) and daily activities of patients, with a substantial and prolonged impact on the indirect costs to the society.1, 2 However, healthcare regulatory authorities and payers focus their resources on diseases with high mortality rates.1
Chronic spontaneous urticaria (CSU) is defined as the spontaneous appearance of hives, angio‐oedema or both for >6 weeks.3 The prevalence of CSU is reported to be between 0.1% and 0.8% in European countries, and it represents over two‐third cases of chronic urticaria (CU).3, 4 CU primarily affects the working population as the peak age of CU onset is between 20 and 40 years.4 Patients with CU experience an underestimated emotional and psychological burden which influences sleep and daily activities and restricts work ability and social life.4, 5, 6, 7, 8, 9 HRQoL impairment in CU increases with disease severity,6, 10 prevalence of comorbid psychological conditions11, 12, 13 and several autoimmune conditions.14, 15 Dimensions of HRQoL impairment caused by CU, such as lack of energy, social isolation and emotional disturbances, are comparable with those caused by severe ischaemic heart disease.16 Compared with atopic dermatitis and psoriasis (PsO) patients, CU patients reported a higher impact on their daily activities and physical discomfort.17 Broadly, the burden of CU translates into costs to healthcare payers and the society. Medication, outpatient costs and loss of productivity due to the absence from work are major cost drivers and increase with disease severity.8, 18 Work and activity impairment in CU patients is twice as high as reported in non‐CU patients.6, 10 Although the burden of CU has been described in previous research, it may fall short of providing sufficient evidence for regulators and payers.
Comparing the burden of CU with a recognized burden of other dermatological conditions will contribute to a better understanding and to an increased awareness of the true impact of CU on patients, healthcare systems and the society. One such condition is PsO, which is considered one of the serious global diseases by the World Health Organisation (WHO).19 PsO is a chronic, non‐communicable, painful, disfiguring and disabling dermatological disease with a negative impact on the quality of life of patients.19 The prevalence of PsO ranges between 1.5% and 5% in most developed countries.19 Among PsO patients, 71% of the PsO patients have mild PsO and 27% of PsO patients have moderate–severe PsO, remaining 2% could not be catagorized.20 Reduction in the HRQoL due to PsO is comparable with other chronic diseases such as depression, myocardial infarction, hypertension and even some cancers.21 Reported annual total cost per PsO patient was €8372 in Italy and €2866–6707 in Germany.22 PsO contributes to 0.04% of the total global DALY, which is twice the global average DALYs for acute hepatitis C.19 In Germany, mean working days lost per year due to a PsO patient were 4.9.23 The socioeconomic burden of PsO increases with the severity of the disease.24 The rate of hospitalization of patients with severe PsO is twice that of patients with mild PsO.25 Patients with severe PsO miss a greater number of days from work or school than those with mild PsO.26 As the burden of PsO is well established and the degree of disability in PsO is perceived as higher than that in CU,17 the aim of this study was to analyse the burden of illness associated with CU relative to PsO patients.
Methods
Study design and data source
This was a retrospective, cross‐sectional analysis of self‐reported data obtained from National Health and Wellness Survey (NHWS) conducted in 2010, 2011 and 2013 in France, Germany, Italy, Spain and the United Kingdom. The survey was not conducted in 2012. The NHWS is a large, international, self‐reported survey conducted regularly by Kantar Health to assess health conditions in the general population. These surveys are conducted primarily through Internet‐based health questionnaires administered to nationwide samples of adults aged 18 years and above.27 Potential respondents were identified through opt‐in online survey panels using a stratified random sampling framework to ensure representativeness in terms of age and gender. In addition, telephonic recruitment was used in countries such as Spain and Italy, where Internet penetration among the elderly population was not considered sufficient to provide an adequate sample of the elderly population. The protocol and questionnaire for the NHWS were reviewed and approved by the Essex Institutional Review Board (Lebanon, NJ, USA).
Study groups
Data obtained from respondents who reported the diagnosis of either CU (defined as hives lasting for >6 weeks) or PsO, who were able to read and write in the primary language of the country in which the study was conducted, and who provided informed consent, were included in this analysis. Respondents who reported the diagnosis of both conditions were excluded from the analysis. The NHWS questionnaire did not include questions about the exact form of CU particularly CSU and its severity. Hence, respondents with a diagnosis of CU were used as a proxy for CSU but no severity groups could be defined. Severity of PsO was collected in the survey using the affected percentage of body surface area (BSA) as estimated by the patients who were instructed to use the surface of the palm of the hand to represent 1% of BSA. PsO patients were categorized into mild and moderate/severe (BSA > 2% or 3% depending on the year) groups.28 Outcomes of the CU cohort were compared with PsO of all severities (overall PsO), mild PsO and moderate/severe PsO, respectively.
Demographic and general health characteristics
Demographic characteristics included age, gender, country of residence, marital status, level of annual household income, level of education and employment status. General health characteristics included body mass index (BMI) calculated from the reported height and weight, cigarette smoking history, frequency of alcohol use and days of exercise in the past month. The Charlson comorbidity index (CCI) was calculated from self‐reported physician diagnoses of comorbid conditions to represent the level of comorbidity among the respondents.29 Higher total index scores indicated a greater comorbidity burden in these patients.
