Abstract
Objectives
The purpose of this systematic review was to evaluate the impact of the abutment characteristics on peri‐implant tissue health and to identify the most suitable material and surface characteristics.
Methods
A protocol was developed aimed to answer the following focused question: “Which is the effect of the modification of the abutment design in regard to the maintenance of the peri‐implant soft tissue health?” Further subanalysis aimed to investigate the impact of the abutment material, macroscopic design, surface topography and surface manipulation. Randomised controlled trials (RCTs) with a follow‐up of at least 6 months after implant loading were considered as inclusion criteria. Meta‐analyses were performed whenever possible.
Results
Nineteen final publications from thirteen investigations were included. The results from the meta‐analysis indicated that zirconia abutments (Zi) experienced less increase in BOP values over time [n = 3; WMD = −26.96; 95% CI (−45.00; −8.92); p = .003] and less plaque accumulation [n = 1; MD = −20.00; 95% CI (−41.47; 1.47); p = .068] when compared with titanium abutments (Ti). Bone loss was influenced by the method of abutment decontamination [n = 1; MD = −0.44; 95% CI (−0.65; −0.23); p < .001]. The rest of the studied outcomes did not show statistically significant differences.
Conclusions
The macroscopic design, the surface topography and the manipulation of the implant abutment did not have a significant influence on peri‐implant inflammation. In contrast, the abutment material demonstrated increased BOP values over time for Ti when compared to Zi abutments.
Keywords: dental abutment, dental implants, dental‐implant abutment surface, mucositis, systematic review
1. INTRODUCTION
Dental implants are the preferred treatment to restore partially and completely edentulous patients due to their reported long‐term success (Buser et al., 2012; Gotfredsen, 2012). Dental implants anchored in the jaw bones are connected to the prosthetic construction through a transmucosal component, the abutment, which allows the transmission of functional masticatory forces and at the same time protects the implants from the highly contaminated oral environment. This is accomplished by the formation of a biological seal where the soft tissues adhere to the abutment surface, and thus, the peri‐implant hard tissues are protected from resorption (Salvi et al., 2015).
Preclinical in vivo research has indicated that the dimension of this soft tissue seal, the so‐called biological width, is compromised of 1.2–2 mm of barrier epithelium and 1–1.5 mm of a connective tissue (Abrahamsson, Berglundh, Wennstrom & Lindhe, 1996; Berglundh, Abrahamsson, Welander, Lang & Lindhe, 2007). The establishment of the biological width is usually coupled with varying degrees of bone remodelling occurring after implant‐abutment connection (Abrahamsson, Zitzmann, Berglundh, Wennerberg & Lindhe, 2001; Berglundh, Abrahamsson & Lindhe, 2005). The factors influencing peri‐implant bone remodelling are a matter of debate and research (Schwarz, Hegewald & Becker, 2014) as it is believed that initial bone resorptive changes may influence the health of the peri‐implant soft tissues and may predispose to peri‐implant diseases (Schwarz, Sahm & Becker, 2012). Although initial changes in peri‐implant soft tissue health are difficult to diagnose, it is well established that bleeding on probing is the preferred method to identify peri‐implant mucosal inflammation (Jepsen et al., 2015).
One of these influencing factors which may impact early bone remodelling and soft tissue integration is the characteristics of the prosthetic abutments. Both the abutment material (Welander, Abrahamsson & Berglundh, 2008) and the surface micro‐topography have shown to influence the soft and hard peri‐implant tissue response. Glauser and co‐workers compared in humans the dimensions of junctional epithelium and connective tissue in turned, oxidised and acid‐etched abutments (Glauser, Schupbach, Gottlow & Hammerle, 2005). The dimensions of the biological width were approximately the same for all surfaces; however, the length of the junctional epithelium was higher on smooth titanium (2.9 mm) when compared to rough surfaces (1.4–1.6 mm). Additionally, the use of microgrooved surfaces has been tested in animal experiments. When compared to standard abutments, the microgrooved surface seemed to be associated with a longer connective tissue attachment and less bone resorption (Iglhaut, Becker, Golubovic, Schliephake & Mihatovic, 2013; Kim et al., 2010).
The position of the epithelial and connective tissue attachment has been shown to influence the remodelling processes that occurs around implants (Rompen, Domken, Degidi, Pontes & Piattelli, 2006). Inflammatory infiltrates elicited by the microbial colonisation of the implant‐abutment interface were considered to be one of the factors causing epithelial downgrowth and subsequent peri‐implant bone loss (Iglhaut et al., 2014). In fact, the contamination of the abutment surface has shown to have a negative effect on the soft tissue integration (Rompen, 2012). Several cleaning protocols have been proposed to effectively decontaminate the implant surface and avoid epithelial downgrowth (Canullo, Genova, et al., 2016; Canullo, Tallarico, et al., 2016).
Although new abutment materials, designs and surface manipulations methods are currently under investigation, there is controversy on their real effect on the peri‐implant hard and soft tissues. It is, therefore, the purpose of this systematic review to assess in systemically healthy patients with at least one abutment connected to an implant the effect of the abutment material, design or surface manipulation method on peri‐implant soft tissue health as measured by bleeding or gingival indexes.
2. MATERIAL AND METHODS
Before the start of the systematic review, a protocol was developed, aiming to answer the following focused question (Needleman, 2002): Which is the effect of modifying the abutment characteristics for maintaining peri‐implant soft tissue health?
This question considered the following PICO question:
Population: Systemically healthy patients with at least one abutment connected to an implant.
Intervention: Any change in abutment material, design (macro‐ or micro‐design) or surface manipulation (e.g., cleaning protocols).
Comparison: Any abutment material, design (macro‐ or micro‐design) or the surface manipulation (e.g., cleaning protocols).
Outcomes: Peri‐implant soft tissue health measured by gingival or bleeding indexes.
As secondary outcomes, the following were considered: implant survival, marginal bone levels, probing pocket depth (PPD), plaque index (PI), changes in the position of the peri‐implant soft tissues (changes on the level of mucosal margin, crown length implant, dimension of keratinised mucosa, thickness of the mucosa, level of the papilla), any aesthetic index, colour of the mucosa, technical complications and patient‐related outcomes (PROMs).
2.1. Eligibility criteria
2.1.1. Inclusion criteria
Randomised clinical trials (RCTs), with at least 6 months of follow‐up after abutment connection;
in systemically healthy patients;
with assessment of peri‐implant soft tissue health by gingival or bleeding indexes.
2.1.2. Exclusion criteria
The following studies were excluded:
Those comparing the effect of different implant‐abutment connections (e.g., Switching platform);
Those investigating mini‐implants and/or orthodontic anchorage devices;
Those evaluating implant prostheses directly screwed into the implant head;
Those evaluating the behaviour of abutments used to retain removable prosthesis;
Those evaluating different implant macro‐designs.
2.2. Information sources and search
2.2.1. Electronic search
Two electronic databases were used as sources in the search for studies satisfying the inclusion criteria: (i) The National Library of Medicine (MEDLINE via Pubmed) and (ii) Cochrane Central Register of Controlled Trials. These databases were searched for studies published until December 2016. The search was limited to human subjects. The specific search protocol is found in Appendix S1.
2.3. Study selection
Eligibility was performed by screening titles and abstract and a thorough analysis of the selected full texts. Titles and abstracts were screened for inclusion by two independent reviewers (ISM and ACA). These were calibrated (unweighted k scores) against an expert in systematic reviews (ISS). Abstracts were excluded if they did not fulfil the inclusion criteria listed before. To avoid the exclusion of potentially relevant articles, abstracts providing unclear results or absent were included in the full‐text analysis.
Full text of studies of possible relevance was retrieved for independent assessment by the same reviewers, using the same inclusion criteria. Any disagreement was resolved by discussion between reviewers, who also conducted independently the quality assessment of the selected studies.
