Abstract
Parents of children with autism spectrum disorders (ASD) consistently report executive functioning (EF) deficits. This study investigates the factor structure of the Behavior Rating Inventory of Executive Function (BRIEF) as reported by parents of children with ASD and typically developing children (TDC). BRIEFs for 411 children with ASD and 467 TDC were examined. Confirmatory factor analysis of a nine-factor model met thresholds for goodness-of-fit in TDC, but not in the ASD sample. We found globally elevated EF problems in the ASD sample, especially on the Shift scale. These findings confirm that children with ASD exhibit significant EF deficits. Further investigation is needed to understand the pervasive nature of cognitive inflexibility in children with ASD.
Keywords: Autism spectrum disorder, Cognitive flexibility, Executive functioning, Behavior Rating Inventory of Executive Function, Factor analysis
Introduction
Autism spectrum disorders (ASD) are characterized by deficits in social-communication, and the presence of repetitive, restricted behaviors and interests. Executive functioning (EF) difficulties are correlated with core symptom presentation (e.g., Kenworthy et al. 2009; Lopez et al. 2005; Reed et al. 2013; Yerys et al. 2009a) and adaptive behavior difficulties in ASD (Gilotty et al. 2002) and are linked to decreased independence and poor outcomes in adulthood (Hume et al. 2009). EF deficits are commonly reported in school age children with ASD, who have difficulty with EF tasks within the context of a neuropsychological assessment (Hill 2004; Kenworthy et al. 2008; Pennington and Ozonoff 1996; Sergeant et al. 2002) as well as through informant report of everyday functioning (Gioia et al. 2002a; Mackinlay et al. 2006; Kenworthy et al. 2005; Winsler et al. 2007).
Many definitions and descriptions of EF exist, generally stating that they are a collection of interconnected processes, which are responsible for planning and executing goal-directed behavior (e.g., Lezak 1995; Welsh and Pennington 1988). They are often reported to include the following major components: impulse control, initiation of tasks or activities, working memory, self-monitoring, cognitive flexibility, planning ability and organizational skills, as well as efficient problem-solving (Anderson et al. 2008). The structure of EF in ASD has implications for clinical practice. Both informant report (Gioia et al. 2002a) and laboratory based assessments (Pennington and Ozonoff 1996) identify EF deficits in ASD across many domains and on tasks that make demands on multiple processes. Cognitive and behavioral flexibility is a specific EF impairment for many individuals with ASD (Gioia et al. 2002a; Rosenthal et al. 2013), and laboratory measures also suggest organization and planning weaknesses (Hill 2004; Kenworthy et al. 2005). Difficulty with flexibility, organization and planning are specifically described as features of ASD in the Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (DSM-5; American Psychiatric Association 2013). Impaired flexibility is reflected in core symptoms of insistence on sameness and inflexible patterns of behavior and thinking (D’Cruz et al. 2013; Lopez et al. 2005; Reed et al. 2013, 2011; Yerys et al. 2009a). In addition, impaired planning and organizational skills are now explicitly referenced in the DSM-5 severity levels for ASD, which indicate that “problems of organization and planning hamper independence” in this population.
Researchers have identified limitations with assessing EF skills during neuropsychological assessment (Gioia and Isquith 2004; Silver 2000). For example, the highly structured clinical setting where assessments are often conducted is not representative of real world EF demands (Bernstein and Waber 1990). Consequently, children may perform within the expected levels on EF tasks in the clinic setting but perform poorly in daily activities that require EF abilities (Powell and Voeller 2004). This suggests that gathering information from parents and teachers about EF in everyday situations is critical, because everyday situations provide less structure, and because survey knowledgeable informants can collapse across many experiences and time to provide a more accurate assessment than might be offered by a single neuropsychological assessment (Burgess 1997). The Behavior Rating Inventory of Executive Function (BRIEF, Gioia et al. 2000) is an informant-report measure that was designed to assess EF abilities in the home and school environments. The BRIEF assesses EF problems across multiple domains within one measure. Recent analyses of the BRIEF in a mixed clinical sample (Gioia et al. 2002b) and with typically developing adults (Roth et al. 2013) support a factor structure of nine scales with three higher-order indices.
The purpose of this study was to examine the factor structure of the BRIEF as reported by parents of children with ASD and age and sex matched typically developing children (TDC). It is unknown whether the nine-factor structure identified in TDC groups is also observed in children with ASD. Given evidence of pervasive EF problems across all domains in ASD, as well as peaks of difficulty related to inflexibility, organizational skills, and planning abilities, we tested the hypothesis that the factor structure of parent reported EF problems in ASD might differ from that observed in TDC.