Outcomes
HRQoL was measured using the 4‐week standard recall form of the revised Medical Outcomes Study 12‐item Short Form survey instrument, version 2 (SF‐12v2) for respondents surveyed in 2010 and 2011 and the Medical Outcomes Study 36‐item Short Form survey instrument, version 2 (SF‐36v2) for those surveyed in 2013.30, 31 Mental component summary (MCS) and physical component summary (PCS) scores were calculated to summarize mental and physical health, respectively. MCS and PCS scores in the US population have a mean of 50 and a standard deviation (SD) of 10, and these standards are also used for the non‐US population;4 lower scores mean worse health status. Health utility scores were calculated from the SF‐12v2 and SF‐36v2 using the SF‐6D algorithm which provides a preference‐based single index measure for health using general population values.32 The utility score ranges from 0 to 1 with a higher utility score indicating a better health. The minimally important difference (MID) is estimated to provide a measure of the smallest change in the patient‐reported outcomes which patients perceive as important.33 The MID for MCS and PCS scores is 3 and that for health utilities is 0.03.34
Self‐reported psychological complaints, such as depression, anxiety and sleep difficulties (insomnia and sleep disturbance), in the past 12 months were assessed. Respondents were considered to have anxiety if they reported experiencing general anxiety disorder, panic disorder, phobia, post‐traumatic stress, obsessive–compulsive disorder, social anxiety disorder or anxiety.
Impairment of work and non‐work daily activities was measured using the General Health version of the Work Productivity and Activity Impairment Questionnaire (WPAI) which estimates the percentage of the absence from work or impairment due to health in the 7 days preceding the survey.32 Metrics for employed respondents included absenteeism (percentage of work time missed), presenteeism (degree of impairment while at work) and overall work impairment. All respondents, regardless of their employment status, reported activity impairment, the percentage of impairment related to non‐work activities.
Healthcare utilization was assessed based on the patient‐reported number of visits to different medical practitioners during the past 6 months. Respondents indicated the type of practitioner visited including traditional healthcare practitioners (HCPs) [e.g. general practitioners (GPs), dermatologists and allergists], emergency room (ER) visits and hospitalizations. The number of each type of visit was also reported. Respondents also indicated the type of alternative HCPs they had visited in the past 6 months, including herbalists, acupuncturists, chiropractors, nutritionists and massage therapists, although the number of those visits was not included in the survey.
Statistical analyses
The analysis first evaluated the differences between patients with CU and overall PsO independent of their disease severity; later, the CU group was compared with the mild and moderate/severe PsO, respectively. In the bivariate analysis, chi‐squared tests were used to compare categorical variables, and independent sample t‐tests for continuous variables. In addition, regression analyses were conducted after adjustments for demographic and general health characteristics of patients. The type of regression was specific to the type of the outcome variable. Normal distributions and identified link functions were used for HRQoL variables, generalized linear models with the negative binomial distribution and log‐link function for WPAI variables, Poisson distribution and log‐link function for resource utilization and binary logistic regression for binary outcomes. To aid in interpretation, adjusted means were calculated from the regression models for HRQoL and the number of healthcare visits, with outcome values for each group presented as the mean of the covariates for the respondents included in the model.
Results
In total, 769 CU patients and 7857 overall PsO patients were identified and included in this analysis. Among the overall PsO patients, 5736 (73.1%) had mild PsO and 2121 (26.9%) had moderate/severe PsO. The CU patients were significantly younger than the overall PsO patients [mean (SD) age: 45.4 (15.2) years vs. 47.9 (15.1) years; P < 0.001]. In the CU cohort, the proportion of women was 70.6%, and it was significantly greater than the proportion of women (52.8%) in the overall PsO group (P < 0.001). BMI, smoking history and alcohol use differed across the groups, whereas the frequency of exercise was fairly consistent (Table 1).