2.4. Data extraction
Data were extracted by the reviewers independently (ISM/ACA) using specially designed data extraction forms. Any disagreement was discussed, and a third investigator (ISS) was consulted when necessary. Authors of the primary studies were consulted to obtain any further information not available in the paper. When the study results were published more than once or results were presented in multiple publications, the most complete dataset was identified, and data were included only once.
2.5. Quality assessment (risk of bias in individual studies)
A quality assessment of the included RCTs was performed according to the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0; updated March 2011 by Higgins and Green, 2011). Six main quality criteria were evaluated: sequence generation, allocation concealment, blinding treatment outcomes to outcome examiners, completeness of follow‐up, selective outcome reporting and other sources of bias. Depending on the descriptions given for each individual criteria, they were rated as: low, unclear or high risk of bias.
2.6. Risk of bias across studies
The publication bias was evaluated using a Funnel plot and the Begg′s test for small‐study effects for bleeding index. A sensitivity analysis of the meta‐analysis results was also performed (Tobias & Campbell, 1999).
2.7. Data analyses
The statistical heterogeneity among studies was assessed using the Q test bases on chi‐square statistics (Cochran, 1954) as well as the I 2 index (Higgins, Thompson, Deeks & Altman, 2003) thus reporting the percentage of variation in the global estimate that was attributable to heterogeneity (I 2 = 25%: low; I 2 = 50%: moderate; I 2 = 75%: high heterogeneity).
To summarise and compare studies, mean values of primary and secondary outcomes were directly pooled and analysed with weighted mean differences (WMDs) and 95% confidence intervals (CIs). Study‐specific estimates were pooled with both the fixed and random‐effect models (DerSimonian & Laird, 1986), and the random‐effect model results were presented. In addition, a subgroup analysis was carried out on the selected outcome variables using the type of procedure (material, macroscopic design, surface topography and surface manipulation), as explanatory variable.
A forest plot was created to illustrate the effects in the meta‐analysis of the different studies and the global estimation. STATA® (StataCorp LP, Lakeway Drive, College Station, TX, USA) intercooled software was used to perform all analyses. Statistical significance was defined as a p value < .05.
3. RESULTS
3.1. Search
Figure 1 depicts the study flow chart. The electronic search delivered 534 titles. After the evaluation of titles and abstracts, 487 studies were discarded, resulting in 47 studies, which after adding 32 articles found through manual search, resulted in 79 studies selected for full‐text analysis [agreement = 89.04%; kappa = .735; 95% IC (0.565; 0.906)]. After this analysis, 19 final papers were included reporting data from 13 different investigations, as five groups of papers reported the results of the same material at different time points or different outcomes from the same population in two publications. The reasons for exclusion of the remaining studies are depicted in Table S1.
Figure 1.
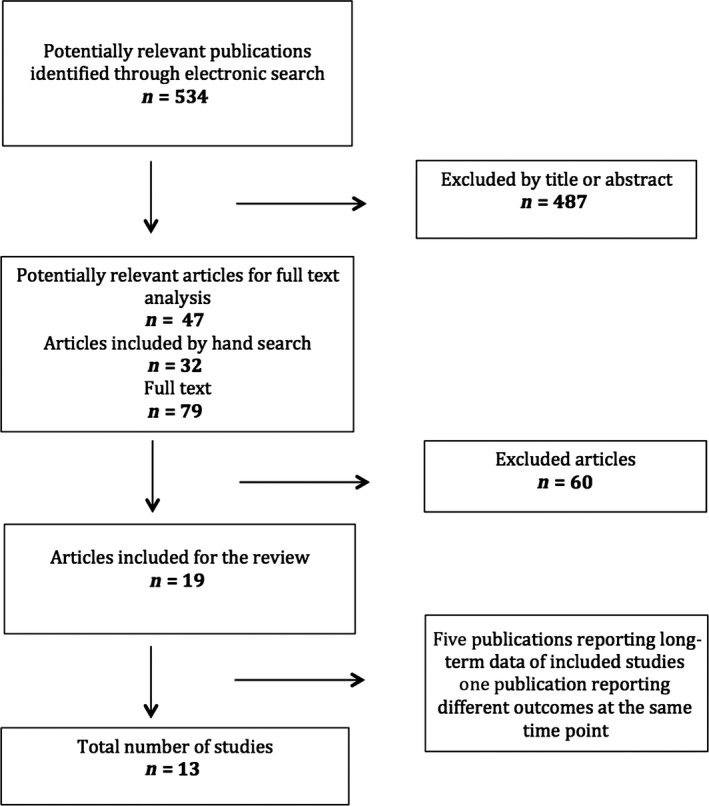
Flow chart depicting the search strategy and selection process
3.2. Description of studies
The methodological characteristics of the selected studies are shown in Table 1. From the 13 investigations, eight investigated the abutment material, three its macroscopic design, one the surface topography and one de surface manipulation.
Table 1.
Methodological characteristics of the studies included
| References | Type RCT | Follow‐up | Test patients baseline (final) /control patients baseline(final) | Test implants/control implants | Type restoration | Interventions test | Interventions control | Study outcomes measured |
|---|---|---|---|---|---|---|---|---|
| Abutment material | ||||||||
| Andersson, Scharer, Simion and Bergstrom (1999) | Parallel | 24 | 16(16)/16(16) | 50/53 | FDP's | Titanium | Ceramic/alum | BOP, PI, BL, IS |
| Andersson, Glauser, Maglione and Taylor (2003) | Parallel | 60 | 16(14)/16(15) | 50/47 | FDP's | Titanium | Ceramic/alum | BOP, PI, BL, IS |
| Andersson et al. (2001) | Parallel | 12 | NR | 34/35 | Single tooth | Titanium | Ceramic/alum | BOP, PI, BL, IS |
| Baldini et al. (2016) | Parallel | 12 | 12(10)/12(12) | 10/12 | Single tooth | Titanium | Zirconia | BOP, PPD, BL, PCO, PA, EST, IS |
| Carrillo de Albornoz et al. (2014) | Parallel | 12 | 12(11)/14(14) | 14/11 | Single tooth | Titanium | Zirconia | BOP, PI, REC, CLI, KT, BL, PCO, PA, EST, IS |
| Fenner et al. (2016) | Parallel | 86.4 | 15(13)/15(15) | 20/16 | Single tooth | Titanium | Ceramic | BOP, PI, PPD, REC, CLI, KT, BL, PCO, PA, IS |
| Gallucci, Grutter, Chuang et al. (2011) | Parallel | 24 | 10(8)/10(9) | 10/10 | Single tooth | Gold | Alumina/GC | BOP, PI, CLI, BL, PCO, PA, EST, IS |
| Gallucci, Grutter, Nedir et al. (2011) | Parallel | 24 | 10(8)/10(9) | 10/10 | Single tooth | Gold | Alumina/GC | BOP, PI, KT, BL, PA, IS |
| Hosseini et al. (2011) | Parallel/split | 12 | 30(30)/29(29) | 37/38 | Single tooth | Titanium | Zirconia | BOP, PI, PPD, BL, PCO, EST, IS |
| Sailer et al. (2009) | Parallel/split | 12 | NR | 12/19 | Single tooth | Titanium | Zirconia | BOP, PI, PPD, STC, PA, IS |
| Zembic et al. (2009) | Parallel/split | 36 | NR | 10/18 | Single tooth | Titanium | Zirconia | BOP, PI, PPD, BL, STC, PA, IS |
| Zembic et al. (2013) | Parallel/split | 67 | NR | 10/18 | Single tooth | Titanium | Zirconia | BOP, PI, PPD, REC, BL, PA, IS |
| Macroscopic design | ||||||||
| Patil et al. (2014) | Split‐mouth | 12 | 26(26)/26(26) | 26/26 | Single tooth | Ti conv. shape | Ti circf. Groove | BOP, PPD, KT, BL, EST, IS |
| Weinlander et al. (2011) | Split‐mouth | 12 | 10(10)/10(10) | 10/10 | Single tooth | Ti conv. shape | Ti circf. Groove | BOP, PI, PPD, BL, PA, EST, IS |
| Wittneben et al. (2017) | Parallel | 12 | 20(20)/20(18) | 20/20 | Single tooth | Prefab Zi. | CAD‐CAM Zi | BOP, PI, PPD, CLI, KT, BL, EST, IS |
| Surface topography | ||||||||
| Van Assche et al. (2012) | Split‐mouth | 12 | 18(18)/18(18) | 43/42 | Full arch | Ti turned | Ti rough | BOP, PI, PPD, REC, BL, IS |
| Nicu et al. (2012) | Split‐mouth | 36 | 14(14)/14(14) | 39/39 | Full arch | Ti turned | Ti rough | BOP, PPD, REC, BL, IS |
| Surface manipulation | ||||||||
| Canullo et al. (2015) | Parallel | 24 | 15(15)/15(15) | 15/15 | Single tooth | Steam cleaned | Plasma argon | BOP, PI, BL, IS |
| Canullo, Genova et al. (2016) and Canullo, Tallarico et al. (2016) | Parallel | 60 | 15(15)/15(15) | 15/15 | Single tooth | Steam cleaned | Plasma argon | BOP, PI, BL, IS |
NR, not reported; FDP's, fixed partial denture; Ti, titanium; Zi, zirconia; GC, glass ceramic; Alum, alumina; Circf, circumferential; BOP, bleeding on probing; PI, plaque index; PPD, pocket probing depth; REC, recession; CLI, crown length index; KT, keratinised mucosa; BL, radiographic bone levels; STC, soft tissue colour; PCO, patient‐centred outcomes; PA, papilla assessment; EST, aesthetic assessment; IS, implant survival.
