Abstract
Purpose
The goal is to investigate the specific contribution of fibroblast‐like synoviocytes (FLS) to the inflammatory milieu of the synovium in juvenile idiopathic arthritis (JIA) through detection of secreted proteins.
Experimental design
Expression of 89 cytokines and chemokines is determined on unprocessed synovial fluid from controls and JIA patients using antibody arrays. Supernatants from pure cell cultures of FLS grown from synovial fluids or tissues from JIA and controls are also examined for protein expression. Ingenuity Pathway Analysis (IPA) is revealed top pathways and upstream regulators of significant proteins.
Results
Protein studies is revealed that JIA FLS release pro‐inflammatory cytokines and chemokines, including IL‐4, IL‐6, IL‐17, CXCL1, and CXCL6, and lose expression of important regulator signals, such as IL‐10 and TIMP2. Of the 84 proteins differentially expressed between controls and JIA in the synovial fluid, 1/3 (29 proteins) are differentially expressed in the cell culture supernatants of JIA and control FLS. ELISA of cell culture supernatants and synovial fluid confirmed seven key proteins.
Conclusion and clinical relevance
JIA FLS are central to perpetuation of inflammation in JIA, including trafficking of inflammatory cells and effects on the extracellular matrix. These cells express key disease‐specific chemokines that, with further refinement, may allow us to tailor therapy appropriately.
Keywords: Chemokines, Cytokines, Fibroblasts, Juvenile idiopathic arthritis, Synovial fluid
Abbreviations
- FLS
fibroblast‐like synoviocyte
- GM‐CSF
granulocyte‐monocyte‐colony stimulating factor
- IFN
interferon
- IL
interleukin
- IPA
Ingenuity Pathway Analysis
- JIA
juvenile idiopathic arthritis
- MMP
matrix metalloproteases
- NF‐κB
nuclear factor kappa‐light‐chain‐enhancer of activated B cells
- OPG
osteoprotegerin
- RA
rheumatoid arthritis
- TBST
Tris‐buffered saline tween 20
- TIMP
tissue inhibitor of metalloproteases
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
1. Introduction
Juvenile idiopathic arthritis (JIA) is the most common rheumatic disease of childhood 1, 2. Despite recent progress, we still lack understanding of the pathogenesis of this disease, thereby halting evolution of targeted therapies. Although there are similarities, rheumatoid arthritis (RA) and JIA are distinct entities 3. The pivotal role of the synovial fibroblast‐like synoviocytes (FLS) in RA has been elegantly investigated 4. RA FLS are known to be major contributors to cartilage destruction through release of matrix metalloprotease 3 (MMP3), with relative decreased expression of tissue inhibitors of metalloproteases (TIMPs). RA FLS are perpetuators of inflammation through release of interleukin (IL)‐6 and granulocyte‐monocyte‐ colony stimulating factor (GM‐CSF), in addition to key regulators of cell trafficking through release of chemokines, such as CXCL1 5. These aggressive cells are thought to be partially transformed. They display loss of contact inhibition and are able to proliferate in an anchorage‐independent manner, express several oncogenes, and exhibit loss of apoptosis with an increase in pro‐survival signal NF‐κB 4, 6. Although RA and JIA are both destructive arthropathies and display Th1 cytokine profiles, they are clinically quite different diseases. Various subtypes and variable outcomes are seen in JIA, whereas a relatively homogeneous phenotype and a poor outcome are typical of RA 3. The role played by the FLS in these two diseases may be distinct. We posit these cells may have an equally important role in the pathogenesis of JIA as joint gatekeepers, perpetuators of inflammation as well as mediators of joint damage and remodeling.
Clinical Relevance
In this study, we utilized antibody arrays for rapid detection of multiple inflammatory mediators in cell culture supernatants and neat synovial fluid from patients with juvenile idiopathic arthritis (JIA) and orthopedic controls. This enabled comparison of the protein content of these samples, shedding light on proteins significantly contributed by the fibroblast‐like synoviocytes, key players in inflammatory arthritis. Further understanding of the contributions of these cells to the perpetuation of inflammation may have long‐term benefits for targeted treatment of inflammatory arthritis.
This is the first analysis of the proteins secreted by JIA FLS in culture and confirmatory examination of the corresponding proteins in synovial fluid. Work has been done on the proteomic profile of synovial fluid from JIA 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, yet we do not have an understanding of the contribution attributable to the FLS in the disease. JIA FLS proved to be active, aggressive cells in the disease process, with profuse release of inflammatory cytokines, chemokines and loss of control over destructive mediators. This paper clarifies the importance of JIA FLS in the disease process and highlights unique features of these cells that must be taken into consideration when developing specific therapies for JIA.
2. Materials and methods
2.1. Selection of samples
Synovial fluid and tissues were obtained from our IRB‐approved tissue and fluid single‐center repository that was established in 2001. Informed consent was obtained from each participant for their sample to be included in the repository. All patients undergoing medically necessary arthrocentesis are offered inclusion in the repository. Appropriate samples were identified by chart review. We used four biological replicates from both persistent oligoarticular JIA and controls selected from the repository. The first available sample from each patient, from steroid‐naïve joints, was used. Control samples were obtained from remnant synovial fluid and tissue obtained from orthopedic procedures performed on non‐arthritic joints. Clinical information on the sampled patients is shown in Table 1.
Table 1.