Table 1.
Patient characteristics of respondents diagnosed with CU and PsO
| CU (n = 769) | Overall PsO (n = 7857) | P value vs. CU | Mild PsO (n = 5736) | P value vs. CU | Moderate‐to‐Severe PsO (n = 2121) | P value vs. CU | |
|---|---|---|---|---|---|---|---|
| Age, mean (SD), years | 45.4 (15.2) | 47.9 (15.1) | <0.001 | 48.1 (15.2) | <0.001 | 47.4 (14.7) | 0.002 |
| Female, n (%) | 543 (70.6) | 4148 (52.8) | <0.001 | 3023 (52.7) | <0.001 | 1125 (53.0) | <0.001 |
| Country, n (%) | <0.001 | <0.001 | <0.001 | ||||
| France | 184 (23.9) | 2461 (31.3) | 1898 (33.1) | 563 (26.5) | |||
| Germany | 185 (24.1) | 1818 (23.1) | 1252 (21.8) | 566 (26.7) | |||
| UK | 114 (14.8) | 1902 (24.2) | 1275 (22.2) | 627 (29.6) | |||
| Italy | 162 (21.1) | 1109 (14.1) | 866 (15.1) | 243 (11.5) | |||
| Spain | 124 (16.1) | 567 (7.2) | 445 (7.8) | 122 (5.8) | |||
| Married/living with partner, n (%) | 469 (61.0) | 5129 (65.3) | 0.017 | 3768 (65.7) | 0.010 | 1361 (64.2) | 0.117 |
| Completed university, n (%) | 300 (39.0) | 3015 (38.4) | 0.728 | 2312 (40.3) | 0.491 | 703 (33.1) | 0.003 |
| Employed, n (%) | 409 (53.2) | 4446 (56.6) | 0.070 | 3279 (57.2) | 0.037 | 1167 (55.0) | 0.381 |
| Household income, n (%) | 0.179 | 0.042 | 0.974 | ||||
| Below median | 405 (52.7) | 3897 (49.6) | 2782 (48.5) | 1115 (52.6) | |||
| Above median | 275 (35.8) | 3075 (39.1) | 2321 (40.5) | 754 (35.5) | |||
| Declined to answer | 89 (11.6) | 885 (11.3) | 633 (11.0) | 252 (11.9) | |||
| CCI, mean (SD) | 0.90 (2.12) | 0.50 (1.11) | <0.001 | 0.47 (1.05) | <0.001 | 0.59 (1.28) | <0.001 |
| BMI categories, n (%) | <0.001 | <0.001 | <0.001 | ||||
| Underweight | 38 (4.9) | 200 (2.5) | 147 (2.6) | 53 (2.5) | |||
| Normal | 336 (43.7) | 3037 (38.7) | 2291 (39.9) | 746 (35.2) | |||
| Overweight | 218 (28.3) | 2709 (34.5) | 1978 (34.5) | 731 (34.5) | |||
| Obese | 165 (21.5) | 1760 (22.4) | 1211 (21.1) | 549 (25.9) | |||
| Declined to answer | 12 (1.6) | 151 (1.9) | 109 (1.9) | 42 (2.0) | |||
| Smoking status, n (%) | <0.001 | 0.001 | <0.001 | ||||
| Current | 237 (30.8) | 2464 (31.4) | 1679 (29.3) | 785 (37.0) | |||
| Former | 244 (31.7) | 2927 (37.3) | 2206 (38.5) | 721 (34.0) | |||
| Never | 288 (37.5) | 2466 (31.4) | 1851 (32.3) | 615 (29.0) | |||
| Alcohol use, n (%) | 0.002 | <0.001 | 0.617 | ||||
| Daily | 61 (7.9) | 767 (9.8) | 596 (10.4) | 171 (8.1) | |||
| Less than daily | 515 (67.0) | 5512 (70.2) | 4057 (70.7) | 1455 (68.6) | |||
| None | 193 (25.1) | 1578 (20.1) | 1083 (18.9) | 495 (23.3) | |||
| Exercise, mean (SD), days in past month | 6.04 (7.78) | 5.70 (7.65) | 0.248 | 5.70 (7.56) | 0.256 | 5.69 (7.88) | 0.298 |
BMI, body mass index; CCI, Charlson comorbidity index; CU, chronic urticaria; PsO, psoriasis; SD, standard deviation.
Compared with the overall PsO patients, the CU patients were more likely to have various comorbidities such as nasal allergies, dermatological conditions other than CU, severe allergic asthma and dyspepsia. The mean (SD) CCI scores were significantly higher in the CU patients than in the overall PsO patients [mean (SD): 0.9 (2.1) vs. 0.5 (1.1), P < 0.001] (Table 1).
HRQoL impairment
The bivariate analysis showed that HRQoL was lower in CU patients compared to overall PsO patients. The mean MCS, PCS and SF‐6D utility scores were 3.7, 2.2 and 0.05 points lower (P < 0.001) in the CU patients when compared to overall PsO patients. Mean difference scores for both MCS and SF‐6D were greater than the MID, except for PCS (Fig. 1a). The regression analysis showed significantly worse HRQoL in the CU patients than in the overall PsO patients, as expressed by lower MCS and PCS scores (−2.4 and −1.6 points difference; P < 0.001) (Fig. 1b). Health utility scores in the CU patients were lower by 0.03 points, which was at the MID for the SF‐6D. MCS, PCS and SF‐6D scores were lower by 3.0, 1.9 and 0.04 points, respectively, in the CU patients in comparison with mild PsO. Compared with moderate/severe PsO patients, CU patients showed a similar reduction in these scores.
Figure 1.
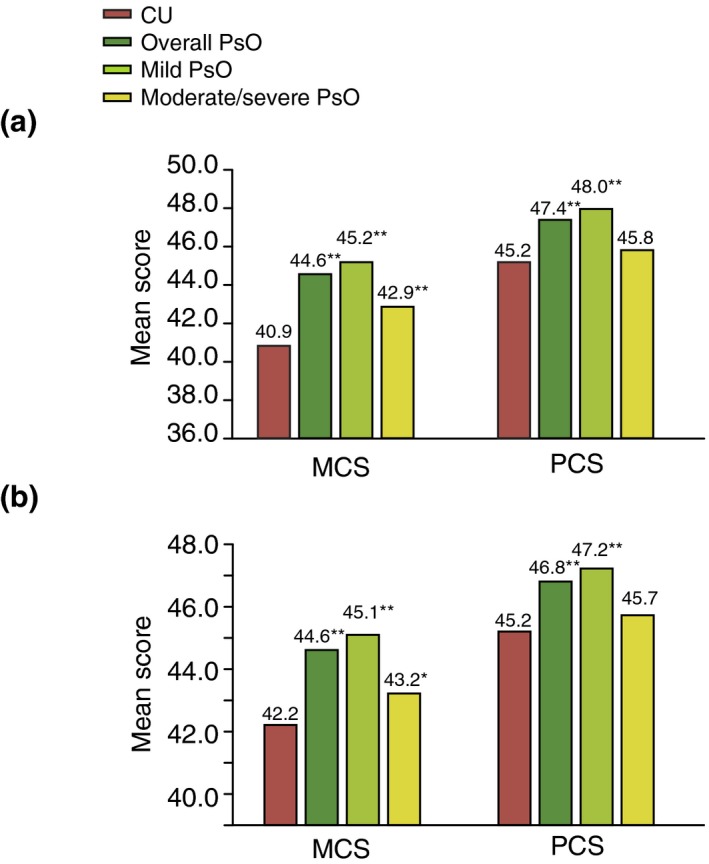
Mean physical (PCS) and mental (MCS) component summary scores in CU patients relative to PsO patients (overall, mild and moderate/severe). (a) Bivariate analysis. **P < 0.05; (b) Multivariate analysis, *P < 0.05; **P < 0.001; CU, chronic urticaria; MCS, Mental Component Summary score; PCS, Physical Component Summary score; PsO, psoriasis.
