Summary
There is strong evidence of roles of the hypothalamus‐pituitary‐adrenal axis and nitric oxide (NO) synthase‐NO system in depression, but the relationship between them is unknown. The aim of this study, therefore, was to elucidate whether there is any correlation between NO and corticotropin‐releasing hormone (CRH) in major depressive disorder (MDD) patients. In 16 outpatients with MDD and 18 healthy controls, the plasma amino acids citrulline (Cit) and arginine (Arg) were determined by high‐performance liquid chromatography, and CRH levels was measured by radioimmunoassay. The Cit/Arg ratio was calculated as an index of NO synthesis. Correlations between NO and CRH were examined with the Spearman test. Before treatment, no significant correlation was observed between the plasma NO level and CRH levels in MDD patients. The plasma NO levels were significantly higher in MDD patients. A significant correlation was found between NO levels and Hamilton Depression Rating Scale (HAMD) scores in MDD patients. The plasma CRH levels were significantly higher in MDD patients than in controls. After monotherapy for 2 months, the NO levels had dramatically declined but were also higher than those in the controls. This study is the first report of the absence of a significant correlation between plasma NO and CRH levels, although both levels are elevated in MDD patients. Furthermore, the strong links between the plasma NO levels and the HAMD scores, as well as the increased NO reduction after remission, suggest that NO plays a key role in depression and may be an indicator of therapeutic success.
Keywords: arginine, citrulline, corticotropin‐releasing hormone, major depressive disorder, nitric oxide
1. INTRODUCTION
Major depressive disorder (MDD) is a prevalent, often recurrent debilitating illness accompanied by severe functional impairment, high mortality, and a heavy health care burden.1 Numerous hypotheses and pathways have been suggested to be involved in MDD, but the underlying mechanism remains unclear.
Nitric oxide (NO) is a highly diffusible and reactive molecule synthesized and released with the assistance of nitric oxide synthases (NOSs), which convert arginine into citrulline, producing NO in the process.2 NO has been shown to modulate the functions of different neurotransmitters, including norepinephrine, serotonin, glutamate and dopamine, and thus plays an important role in the neurobiology of major depression.3 Altered NO levels have been found in depression, not only in different brain regions4, 5 and cerebrospinal fluid (CSF) but also in blood6, 7 and exhaled gas.8 However, there are many inconsistent results in previous studies.9, 10, 11, 12 Our laboratory's previous data have also revealed increased plasma NO levels in patients with first‐episode melancholic MDD.13 Much other work has been done to elucidate the contribution of NO to the pathophysiology of depression. However, there is disagreement regarding its specific function in depression, as has been reviewed by Dhir and Kulkarni.3
Previous animal experiments have indicated the co‐localization of NOS1 with corticotropin‐releasing hormone (CRH) in the hypothalamus paraventricular nucleus (PVN),14 and NO modulates the release of CRH.15 The hypothalamo‐pituitary‐adrenal (HPA) axis is the key regulating system for stress responses.16 Activity of the CRH neurons in the PVN forms the basis of HPA‐axis activity.17 Several findings have suggested a central role of CRH in the neurobiology of depression. However, to date, there are inconsistent findings concerning the CRH levels (whether central or peripheral) in clinical depression patients. Decreased,18, 19 increased,20 or unchanged21 CRH levels have been reported in depression. A series of studies have reported a decreased number of nNOS neurons in the PVN in patients with depression22 but increased numbers of CRH neurons23 and higher CRH‐mRNA levels in the PVN in depressed patients.24 Hence, the hypothesis that the NOS‐NO system modulates HPA‐axis function has been proposed in the past decade. Unfortunately, those data have come from various laboratories using different methods. Most conclusions have been derived from postmortem human brain slices or animal studies, and no studies of the relationship between NO and CRH have been conducted on special subtypes of depression in clinical patients. In fact, our previous data have yielded different results, showing no co‐localization of NOS1 and CRH in the rat PVN;25 moreover, no significant correlations were found between plasma NO and cortisol levels in either the control or the MDD group, and no significant correlations were found between plasma NO and corticosterone levels in the animal control group or in the CUS group.25
In the present study, we aimed to further elucidate the relationship between plasma NO and plasma CRH in clinical MDD patients. To do so, we analyzed the changes in plasma CRH levels, NO levels and NO in pre‐ and post‐treatment MDD patients.