Clinical information on samples
| Sample | Source of FLS | Joint | Diagnosis | Duration of disease | Age of patient | Gender of patient | Concurrent medications | Used for |
|---|---|---|---|---|---|---|---|---|
| C1 | Synovial tissue | R knee | Skeletal dysplasia | N/A | 15 y.o. | Male | None | A‐CS, E‐CS |
| C2 | Synovial fluid | Knee | Torn meniscus | N/A | >16 y.o. | Female | Unknown | A‐SF, E‐SF |
| C3 | Synovial tissue | L hip | Hip dysplasia | N/A | 4 y.o. | Female | None | A‐CS, E‐CS |
| C4 | Synovial fluid | R knee | ACL tear | N/A | 17 y.o. | Male | None | A‐SF, E‐SF |
| C5 | Synovial fluid | R knee | Discoid meniscus | N/A | 7 y.o. | Female | None | A‐SF, E‐SF |
| C6 | Synovial fluid | R knee | Discoid meniscus | N/A | 15 y.o. | Male | None | A‐CS, E‐CS |
| C7 | Synovial fluid | R knee | ACL tear | N/A | 15 y.o. | Male | None | A‐CS, E‐CS |
| C8 | Synovial fluid | R knee | ACL tear | N/A | 16 y.o. | Female | None | A‐SF, E‐SF |
| J1 | Synovial fluid | R knee | Oligo JIA | 9 months | 14 y.o. | Female | None | A‐CS |
| J2 | Synovial fluid | R knee | Oligo JIA | 10 months | 12 y.o. | Female | None | A‐CS |
| J3 | Synovial fluid | R knee | Oligo JIA | 3 months | 6 y.o. | Male | Methotrexate, naproxen, folic acid | A‐SF, E‐SF, E‐CS |
| J4 | Synovial fluid | L knee | Oligo JIA (extended) | 6 weeks | 11 y.o. | Female | Naproxen, doxycycline | A‐SF, E‐SF, E‐CS |
| J5 | Synovial fluid | L knee | Oligo JIA | 1 month | 12 y.o. | Male | Naproxen | A‐SF, E‐SF |
| J6 | Synovial fluid | L knee | Oligo JIA | 6 months | 17 y.o. | Male | Naproxen | A‐CS |
| J7 | Synovial fluid | L knee | Oligo JIA | 7 years | 12 y.o. | Male | Naproxen | A‐SF, E‐SF |
| J8 | Synovial fluid | L knee | Oligo JIA | 12 years | 14 y.o. | Female | Naproxen | A‐CS |
| J9 | Synovial fluid | L knee | Oligo JIA | 14 years | 15 y.o. | Female | Naproxen | E‐CS |
| J10 | Synovial fluid | L knee | Oligo JIA (psoriatic) | 1 month | 3 y.o. | Female | Naproxen | E‐CS |
A‐CS = used for arrays of cell culture supernatants
E‐CS = used for ELISA of cell culture supernatants
A‐SF = used for arrays of synovial fluids
E‐SF = used for ELISA of synovial fluids
2.2. Cell culture
All samples were processed immediately upon collection. Tissue was finely minced, digested for 30 min at 37°C in phosphate‐buffered saline (PBS) containing 0.1% trypsin. Minced tissue and released cells were then re‐suspended in Dulbecco's modified Eagle medium (DMEM)/15% fetal calf serum (FCS), filtered to remove debris and the cells were collected by centrifugation. Synovial fluid was centrifuged and cells were re‐suspended in cell media. Cells isolated from either synovial fluid or tissue were then plated in 6‐well plates with 15% fetal bovine serum/Dulbecco's modified Eagle's medium. Initial primary cultures were passaged into T‐75 flasks and, at confluence, were passaged 3–6 more times, and then harvested at confluence. It is well established that by the third passage, FLS are the predominant cell type in culture 18. We have previously published confirmation of the phenotype of these FLS grown in culture through cell staining and gene expression 19. Due to the scarcity of control samples, some control FLS were grown from synovial tissue while others were grown from synovial fluid. All JIA FLS were obtained from synovial fluid. All cell culture supernatant samples were taken in 10 mL volumes in Falcon 15 mL conical centrifuge tubes at room temperature before cells were harvested. Supernatants were then centrifuged at 1500 rpm for 5 min at room temperature to remove any debris. Cell supernatant samples were then distributed into 1 mL aliquots in 1.5 mL Eppendorf microcentrifuge tubes and stored at −20°C until use in assays.
2.3. Antibody arrays and proteomic analysis
Using cell culture supernatants and synovial fluid samples, antibody arrays were completed using RayBiotech Membrane‐based Antibody Arrays (Human Cytokine Array C5 AAH‐CYT‐C5 and Human Inflammation Array AAH‐INF‐C3) using RayBiotech protocols. Both cell culture supernatants and synovial fluid samples protein concentrations were calculated using a Bradford assay. Once the amount of protein was determined, all samples were diluted to 500 ug/mL as per RayBiotech instructions. Membrane arrays were first blocked using a BSA blocking solution provided by manufacturer for 30 min at room temperature. Either cell culture supernatant or synovial fluid samples were incubated in 1 mL aliquots per membrane overnight at 4°C. Membranes were then washed using 2 mL of TBST solution provided by manufacturer for 5 min at room temperature for five washes after sample incubations. Each membrane was then incubated with diluted biotinylated antibody cocktail that was prepared by pipetting 2 mL of Blocking Buffer into each vial of antibody and incubated at room temperature for 2 h. After incubations membranes were washed as previously described. HRP‐streptavidin was prepared in blocking buffer by diluting it 1:1000 and incubated on each membrane for 2 h at room temperature. Membranes were washed again as previously described. Membranes were transferred, printed side up, onto a sheet of blotting paper lying on a flat surface to remove excess wash buffer by blotting the membrane edges with another piece of paper. Membranes were then transferred and placed, printed side up, onto a plastic sheet provided by manufacturer and 500 μL of the Detection Buffer mixture (a 1:1 mixture of buffers provided) onto each membrane and incubated for 2 min at room temperature. To ensure that our cell culture media did not interfere with our array signal, one inflammatory array and one cytokine array were incubated with only prepared cell culture media. There was no detectible evidence that the serum contained any of the proteins of interest (Supporting Data). Films of membrane arrays were scanned and intensities of protein signals were measured using ImageJ. Each spot was outlined using ImageJ software and readout was set to intensity measurement. All intensity measurements were normalized to positive controls. Each membrane contained Positive Control Spots that were a controlled amount of biotinylated antibody printed onto the array and were used for normalization and to orientate the arrays. Negative Control Spots are buffer printed (no antibodies) and used to measure the baseline responses. They were also used for determining the level of non‐specific binding of the samples. Blank Spots had nothing printed and were used to measure the background response. Statistical data (average, standard error, and p‐value) were calculated using SigmaStat.