Self‐reported psychological complaints
The bivariate analysis showed that significantly more CU patients reported anxiety, depression and sleep difficulties in the past 12 months compared with overall PsO and mild PsO patients. The difference was still significant compared with moderate/severe PsO patients on depression and sleep difficulty but not on anxiety (Fig. 2).
Figure 2.
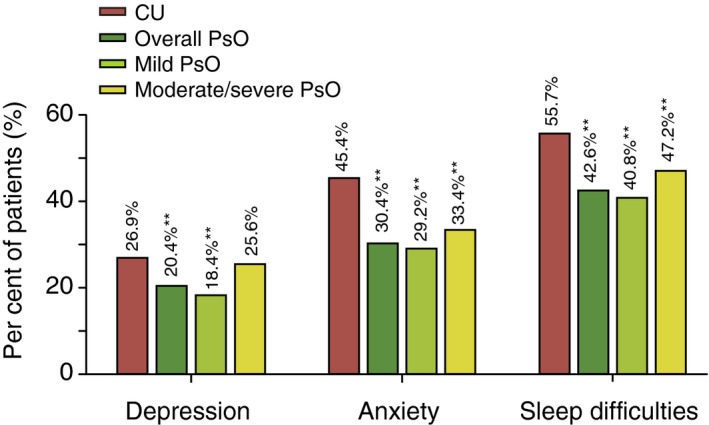
Self‐reported psychological complaints in CU patients relative to PsO patients (overall, mild and moderate/severe): bivariate analysis. **P < 0.001; CU, chronic urticaria; PsO, psoriasis.
The regression analysis showed that the CU patients had significantly higher adjusted odds of anxiety, depression and sleep difficulties in comparison with overall PsO. Compared with mild PsO, these differences were even higher. The CU and moderate/severe PsO patients showed no significant difference in the odds of psychological complaints (Table 2).
Table 2.
Risk of psychological complaints in the CU vs. PsO patients (overall, mild and moderate/severe): Multivariate analysis
| CU vs. Overall PsO OR (95% CI) | P value | CU vs. Mild PsO OR | P value | CU vs. Moderate/Severe PsO OR | P value | |
|---|---|---|---|---|---|---|
| Anxiety | 1.63 (1.39–1.92) | <0.001 | 1.70 | <0.001 | 1.45 | 0.109 |
| Depression | 1.34 (1.12–1.60) | 0.002 | 1.44 | <0.001 | 1.12 | 0.315 |
| Sleep difficulties | 1.56 (1.33–1.82) | <0.001 | 1.63 | <0.001 | 1.36 | 0.329 |
CU, chronic urticaria; OR, odds ratio; PsO, psoriasis.
Work and activity impairment
No difference in the labour force participation was observed between the CU and overall PsO patients (Table 1). Results of the bivariate analysis showed that the CU patients reported significantly higher presenteeism, overall work impairment and activity impairment than the overall PsO and mild PsO patients. Compared with moderate/severe PsO, all scores were not significantly different (Fig. 3). Results of the regression analysis showed that all work‐related scores were comparable among CU, overall PsO and its subgroups. The activity impairment score was significantly higher for CU patients compared to that reported by overall PsO and mild PsO patients, but was similar to that reported by moderate/severe PsO patients (Table 3).
Figure 3.
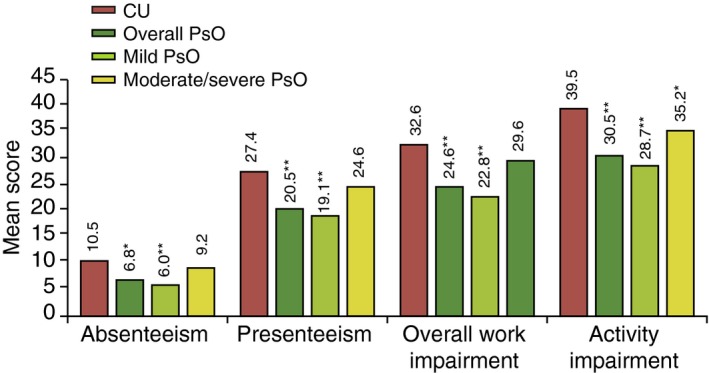
Work Productivity and Activity Impairment (WPAI) scores in CU patients relative to PsO patients (overall, mild and moderate/severe): bivariate analysis. **P < 0.05; *P < 0.001; CU, chronic urticaria; PsO, psoriasis.
Table 3.
Work Productivity and Activity Impairment (WPAI) results in the CU patients vs. PsO patients (overall, mild and moderate/severe): Multivariate analysis
| CU vs. overall PsO RR (95% CI) | P value | CU vs. Mild PsO RR | P value | CU vs. Moderate/Severe PsO RR | P value | |
|---|---|---|---|---|---|---|
| Absenteeism | 1.31 (0.78–2.19) | 0.305 | 1.45 | 0.163 | 0.99 | 0.972 |
| Presenteeism | 1.15 (0.92–1.42) | 0.223 | 1.22 | 0.077 | 0.97 | 0.808 |
| Overall work impairment | 1.15 (0.93–1.42) | 0.190 | 1.22 | 0.061 | 0.97 | 0.830 |
| Activity impairment | 1.21 (1.07–1.37) | 0.002 | 1.26 | <0.001 | 1.08 | 0.259 |
CI, confidence interval; CU, chronic urticaria; PsO, psoriasis; RR, relative risk.