Methods
Participants
Four hundred and eleven children with ASD (348 males, 63 females, age range 5–18 years, mean = 10.6, SD = 3.2) were assessed at Children’s National Medical Center, the National Institute of Mental Health, and the Children’s Hospital of Philadelphia. Included participants received an ASD diagnosis from trained and experienced clinicians applying DSM-IV-TR criteria (APA 2000) and met diagnostic criteria established by the NICHD/NIDCD Collaborative Programs for Excellence in Autism (Lainhart et al. 2006) using expert clinical judgment based on all available clinical data including the Autism Diagnostic Interview/Autism Diagnostic Interview-Revised (ADI/ADI-R; Le Couteur et al. 1989; Lord et al. 1994) and the Autism Diagnostic Observation Schedule (Lord et al. 2000), or met the revised criterion score (ASD threshold ≥12: Corsello et al. 2007) for an ASD on the Social Communication Questionnaire (SCQ; Rutter et al. 2003). All ASD participants had a Full Scale IQ score ≥ 70, which was assessed using the Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV; The Psychological Corporation 2003), Wechsler Abbreviated Scale of Intelligence (WASI; The Psychological Corporation 1999), Wechsler Preschool and Primary Scale of Intelligence-Third Edition (WPPSI-III; The Psychological Corporation 2002), and Differential Ability Scales (DAS, The Psychological Corporation 1990). Participants were excluded if they had any parent reported history of co-morbid genetic or neurological disorders (e.g., Fragile X syndrome, Tourette’s syndrome, epilepsy).
Data from 467 age and sex matched TDC (394 males, 73 females, age range 5–18 years, mean = 10.2, SD = 3.2) were selected from the larger BRIEF normative sample of 1,419 children. The normative data were obtained through public and private school recruitment in urban, suburban, and rural parts of Maryland. All TDC had no history of special education or psychotropic medication usage (Gioia et al. 2000).
See Table 1 for demographic information.
Table 1.
Demographics (mean (SD))
| Participants | ASD | TDC |
|---|---|---|
| Total N | 411 | 467 |
| Age | 10.7 (3.2) | 10.2 (3.2) |
| % Male | 84.7 | 84.4 |
| SCQ (N = 290) | 19.6 (6.2) | – |
| ADI (N = 332) | ||
| Reciprocal social interactions | 18.6 (6.3) | – |
| Communication | 15.0 (4.9) | – |
| Restricted, repetitive, and stereotyped behavior | 5.7 (2.6) | – |
| ADOS communication + social (N = 328) | 11.7 (4.4) | – |
Procedures
Approval of the present study was obtained from Institutional Review Boards at Children’s National Medical Center, the National Institutes of Health, and the Children’s Hospital of Philadelphia. The BRIEF, intelligence and diagnostic assessments of ASD participants were completed as part of larger protocols.
Measures
The Behavior Rating Inventory of Executive Functioning (BRIEF; Gioia et al. 2000) is an 86-item, parent-report inventory that measures EF skills in children ages 5–18 years. Each item is scored on a Likert scale from 1 (Never) to 3 (Often). The BRIEF contains eight scales corresponding to the following EF subdomains: inhibition, shift, emotional control, initiation, working memory, planning and organization, organization of materials, self-monitoring. In addition, there are two higher-order indices: the Behavioral Regulation Index (comprised of the Inhibition, Shift, and Emotional Control scales) and the Metacognition Index (comprised of the Initiate, Working Memory, Planning and Organization, Organization of Materials, and Monitor scales), and an overall Global Executive Composite score. Raw scores on each of the domains are converted to T-scores; T-scores ≥65 indicate “potential clinical significance” (Gioia et al. 2000, p. 14). In addition to the eight scales in the published instrument, a nine scale factor structure has been proposed, in which the Monitor scale is split into Self-Monitor and Task-Monitor and the nine scales load onto three indices, Behavioral Regulation, Emotional Regulation, and Metacognition (Gioia et al. 2002b; Roth et al. 2013). There were no missing data from the BRIEFs of either the ASD or TDC groups.
The Autism Diagnostic Interview/Autism Diagnostic Interview-Revised (ADI/ADI-R; Le Couteur et al. 1989; Lord et al. 1994) is an interview conducted with the primary caregiver for differential diagnosis of ASD. The domains assessed in the algorithm match the criteria for diagnosis in the DSM-IV (American Psychiatric Association 1994). The three main diagnostic domains are assessed in the diagnostic algorithm, including Reciprocal Social Interaction, Communication, and Restricted, Repetitive, and Stereotyped Patterns of Behavior.