2. RESULTS
No significant differences were found in age, sex, race, years of education, body mass index (BMI) and Hamilton Depression Rating Scale (HAMD) scores between the MDD group and the control group (Table 1). Moreover, these demographic and clinical characteristics were similar between MDD and control patients in both male and female subgroups. After the first blood collection, all patients were treated with the selective serotonin reuptake inhibitor (SSRI) escitalopram at 10‐20 mg/day. Thirteen patients had monotherapy, and another three patients underwent combination therapy with alprazolam (0.4‐0.8 mg/day) because of insomnia and anxiety. Visits were made once per 2 weeks until 2 months after the first visit. The patients voluntarily joined the follow‐up study. At the last visit, the patients’ symptoms and HAMD scores were evaluated again, together with their blood samples being taken. Five patients dropped out for various reasons (1 for severe insomnia, 2 for nausea, and 2 for restlessness).
Table 1.
Sociodemographic and clinical profiles
| Variables | MDD (n = 16) | CONT (n = 18) | P valuea |
|---|---|---|---|
| Age/years | 50.63 ± 2.975 | 46.17 ± 2.801 | .2833 |
| Gender (male/female) | 7/9 | 8/10 | .9118 |
| Race (Han%) | 100% | 100% | 1 |
| Education/years | 10.69 ± 0.9207 | 10.56 ± 0.7930 | .9137 |
| Body mass index | 22.81 ± 0.5777 | 22.46 ± 0.5131 | .6460 |
| HAMD | 30.50 ± 2.564 | 5.889 ± 0.6151 | <.0001 |
Significance at P < .05.
MDD, major depressive disorder; CONT, Control.
The total MDD group showed a significant increase in NO content compared with that in the healthy control group (median 1.45 vs 0.96, Z = −4.28, P = .000, Figure 1). Both male and female subgroup analyses revealed that MDD patients had a significantly higher plasma NO content than did healthy participants (males: median 1.36 vs 1.11, Z = −3.00, P = .003; females: median 1.52 vs 0.95, Z = −2.94, P = .003). A strong association was found between the plasma NO levels and HAMD scores in the MDD group (rho = 0.63, P = .008; Figure 2). After treatment, the NO level markedly decreased compared with pretreatment levels (median 1.33 vs 1.39, Z = −2.05, P = .041; Figure 3).
Figure 1.
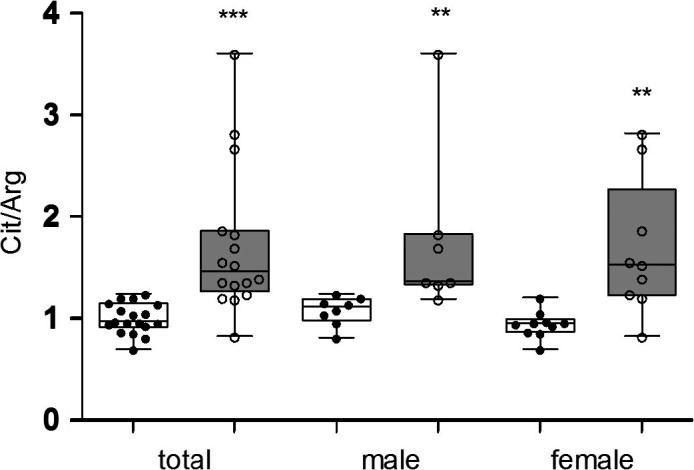
Plasma concentrations of nitric oxide (citrulline/arginine ratio) in ( ) major depressive disorder (MDD) and (
) major depressive disorder (MDD) and ( ) healthy controls. Both MDD patients and healthy participants were divided into male and female subgroups. In the total analysis, MDD patients showed a significant increase in the plasma NO (Cit/Arg ratio) content compared with that in the healthy control group. Both male and female subgroup analyses showed that MDD patients had significantly higher plasma NO content than that in healthy participants. The data are shown as the median, 25th‐75th percentiles, and the range. Cit, citrulline; Arg, arginine; MDD, major depressive disorder. **P < .01, ***P < .001
) healthy controls. Both MDD patients and healthy participants were divided into male and female subgroups. In the total analysis, MDD patients showed a significant increase in the plasma NO (Cit/Arg ratio) content compared with that in the healthy control group. Both male and female subgroup analyses showed that MDD patients had significantly higher plasma NO content than that in healthy participants. The data are shown as the median, 25th‐75th percentiles, and the range. Cit, citrulline; Arg, arginine; MDD, major depressive disorder. **P < .01, ***P < .001
Figure 2.