Healthcare resource use
Results of the bivariate analysis showed that significantly more CU patients had HCPs visits and ER visits or were hospitalized in the past 6 months than the overall PsO patients. The mean number of visits to any type of HCPs (overall HCP) was significantly higher in the CU patients [mean (SD): 9.05 (9.83) vs. 7.66 (9.38), P = 0.001] than in the overall PsO patients. The number of visits to allergists and psychiatrists was also significantly higher in the CU patients than that in the overall PsO patients (Table 4a).
Table 4.
Healthcare visits in CU patients vs. PsO patients (overall, mild and moderate/severe) (a) Bivariate analysis (b) Multivariate analysis
| (a) | CU (n = 769) | Overall PsO (n = 7857) | P value | Mild PsO (n = 3468) | P value | Moderate/severe PsO (n = 2121) | P value |
|---|---|---|---|---|---|---|---|
| Traditional HCP | |||||||
| Number of visits, mean (SD) | 9.05 (9.83) | 6.85 (8.46) | <0.001 | 6.55 (8.08) | <0.001 | 7.66 (9.38) | 0.001 |
| Visited, n (%) | 735 (95.6) | 7160 (91.1) | <0.001 | 5234 (91.2) | <0.001 | 1926 (90.8) | <0.001 |
| General practitioner | |||||||
| Number of visits, mean (SD) | 3.08 (3.93) | 2.52 (3.21) | <0.001 | 2.47 (3.17) | <0.001 | 2.68 (3.32) | 0.007 |
| Visited, n (%) | 601 (78.2) | 5998 (76.3) | 0.258 | 4396 (76.6) | 0.350 | 1602 (75.5) | 0.143 |
| Allergist | |||||||
| Number of visits, mean (SD) | 0.21 (0.59) | 0.06 (0.50) | <0.001 | 0.06 (0.52) | <0.001 | 0.08 (0.44) | <0.001 |
| Visited, n (%) | 103 (13.4) | 273 (3.5) | <0.001 | 173 (3.0) | <0.001 | 100 (4.7) | <0.001 |
| Dermatologist | |||||||
| Number of visits, mean (SD) | 0.38 (0.97) | 0.37 (1.31) | 0.818 | 0.27 (0.92) | 0.002 | 0.64 (1.99) | 0.001 |
| Visited, n (%) | 164 (21.3) | 1474 (18.8) | 0.083 | 918 (16.0) | <0.001 | 556 (26.2) | 0.007 |
| Psychiatrist | |||||||
| Number of visits, mean (SD) | 0.33 (1.75) | 0.25 (1.75) | 0.273 | 0.22 (1.49) | 0.073 | 0.34 (2.33) | 0.879 |
| Visited, n (%) | 64 (8.3) | 400 (5.1) | <0.001 | 273 (4.8) | <0.001 | 127 (6.0) | 0.026 |
| Psychologist/psychotherapist | |||||||
| Number of visits, mean (SD) | 0.57 (2.96) | 0.36 (2.34) | 0.018 | 0.34 (2.35) | 0.013 | 0.41 (2.28) | 0.116 |
| Visited, n (%) | 56 (7.3) | 375 (4.8) | 0.002 | 244 (4.3) | <0.001 | 131 (6.2) | 0.286 |
| Other traditional HCP | |||||||
| Number of visits, mean (SD) | 4.49 (6.28) | 3.28 (5.08) | <0.001 | 3.19 (4.92) | <0.001 | 3.51 (5.47) | <0.001 |
| Visited, n (%) | 624 (81.1) | 5738 (73.0) | <0.001 | 4213 (73.4) | <0.001 | 1525 (71.9) | <0.001 |
| Non‐traditional HCP, % | |||||||
| Visited, n (%) | 217 (28.2) | 1397 (17.8) | <0.001 | 1016 (17.7) | <0.001 | 381 (18) | <0.001 |
| ER | |||||||
| Number of visits, mean (SD) | 0.75 (3.11) | 0.25 (1.04) | <0.001 | 0.23 (1.02) | <0.001 | 0.30 (1.10) | <0.001 |
| Visited, n (%) | 198 (25.7) | 1070 (13.6) | <0.001 | 736 (12.8) | <0.001 | 334 (15.7) | <0.001 |
| Hospitalization | |||||||
| Number of visits, mean (SD) | 0.43 (2.37) | 0.17 (0.75) | <0.001 | 0.15 (0.64) | <0.001 | 0.23 (0.99) | 0.001 |
| Visited, n (%) | 117 (15.2) | 841 (10.7) | <0.001 | 552 (9.6) | <0.001 | 289 (13.6) | 0.277 |
| (b) |
CU vs. overall PsO OR (95% CI) |
P value |
CU vs. Mild PsO OR |
P value |
CU vs. Moderate/Severe PsO OR |
P value |
|---|---|---|---|---|---|---|
| Traditional HCP | 1.74 (1.22–2.50) | 0.003 | 1.74 | 0.012 | 1.75 | 0.020 |
| GP | 1.12 (0.93–1.36) | 0.218 | 1.11 | 0.715 | 1.17 | 0.130 |
| Allergist | 3.22 (2.49–4.15) | <0.001 | 3.79 | <0.001 | 2.20 | 0.328 |
| Dermatologist | 1.03 (0.85–1.24) | 0.765 | 1.27 | <0.001 | 0.63 | <0.001 |
| Psychiatrist | 1.37 (1.03–1.84) | 0.032 | 1.44 | 0.013 | 1.23 | 0.836 |
| Psychologist/psychotherapist | 1.15 (0.85–1.57) | 0.363 | 1.27 | 0.013 | 0.93 | 0.129 |
| ER | 1.72 (1.43–2.08) | <0.001 | 1.82 | <0.001 | 1.50 | 0.184 |
| Hospitalization | 1.31 (1.05–1.65) | 0.018 | 1.45 | <0.001 | 1.05 | 0.119 |
| Alternative HCP | 1.40 (1.18–1.68) | <0.001 | 1.43 | <0.001 | 1.32 | 0.192 |
CU, chronic urticaria; ER, emergency room; GP, general practitioner; HCP, healthcare professionals; OR, odds ratio; PsO, psoriasis; SD, standard deviation.