The Autism Diagnostic Observation Schedule (ADOS; Lord et al. 2000) is a semi-structured assessment that includes play and conversational interviews designed to assist with diagnosis of ASD. Like the ADI/ADI-R, the ADOS algorithm was also created to align with the DSM-IV criteria for an ASD, and the diagnostic algorithm included items related to reciprocal social interaction and communication skills.
The Social Communication Questionnaire (SCQ; Rutter et al. 2003) is a parent report questionnaire based on the ADI that evaluates social functioning, communication skills, and repetitive behaviors, both historically and currently. The SCQ Total score shows strong correlations with the ADI-R (r = 0.71) (Berument et al. 1999).
Data Analyses
Confirmatory factor analysis (CFA) using a robust weighted least squares estimation method was run to assess three, eight, and nine factor models representing BRIEF scores in children with ASD and TDC. The models were chosen based on previous findings in support of a three index, nine-scale factor structure (Gioia et al. 2002b; Roth et al. 2013) and the original BRIEF analyses published with the measure indicating an eight-scale factor structure (Gioia et al. 2000). In addition, second-order CFAs were conducted in both the ASD and TDC groups, specifically a model evaluating the fit of the nine scales (i.e., Inhibition, Shift, Emotional Control, Initiate, Working Memory, Planning and Organization, Organization of Materials, Self-Monitor, and Task-Monitor) nested in the three higher-order indices (i.e., Behavioral Regulation, Emotional Regulation, and Metacognition). The data from the BRIEF are ordinal and therefore CFAs were conducted using polychoric correlations and asymptotic covariance matrices (DiStefano 2002; Flora and Curran 2004). Criteria for each index of fit were as follows: root mean squared error of approximation (RMSEA) <0.06; comparative fit index (CFI) and Tucker-Lewis Index (TLI) ≥0.95 (Hu and Bentler 1999). We explored the data further by conducting a two (ASD, TDC) × eight (each BRIEF scale) repeated measures analysis of variance (ANOVA) to compare the ASD BRIEF scores to the TDC BRIEF scores. Post-hoc independent and paired t-tests were used to assess differences on specific BRIEF scale scores.
Results
Table 2 describes the summary of fit indices for the CFA. The three-factor and eight-factor models were relatively poor fits for BRIEF ratings in the ASD and TDC groups. The CFA of the nine-factor model met thresholds for goodness-of-fit in the TDC group, but not the ASD group. Exploratory model modification indices revealed that a cognitive flexibility item (#8) from the Shift subscale: “Tries the same approach to a problem over and over even when it does not work,” loaded strongly across all scales contributing to the Metacognition Index in the ASD, but not the TDC group. Further exploration of this item was completed. We attempted to free the parameters as well as remove the item entirely from the analysis and both methods led to other areas of strain in the model. We also tested other theoretically based model modifications, but none of the attempts to improve model fit resulted in acceptable fit indices.
Table 2.
CFA fit indices
| TDC
|
ASD
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 3 factors | 8 factors | 9 factors | Second order CFA | 3 factors | 8 factors | 9 factors | Second order CFA | |
| CFI | .930 | .941 | .953a | .953a | .863 | .861 | .829 | .917 |
| TLI | .928 | .939 | .951a | .951a | .859 | .856 | .822 | .914 |
| RMSEA | .046a | .043a | .038a | .038a | .052a | .052a | .048a | .041a |
CFI comparative fit index, TLI Tucker-Lewis Index, RMSEA root mean square of approximation
Met criteria for goodness of fit
Given the influence of item 8 (related to cognitive flexibility) in the exploratory model modification indices as well as previous literature that describes elevated inflexibility in children with ASD (e.g., Kenworthy et al. 2008; Rosenthal et al. 2013), we explored the frequency of item 8 endorsement in the TDC and ASD groups and the profile of BRIEF scales. Scores on item 8 were higher in the ASD than the TDC group. Seventy-two percent of parents rated children in the ASD group as having difficulty with item 8, compared to 32 % in the TDC group. (See Table 3 for frequency scores.) A repeated measures ANOVA showed a significant main effect of group across BRIEF scales (F(1,876) = 484.61, p <.001, ). Post-hoc t-tests showed that the ASD group had significantly higher scores on all BRIEF scales as compared to the TDC group (ts > 11.8, ps < 0.001). See Fig. 1. The ASD group’s Shift scale had the highest mean T-score (M = 70.1). An additional repeated measures ANOVA within the ASD group showed a significant main effect of scale (F(5.2,2151) = 75.79, p < 0.001, ). Post-hoc paired-samples t-tests revealed that the Shift scale was significantly higher than all other BRIEF scales in the ASD group (ts > 5.1, ps < 0.001).