This systematic review pooled data of 372 patients at baseline, with a total of 608 implants placed. The mean follow‐up period was of 36.69 months, with a minimum of 12 months in eight studies and a maximum of 86.4 months in one study. At the end of the study, 353 patients were followed with a total of 587 remaining implants. When the data were divided according to the treatment modality, 248 patients were treated to evaluate the abutment material (381 implants), 76 patients to study different macroscopic designs (112 implants), 18 patients to examine different surfaces topography (85 implants) and 30 patients to analyse the effects of the surface manipulation (30 implants).
3.3. Risk of bias in individual studies
Table 2 depicts the scores for each criterion in all the included studies individually. There was not a single study that had a low risk of bias for all the fields. However, six studies had a low risk of bias for the five main criteria (except other sources of bias; Carrillo de Albornoz et al., 2014; Gallucci, Grutter, Chuang & Belser, 2011; Wittneben et al., 2017) or for five different domains (Canullo, Penarrocha, Clementini, Iannello & Micarelli, 2015; Canullo, Genova, et al., 2016; Canullo, Tallarico, et al., 2016; Hosseini, Worsaae, Schiodt & Gotfredsen, 2011). The remaining studies had a high or unclear risk of bias in two or more fields.
Table 2.
Risk of bias assessment according to the Cochrane Collaboration recommendations (Higgins and Green, 2011)
| References | Selection bias sequence generation | Selection bias allocation concealment | Performance bias | Detection bias | Attrition bias | Selective reporting bias | Other potential risk of bias |
|---|---|---|---|---|---|---|---|
| Andersson et al. (1999) | Low | High | High | High | Low | Low | High |
| Andersson et al. (2003) | Low | High | High | High | Low | Low | Low |
| Andersson et al. (2001) | Low | High | High | High | Low | High | High |
| Gallucci, Grutter, Chuang et al. (2011) | Unclear | High | High | High | Unclear | Low | High |
| Gallucci, Grutter, Nedir et al. (2011) | Low | Low | Low | Low | Low | Low | High |
| Patil et al. (2014) | Unclear | High | High | Low | High | High | Low |
| Canullo, Genova et al. (2016) and Canullo, Tallarico et al. (2016) | Low | Low | Low | Low | Low | High | Low |
| Baldini et al. (2016) | Low | Low | Low | Low | Unclear | Low | High |
| Carrillo de Albornoz et al. (2014) | Low | Low | Low | Low | Low | Low | High |
| Fenner et al. (2016) | Unclear | High | High | High | Low | Low | Low |
| Hosseini et al. (2011) | Low | Low | High | Low | Low | Low | Low |
| Van Assche et al. (2012) | Low | High | High | Unclear | Low | Low | High |
| Nicu et al. (2012) | Low | High | High | High | Low | Low | Low |
| Weinlander et al. (2011) | Low | High | High | High | Low | High | High |
| Sailer et al. (2009) | Unclear | High | High | High | Low | High | High |
| Zembic et al. (2009) | Low | High | High | High | Low | High | Low |
| Zembic et al. (2013) | Unclear | High | High | High | Low | Low | Low |
| Wittneben et al. (2017) | Low | Low | Low | Low | Low | Low | High |
3.4. Risk of bias across studies
A statistically significant publication bias was observed when combining all studies for BOP (p = .032). No significant publication bias was detected for changes in BOP in studies dealing with material (p = .072).
3.5. Effects of interventions
3.5.1. Main outcome: mucosal inflammation
Authors were contacted when additional data were required and some of the bleeding or gingival indexes were transformed to bleeding on probing (BOP) values. Three studies were not included in the meta‐analysis, one because the modified bleeding index was used (Wittneben et al., 2017), another because no data were reported although it was mentioned to be recorded (Patil et al., 2014) and one because data were reported as medians (Hosseini et al., 2011). The longest follow‐up from the same study was included in the meta‐analysis, except for (Canullo, Genova, et al., 2016; Canullo, Tallarico, et al., 2016) where no baseline data were provided. Changes in BOP percentages for all treatments modalities were not significant when comparing test to control abutments [n = 10; WMD = −3.34%; 95% CI (−9.84%; 3.16%); p < .314] and there was a high heterogeneity (I 2 = 64.1%; p = .003) (Table 3). When comparing the four different subgroups, no statistically significant differences were observed between the test and control groups. Within the abutment material group, there was a statistically significant greater increase in BOP values for titanium abutments when compared to zirconia abutments [n = 3; WMD = −26.96%; 95% CI (−45.00%; −8.92%); p < .003]. There was a trend towards significance for a greater increase in the percentage of BOP for gold abutments when compared to alumina abutments, although there was only one study comparing these materials [n = 1; mean difference = −4.24%; 95% CI (−8.86%; 0.38%); p < .072]. Figure 2 depicts the forest plots of the meta‐analysis performed for BOP.
Table 3.
Meta‐analysis for BOP (%)
| Group | Subgroups | n | Weighted mean difference (WMD) | Heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|
| DL (%) | 95% CI (%) | p‐Value | I 2 (%) | p‐Value | ||||
| Upper | Lower | |||||||
| All | 10 | −3.338 | −9.840 | 3.165 | .314 | 64.1 | .003 | |
| Material (All) | 7 | −6.842 | −16.702 | 3.017 | .174 | 72.2 | <.001 | |
| Metal vs. Alu | ||||||||
| All | 4 | 11.041 | −7.242 | 9.270 | .810 | 62.5 | .046 | |
| Ti vs. Alu | 3 | 4.833 | −3.984 | 13.650 | .283 | 29.2 | .244 | |
| Gold vs. Alu | 1a | −4.240 | −8.867 | 0.387 | .072 | |||
| Ti vs. Zir | 3 | −26.961 | −45.000 | −8.922 | .003 | 33.8 | .221 | |
| Macroscopic design | 1a | 15.000 | −44.468 | 14.468 | .318 | |||
| Surface topography | 1a | 14.880 | −10.057 | 39.817 | .242 | |||
| Surface manipulation | 1a | 0.000 | −4.452 | 4.452 | 1.0 | |||
N, number of studies; Ti, titanium; Alu, alumina; CI, confidence interval; vs., versus; DL, DerSimonian & Laird method; I2, heterogeneity index.
Mean difference instead of weighted mean difference, as it is based on only one study.