The regression analysis showed that the CU patients were more likely to visit HCPs, ER and be hospitalized compared with the overall PsO patients [odds ratio (OR): 1.74, 1.72, 1.31, respectively, all P < 0.05] and mild PsO patients (Table 4b). The CU and moderate/severe PsO patients had similar odds of hospitalization. The CU patients had a higher but non‐significant odds of ER visits and higher odds of HCP visits overall (OR: 1.74, P = 0.02) than the moderate/severe PsO patients (Table 4b). The odds of visiting an allergist were significantly higher in the CU patients than in the overall PsO patients; no significant difference was observed for other specialties. Except for GP visits, the odds of visiting other specialists were significantly higher in the CU patients than in the mild PsO patients. No significant differences in specialists visited between the CU and moderate/severe PsO patients, except for dermatologist visits which were significantly higher in the moderate/severe PsO patients.
According to the bivariate analysis, the frequency of alternative HCP visits was higher in the CU patients than in the overall PsO patients. However, visits to an acupuncturist, chiropractor and massage therapist were similar between the CU and moderate/severe PsO patients (Fig. 4). Odds of visiting alternative HCPs were significantly higher in the CU patients than in both overall PsO and mild PsO patients but were not different when compared with the moderate/severe PsO patients (Table 4b).
Figure 4.
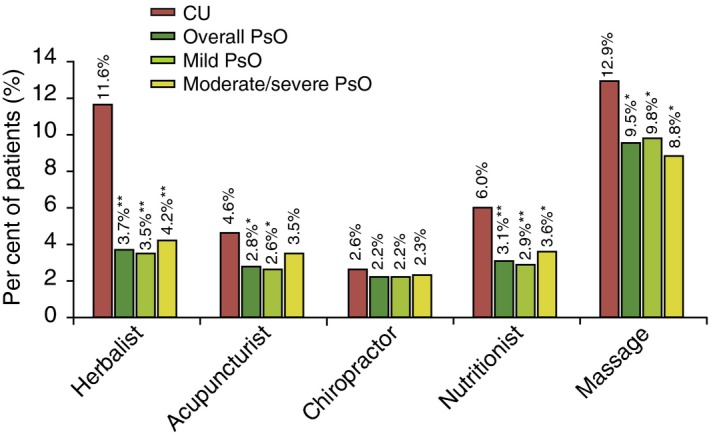
Alternative HCP visits in CU patients relative to PsO patients (overall, mild and moderate/severe): bivariate analysis. **P < 0.05; *P < 0.001; CU, chronic urticaria; HCP, healthcare practitioner; PsO, psoriasis.
Discussion
This is one of the first analyses of patient‐reported real‐world data comparing the burden of illness in CU and PsO from the humanistic and economic perspective in five European countries. The burden of illness measured in terms of HRQoL, psychological complaints, work and non‐work daily activities, and healthcare utilization presents a holistic view on how patients report the impact of their disease and the economic consequences. This analysis suggests that patients with CU and PsO report an overall negative impact of their disease confirmed by the bivariate and multivariate analyses. These findings are consistent with the results of a recent comparative analysis of CU and PsO patients in US NHWS.6
There were significant differences between the CU and PsO patients of all severities on mental and physical health status. The scores were significantly lower (i.e. worse) in the CU patients than the overall PsO patients or mild PsO, but similar to the scores reported by moderate/severe PsO patients. These findings are consistent with the results of previous studies which showed that CU patients have a poorer HRQoL in certain dimensions than patients with PsO6, 10, 17 and atopic dermatitis.17 CU patients report worse scores than PsO patients on mood and physical discomfort in a comparative study published by Grob et al.17 In our study, the risk of having anxiety, depression and sleep difficulties in CU patients was greater when compared with overall and mild PsO and similar to moderate/severe PsO.
The impact of dermatological conditions such as PsO on work, including absenteeism, loss of productivity while at work and the impact on daily activity, increases with the disease severity and is well documented.35 The results of absenteeism, presenteeism and overall work impairment scores in CU patients suggest that the impact of CU is comparable to PsO in these aspects. Therefore, the extent of economic impact from the productivity loss due to CU is likely to be significant, similar to that seen with PsO. In this study, both CU and overall PsO patients reported having at least one HCP visit (visits to GPs, allergists, or ER or hospitalization) in the past 6 months, and CU patients had more frequent visits with an additional three HCP visits per patient within a 6‐month period. Overall, when comparing CU cohort with PsO patient cohort of all severity levels, CU patients have consistently and significantly lower scores on all outcomes, but when comparing only with the moderate/severe PsO group, the scores reflected similar impairment and impact.