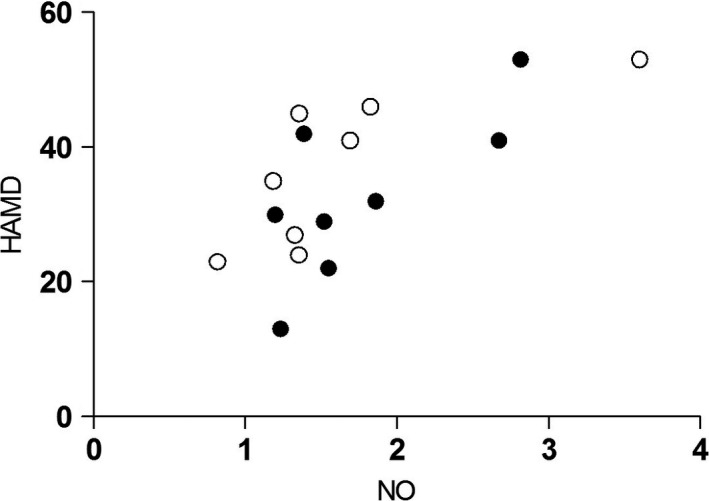
Correlation between plasma nitric oxide levels and Hamilton Depression Scale scores in major depressive disorder. A strong association between plasma NO levels and HAMD scores was observed in the MDD group. NO, nitric oxide; HAMD, Hamilton Depression Scale. (○) Male; (●) Female
Figure 3.
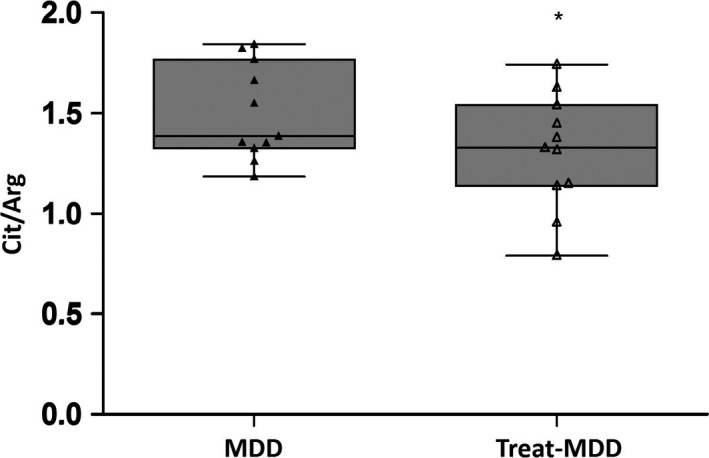
Change in the plasma concentrations of nitric oxide (citrulline/arginine ratio) between pre‐ and post‐ treatment in major depressive disorder. After treatment, the NO levels markedly decreased compared with pretreatment levels but were still higher than those in the control group. Cit, citrulline; Arg, arginine; MDD, major depressive disorder; treat‐MDD, post‐treatment in major depressive disorder. *P < .05, **P < .01, ***P < .001
The plasma CRH level also showed a significant increase in the total MDD group compared with the control group (median 19.48 pg/mL vs 10.55 pg/mL, Z = −3.20, P = .001, Figure 4). The change in CRH in female patients was more pronounced than that in male patients (male: median 18.08 pg/mL vs 11.64 pg/mL, Z = −1.77, P = .077; female: median 20.45 pg/mL vs 11.24 pg/mL, Z = −2.37, P = .018).
Figure 4.