Figure 2.
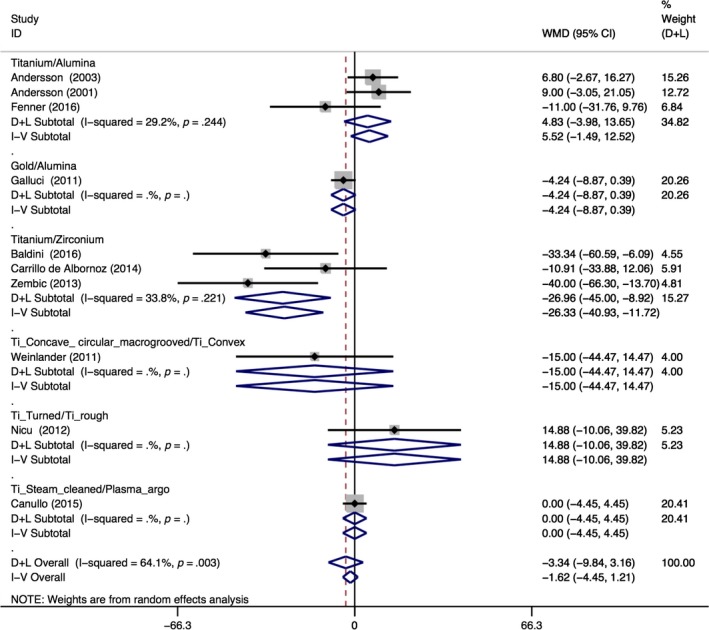
Forest plots for the BOP meta‐analysis
3.5.2. Secondary outcomes
Implant survival was given in all the studies except one (Patil et al., 2014). The mean implant survival rate was 98.61% (min: 89%; max: 100%), and there were no differences between the test and control groups (98.6% and 98.62%, respectively).
Radiographic changes in crestal bone levels were assessed in all the studies except two (Gallucci, Grutter, Nedir, Bischof & Belser, 2011; Sailer et al., 2009), both as mesial and distal values or as its average. Two additional studies only reported the final values (Fenner, Hammerle, Sailer & Jung, 2016; Van Assche et al., 2012) and another just reported the cumulative bone loss using a graphic (Nicu, Van Assche, Coucke, Teughels & Quirynen, 2012), so they were not included in the meta‐analysis. The meta‐analysis revealed no significant differences between the test and control abutments when compared all together or when comparing the abutment material or the macroscopic design. The only statistically significant difference was seen for the surface manipulation (cleaning method), with one study showing a greater amount of bone loss when using steam compared to argon plasma [n = 1; MD = −0.44 mm; 95% CI (−0.65 mm; −0.23 mm); p < .001] (Table 4).
Table 4.
Meta‐analysis for bone levels, probing pocket depth (PPD) and plaque index (PI)
| Group | Subgroups | n | Weighted mean difference (WMD) | Heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|
| DL | 95% CI | p‐Value | I 2 (%) | p‐Value | ||||
| Upper | Lower | |||||||
| BL (mm) | ||||||||
| All | 10 | −0.105 | −0.264 | 0.055 | .195 | 67.4 | .001 | |
| Material | ||||||||
| All | 7 | −0.008 | −0.185 | 0.168 | .925 | 51.8 | .053 | |
| Ti vs. Alu | 3 | 0.151 | −0.028 | 0.330 | .099 | 0.0 | .495 | |
| Ti vs. Zir | 4 | −0.078 | −0.344 | 0.188 | .566 | 60.4 | .056 | |
| Macroscopic design | 2 | −0.131 | −0.295 | 0.034 | .120 | 0.0 | .699 | |
| Surface topography | 0 | |||||||
| Surface manipulation | 1a | −0.440 | −0.651 | −0.229 | <.001 | |||
| PPD (mm) | ||||||||
| All | 6 | 0.097 | −0.144 | 0.339 | .428 | 33.4 | .186 | |
| Material, All (Ti vs. Zir) | 3 | −0.137 | −0.616 | 0.343 | .576 | 30.6 | .237 | |
| Macroscopic design | 2 | 0.191 | −0.209 | 0.591 | .350 | 67.0 | .082 | |
| Surface topography | 1a | 0.350 | −0.309 | 1.009 | .298 | |||
| Surface manipulation | 0 | |||||||
| PI (%) | ||||||||
| All | 6 | −0.095 | −3.079 | 2.889 | .950 | 0.0 | .601 | |
| Material , All | 4 | −1.231 | −7.771 | 5.309 | .712 | 17.5 | .303 | |
| Metal vs. Alu | ||||||||
| All | 3 | 0.864 | −4.276 | 6.003 | .742 | |||
| Ti vs. Alu | 2 | −1.306 | −12.235 | 9.623 | .815 | 0.0 | 0.907 | |
| Gold vs. Alu | 1a | 1.480 | −4.344 | 7.304 | .618 | |||
| Ti vs. Zir | 1a | −20.000 | −41.472 | 1.472 | .068 | |||
| Macroscopic design | 1a | 0.000 | −29.654 | 29.654 | 1.000 | |||
| Surface topography | 0 | |||||||
| Surface manipulation | 1a | 0.000 | −3.749 | 3.749 | 1.000 | |||
N, number of studies; DL, DerSimonian & Laird method; CI, confidence interval; I2, heterogeneity index; BL, bone loss; Ti, titanium; Alu, alumina; Zir, zirconia; vs., versus; PPD, probing pocket depth; PI, plaque index.
Mean difference instead of weighted mean difference, as it is based on only one study.
Regarding other peri‐implant health outcomes, PPD was assessed in all studies except six. Three additional studies only reported the final values, so they were not included in the meta‐analysis (Canullo et al., 2015; Fenner et al., 2016; Weinlander et al., 2011). The meta‐analysis revealed no significant differences, neither for the global nor for the subgroups comparisons (Table 4). Plaque index (PI) was recorded at the implant level as the percentage of sites exhibiting plaque in all the studies except six, where plaque was registered as full‐mouth levels (Baldini et al., 2016; Carrillo de Albornoz et al., 2014; Gallucci, Grutter, Nedir, et al., 2011) or as the modified plaque index (Hosseini et al., 2011; Nicu et al., 2012; Wittneben et al., 2017). Additionally, two studies reported only the final plaque values (Fenner et al., 2016; Sailer et al., 2009) and one stated that plaque was recorded but no data were given in the article (Patil et al., 2014), so they were not included in the meta‐analysis. The meta‐analysis revealed no significant differences for any of the comparisons, although there was a trend for a higher increase in plaque levels for titanium abutments compared to zirconia abutments [n = 1; Mean difference = −20.00%; 95% CI (−41.47%; 1.47%); p < .068] (Table 4). The incidence of peri‐implantitis was assessed in six studies. Five reported an incidence of 0%, both at the implant and at the subject level (Carrillo de Albornoz et al., 2014; Fenner et al., 2016; Hosseini et al., 2011; Weinlander et al., 2011; Zembic, Sailer, Jung & Hammerle, 2009). However, in the study by Hosseini et al. (2011); three patients in the test group presented positive suppuration without bone loss and three patients in the control group presented positive suppuration with PPD ≥ 5 mm without bone loss. In contrast, the study by Zembic, Bosch, Jung, Hammerle & Sailer (2013) reported an incidence of peri‐implantitis of 5.5% at the patient level and 7.1% at the implant level.
The peri‐implant soft tissue evaluation included the level of the mucosal margin, the crown length of the implant restoration (CLI), the apico‐coronal dimension of the keratinised mucosa on the mid‐buccal aspect of the implant crown (KM), the thickness of the mucosa and the level of the papilla. For these outcomes, no meta‐analysis was performed due to the small number of studies assessing them or because the variable was registered only at the end of the study.