Although the NHWS is representative of the adult population from the included countries in terms of age and gender, the panel‐based recruitment may introduce a potential bias for variables, such as education, which were not incorporated into the sampling strata. However, because cases of both CU and PsO were drawn from the same panel, this limitation would be expected to have a minimal impact on the estimated comparative burden of these conditions. The major limitations of the NHWS are the self‐reported data, which are based on patient recall of diagnoses and self‐reporting of outcomes which cannot be confirmed. One of the influencing factors which affect QoL is patient–physician relationship in both CU and PsO patients, and this has not been accounted for in this study.36, 37, 38 Another limitation might be that medical resource use was not collected for each individual comorbidity, but for the general health of the respondent. As the exact type of CU was not collected, the study was conducted in respondents diagnosed with CU and used as a proxy for CSU. Patients with confirmed CSU may have different specific characteristics than CU, and therefore, these study results may underestimate the full impact of CSU on patients. In addition, this study did not include data on severity of CU; moreover, diagnosis of CU and its severity levels are more challenging than that of PsO.3 A quick self‐assessment method of disease activity/severity does not exist yet in CU. The sample size of PsO was much larger than that of CU in this study. Even if this might be due to variation in the NHWS sampling method, it reflects the difference of prevalence between these diseases.4, 19 Self‐assessment of severity in PsO based on a validated, accepted method reflected a higher proportion of mild PsO patients compared to moderate/severe, but this is consistent with real‐world data.20 Other possible reason for this might be, as NHWS is a 30‐min online survey, PsO patients with high disease severity might be less able to participate in the survey. However, there is no reason to believe our study has unusually mild psoriasis representation or unusually severe urticaria patients, especially as both patient populations are obtained from the same survey (NHWS) representative for the general population in the respective countries. Advanced and efficacious medications are available for PsO; hence, many PsO patients may report current severity as mild.
In conclusion, the results of the current study provide further evidence that CU is associated not only with a significant detrimental impact on the HRQoL but also with a considerable impairment of productivity and high use of healthcare resources as PsO patients. Better management of CU will benefit the well‐being of patients and potentially reduce its impact on employers, payers and the healthcare system.
Acknowledgement
Medical writing and editorial assistance were provided by Mrs. Vijayalakshmi Vasanthaprasad of Novartis Healthcare Pvt Ltd, Hyderabad.
Conflicts of interest
Torsten Zuberbier has acted as a consultant for AnseIl, Bayer Schering, DST, FAES, Fujisawa, HAL, Henkel, Kryolan, Leti, Menarini, Merck, MSD, Novartis, Procter and Gamble, Ranbaxy, Sanofi‐Aventis, Schering Plough, Stallergenes, Takeda and UCB. Maria‐Magdalena Balp and Sam Khalil are employees of Novartis Pharma AG. Haijun Tian is an employee of Novartis Pharmaceutical Corporation, and Susan Gabriel was an employee of Novartis Pharmaceutical Corporation, at the time of study conduct. At the time of the analysis, Jeffrey Vietri was an employee of Kantar Health, to which Novartis paid fees for analysis and reporting.
Funding sources
The study was supported by Novartis Pharma AG, Basel, Switzerland, and Genentech, Inc., South San Francisco, CA, USA.
The results of this study have been partly presented as poster presentations at the 24th European Academy of Dermatology and Venereology Congress in Copenhagen, Denmark (7–11 October 2015).
The copyright line for this article was changed on 27 July 2018 after original online publication.
References
- 1. Hay RJ, Johns NE, Williams HC et al The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol 2014; 134: 1527–1534. [DOI] [PubMed] [Google Scholar]
- 2. Zuberbier T, Asero R, Bindslev‐Jensen C et al EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy 2009; 64: 1417–1426. [DOI] [PubMed] [Google Scholar]
- 3. Zuberbier T, Aberer W, Asero R et al The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy 2014; 69: 868–887. [DOI] [PubMed] [Google Scholar]
- 4. Maurer M, Weller K, Bindslev‐Jensen C et al Unmet clinical needs in chronic spontaneous urticaria. A GA(2)LEN task force report. Allergy 2011; 66: 317–330. [DOI] [PubMed] [Google Scholar]
- 5. Lewis V, Finlay AY. 10 years experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc 2004; 9: 169–180. [DOI] [PubMed] [Google Scholar]
- 6. Vietri J, Turner SJ, Tian H, Isherwood G, Balp MM, Gabriel S. Effect of chronic urticaria on US patients: analysis of the National Health and Wellness Survey. Ann Allergy Asthma Immunol 2015; 115: 306–311. [DOI] [PubMed] [Google Scholar]
- 7. Weller K, Viehmann K, Brautigam M et al Cost‐intensive, time‐consuming, problematical? How physicians in private practice experience the care of urticaria patients. J Dtsch Dermatol Ges 2012; 10: 341–347. [DOI] [PubMed] [Google Scholar]
- 8. Delong LK, Culler SD, Saini SS, Beck LA, Chen SC. Annual direct and indirect health care costs of chronic idiopathic urticaria: a cost analysis of 50 nonimmunosuppressed patients. Arch Dermatol 2008; 144: 35–39. [DOI] [PubMed] [Google Scholar]