2.4. Image J
The integrated density of each dot is measured by outlining it and using the Analyze/Measure command in ImageJ. This method requires background correction of the image, which can be done using the Process/Subtract Background command in ImageJ. Profile plots are obtained for each row of dots (Analyze/Plot Profile) before and after background correction. After correcting the background, "Integrated Density" was enabled in Analyze/Set Measurements. This created a circular selection that was dragged over the dots. "M" (Analyze/Measure) was pressed to measure intensity. This step was repeated for each dot. Data was exported into Excel for calculations. Statistical data was calculated using SigmaStat. A standard t‐test was performed to determine significance (p < 0.05). To account for 89 comparisons, false discovery rate and corrected p‐value for multiple testing was calculated using Benjamini‐Hochberg (p < 0.048) 20.
2.5. Enzyme‐linked immunosorbent assay (ELISA)
Using cell culture supernatants and synovial fluid samples, ELISAs were completed using Quantikine Colormetric ELISA kits from R&D Systems. For each sample, 500 μg of total protein sample was incubated on the manufacturer provided 96‐well plates. All assays were carried out following the manufacturer protocols. Each plate contained a standard curve created by using a recombinant protein provided by the manufacturer in serial dilutions according to manufacturer protocols. Each standard and sample were plated in triplicate. Additionally, controls including primary antibody only controls, primary and secondary antibody only controls, and dilution buffer controls incubated with primary antibody were used to measure background and specificity. A detailed protocol is as follows: 100 μL of Assay Diluent (manufacturer) was added to each well plus 100 μL of Standard, sample, or control. The plates were incubated overnight at 4°C. After incubations, each well was aspirated and washed using wash buffer provided by manufacturer. Plates were washed by filling each well with 400 μL of wash buffer using a squirt bottle. After the last wash, any remaining Wash Buffer was removed by aspirating the wells then the plate was inverted and blotted against clean paper towels. After wash, 200 μL of Conjugate (manufacturer) was added to each well and incubated for 2 h at room temperature. The plates were then washed as previously described. and 200 μL of Substrate Solution (manufacturer) was added to each well and incubated for 20 min at room temperature, protected from light. After incubations, 50 μL of Stop Solution (manufacturer) was added to each well. The color in the wells changed from blue to yellow. Last, we determined the optical density of each well within 30 min, using a microplate reader set to 450 nm. Wavelength correction was set to 540 or 570 nm to correct for optical imperfections in the plate. Quantikine kits were as follows: IL‐6 D6050, IL‐10 D1000B, IL‐17 D1700, IL‐4 D4050, CXCL1 DGR00, CXCL6 DGC00, and CXCL8 D8000C. All data were analyzed using Excel. Averages were obtained for each sample group using Excel, while other statistical data (standard error, and p‐value) were calculated using SigmaStat.
3. Results and discussion
3.1. JIA synovial fluid contains an abundance of proteins
Of the 89 proteins tested by the arrays, 84 proteins were differentially expressed in the synovial fluid between the controls and the JIA samples. Among this extensive panel of proteins tested on the synovial fluid, there were several important findings, including a decrease in osteoprotegerin (OPG) and TIMP‐1 in the JIA synovial fluid. Not surprisingly, JIA synovial fluid had increased levels of tumor necrosis factor (TNF‐α), sTNFR1, vascular endothelial growth factor (VEGF), interferon (IFNγ), IL‐1α and IL‐1β. Chemokines and cytokines, including CX3CL1, which attracts T cells and monocytes, CCL18, which recruits the adaptive immune system, CCL26, which is stimulated by IL4, and CCL1, which is secreted by activated T cells and attracts monocytes, natural killer cells, immature B cells and dendritic cells, were all increased in JIA synovial fluid. Interestingly, CCL20, the ligand that recruits Th17 cells through CCR6, was significantly higher in the synovial fluid from JIA when compared to controls.
3.2. JIA FLS display a distinct inflammatory cytokine repertoire
The characteristics of these important indigenous cells have not been explored, so we examined the proteins secreted into the supernatants of a pure culture of FLS from both JIA and control subjects and compared them to the findings in synovial fluid. To determine which of these proteins were contributed, at least in part, by the FLS, we tested cell culture supernatants from control and JIA FLS by antibody arrays. There were many proteins that were differentially expressed in the synovial fluid when we compared JIA with controls. Some proteins did not reach significance in the comparison of FLS cell culture supernatants, indicating that these proteins are contributed by various other cell sources in the joint. Of the 84 differentially expressed synovial fluid proteins, 29 proteins were differentially expressed by the FLS in cell culture (Fig. 1). The remaining 55 proteins did not reach significance when the cell culture supernatants were compared (Supporting Data). JIA FLS preferentially secrete pro‐inflammatory proteins that play a central role in immunomodulation (Fig. 1). Additionally, there is decreased secretion of the anti‐inflammatory cytokine IL‐10 as compared to controls. This profile was confirmed in synovial fluid, demonstrating that FLS are truly inflammatory cells making significant contributions to disease pathogenesis in JIA.