Table 3.
Cognitive inflexibility item (BRIEF item 8) frequency in ASD and TDC
| TDC (%) | ASD (%) | |
|---|---|---|
| Never | 68.1 | 28.0 |
| Sometimes | 30.0 | 48.9 |
| Often | 1.9 | 23.1 |
Fig. 1.
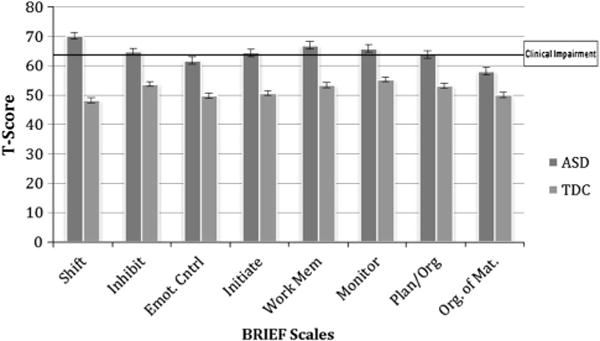
BRIEF Scale mean T-scores with standard error for ASD and TDC group
Discussion
To the best of our knowledge, this is the first study investigating the factor structure of EF in everyday settings with both TDC and children with ASD. In addition, this is the largest sample of children with ASD yet investigated using the BRIEF. We confirmed the nine-factor model of the BRIEF in our TDC group (i.e., Inhibition, Shift, Emotional Control, Initiate, Working Memory, Planning and Organization, Organization of Materials, Self-Monitor, and Task-Monitor) reported previously (e.g., Roth et al. 2013), and the second-order model where the nine BRIEF scales were nested in three higher-order indices (i.e., Behavioral Regulation, Emotional Regulation, and Metacognition). However, the three-factor model alone (Gioia et al. 2002b; Roth et al. 2013) was not confirmed among TDC in this study. In the ASD group none of the models met goodness of fit criteria and further analysis that involved attempts to free parameters and remove items, continued to lead to other areas of strain in the model.
We explored potential explanations for why the ASD group did not fit the same factor structure as the TDC group. Consistent with previous research (e.g., Gioia et al. 2002a), the BRIEF in children with ASD was elevated across scales, perhaps contributing to the poor goodness-of-fit. However, we utilized a robust estimation method to account for the ordinal nature of the data and the nonnormal distribution of the scores in the ASD group. Thus, the poor model fit in the ASD group is likely not attributable to higher BRIEF scores. An alternative explanation for the failure to replicate the TDC factor structure in the ASD data is the unusual dominance of flexibility problems in the ASD EF profile. In the ASD, but not the TDC sample, a single cognitive flexibility item (“Tries the same approach to a problem over and over even when it does not work,”) loaded highly on all BRIEF Metacognitive Index scales, and this item was endorsed as a problem by more than two-thirds of ASD parents but only a third of TDC parents. We also confirmed previous reports that the Shift scale is a significant peak in EF problems within the ASD group when compared to the other BRIEF scales (Gioia et al. 2002a; Kenworthy et al. 2005; Rosenthal et al. 2013). These findings are consistent with performance-based measures identifying cognitive flexibility deficits in ASD (Hill 2004; Reed et al. 2013; Yerys et al. 2009a) and raise the possibility that the predominance of flexibility problems within the profile of EF domains in ASD interferes with observation of the expected factor structure of EF in this group. Although this is an exploratory analysis, it reveals theoretically consistent findings, which has been previously identified as justification for interpreting the model modification indices (Schreiber et al. 2006).
Contrary to reports from laboratory based assessments, we did not find that parents reported problems with planning and organization to a greater degree than problems in other EF domains. This is consistent with another recent study involving the BRIEF from our lab (Rosenthal et al. 2013). The Plan/Organize scale was significantly elevated for the ASD group compared to TDC, but it was not in the clinically significant range and was lower than several other scales in addition to Shift. The divergence of planning and organization deficits between informant report and performance-based findings could reflect poor overlap between the two sources of information. Alternatively, the BRIEF Plan/Organize questions may not fully capture the impaired planning and organization that is so evident to clinicians in high functioning children with ASD (American Psychiatric Association 2013).