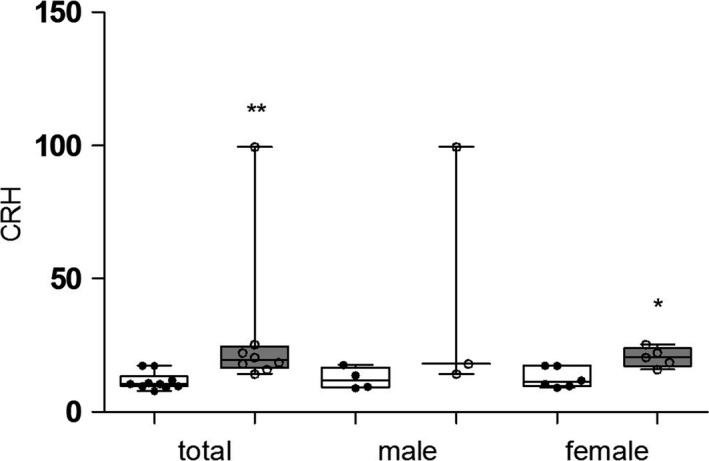
Plasma concentrations of corticotropin‐releasing hormone in ( ) major depressive subjects (MDD) and (
) major depressive subjects (MDD) and ( ) healthy controls. The plasma CRH level also showed a significant increase in the MDD group compared with the control group. The changes in female patients were more pronounced than those in male patients. CRH, corticotropin‐releasing hormone; MDD, major depressive disorder. *P < .05, **P < .01
) healthy controls. The plasma CRH level also showed a significant increase in the MDD group compared with the control group. The changes in female patients were more pronounced than those in male patients. CRH, corticotropin‐releasing hormone; MDD, major depressive disorder. *P < .05, **P < .01
No significant correlation was observed between plasma NO levels and CRH levels (r = .05, P = .91, Figure 5).
Figure 5.
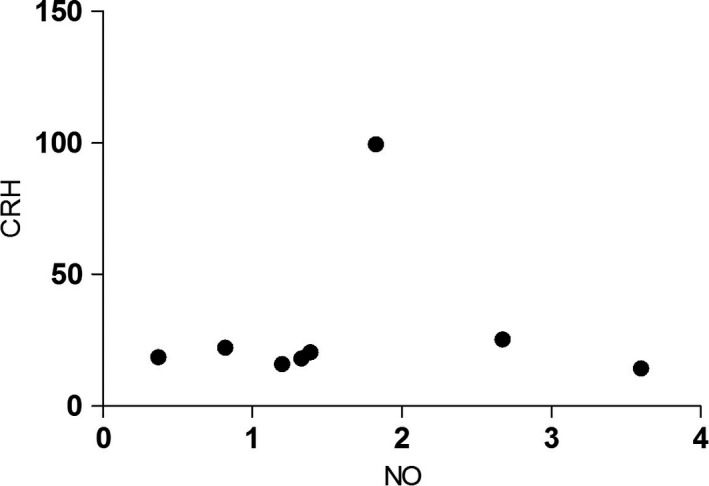
Correlation between plasma nitric oxide levels and corticotropin‐releasing hormone levels in patients with major depressive disorder. No significant correlation was observed between the plasma NO levels and CRH level. CRH, corticotropin‐releasing hormone; NO, nitric oxide
3. DISCUSSION
We found no significant correlation between plasma NO levels and CRH levels, although they both were significantly increased in the MDD group compared with the control group. There was a strong association between plasma NO levels and HAMD scores before treatment.
Herein, we provide the first report of the lack of a significant correlation between plasma NO and CRH levels, in accordance with our previous data showing no co‐localization for NOS1 and CRH in the CUS rat PVN; no significant correlations were found between plasma NO and corticosterone levels either in the CUS rat model or in the control group, and there was no significant correlation between plasma NO and cortisol levels in MDD patients.25 Our results indicated that the NOS‐NO system and HPA axis may function independently, contrary to some previous reports.14, 15, 22, 23, 24 However, most of the previous data have come from various laboratories using different methods. Furthermore, most of the conclusions came not from clinical patients but from postmortem human brain slices or animal studies, and most of those studies did not focus on special subtypes of depression.
In the present study, the levels of NO were also significantly higher in the MDD patients and declined after antidepressant treatment. In this study, the ratio of the amino acids citrulline and arginine (Cit‐Arg ratio) was calculated as an index of NO synthesis. This method has been reported to be sufficiently accurate and reproducible,5 and it can be used as an effective index to reflect the NOS‐NO system activity.26, 27, 28 Furthermore, we selected a special subtype of depression (MDD) for study, using the same SSRI monotherapy. In addition, we collected blood samples before treatment. Our result was consistent with those from other laboratories.7, 29 Our published data revealed increased plasma NO levels both in a male rat model of chronic unpredictable stress25 and in first‐episode MDD patients.13 Plasma levels of NO metabolites, i.e., nitrite and nitrate, which reflect plasma NO concentrations, have also been reported to increase depression.6, 30 Of course, there are still some inconsistent findings in previous studies,9, 10, 11, 12 possibly because of different methods, different subtypes of depression or the different treatments the patients received, and multiple depression comorbidities. Therefore, in this study, we excluded the above factors.