Figure 1.
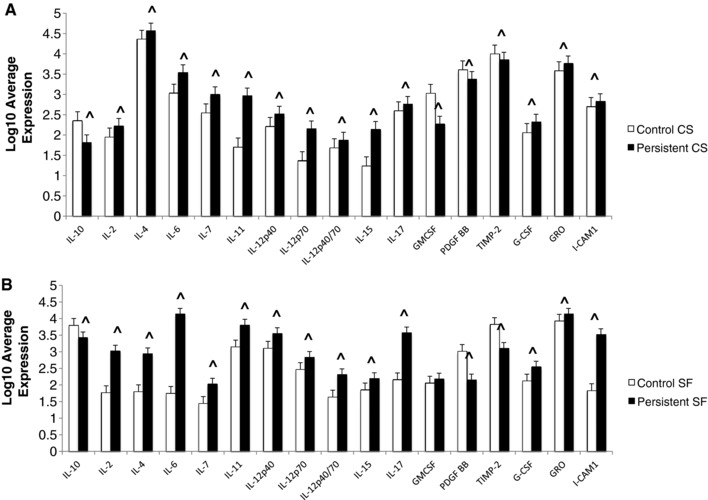
Antibody Arrays. (A) Log10 Average expression of differentially expressed proteins in the cell culture supernatants comparing controls and JIA. (B) Log10 Average expression of differentially expressed proteins in the synovial fluids comparing controls and JIA. ^ designates significantly significant by Benjamini and Hochberg method (p < 0.048). n = 4 controls and 4 JIA biological replicates.
ELISA was used to confirm these differences in both the cell culture supernatants and synovial fluid samples for IL‐6, IL‐4, IL‐17, and IL‐10 (Fig. 2). These data revealed that JIA FLS are secreting IL‐6, IL‐4, and IL‐17 in larger amounts than control FLS in culture, which is reflected in the relationship of these proteins in the synovial fluid. IL‐10 production is decreased in JIA FLS and the synovial fluid from JIA. ELISA data correlated with antibody array findings, with validation of the relative expression.
Figure 2.
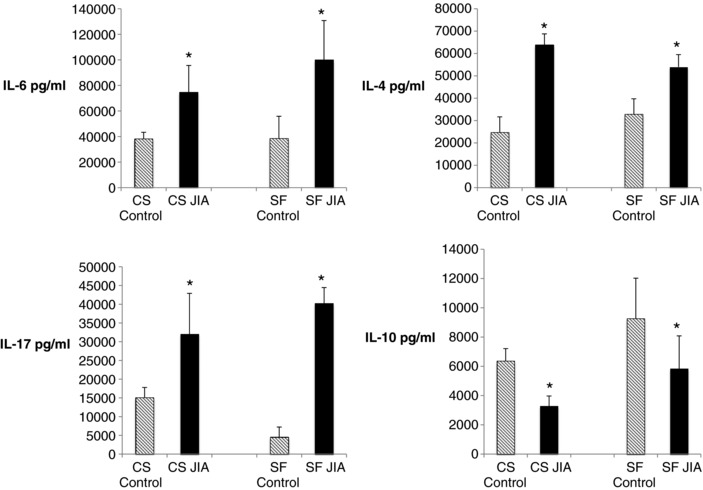
ELISAs of cell culture supernatants and synovial fluid samples from fibroblast‐like synoviocytes (FLS) from juvenile idiopathic arthritis (JIA) and controls. IL‐6, IL‐17, and IL‐4 have higher levels in JIA, both in cell culture supernatants and synovial fluid samples. IL‐10 demonstrates lower levels in JIA in both the cell culture supernatant and the synovial fluid. P‐values ≤ 0.005. n = 4 controls and 4 JIA biological replicates, each run in triplicate.
This is the first study to identify cytokines produced by a pure culture of JIA FLS. Serum levels of cytokines have been investigated in JIA 7, 21 yet synovial fluid profiles may reflect more accurately the condition in the inflamed joint. Synovial fluid from patients with JIA revealed presence of IL‐2, IL‐4, IL‐6, IL‐12, IL‐15, IL‐17, CCL17, and CXCL9 7, consistent with our findings in JIA FLS cell culture supernatants and synovial fluid, however, there were no control synovial fluid samples tested for comparison in the published study. IL‐4 is known to be elevated in JIA, especially in remitting oligoarticular disease 22, which in turn may influence remission in oligoarticular JIA. It is possible that synovial FLS play a dual role in the homeostasis of the inflamed synovium. In sum, FLS may play both roles, i.e. pro‐ and anti‐inflammatory, depending on disease state and other local influences.