The recession of the mucosal margin was assessed in six studies. When comparing zirconia to titanium abutments, minimal or no changes were observed in two studies (Carrillo de Albornoz et al., 2014; Zembic et al., 2013), whereas in one study, the titanium abutment experienced a greater amount of recession (0.29 mm), compared to the mucosal position of the zirconia abutment (−0.31 mm; Fenner et al., 2016). When evaluating the roughness of the abutment, minor recession was observed after 1 (0.25 mm) and 3 years (0.3 mm), without differences between groups (Nicu et al., 2012; Van Assche et al., 2012). For the surface decontamination method, abutments cleaned with plasma Argon had higher recession when compared to the conventional cleaning (0.58 mm vs. 0.22). The CLI was evaluated in three studies comparing different abutment materials (Carrillo de Albornoz et al., 2014; Fenner et al., 2016; Gallucci, Grutter, Nedir, et al., 2011) and one study comparing different macroscopic designs (Wittneben et al., 2017). In all, except one, where the titanium abutment experienced a greater increase in the CLI (0.86 mm vs. 0.19 mm; Fenner et al., 2016), the changes were minimal (<0.4 mm) and without any difference between groups.
The KM height was recorded in five studies, three comparing different abutment materials (Carrillo de Albornoz et al., 2014; Fenner et al., 2016; Gallucci, Grutter, Nedir, et al., 2011) and two evaluating different macroscopic designs (Patil et al., 2014; Wittneben et al., 2017). The changes over time were minimal within groups (0–0.7 mm), and no significant differences were observed between test and control (0–0.6 mm). At the end of the study periods, the mean values varied between 2.85 mm (SD = 0.37) and 5.4 mm (SD = 1.7). The thickness of the mucosa was evaluated in five studies. In four studies different abutment materials were compared, and an endodontic file was used to assess this outcome. In two of these studies, no changes were observed within groups over time (Baldini et al., 2016; Zembic et al., 2009), whereas in one study, both groups experienced a minor increase in the thickness (0.4–0.6 mm; Carrillo de Albornoz et al., 2014). In the remaining study, the outcome was only assessed at the end (Sailer et al., 2009). For all of them, the differences between groups were minimal (0–0.4 mm). Additionally, one study comparing concave to convex abutments showed that the thickness was greater in the concave group (2.1 vs. 1.3 mm; Weinlander et al., 2011).
The position of the interproximal papilla was assessed with the Jemt index (Jemt 1997) in seven studies (Baldini et al., 2016; Carrillo de Albornoz et al., 2014; Fenner et al., 2016; Sailer et al., 2009; Weinlander et al., 2011; Zembic et al., 2009, 2013) and by measuring the papilla height in study casts in one investigation (Gallucci, Grutter, Nedir, et al., 2011), or with the use of a stent (Canullo, Genova, et al., 2016; Canullo, Tallarico, et al., 2016). When evaluating the changes on the papilla index, there was a significant increase after one year for the mesial, distal or the average of both measurements (Baldini et al., 2016; Carrillo de Albornoz et al., 2014; Weinlander et al., 2011). On the other hand, when evaluating the changes between 3 and 5 years, the papilla index remained stable or slightly increased (Zembic et al., 2013). When comparing the differences between groups, one study found that the papilla index was higher when using a titanium abutment compared to a zirconia abutment (Baldini et al., 2016), another study observed the opposite (Carrillo de Albornoz et al., 2014) and two other studies did not find any differences among the abutment materials (Zembic et al., 2009, 2013). The remaining two studies combined the results for both treatment groups, so no comparisons could be done (Fenner et al., 2016; Sailer et al., 2009). For the papilla height, there was a significant and similar increase after two and five years in test and control groups (Canullo, Genova, et al., 2016; Canullo, Tallarico, et al., 2016; Gallucci, Grutter, Nedir, et al., 2011).
The analysis of aesthetics results, mucosal colour and technical complications is depicted in Appendix S2.
Finally, PROM's were reported in five articles by means of a visual analogue scale (VAS). In three of the studies, patients were equally satisfied regarding the aesthetic outcome when comparing zirconia to titanium abutments, with values from 8.3 to 9.1 out of 10 (Baldini et al., 2016; Carrillo de Albornoz et al., 2014; Hosseini et al., 2011), whereas in another study, the results were combined for both groups, with an average of 9.7 out of 10 (Fenner et al., 2016). In the last study, patients reported similar satisfaction when comparing gold to alumnina abutments (87.71 vs. 91.91 out of 100; Gallucci, Grutter, Chuang, et al., 2011).
4. DISCUSSION
The present investigation found that there was a statistically significant greater increase in mucosal inflammation (BOP) for titanium abutments when compared to zirconia abutments. The macroscopic design, surface topography or surface manipulation, however, did not have a significant influence in soft tissue inflammation. In regard to the peri‐implant hard tissues evaluated by the radiographic changes in marginal bone levels, no differences were encountered for the different abutment materials, their macroscopic design or surface topography although the method of their decontamination seemed to influence marginal bone loss.
The lesser BOP reported for the zirconia abutments may be explained by its lesser plaque retention, compared to titanium, due to the surface properties of this material, hence inducing a lesser degree of inflammation (Degidi et al., 2006; Nakamura, Kanno, Milleding & Ortengren, 2010). In fact, the meta‐analysis found lesser plaque accumulation in the zirconia abutment group. This finding has been confirmed in in vivo investigations where bacterial colonisation was compared between zirconia and titanium discs attached to removable dentals prosthesis. Zirconia discs harboured less overall bacteria (Rimondini, Cerroni, Carrassi & Torricelli, 2002; Scarano, Piattelli, Caputi, Favero & Piattelli, 2004). However, when zirconia and titanium implant abutments were compared in a 3‐month split‐mouth clinical trial, these findings were not confirmed and no differences in soft tissue health and bacterial composition were reported (van Brakel et al., 2011, 2012).
Not only the different plaque accumulation but also the quality of soft tissue attachment may play a role in the degree of inflammation. Zirconia has been shown to promote in vitro a higher degree of fibroblast proliferation when compared to titanium (Nothdurft et al., 2015). This property, however, did not translate to differential histological outcomes in experimental studies comparing abutments made of zirconium, titanium and gold alloys (Welander et al., 2008). This study reported similar soft tissue dimensions when titanium and zirconia abutments were compared. In gold alloy abutments, however, there was an apical shift of the barrier epithelium followed by marginal bone loss.
In regard to the macroscopic design, two studies used a commercially available abutment with a macro‐grove at the level of the implant shoulder compared to a conventional straight abutment. The tested design left more space for the soft tissue, increasing its thickness with the hope of improving the marginal seal. However, this modification did not seem to impact the peri‐implant soft tissues or radiographic outcomes (Patil et al., 2014; Weinlander et al., 2011).
The fact that surface topography did not have an effect in BOP or bone levels must not be overlooked. This finding is not in line with the hypothesis that an increase surface roughness would facilitate biofilm formation and therefore has a negative influence in clinical parameters (Teughels, Van Assche, Sliepen & Quirynen, 2006). Regarding the impact of surface roughness on soft tissue attachment, classic experimental studies have shown contradictory results. Abrahamsson et al. (2002) compared the soft tissue response to turned and acid‐etched titanium abutments. They demonstrated that the soft tissue adhesion was not significantly influenced by the material roughness. On the contrary, other histological investigations in animal and humans have demonstrated that soft tissue attachment is influenced by the surface roughness and that moderately rough surfaces can be beneficial for soft tissue integration (Glauser et al., 2005; Hermann et al., 2011; Schwarz et al., 2013). Considering the heterogeneity of the evidence, it must be taken into consideration that the lack of differences found in the surface topography subgroup analysis was based on two studies from one investigation with a 3‐year follow‐up, which may be insufficient to draw robust conclusions.