A strong association between plasma NO levels and HAMD scores was also revealed, thus indicating that the NO alterations were consistent with the severity of depressive symptoms. After antidepressant treatment, the concentrations of NO declined, as we have previously reported.13 Therefore, the plasma NO level may be a monitor of depressive systems or may forecast the outcome of anti‐depression treatment. Interestingly, multiple antidepressants have been reported to change the NO levels in an animal's body,3, 31, 32 and clinical studies have also confirmed the NO modulatory activity of various antidepressants, particularly those belonging to the SSRI class.3 Hence, it is estimated that future antidepressants that may act partly on the NO signaling pathway might be helpful for the treatment of drug‐resistant depression.
Our results indicated that the plasma CRH levels increased in MDD patients, in agreement with previous clinical results33, 34 and postmortem human brain studies,23, 24, 35 thus revealing that hyperactivity of hypothalamic CRH neurons is involved in the progression of depression. It has also been shown that administration of CRH‐R1 antagonists has clear antidepressant‐like effects in depression.36, 37 We observed a more significantly increased CRH level in female patients than in males, thus revealing a closer relationship between the HPA axis and female depression.
Several limitations should be noted in the present study. First, we collected only blood samples and not CSF samples of the patients, for ethical reasons. Some researchers have proposed that the plasma levels of these neuroactive amino acids might, to a certain degree, reflect their brain levels.4 Moreover, some researchers have interpreted this finding as evidence that the plasma CRH measured had a hypothalamic origin.33, 38 Second, our results came from a sample that was relatively small because we had to strictly control many factors. For example, the MDD patients had decreased appetites and decreased food intake, thus leading to a decreased metabolism and decreased synthesis of these amino acids. Consequently, we limited the BMI range in the present study.
In conclusion, this is the first report of a lack of a significant correlation between increased plasma NO and CRH levels, thus indicating that these two systems may function independently. Furthermore, the strong links between the plasma NO levels and the HAMD scores suggested that NO might be correlated with the severity of depression. The decreased NO levels after remission suggested that NO may be an indicator of therapeutic success and is useful for monitoring during therapy in MDD patients.
4. METHODS
4.1. Study design
This was a retrospective case‐control study that included three parts: The first part was to analyze the plasma NO levels in MDD patients and the changes in NO levels in pre‐ and post‐treatment patients. The second part was to evaluate the plasma CRH levels in MDD patients. The third part was to elucidate the relationship between NO and CRH in MDD patients. All subjects were evaluated through standard physical examinations, routine clinical laboratory tests and psychological assessments (including HAMD scores) when recruited. They had a follow‐up once every 2 weeks; after 2 months of SSRI treatment, at the last visit, the patients’ symptoms and HAMD scores were evaluated again, together with their blood samples being taken. The investigation was conducted in accordance with the latest version of the Declaration of Helsinki. All subjects signed informed consent forms, and the study was approved by the Medical Ethics Committee of the Fourth Affiliated Hospital of Zhejiang University School of Medicine.
4.2. Subjects
Sixteen Chinese Han untreated outpatients with MDD (7 males and 9 females, mean age of 47 years) and 18 age‐ and sex‐matched control subjects (8 males and 10 females, mean age of 45 years) were recruited to the study by the Department of Psychiatry of the Fourth Affiliated Hospital of Zhejiang University School of Medicine from Oct 2014 to Oct 2016. They were diagnosed with MDD according to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM‐V) by qualified psychiatrists. The Mini International Neuropsychiatric Interview (Chinese modified version) was used to confirm the DSM‐V diagnosis. The 24‐item Hamilton Depression Scale (HAMD) was used to rate the severity of depression.
Exclusion criteria were pregnancy; a serious suicide attempt; current use of another medication that could interact with an SSRI; current substance or alcohol abuse disorders; previous attendance at or completion of one of the study treatments; a primary diagnosis of schizophrenia, bipolar disorder, eating disorders or other psychiatric diagnoses, involving Axis I and II disorders; other physical illnesses (diabetes, endocrine disorders, hepatitis, cancer, or chronic infections); or an abnormal body mass index (BMI ≤ 18 or BMI ≥ 25).
4.3. Blood sample collection and management
Fasting venous blood samples were collected into anticoagulated tubes (containing EDTA) in the morning (08.00 hours). After being centrifuged (4°C, 12 000 g) for 15 minutes, the plasma samples were divided into aliquots and immediately stored at −80°C in the laboratory for further measurement of NO and CRH levels.