3.3. JIA FLS display increased chemokine production
JIA FLS express higher levels of the several members of the C‐C family of chemokines, which recruit monocytes, macrophages, T cells and neutrophils, and the C‐X‐C family of chemokines, which recruit and activate myeloid cells (Fig. 3). We have shown CXCL6 to be one of the most highly differentially expressed genes between control and JIA FLS with 70‐fold higher expression in JIA on the RNA level 19. Although not significantly different on the antibody arrays of the cell culture supernatants, CXCL6 protein level was higher in the JIA cell culture supernatant when tested by ELISA, likely due to higher sensitivity of this method (Fig. 4). JIA FLS also highly secreted XCL1 (lymphotaxin), which is chemotactic for T cells and NK cells, and ICAM1, which allows leukocytes to transmigrate into tissues. The majority of these chemokines remained significantly increased in the synovial fluid analysis, implying an influential role of JIA FLS in controlling trafficking into the joint. Three proteins, CXCL5, CXCL10, and CXCL13, had decreased secretion in cultured JIA FLS, but were increased in JIA synovial fluid, indicating an alternative cell source for these chemokines. XCL1, CCL16, CCL25, CCL27, and CXCL11 were increased in cell culture supernatants of JIA FLS, but due to differences in antibody array map, were not tested in synovial fluid (Fig. 3). Confirmation of FLS as major sources of CXCL1 and CXCL6 was done by ELISA (Fig. 4). ELISA was used to confirm the relationships of CXCL8 on both cell culture supernatants and synovial fluid samples (Fig. 4).
Figure 3.
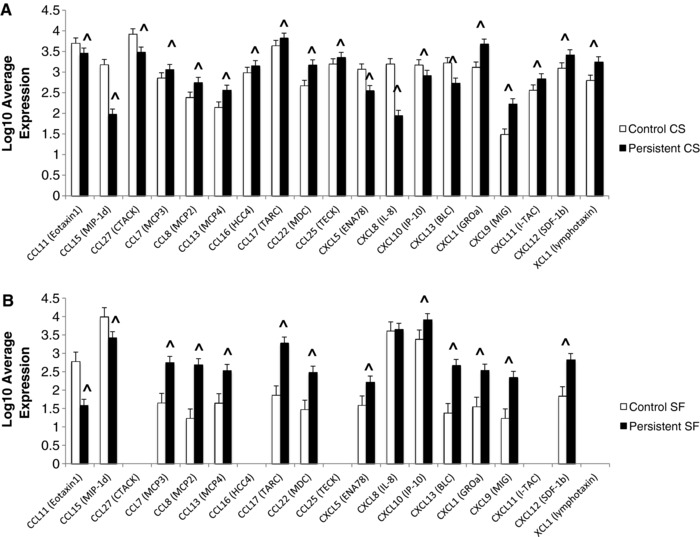
Antibody Arrays. (A) Log10 Average expression of differentially expressed proteins in the cell culture supernatants comparing controls and JIA. (B) Log10 Average expression of differentially expressed proteins in the synovial fluids comparing controls and JIA. ^ designates significantly significant by Benjamini and Hochberg method (p < 0.048). n = 4 controls and 4 JIA biological replicates.
Figure 4.
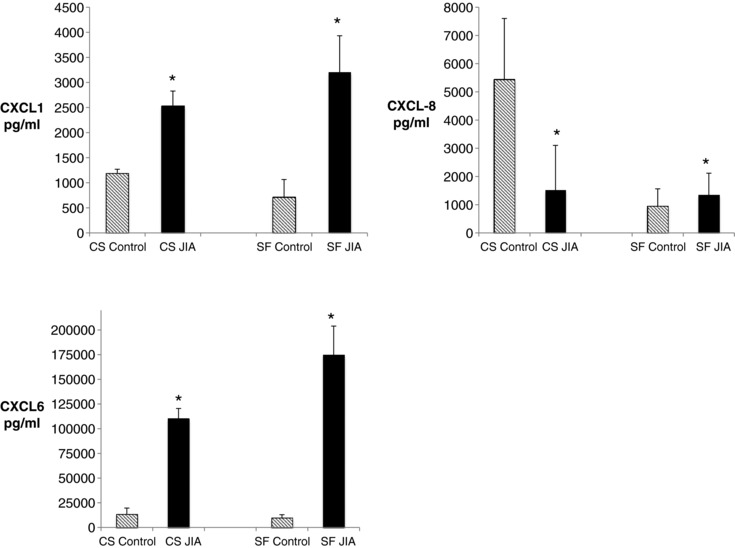
ELISAs of cell culture supernatants and synovial fluid samples from fibroblast‐like synoviocytes (FLS) from juvenile idiopathic arthritis (JIA) and controls. CXCL1 and CXCL6 have higher levels in JIA. CXCL8 has higher levels in JIA cell culture supernatants but no difference in levels in the synovial fluid. P‐values ≤ 0.005. n = 4 controls and 4 JIA biological replicates, each run in triplicate.
JIA FLS may contribute to the inflow of inflammatory cells into the joint space via secretion of a large number of chemokine ligands (Fig. 3). The contribution of FLS to inflammatory cell trafficking has been shown in RA, although likely through different sets of chemokines. In line with the findings in RA synovial tissue 23, JIA FLS had increased expression of CXCL1, CXCL9, XCL1 (lymphotaxin), and CCL7. In contrast, RA synovial tissue shows expression of CXCL5, CXCL8, CXCL10, and CXCL13 23, all of which we found decreased in JIA FLS at the protein level. CXCL12 was increased in JIA FLS, but is reportedly decreased in RA synovial tissue 23, suggesting differences in pathways utilized for cell trafficking. CXCL8 has been observed in RA synovial tissue 23, but specifically in many cell types from the RA joint, including dendritic cells, synovial macrophages, neutrophils, and synovial fibroblasts 23, 24, 25, 26, 27, 28, in addition to synovial fluid of JIA 21, but had the largest fold‐change decrease in expression in cultured JIA FLS. When tested on synovial fluid there was no significant difference in expression, suggesting that there are likely other cell sources of CXCL8 in JIA as well. Distinct chemokine profiles imply that therapeutic targets aimed at chemokine ligands are likely to have varying degrees of efficacy in JIA as compared to RA.