The only factor that seemed to influence the peri‐implant bone levels was the abutment surface decontamination. It is well known that the strong affinity of titanium to proteins and amino acids makes the complete cleaning of its surface rather difficult (Rowland, Shalaby, Latour & von Recum, 1995). Recently, plasma argon cleaning has shown to effectively decontaminate titanium surfaces in vitro (Canullo, Micarelli, Lembo‐Fazio, Iannello & Clementini, 2014). Human histologies have revealed that plasma of argon may promote cell adhesion and positively influence collagen fibre orientation (Garcia et al., 2017). The results of the publication included in the analysis revealed a difference of 0.44 mm between plasma argon and steamed cleaned abutments. Although statistically significant, these differences may not be clinically relevant.
The lack of differences for some of the comparisons analysed may be due to the questioned reliability of periodontal parameters to assess peri‐implant health (Coli, Christiaens, Sennerby & Bruyn, 2017). It has been shown that several factors such as gender or implant position can influence BOP values around implants (Farina, Filippi, Brazzioli, Tomasi & Trombelli, 2017). Moreover, excessive probing forces may induce false‐positive BOP readings (Gerber, Tan, Balmer, Salvi & Lang, 2009). Additionally, the access to insert the periodontal probe in cases of overhanging restorations may underestimate PPD values (Serino, Turri & Lang, 2013) and may induce trauma in the soft tissues, increasing false‐positive BOP.
Concerning the appearance of the tissues (i.e., soft tissue dimensions and colour of the mucosa), the systematic review failed to find differences among the tested abutments. Nevertheless, the scope of this review focused on clinical parameters that assessed inflammation, which excluded a number of investigations in which their main focus was the evaluation of the aesthetic outcomes. In this sense, a recent systematic review has shown a tendency for zirconia abutments to evoke a better colour response and superior aesthetic outcomes when compared to titanium (Linkevicius & Vaitelis, 2015).
Despite our efforts to study the impact of abutment characteristics in clinical parameters, very few investigations have actually evaluated the incidence of peri‐implant diseases. Although the onset of peri‐implantitis has been reported to occur as soon as three years after loading (Derks et al., 2016), there is a need of longer follow‐up evaluations, as the shift from mucositis to peri‐implantitis requires the detection of early signs of bone loss, which demands a longitudinal evaluation. From the methodological point of view, the statistical analysis presented a clear limitation based on the small number of included studies in some comparisons, which had an influence on the analysis of the publications bias. This precluded the subgrouping analysis based on methodological issues such as the study design (split vs. parallel) or on the unit of analyses (patient or implant level) which might have an impact on results and should be interpreted with caution. Two investigations that used one‐piece implants were also included in the analysis. One‐piece implants have a transmucosal portion in the form of a polished collar fixed to the body of the implant minimising the subgingival component of the prosthetic restoration and limiting the possible effect of changes in its surface characteristics. The studies that used this implant design (Fenner et al., 2016; Gallucci, Grutter, Chuang, et al., 2011; Gallucci, Grutter, Nedir, et al., 2011) treated mainly aesthetic areas countersinking the metal collar to allow for enough space for the subgingival component and concealment of the metal portion. Although these implants are not ideal for the study of changes in the subgingival composition of the abutment or prosthetic component, they were still included taking the limited evidence available. It is therefore recommended to conduct further investigations in the field of abutment design, surface manipulation and surface topography.
In conclusion, the results of this systematic review and meta‐analysis have shown that the macroscopic design, the surface topography and the manipulation of the implant abutment did not have an influence on peri‐implant BOP. In contrast, the abutment material had a significant impact, with a greater increase in BOP values over time for the Ti abutments when compared to Zi abutments.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
ACKNOWLEDGEMENTS
This investigation was performed as part of the OR Foundation consensus conference. The authors would like to thank all the authors who have been contacted to provide additional data on the published manuscripts.
Sanz‐Martín I, Sanz‐Sánchez I, Carrillo de Albornoz A, Figuero E, Sanz M. Effects of modified abutment characteristics on peri‐implant soft tissue health: A systematic review and meta‐analysis. Clin Oral Impl Res. 2018;29:118–129. 10.1111/clr.13097
Funding information
This systematic review was partially funded by the ORAL RECONSTRUCTION Foundation.
[The copyright line for this article was changed on 28 July 2018 after original online publication.]
REFERENCES
- Abrahamsson, I. , Berglundh, T. , Wennstrom, J. , & Lindhe, J. (1996). The peri‐implant hard and soft tissues at different implant systems. A comparative study in the dog. Clinical Oral Implants Research, 7, 212–219. [DOI] [PubMed] [Google Scholar]
- Abrahamsson, I. , Zitzmann, N. U. , Berglundh, T. , Linder, E. , Wennerberg, A. , & Lindhe, J. (2002). The mucosal attachment to titanium implants with different surface characteristics: An experimental study in dogs. Journal of Clinical Periodontology, 29, 448–455. [DOI] [PubMed] [Google Scholar]
- Abrahamsson, I. , Zitzmann, N. U. , Berglundh, T. , Wennerberg, A. , & Lindhe, J. (2001). Bone and soft tissue integration to titanium implants with different surface topography: An experimental study in the dog. International Journal of Oral and Maxillofacial Implants, 16, 323–332. [PubMed] [Google Scholar]
- Andersson, B. , Glauser, R. , Maglione, M. , & Taylor, A. (2003). Ceramic implant abutments for short‐span fpds: A prospective 5‐year multicenter study. International Journal of Prosthodontics, 16, 640–646. [PubMed] [Google Scholar]
- Andersson, B. , Scharer, P. , Simion, M. , & Bergstrom, C. (1999). Ceramic implant abutments used for short‐span fixed partial dentures: A prospective 2‐year multicenter study. International Journal of Prosthodontics, 12, 318–324. [PubMed] [Google Scholar]
- Andersson, B. , Taylor, A. , Lang, B. R. , Scheller, H. , Scharer, P. , Sorensen, J. A. , & Tarnow, D. (2001). Alumina ceramic implant abutments used for single‐tooth replacement: A prospective 1‐ to 3‐year multicenter study. International Journal of Prosthodontics, 14, 432–438. [PubMed] [Google Scholar]
- Baldini, N. , D'Elia, C. , Clementini, M. , Carrillo de Albornoz, A. , Sanz, M. , & De Sanctis, M. (2016). Esthetic outcomes of single‐tooth implant‐supported restorations using metal‐ceramic restorations with zirconia or titanium abutments: A randomized controlled clinical study. International Journal of Periodontics & Restorative Dentistry, 36, e59–e66. [DOI] [PubMed] [Google Scholar]
- Berglundh, T. , Abrahamsson, I. , & Lindhe, J. (2005). Bone reactions to longstanding functional load at implants: An experimental study in dogs. Journal of Clinical Periodontology, 32, 925–932. [DOI] [PubMed] [Google Scholar]