4.4. High‐performance liquid chromatography (HPLC) with fluorescence detection (FLD) for plasma amino acid analysis
The protocol was the same as that previously published.13 The plasma samples were deproteinized with acetonitrile (1:1 v/v), lyophilized and diluted with 60% (v/v) methanol before analysis. The plasma amino acid levels were determined by HPLC, which included pre‐column derivatization with o‐phthalaldehyde (Cat: O120; Pickering Laboratories, Inc., Mountain View, CA, USA) and 3‐mercaptopropionic acid (MPA, CAS: 107‐96‐0, Acros Organics, Somerville, NJ, USA), reverse phase separation with an Agilent 1100 series HPLC system and FLD (Agilent Technologies, Santa Clara, CA, USA). Two mobile phases were used in the detection. The linearity of the detector response to standards was in the range of 0.9‐158.2 μmol/L (Cit) and 1.8‐178.0 μmol/L (Arg). The intraday relative standard deviations for the peak area were as follows: Cit, 0.97% and Arg, 0.77%, respectively. The interday relative standard deviations for the peak area were as follows: Cit, 4.29% and Arg, 3.18%, respectively.
4.5. Radioimmunoassay for CRH
Plasma samples were extracted and concentrated to 1 ml and analyzed using a competitive RIA kit (Phoenix Pharmaceutical Company, Harbor Boulevard, Belmont, CA, USA). Anti‐CRH serum can elicit a 100% cross reaction with CRH (1‐41), but no cross response with prepro‐CRH (125‐151), cortisol, adrenocorticotropic hormone, vasopressin and brain natriuretic peptide Acros Organics 45. The limit of detection (LOD) was 10 pg/mL. The 50% binding intercept was 145 pg/mL. The CRH recovery from plasma was 62%,and the intra‐ and inter‐ batch variation coefficient were 6.7% and 10.8%, respectively.
4.6. Statistical analysis
The results of the measurements are presented as the median (quartile). The comparisons between the groups were conducted on the basis of nonparametric statistics, because the small sample size did not provide enough data to discriminate between Gaussian and non‐Gaussian distributions, thereby increasing the risk of an incorrect P value if running parametric tests. A Mann‐Whitney U test was used to measure the differences between the two groups. The comparison between two related samples was analyzed using the Wilcoxon test. Correlations between binary variables were examined with the Spearman test. Pearson correlation analysis was used to assess the correlation between continuous variables. SPSS version 11.5 software was used for the statistical analyses. Differences were considered significant if the two‐sided P value was .05 or less.
DISCLOSURE
All authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
This study was supported by grants from the Education Department Foundation of Zhejiang Province (Y201432662) and the Medical Science and Technology Project of Zhejiang Province (2015KYB232) to Yunrong Lu.
AUTHORS’ CONTRIBUTIONS
Author Yunrong Lu designed the study and wrote the protocol. Authors Yan Zhang, Yingbo Rao and Yu Zhang performed the sample collection, HPLC and RIA analysis. Authors Xi Chen and Han‐fen Lou conducted the psychological tests. Authors Haiyan Xie and Ping Fang were responsible for the patient recruitment and follow‐up. Author Li‐wei Hu undertook the statistical analysis, and author Yunrong Lu wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Lu Y‐R, Zhang Y, Rao Y‐B, et al. The changes in, and relationship between, plasma nitric oxide and corticotropin‐releasing hormone in patients with major depressive disorder. Clin Exp Pharmacol Physiol. 2018;45:10–15. 10.1111/1440-1681.12826
*The copyright line for this article was changed on 6 August 2018 after original online publication.