JIA FLS are likely influenced by and are influencing Th17 cells. Th17 cells and IL‐17 are present in increased levels in the synovial fluid of patients with JIA 8, 29, 30. Importantly, Th17 cells express CCR2 and CCR6. The CCR2 ligands are CCL7, CCL8, CCL13, and CCL16, are being secreted by JIA FLS in culture and are increased in JIA synovial fluid (CCL16 was not tested in synovial fluid), underscoring the high likelihood that there is cross‐talk in the joint between the JIA FLS and the Th17 cells.
The Th17 cells in the joint could be influencing the FLS to release CXCL1 and CXCL6, thereby directing trafficking of neutrophils into the joint space with the help of the FLS. IL‐17 is also secreted by JIA FLS, which may be working in an autocrine manner to stimulate the FLS to then release CXCL1 and CXCL6. Additionally, IL‐17 increases expression of IL‐6 by JIA FLS 8, which may occur in a paracrine manner from Th17 cells in the synovial fluid or by autocrine signaling, thereby resulting in perpetuation of the inflammatory response.
The increase in T cells expressing CCR5 and CXCR3 seen in the inflamed joint in JIA has been attributed to selective recruitment 31. JIA FLS release CCL16, the chemokine ligand for CCR5, and CXCL9 and CXCL11, ligands for CXCR3, suggesting FLS contribution to the recruitment of these cells into the joint. CXCL9 remained significantly more highly expressed in JIA synovial fluid. CCL16 and CXCL11 were not tested on synovial fluid.
3.4. Dysregulated pathways reveal that FLS are “master traffickers”
To uncover connections between these dysregulated proteins, we looked at the top pathways uniting our proteins using QIAGEN'S Ingenuity Pathway Analysis (IPA, QIAGEN Redwood City, http://www.qiagen.com/ingenuity). The top canonical pathways these proteins are involved in are (i) Granulocyte adhesion and diapedesis (p‐value = 3.1 × 10−32), (ii) Agranulocyte (mononuclear leukocyte) adhesion and diapedesis (p‐value = 1.48 × 10−29), (iii) Hematopoiesis from pluripotent stem cells (p‐value = 2.81 × 10−21), (iv) Role of cytokines in mediating communication between immune cells (p‐value = 2.19 × 10−20) and (v) Communication between innate and adaptive immune cells (p‐value = 4.89 × 10−18). The activity of these pathways highlights the central role the FLS play in directing traffic and influencing the inflammatory milieu of the joint.
3.5. Proteins are unified by upstream regulation through nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB)
We used IPA to investigate the upstream regulators of dysregulated proteins in the FLS. The top six upstream regulators for the FLS include transcription regulators JUN, EZH2, RELA, IRF3, NFKB1A, and the NFKB complex. Of the top six upstream regulators of the proteins expressed by JIA FLS in culture, four were involved in NF‐κB signaling. This highlights the importance of NF‐κB signaling in the pathogenesis of JIA, as has been established in RA 32. NF‐κB is a major inflammatory regulator and has a role in activation and proliferation of RA FLS and the development of the Th1 helper subset 32, 33, 34. TNF has been shown to induce a TNFR2/NF‐κB‐dependent proinflammatory program in Tregs derived from JIA synovial fluid 35, but ours is the first work to show the activity of NF‐κB signaling in JIA FLS.
3.6. Limitations of study
One of the strengths of our work is also one of its limitations. While the protein findings in cell culture supernatants can certainly be attributed to the cells in question (FLS) we are also limiting our ability to observe the behavior of these cells within the inflammatory milieu containing other cells of the inflamed synovial membrane. FLS could, in fact, exhibit a different phenotype in situ in the joint. Of particular interest would be the interaction between the FLS and Th17 cells in JIA. Through paired examination of the synovial fluid, however, we are able to demonstrate that FLS are secreting cytokines and chemokines and hence contributing to the inflammatory milieu of the joint space. Additionally, we do have a small sample cohort, however, there were detectible differences between controls and JIA. FBS was not removed from samples before antibody arrays were done. This should not influence the detectible differences since all samples, controls and JIA, were treated exactly the same way and FBS does not contain any of the studied proteins, as demonstrated by us (Supporting Data) and others 36.
4. Concluding remarks
The present study demonstrates a central role for FLS in JIA. These cells seem pivotal in perpetuating inflammation and directing cell traffic into the joint. With prolific outpouring of cytokines and chemokines, they are influencing the inflammatory response, making them potential therapeutic targets.
This is the first study to look at a large panel of proteins in synovial fluid and released from a pure culture of FLS in JIA. JIA FLS are performing many essential activities in common with RA, albeit through different messengers. It is essential to delineate this distinction when developing targeted therapies, such as anti‐chemokine agents, likely to be efficacious in JIA. In vitro experiments blocking upstream regulators could also give great insight into disease pathogenesis and provide the basis for new therapeutic modalities. Through select protein analysis of pure culture of FLS and the corresponding proteins in synovial fluid, we have shown that JIA FLS are major contributors to inflammatory response and “master traffickers” into the joint in juvenile idiopathic arthritis. Knowledge of the key disease‐specific chemokines may allow us to tailor therapy appropriately.
The authors have declared no conflict of interest.
Supporting information
Supplementary Material
Acknowledgments
We would like to thank the Nemours/AI duPont Hospital for Children Orthopedics Department for control samples and the Nemours BioBank for assistance with obtaining samples. We thank Edward M. Behrens, MD and Suzanne M. McCahan, PhD for helpful discussions. In addition, we thank Paul T. Fawcett, PhD for maintaining the sample repository. We are grateful for the technical support of the COBRE‐funded Biomolecular Core Laboratory of Nemours Biomedical Research.