- 9. Tian H, Chambenoit O, Chiva‐Razavi S et al Healthcare resource utilisation among chronic spontaneous/idiopathic urticaria patients‐ findings from the first international burden of illness Study (Assure‐CSU). Value Health 2015; 18: A424. [Google Scholar]
- 10. Balp MM, Vietri J, Tian H, Isherwood G. The impact of chronic urticaria from the patient's perspective: a survey in five European countries. Patient 2015; 8: 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ben‐Shoshan M, Blinderman I, Raz A. Psychosocial factors and chronic spontaneous urticaria: a systematic review. Allergy 2013; 68: 131–141. [DOI] [PubMed] [Google Scholar]
- 12. Ozkan M, Oflaz SB, Kocaman N et al Psychiatric morbidity and quality of life in patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol 2007; 99: 29–33. [DOI] [PubMed] [Google Scholar]
- 13. Staubach P, Dechene M, Metz M et al High prevalence of mental disorders and emotional distress in patients with chronic spontaneous urticaria. Acta Derm Venereol 2011; 91: 557–561. [DOI] [PubMed] [Google Scholar]
- 14. Kolkhir P, Pogorelov D, Olisova O, Maurer M. Comorbidity and pathogenic links of chronic spontaneous urticaria and systemic lupus erythematosus – a systematic review. Clin Exp Allergy 2015; 46: 275–287. [DOI] [PubMed] [Google Scholar]
- 15. Sugiyama A, Nishie H, Takeuchi S, Yoshinari M, Furue M. Hashimoto's disease is a frequent comorbidity and an exacerbating factor of chronic spontaneous urticaria. Allergol Immunopathol (Madr) 2015; 43: 249–253. [DOI] [PubMed] [Google Scholar]
- 16. O'Donnell BF, Lawlor F, Simpson J, Morgan M, Greaves MW. The impact of chronic urticaria on the quality of life. Br J Dermatol 1997; 136: 197–201. [PubMed] [Google Scholar]
- 17. Grob JJ, Revuz J, Ortonne JP, Auquier P, Lorette G. Comparative study of the impact of chronic urticaria, psoriasis and atopic dermatitis on the quality of life. Br J Dermatol 2005; 152: 289–295. [DOI] [PubMed] [Google Scholar]
- 18. Zazzali JL, Broder MS, Chang E, Chiu MW, Hogan DJ. Cost, utilization, and patterns of medication use associated with chronic idiopathic urticaria. Ann Allergy Asthma Immunol 2012; 108: 98–102. [DOI] [PubMed] [Google Scholar]
- 19. Parisi R, Symmons DPM, Griffiths CEM, Ashcroft DM, Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team . Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013; 133: 377–385. [DOI] [PubMed] [Google Scholar]
- 20. Takeshita J, Gelfand JM, Li P et al Psoriasis in the US medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol 2015; 135: 2955–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol 1999; 41: 401–407. [DOI] [PubMed] [Google Scholar]
- 22. Feldman SR, Burudpakdee C, Gala S, Nanavaty M, Mallya UG. The economic burden of psoriasis: a systematic literature review. Expert Rev Pharmacoecon Outcomes Res 2014; 14: 685–705. [DOI] [PubMed] [Google Scholar]
- 23. Augustin M, Kruger K, Radtke MA, Schwippl I, Reich K. Disease severity, quality of life and health care in plaque‐type psoriasis: a multicenter cross‐sectional study in Germany. Dermatology 2008; 216: 366–372. [DOI] [PubMed] [Google Scholar]
- 24. Dubertret L, Mrowietz U, Ranki A et al European patient perspectives on the impact of psoriasis: the EUROPSO patient membership survey. Br J Dermatol 2006; 155: 729–736. [DOI] [PubMed] [Google Scholar]
- 25. Mattei PL, Corey KC, Kimball AB. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI): the correlation between disease severity and psychological burden in patients treated with biological therapies. J Eur Acad Dermatol Venereol 2014; 28: 333–337. [DOI] [PubMed] [Google Scholar]
- 26. Navarini AA, Laffitte E, Conrad C et al Estimation of cost‐of‐illness in patients with psoriasis in Switzerland. Swiss Med Wkly 2010; 140: 85–91. [DOI] [PubMed] [Google Scholar]
- 27. Kantar Health . The National Health and Wellness Survey. [WWW document] 2017. URL http://www.kantarhealth.com/docs/datasheets/kh-national-health-and-wellness-survey.pdf (last accessed: 2 February 2017).
- 28. National Psoriasis Foundation . Psoriasis is an immune‐mediated disease that causes raised, red, scaly patches to appear on the skin. [WWW document] 2017. URL https://www.psoriasis.org/about-psoriasis (last accessed: 2 February 2017).
- 29. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 30. Maruish ME. User's Manual for the SF‐36v2 Health Survey, 3rd edn Quality Metric Inc, Lincoln, RI, 2011. [Google Scholar]
- 31. Ware J, Kosinski M, Turner‐Bowker DM, Gandek B. SF‐12v2: How to Score Version 2 of the SF‐12 Health Survey. Quality Metric Inc.; Health Assessment Lab, Lincoln, RI; Boston, MA, 2002. [Google Scholar]
- 32. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993; 4: 353–365. [DOI] [PubMed] [Google Scholar]
- 33. Johnston BC, Ebrahim S, Carrasco‐Labra A et al Minimally important difference estimates and methods: a protocol. BMJ Open 2015; 5: e007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ‐5D and SF‐6D. Qual Life Res 2005; 14: 1523–1532. [DOI] [PubMed] [Google Scholar]
- 35. Pearce DJ, Singh S, Balkrishnan R, Kulkarni A, Fleischer AB, Feldman SR. The negative impact of psoriasis on the workplace. J Dermatolog Treat 2006; 17: 24–28. [DOI] [PubMed] [Google Scholar]
- 36. Patruno C, Ayala F, Megna M, Napolitano M, Balato N. Patient‐physician relationship in patients with psoriasis. Indian J Dermatol Venereol Leprol 2012; 78: 228. [DOI] [PubMed] [Google Scholar]
- 37. Linder D, Sampogna F, Torreggiani A, Balato N, Bianchi L, Cassano N. Psodisk, a new visual method for assessing the burden of psoriasis on patients. J Eur Acad Dermatol Venereol 2012; 26: 1163–1166. [DOI] [PubMed] [Google Scholar]
- 38. Maurer M, Ortonne JP, Zuberbier T. Patient–physician relationship in patients with psoriasis. Allergy 2009; 64: 581–588. [DOI] [PubMed] [Google Scholar]