REFERENCES
- 1. Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS‐R). JAMA. 2003;289:3095‐3105. [DOI] [PubMed] [Google Scholar]
- 2. Griffith OW, Stuehr DJ. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol. 1995;57:707‐736. [DOI] [PubMed] [Google Scholar]
- 3. Dhir A, Kulkarni SK. Nitric oxide and major depression. Nitric Oxide. 2011;24:125‐131. [DOI] [PubMed] [Google Scholar]
- 4. Gao SF, Bao AM. Corticotropin‐releasing hormone, glutamate, and gamma‐aminobutyric acid in depression. Neuroscientist. 2011;17:124‐144. [DOI] [PubMed] [Google Scholar]
- 5. Sethuraman R, Lee TL, Chui JW, Tachibana S. Changes in amino acids and nitric oxide concentration in cerebrospinal fluid during labor pain. Neurochem Res. 2006;31:1127‐1133. [DOI] [PubMed] [Google Scholar]
- 6. Kim YK, Paik JW, Lee SW, Yoon D, Han C, Lee BH. Increased plasma nitric oxide level associated with suicide attempt in depressive patients. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1091‐1096. [DOI] [PubMed] [Google Scholar]
- 7. Akpinar A, Yaman GB, Demirdas A, Onal S. Possible role of adrenomedullin and nitric oxide in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:120‐125. [DOI] [PubMed] [Google Scholar]
- 8. Cepeda MS, Stang P, Makadia R. Depression is associated with high levels of C‐reactive protein and low levels of fractional exhaled nitric oxide: results from the 2007–2012 national health and nutrition examination surveys. J Clin Psychiatry. 2016;77:1666‐1671. [DOI] [PubMed] [Google Scholar]
- 9. Garcia RG, Zarruk JG, Barrera C, et al. Plasma nitrate levels and flow‐mediated vasodilation in untreated major depression. Psychosom Med. 2011;73:344‐349. [DOI] [PubMed] [Google Scholar]
- 10. Chrapko W, Jurasz P, Radomski MW, et al. Alteration of decreased plasma NO metabolites and platelet NO synthase activity by paroxetine in depressed patients. Neuropsychopharmacology. 2006;31:1286‐1293. [DOI] [PubMed] [Google Scholar]
- 11. Chrapko WE, Jurasz P, Radomski MW, Lara N, Archer SL, Le Melledo JM. Decreased platelet nitric oxide synthase activity and plasma nitric oxide metabolites in major depressive disorder. Biol Psychiatry. 2004;56:129‐134. [DOI] [PubMed] [Google Scholar]
- 12. Selley ML. Increased (E)‐4‐hydroxy‐2‐nonenal and asymmetric dimethylarginine concentrations and decreased nitric oxide concentrations in the plasma of patients with major depression. J Affect Disord. 2004;80:249‐256. [DOI] [PubMed] [Google Scholar]
- 13. Lu YR, Fu XY, Shi LG, et al. Decreased plasma neuroactive amino acids and increased nitric oxide levels in melancholic major depressive disorder. BMC Psychiatry. 2014;14:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yamada K, Emson P, Hokfelt T. Immunohistochemical mapping of nitric oxide synthase in the rat hypothalamus and colocalization with neuropeptides. J Chem Neuroanat. 1996;10:295‐316. [DOI] [PubMed] [Google Scholar]
- 15. Costa A, Trainer P, Besser M, Grossman A. Nitric oxide modulates the release of corticotropin‐releasing hormone from the rat hypothalamus in vitro. Brain Res. 1993;605:187‐192. [DOI] [PubMed] [Google Scholar]
- 16. Bao AM, Ruhe HG, Gao SF, Swaab DF. Neurotransmitters and neuropeptides in depression. Handb Clin Neurol. 2012;106:107‐136. [DOI] [PubMed] [Google Scholar]
- 17. Bao AM, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res Rev. 2008;57:531‐553. [DOI] [PubMed] [Google Scholar]
- 18. Geracioti TD Jr, Orth DN, Ekhator NN, Blumenkopf B, Loosen PT. Serial cerebrospinal fluid corticotropin‐releasing hormone concentrations in healthy and depressed humans. J Clin Endocrinol Metab. 1992;74:1325‐1330. [DOI] [PubMed] [Google Scholar]
- 19. Geracioti TD Jr, Loosen PT, Orth DN. Low cerebrospinal fluid corticotropin‐releasing hormone concentrations in eucortisolemic depression. Biol Psychiatry. 1997;42:165‐174. [DOI] [PubMed] [Google Scholar]
- 20. Banki CM, Karmacsi L, Bissette G, Nemeroff CB. CSF corticotropin‐releasing hormone and somatostatin in major depression: response to antidepressant treatment and relapse. Eur Neuropsychopharmacol. 1992;2:107‐113. [DOI] [PubMed] [Google Scholar]