There was no financial support from commercial sources.
Supported by: NIH 1K23AR066724‐01, NIH 8P20GM103464, Arthritis Foundation Innovative Research Grant, NIH ARRA supplement to P20 RR020173, The Nancy Taylor Foundation for Chronic Diseases, Nemours Biomedical Research, Delaware Community Foundation and Open Net Foundation.
Brescia A. C., Simonds M. M., Sullivan K. E., Rose C. D., Prot. Clin. Appl. 2017, 11, 1600088.
5 References
- 1. Lawrence, R. C. , Helmick, C. G. , Arnett, F. C. , Deyo, R. A. et al., Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthr. Rheumat. 1998, 41, 778–799. [DOI] [PubMed] [Google Scholar]
- 2. Manners, P. J. , Bower, C. , Worldwide prevalence of juvenile arthritis why does it vary so much? J. Rheumatol. 2002, 29, 1520–1530. [PubMed] [Google Scholar]
- 3. Prahalad, S. , Glass, D. N. , Is juvenile rheumatoid arthritis/juvenile idiopathic arthritis different from rheumatoid arthritis? Arthr. Res. Ther. 2002, 4, 303–310. [Google Scholar]
- 4. Firestein, G. S. Invasive fibroblast‐like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthr. Rheumat. 1996, 39, 1781–1790. [DOI] [PubMed] [Google Scholar]
- 5. Bartok, B. , Firestein, G. S. , Fibroblast‐like synoviocytes: key effector cells in rheumatoid arthritis. Immunological Rev. 2010, 233, 233–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Firestein, G. S. , Nguyen, K. , Aupperle, K. R. , Yeo, M. et al., Apoptosis in rheumatoid arthritis: p53 overexpression in rheumatoid arthritis synovium. Am. J. Pathol. 1996, 149, 2143–2151. [PMC free article] [PubMed] [Google Scholar]
- 7. de Jager, W. , Hoppenreijs, E. P. , Wulffraat, N. M. , Wedderburn, L. R. et al., Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: a cross‐sectional study. Annal. Rheumat. Dis. 2007, 66, 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Agarwal, S. , Misra, R. , Aggarwal, A. , Interleukin 17 levels are increased in juvenile idiopathic arthritis synovial fluid and induce synovial fibroblasts to produce proinflammatory cytokines and matrix metalloproteinases. J. Rheumatol. 2008, 35, 515–519. [PubMed] [Google Scholar]
- 9. Madson, K. L. , Moore, T. L. , Lawrence, J. M., 3rd , Osborn, T. G., Cytokine levels in serum and synovial fluid of patients with juvenile rheumatoid arthritis. J. Rheumatol. 1994, 21, 2359–2363. [PubMed] [Google Scholar]
- 10. Gibson, D. S. , Finnegan, S. , Jordan, G. , Scaife, C. et al., Stratification and monitoring of juvenile idiopathic arthritis patients by synovial proteome analysis. J. Proteome Res. 2009, 8, 5601–5609. [DOI] [PubMed] [Google Scholar]
- 11. Rosenkranz, M. E. , Wilson, D. C. , Marinov, A. D. , Decewicz, A. et al., Synovial fluid proteins differentiate between the subtypes of juvenile idiopathic arthritis. Arthr. Rheumat. 2010, 62, 1813–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agarwal, S. , Misra, R. , Aggarwal, A. , Synovial fluid RANKL and matrix metalloproteinase levels in enthesitis related arthritis subtype of juvenile idiopathic arthritis. Rheumatol. Internat. 2009, 29, 907–911. [DOI] [PubMed] [Google Scholar]
- 13. Kutukculer, N. , Caglayan, S. , Aydogdu, F. , Study of pro‐inflammatory (TNF‐alpha, IL‐1alpha, IL‐6) and T‐cell‐derived (IL‐2, IL‐4) cytokines in plasma and synovial fluid of patients with juvenile chronic arthritis: correlations with clinical and laboratory parameters. Clin. Rheumatol. 1998, 17, 288–292. [DOI] [PubMed] [Google Scholar]
- 14. Pharoah, D. S. , Varsani, H. , Tatham, R. W. , Newton, K. R. et al., Expression of the inflammatory chemokines CCL5, CCL3 and CXCL10 in juvenile idiopathic arthritis, and demonstration of CCL5 production by an atypical subset of CD8+ T cells. Arthr. Res. Ther. 2006, 8, R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saxena, N. , Aggarwal, A. , Misra, R. , Elevated concentrations of monocyte derived cytokines in synovial fluid of children with enthesitis related arthritis and polyarticular types of juvenile idiopathic arthritis. J. Rheumatol. 2005, 32, 1349–1353. [PubMed] [Google Scholar]
- 16. De Benedetti, F. , Pignatti, P. , Gerloni, V. , Massa, M. et al., Differences in synovial fluid cytokine levels between juvenile and adult rheumatoid arthritis. J. Rheumatol. 1997, 24, 1403–1409. [PubMed] [Google Scholar]
- 17. De Benedetti, F. , Pignatti, P. , Bernasconi, S. , Gerloni, V. et al., Interleukin 8 and monocyte chemoattractant protein‐1 in patients with juvenile rheumatoid arthritis. Relation to onset types, disease activity, and synovial fluid leukocytes. J. Rheumatol. 1999, 26, 425–431. [PubMed] [Google Scholar]
- 18. Stebulis, J. A. , Rossetti, R. G. , Atez, F. J. , Zurier, R. B. , Fibroblast‐like synovial cells derived from synovial fluid. J. Rheumatol. 2005, 32, 301–306. [PubMed] [Google Scholar]