- Berglundh, T. , Abrahamsson, I. , Welander, M. , Lang, N. P. , & Lindhe, J. (2007). Morphogenesis of the peri‐implant mucosa: An experimental study in dogs. Clinical Oral Implants Research, 18, 1–8. [DOI] [PubMed] [Google Scholar]
- van Brakel, R. , Meijer, G. J. , Verhoeven, J. W. , Jansen, J. , de Putter, C. , & Cune, M. S. (2012). Soft tissue response to zirconia and titanium implant abutments: An in vivo within‐subject comparison. Journal of Clinical Periodontology, 39, 995–1001. [DOI] [PubMed] [Google Scholar]
- van Brakel, R. , Noordmans, H. J. , Frenken, J. , de Roode, R. , de Wit, G. C. , & Cune, M. S. (2011). The effect of zirconia and titanium implant abutments on light reflection of the supporting soft tissues. Clinical Oral Implants Research, 22, 1172–1178. [DOI] [PubMed] [Google Scholar]
- Buser, D. , Janner, S. F. , Wittneben, J. G. , Bragger, U. , Ramseier, C. A. , & Salvi, G. E. (2012). 10‐year survival and success rates of 511 titanium implants with a sandblasted and acid‐etched surface: A retrospective study in 303 partially edentulous patients. Clinical Implant Dentistry and Related Research, 14, 839–851. [DOI] [PubMed] [Google Scholar]
- Canullo, L. , Genova, T. , Tallarico, M. , Gautier, G. , Mussano, F. , & Botticelli, D. (2016). Plasma of argon affects the earliest biological response of different implant surfaces: An in vitro comparative study. Journal of Dental Research, 95, 566–573. [DOI] [PubMed] [Google Scholar]
- Canullo, L. , Micarelli, C. , Lembo‐Fazio, L. , Iannello, G. , & Clementini, M. (2014). Microscopical and microbiologic characterization of customized titanium abutments after different cleaning procedures. Clinical Oral Implants Research, 25, 328–336. [DOI] [PubMed] [Google Scholar]
- Canullo, L. , Penarrocha, D. , Clementini, M. , Iannello, G. , & Micarelli, C. (2015). Impact of plasma of argon cleaning treatment on implant abutments in patients with a history of periodontal disease and thin biotype: Radiographic results at 24‐month follow‐up of a rct. Clinical Oral Implants Research, 26, 8–14. [DOI] [PubMed] [Google Scholar]
- Canullo, L. , Tallarico, M. , Penarrocha‐Oltra, D. , Monje, A. , Wang, H. L. , & Penarrocha‐Diago, M. (2016). Implant abutment cleaning by plasma of argon: 5‐year follow‐up of a randomized controlled trial. Journal of Periodontology, 87, 434–442. [DOI] [PubMed] [Google Scholar]
- Carrillo de Albornoz, A. , Vignoletti, F. , Ferrantino, L. , Cardenas, E. , De Sanctis, M. , & Sanz, M. (2014). A randomized trial on the aesthetic outcomes of implant‐supported restorations with zirconia or titanium abutments. Journal of Clinical Periodontology, 41, 1161–1169. [DOI] [PubMed] [Google Scholar]
- Cochran, W. G. (1954). The combination of estimates from different experiments. Biometrics, 10, 101–129. [Google Scholar]
- Coli, P. , Christiaens, V. , Sennerby, L. , & Bruyn, H. (2017). Reliability of periodontal diagnostic tools for monitoring peri‐implant health and disease. Periodontology 2000, 73, 203–217. [DOI] [PubMed] [Google Scholar]
- Degidi, M. , Artese, L. , Scarano, A. , Perrotti, V. , Gehrke, P. , & Piattelli, A. (2006). Inflammatory infiltrate, microvessel density, nitric oxide synthase expression, vascular endothelial growth factor expression, and proliferative activity in peri‐implant soft tissues around titanium and zirconium oxide healing caps. Journal of Periodontology, 77, 73–80. [DOI] [PubMed] [Google Scholar]
- Derks, J. , Schaller, D. , Hakansson, J. , Wennstrom, J. L. , Tomasi, C. , & Berglundh, T. (2016). Peri‐implantitis – Onset and pattern of progression. Journal of Clinical Periodontology, 43, 383–388. [DOI] [PubMed] [Google Scholar]
- DerSimonian, R. , & Laird, N. (1986). Meta‐analysis in clinical trials. Controlled Clinical Trials, 7, 177–188. [DOI] [PubMed] [Google Scholar]
- Farina, R. , Filippi, M. , Brazzioli, J. , Tomasi, C. , & Trombelli, L. (2017). Bleeding on probing around dental implants: A retrospective study of associated factors. Journal of Clinical Periodontology, 44, 115–122. [DOI] [PubMed] [Google Scholar]
- Fenner, N. , Hammerle, C. H. , Sailer, I. , & Jung, R. E. (2016). Long‐term clinical, technical, and esthetic outcomes of all‐ceramic vs. titanium abutments on implant supporting single‐tooth reconstructions after at least 5 years. Clinical Oral Implants Research, 27, 716–723. [DOI] [PubMed] [Google Scholar]
- Gallucci, G. O. , Grutter, L. , Chuang, S. K. , & Belser, U. C. (2011). Dimensional changes of peri‐implant soft tissue over 2 years with single‐implant crowns in the anterior maxilla. Journal of Clinical Periodontology, 38, 293–299. [DOI] [PubMed] [Google Scholar]
- Gallucci, G. O. , Grutter, L. , Nedir, R. , Bischof, M. , & Belser, U. C. (2011). Esthetic outcomes with porcelain‐fused‐to‐ceramic and all‐ceramic single‐implant crowns: A randomized clinical trial. Clinical Oral Implants Research, 22, 62–69. [DOI] [PubMed] [Google Scholar]
- Garcia, B. , Camacho, F. , Penarrocha, D. , Tallarico, M. , Perez, S. , & Canullo, L. (2017). Influence of plasma cleaning procedure on the interaction between soft tissue and abutments: A randomized controlled histologic study. Clinical Oral Implants Research. 28, 1269–1277. [DOI] [PubMed] [Google Scholar]
- Gerber, J. A. , Tan, W. C. , Balmer, T. E. , Salvi, G. E. , & Lang, N. P. (2009). Bleeding on probing and pocket probing depth in relation to probing pressure and mucosal health around oral implants. Clinical Oral Implants Research, 20, 75–78. [DOI] [PubMed] [Google Scholar]
- Glauser, R. , Schupbach, P. , Gottlow, J. , & Hammerle, C. H. (2005). Periimplant soft tissue barrier at experimental one‐piece mini‐implants with different surface topography in humans: A light‐microscopic overview and histometric analysis. Clinical Implant Dentistry and Related Research, 7(Suppl 1), S44–S51. [DOI] [PubMed] [Google Scholar]
- Gotfredsen, K. (2012). A 10‐year prospective study of single tooth implants placed in the anterior maxilla. Clinical Implant Dentistry and Related Research, 14, 80–87. [DOI] [PubMed] [Google Scholar]
- Hermann, J. S. , Jones, A. A. , Bakaeen, L. G. , Buser, D. , Schoolfield, J. D. , & Cochran, D. L. (2011). Influence of a machined collar on crestal bone changes around titanium implants: A histometric study in the canine mandible. Journal of Periodontology, 82, 1329–1338. [DOI] [PubMed] [Google Scholar]
- Higgins J. P. T., & Green S. (2011). Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. London: The Cochrane Collaboration; Retrieved from http://handbook.cochrane.org. [Google Scholar]
- Higgins, J. P. T. , Thompson, S. G. , Deeks, J. J. , & Altman, D. G. (2003). Measuring inconsistency in meta‐analyses. British Medical Journal, 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini, M. , Worsaae, N. , Schiodt, M. , & Gotfredsen, K. (2011). A 1‐year randomised controlled trial comparing zirconia versus metal‐ceramic implant supported single‐tooth restorations. European Journal of Oral Implantology, 4, 347–361. [PubMed] [Google Scholar]
- Iglhaut, G. , Becker, K. , Golubovic, V. , Schliephake, H. , & Mihatovic, I. (2013). The impact of dis‐/reconnection of laser microgrooved and machined implant abutments on soft‐ and hard‐tissue healing. Clinical Oral Implants Research, 24, 391–397. [DOI] [PubMed] [Google Scholar]
- Iglhaut, G. , Schwarz, F. , Winter, R. R. , Mihatovic, I. , Stimmelmayr, M. , & Schliephake, H. (2014). Epithelial attachment and downgrowth on dental implant abutments–a comprehensive review. Journal of Esthetic and Restorative Dentistry, 26, 324–331. [DOI] [PubMed] [Google Scholar]