- 21. Carpenter LL, Schecter JM, Tyrka AR, et al. Open‐label tiagabine monotherapy for major depressive disorder with anxiety. J Clin Psychiatry. 2006;67:66‐71. [DOI] [PubMed] [Google Scholar]
- 22. Bernstein HG, Stanarius A, Baumann B, et al. Nitric oxide synthase‐containing neurons in the human hypothalamus: reduced number of immunoreactive cells in the paraventricular nucleus of depressive patients and schizophrenics. Neuroscience. 1998;83:867‐875. [DOI] [PubMed] [Google Scholar]
- 23. Bao AM, Hestiantoro A, Van Someren EJ, Swaab DF, Zhou JN. Colocalization of corticotropin‐releasing hormone and oestrogen receptor‐alpha in the paraventricular nucleus of the hypothalamus in mood disorders. Brain. 2005;128:1301‐1313. [DOI] [PubMed] [Google Scholar]
- 24. Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF. Corticotropin‐releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer's disease and depression. Am J Psychiatry. 1995;152:1372‐1376. [DOI] [PubMed] [Google Scholar]
- 25. Gao SF, Lu YR, Shi LG, et al. Nitric oxide synthase and nitric oxide alterations in chronically stressed rats: a model for nitric oxide in major depressive disorder. Psychoneuroendocrinology. 2014;47:136‐140. [DOI] [PubMed] [Google Scholar]
- 26. Fekkes D, Bannink M, Kruit WH, et al. Influence of pegylated interferon‐alpha therapy on plasma levels of citrulline and arginine in melanoma patients. Amino Acids. 2007;32:121‐126. [DOI] [PubMed] [Google Scholar]
- 27. Schulz R, Nava E, Moncada S. Induction and potential biological relevance of a Ca(2 + )‐independent nitric oxide synthase in the myocardium. Br J Pharmacol. 1992;105:575‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoekstra R, Fekkes D, Pepplinkhuizen L, Loonen AJ, Tuinier S, Verhoeven WM. Nitric oxide and neopterin in bipolar affective disorder. Neuropsychobiology. 2006;54:75‐81. [DOI] [PubMed] [Google Scholar]
- 29. Suzuki E, Yagi G, Nakaki T, Kanba S, Asai M. Elevated plasma nitrate levels in depressive states. J Affect Disord. 2001;63:221‐224. [DOI] [PubMed] [Google Scholar]
- 30. Lee BH, Lee SW, Yoon D, et al. Increased plasma nitric oxide metabolites in suicide attempters. Neuropsychobiology. 2006;53:127‐132. [DOI] [PubMed] [Google Scholar]
- 31. Tomaz VS, Cordeiro RC, Costa AM, et al. Antidepressant‐like effect of nitric oxide synthase inhibitors and sildenafil against lipopolysaccharide‐induced depressive‐like behavior in mice. Neuroscience. 2014;268:236‐246. [DOI] [PubMed] [Google Scholar]
- 32. Krass M, Wegener G, Vasar E, Volke V. The antidepressant action of imipramine and venlafaxine involves suppression of nitric oxide synthesis. Behav Brain Res. 2011;218:57‐63. [DOI] [PubMed] [Google Scholar]
- 33. Galard R, Catalan R, Castellanos JM, Gallart JM. Plasma corticotropin‐releasing factor in depressed patients before and after the dexamethasone suppression test. Biol Psychiatry. 2002;51:463‐468. [DOI] [PubMed] [Google Scholar]
- 34. Catalan R, Gallart JM, Castellanos JM, Galard R. Plasma corticotropin‐releasing factor in depressive disorders. Biol Psychiatry. 1998;44:15‐20. [DOI] [PubMed] [Google Scholar]
- 35. Banki CM, Karmacsi L, Bissette G, Nemeroff CB. Cerebrospinal fluid neuropeptides in mood disorder and dementia. J Affect Disord. 1992;25:39‐45. [DOI] [PubMed] [Google Scholar]
- 36. Kunzel HE, Ising M, Zobel AW, et al. Treatment with a CRH‐1‐receptor antagonist (R121919) does not affect weight or plasma leptin concentration in patients with major depression. J Psychiatr Res. 2005;39:173‐177. [DOI] [PubMed] [Google Scholar]
- 37. Kunzel HE, Zobel AW, Nickel T, et al. Treatment of depression with the CRH‐1‐receptor antagonist R121919: endocrine changes and side effects. J Psychiatr Res. 2003;37:525‐533. [DOI] [PubMed] [Google Scholar]
- 38. Liposits Z, Phelix C, Paull WK. Synaptic interaction of serotonergic axons and corticotropin releasing factor (CRF) synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. A light and electron microscopic immunocytochemical study. Histochemistry. 1987;86:541‐549. [DOI] [PubMed] [Google Scholar]