- 19. Brescia, A. C. , Simonds, M. M. , McCahan, S. M. , Fawcett, P. T. , Rose, C. D. , The role of transforming growth factor beta signaling in fibroblast‐like synoviocytes from patients with oligoarticular juvenile idiopathic arthritis: dysregulation of transforming growth factor beta signaling, including overexpression of bone morphogenetic protein 4, may lead to a chondrocyte phenotype and may contribute to bony hypertrophy. Arthr. Rheumatol. 2014, 66, 1352–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Benjamini YaH, Y. , Controlling the false discovery rate: a pracxtical and powerful approach to multiple testing. J. Royal Statist. Soc. Series B (Methodological). 1995, 57, 289–300. [Google Scholar]
- 21. Prahalad, S. , Martins, T. B. , Tebo, A. E. , Whiting, A. et al., Elevated serum levels of soluble CD154 in children with juvenile idiopathic arthritis. Pediatric Rheumatol. Online J. 2008, 6, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murray, K. J. , Grom, A. A. , Thompson, S. D. , Lieuwen, D. et al., Contrasting cytokine profiles in the synovium of different forms of juvenile rheumatoid arthritis and juvenile spondyloarthropathy: prominence of interleukin 4 in restricted disease. J. Rheumatol. 1998, 25, 1388‐1398. [PubMed] [Google Scholar]
- 23. Schmutz, C. , Hulme, A. , Burman, A. , Salmon, M. , Ashton, B. et al., Chemokine receptors in the rheumatoid synovium: upregulation of CXCR5. Arthr. Res. Ther. 2005, 7, R217–R229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Auer, J. , Blass, M. , Schulze‐Koops, H. , Russwurm, S. et al., Expression and regulation of CCL18 in synovial fluid neutrophils of patients with rheumatoid arthritis. Arthr. Res. Ther. 2007, 9, R94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Radstake, T. R. , van der Voort, R. , ten Brummelhuis, M. , de Waal Malefijt, M. et al., Increased expression of CCL18, CCL19, and CCL17 by dendritic cells from patients with rheumatoid arthritis, and regulation by Fc gamma receptors. Annal. Rheumat. Dis. 2005, 64, 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cho, M. L. , Ju, J. H. , Kim, H. R. , Oh, H. J. et al., Toll‐like receptor 2 ligand mediates the upregulation of angiogenic factor, vascular endothelial growth factor and interleukin‐8/CXCL8 in human rheumatoid synovial fibroblasts. Immunol. Lett. 2007, 108, 121–128. [DOI] [PubMed] [Google Scholar]
- 27. Huang, Q. , Ma, Y. , Adebayo, A. , Pope, R. M. , Increased macrophage activation mediated through toll‐like receptors in rheumatoid arthritis. Arthr. Rheumat. 2007, 56, 2192–2201. [DOI] [PubMed] [Google Scholar]
- 28. Pelletier, M. , Maggi, L. , Micheletti, A. , Lazzeri, E. et al., Evidence for a cross‐talk between human neutrophils and Th17 cells. Blood 2010, 115, 335–343. [DOI] [PubMed] [Google Scholar]
- 29. Nistala, K. , Moncrieffe, H. , Newton, K. R. , Varsani, H. et al., Interleukin‐17‐producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthr. rheumat. 2008, 58, 875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mahendra, A. , Misra, R. , Aggarwal, A. , Th1 and Th17 predominance in the enthesitis‐related arthritis form of juvenile idiopathic arthritis. J. Rheumatol. 2009, 36, 1730–1736. [DOI] [PubMed] [Google Scholar]
- 31. Wedderburn, L. R. , Robinson, N. , Patel, A. , Varsani, H. , Woo, P. , Selective recruitment of polarized T cells expressing CCR5 and CXCR3 to the inflamed joints of children with juvenile idiopathic arthritis. Arthr. Rheumat. 2000, 43, 765–774. [DOI] [PubMed] [Google Scholar]
- 32. Makarov, S. S. NF‐kappa B in rheumatoid arthritis: a pivotal regulator of inflammation, hyperplasia, and tissue destruction. Arthritis Res. 2001, 3, 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang, H. G. , Huang, N. , Liu, D. , Bilbao, L. et al., Gene therapy that inhibits nuclear translocation of nuclear factor kappaB results in tumor necrosis factor alpha‐induced apoptosis of human synovial fibroblasts. Arthritis Rheumat. 2000, 43, 1094–1105. [DOI] [PubMed] [Google Scholar]
- 34. Li, B. , Yu, H. , Zheng, W. , Voll, R. et al., Role of the guanosine triphosphatase Rac2 in T helper 1 cell differentiation. Science 2000, 288, 2219–2222. [DOI] [PubMed] [Google Scholar]
- 35. Nagar, M. , Jacob‐Hirsch, J. , Vernitsky, H. , Berkun, Y. et al., TNF activates a NF‐kappaB‐regulated cellular program in human CD45RA‐ regulatory T cells that modulates their suppressive function. J. Immunol. 2010, 184, 3570–3781. [DOI] [PubMed] [Google Scholar]
- 36. Zheng, X. , Baker, H. , Hancock, W. S. , Fawaz, F. et al., Proteomic analysis for the assessment of different lots of fetal bovine serum as a raw material for cell culture. Part IV. Application of proteomics to the manufacture of biological drugs. Biotechnol. Progress 2006, 22, 1294–1300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