- Jemt, T. (1997). Regeneration of gingival papillae after single‐implant treatment. International Journal of Periodontics & Restorative Dentistry, 17, 326–333. [PubMed] [Google Scholar]
- Jepsen, S. , Berglundh, T. , Genco, R. , Aass, A. M. , Demirel, K. , Derks, J. , … Zitzmann, N. U. (2015). Primary prevention of peri‐implantitis: Managing peri‐implant mucositis. Journal of Clinical Periodontology, 42(Suppl 16), S152–S157. [DOI] [PubMed] [Google Scholar]
- Kim, S. , Oh, K. C. , Han, D. H. , Heo, S. J. , Ryu, I. C. , Kwon, J. H. , & Han, C. H. (2010). Influence of transmucosal designs of three one‐piece implant systems on early tissue responses: A histometric study in beagle dogs. International Journal of Oral and Maxillofacial Implants, 25, 309–314. [PubMed] [Google Scholar]
- Linkevicius, T. , & Vaitelis, J. (2015). The effect of zirconia or titanium as abutment material on soft peri‐implant tissues: A systematic review and meta‐analysis. Clinical Oral Implants Research, 26(Suppl 11), 139–147. [DOI] [PubMed] [Google Scholar]
- Nakamura, K. , Kanno, T. , Milleding, P. , & Ortengren, U. (2010). Zirconia as a dental implant abutment material: A systematic review. International Journal of Prosthodontics, 23, 299–309. [PubMed] [Google Scholar]
- Needleman, I. G. (2002). A guide to systematic reviews. Journal of Clinical Periodontology, 29(Suppl 3), 6–9; discussion 37–38. Review. [DOI] [PubMed] [Google Scholar]
- Nicu, E. A. , Van Assche, N. , Coucke, W. , Teughels, W. , & Quirynen, M. (2012). Rct comparing implants with turned and anodically oxidized surfaces: A pilot study, a 3‐year follow‐up. Journal of Clinical Periodontology, 39, 1183–1190. [DOI] [PubMed] [Google Scholar]
- Nothdurft, F. P. , Fontana, D. , Ruppenthal, S. , May, A. , Aktas, C. , Mehraein, Y. , … Kaestner, L. (2015). Differential behavior of fibroblasts and epithelial cells on structured implant abutment materials: A comparison of materials and surface topographies. Clinical Implant Dentistry and Related Research, 17, 1237–1249. [DOI] [PubMed] [Google Scholar]
- Patil, R. C. , den Hartog, L. , van Heereveld, C. , Jagdale, A. , Dilbaghi, A. , & Cune, M. S. (2014). Comparison of two different abutment designs on marginal bone loss and soft tissue development. International Journal of Oral and Maxillofacial Implants, 29, 675–681. [DOI] [PubMed] [Google Scholar]
- Rimondini, L. , Cerroni, L. , Carrassi, A. , & Torricelli, P. (2002). Bacterial colonization of zirconia ceramic surfaces: An in vitro and in vivo study. International Journal of Oral and Maxillofacial Implants, 17, 793–798. [PubMed] [Google Scholar]
- Rompen, E. (2012). The impact of the type and configuration of abutments and their (repeated) removal on the attachment level and marginal bone. European Journal of Oral Implantology, 5(Suppl), S83–S90. [PubMed] [Google Scholar]
- Rompen, E. , Domken, O. , Degidi, M. , Pontes, A. E. , & Piattelli, A. (2006). The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clinical Oral Implants Research, 17(Suppl 2), 55–67. [DOI] [PubMed] [Google Scholar]
- Rowland, S. A. , Shalaby, S. W. , Latour, R. A. Jr , & von Recum, A. F. (1995). Effectiveness of cleaning surgical implants: Quantitative analysis of contaminant removal. Journal of Applied Biomaterials, 6, 1–7. [DOI] [PubMed] [Google Scholar]
- Sailer, I. , Zembic, A. , Jung, R. E. , Siegenthaler, D. , Holderegger, C. , & Hammerle, C. H. (2009). Randomized controlled clinical trial of customized zirconia and titanium implant abutments for canine and posterior single‐tooth implant reconstructions: Preliminary results at 1 year of function. Clinical Oral Implants Research, 20, 219–225. [DOI] [PubMed] [Google Scholar]
- Salvi, G. E. , Bosshardt, D. D. , Lang, N. P. , Abrahamsson, I. , Berglundh, T. , Lindhe, J. , … Donos, N. (2015). Temporal sequence of hard and soft tissue healing around titanium dental implants. Periodontology 2000, 68, 135–152. [DOI] [PubMed] [Google Scholar]
- Scarano, A. , Piattelli, M. , Caputi, S. , Favero, G. A. , & Piattelli, A. (2004). Bacterial adhesion on commercially pure titanium and zirconium oxide disks: An in vivo human study. Journal of Periodontology, 75, 292–296. [DOI] [PubMed] [Google Scholar]
- Schwarz, F. , Hegewald, A. , & Becker, J. (2014). Impact of implant‐abutment connection and positioning of the machined collar/microgap on crestal bone level changes: A systematic review. Clinical Oral Implants Research, 25, 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, F. , Mihatovic, I. , Becker, J. , Bormann, K. H. , Keeve, P. L. , & Friedmann, A. (2013). Histological evaluation of different abutments in the posterior maxilla and mandible: An experimental study in humans. Journal of Clinical Periodontology, 40, 807–815. [DOI] [PubMed] [Google Scholar]
- Schwarz, F. , Sahm, N. , & Becker, J. (2012). Impact of the outcome of guided bone regeneration in dehiscence‐type defects on the long‐term stability of peri‐implant health: Clinical observations at 4 years. Clinical Oral Implants Research, 23, 191–196. [DOI] [PubMed] [Google Scholar]
- Serino, G. , Turri, A. , & Lang, N. P. (2013). Probing at implants with peri‐implantitis and its relation to clinical peri‐implant bone loss. Clinical Oral Implants Research, 24, 91–95. [DOI] [PubMed] [Google Scholar]
- Teughels, W. , Van Assche, N. , Sliepen, I. , & Quirynen, M. (2006). Effect of material characteristics and/or surface topography on biofilm development. Clinical Oral Implants Research, 17(Suppl 2), 68–81. [DOI] [PubMed] [Google Scholar]
- Tobias, A. , & Campbell, M. J. (1999). Modelling influenza epidemics in the relation between black smoke and total mortality. A sensitivity analysis. Journal of Epidemiology and Community Health, 53, 583–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Assche, N. , Coucke, W. , Teughels, W. , Naert, I. , Cardoso, M. V. , & Quirynen, M. (2012). Rct comparing minimally with moderately rough implants. Part 1: Clinical observations. Clinical Oral Implants Research, 23, 617–624. [DOI] [PubMed] [Google Scholar]
- Weinlander, M. , Lekovic, V. , Spadijer‐Gostovic, S. , Milicic, B. , Wegscheider, W. A. , & Piehslinger, E. (2011). Soft tissue development around abutments with a circular macro‐groove in healed sites of partially edentulous posterior maxillae and mandibles: A clinical pilot study. Clinical Oral Implants Research, 22, 743–752. [DOI] [PubMed] [Google Scholar]
- Welander, M. , Abrahamsson, I. , & Berglundh, T. (2008). The mucosal barrier at implant abutments of different materials. Clinical Oral Implants Research, 19, 635–641. [DOI] [PubMed] [Google Scholar]
- Wittneben, J. G. , Gavric, J. , Belser, U. C. , Bornstein, M. M. , Joda, T. , Chappuis, V. , … Bragger, U. (2017). Esthetic and clinical performance of implant‐supported all‐ceramic crowns made with prefabricated or cad/cam zirconia abutments. Journal of Dental Research, 96, 163–170. [DOI] [PubMed] [Google Scholar]
- Zembic, A. , Bosch, A. , Jung, R. E. , Hammerle, C. H. , & Sailer, I. (2013). Five‐year results of a randomized controlled clinical trial comparing zirconia and titanium abutments supporting single‐implant crowns in canine and posterior regions. Clinical Oral Implants Research, 24, 384–390. [DOI] [PubMed] [Google Scholar]
- Zembic, A. , Sailer, I. , Jung, R. E. , & Hammerle, C. H. (2009). Randomized‐controlled clinical trial of customized zirconia and titanium implant abutments for single‐tooth implants in canine and posterior regions: 3‐year results. Clinical Oral Implants Research, 20, 802–808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