There were several limitations to this study. It excluded children with intellectual disability and children with medical and/or genetic conditions and therefore the findings may not generalize to all children with ASD. It also relied solely on parent report of EF. Future studies are needed to investigate teacher rating measures and performance-based measures in order to replicate these findings across settings and methods of assessment. Future studies could investigate the factor structure of performance-based EF measures to better understand the nature of cognitive inflexibility in children with ASD. Finally, under the new DSM-V criteria, clinicians have the opportunity to diagnose comorbid Attention Deficit/Hyperactivity Disorder (ADHD), which was not allowed utilizing the diagnostic criteria of Autistic Disorder, Asperger’s Disorder, or Pervasive Developmental Disorder-Not Otherwise Specified in the DSM-IV-TR. Since previous research has shown that comorbid ADHD negatively impacts EF in children with ASD (Corbett et al. 2009; Sinzig et al. 2008; Yerys et al. 2009b), future studies should consider the effect of co-morbid ADHD on the factor structure of EF in ASD. More generally, investigating whether children with ADHD, ASD, and comorbid ASD and ADHD have different BRIEF profiles would be a useful extension of earlier work (Gioia et al. 2002a) examining the discriminative validity of the BRIEF in clinical populations.
The current study confirms that children with ASD experience significant EF difficulties in everyday settings—with an emphasis on cognitive flexibility. Our results suggest that cognitive inflexibility is pervasive across metacognitive areas of EF within this population. These results have clinical implications. For example, due to the perseverative nature of cognitive flexibility in children with ASD, an intervention that focuses specifically on flexibility training (e.g., Cannon et al. 2011; Kenworthy et al. 2014), may lead to direct improvement in other metacognitive EF domains. By focusing on the EF area of most difficulty for children with ASD (i.e., cognitive inflexibility), working memory, planning, and organizational skills may also benefit. This will be an important hypothesis to test in future studies. In addition, these data confirm that the BRIEF is sensitive to EF deficits in ASD and also identify a unique profile of EF deficits, which emphasizes problems with flexibility. This makes the BRIEF a useful clinical tool in ASD (Leung and Zakzanis 2014; Tunc et al. 2014), but assumptions about the factor structure of the BRIEF in ASD populations should not be premised on findings from TDC. Finally, the fact that we replicated the normative factor structure in TDC but not those with ASD underscores the need to directly investigate the factor structure of complex constructs such as EF with other clinical populations before assuming that it matches that seen in TDC.
Acknowledgments
The authors thank the children and their families who contributed data to this study and also the authors of the BRIEF, Gerry Gioia, Peter Isquith and Steven Guy, for allowing access to the BRIEF normative data for this study. This research was supported by the Intramural Research Program of the NIH, National Institute of Mental Health, the Isadore and Bertha Gudelsky Foundation, registry grant NIMH 1RC1MH088791 to R. Schultz, a grant from the Pennsylvania Department of Health (SAP # 4100042728) to R. Schultz, a grant from the Pennsylvania Department of Health (SAP # 4100047863) to R. Schultz, a grant from Pfizer to R. Schultz, and a grant from the Robert Wood Johnson Foundation, #6672 to R. Schultz.
Footnotes
Conflict of interest Lauren Kenworthy receives financial compensation for use of the BRIEF. The other authors have no conflicts of interest.
Contributor Information
Yael Granader, Children’s National Medical Center, 15245 Shady Grove Road, Suite 350, Rockville, MD 20850, USA.
Gregory L. Wallace, Laboratory of Brain and Cognition, National Institute of Mental Health, Bethesda, MD, USA Department of Speech and Hearing Sciences, George Washington University, Washington, DC, USA.
Kristina K. Hardy, Children’s National Medical Center, 15245 Shady Grove Road, Suite 350, Rockville, MD 20850, USA
Benjamin E. Yerys, Center for Autism Research, Children’s Hospital of Philadelphia and the University of Pennsylvania, Philadelphia, PA, USA
Rachel A. Lawson, Children’s National Medical Center, 15245 Shady Grove Road, Suite 350, Rockville, MD 20850, USA
Michael Rosenthal, Children’s National Medical Center, 15245 Shady Grove Road, Suite 350, Rockville, MD 20850, USA.
Meagan C. Wills, Children’s National Medical Center, 15245 Shady Grove Road, Suite 350, Rockville, MD 20850, USA
Eunice Dixon, Laboratory of Brain and Cognition, National Institute of Mental Health, Bethesda, MD, USA.
Juhi Pandey, Center for Autism Research, Children’s Hospital of Philadelphia and the University of Pennsylvania, Philadelphia, PA, USA.
Rebecca Penna, Center for Autism Research, Children’s Hospital of Philadelphia and the University of Pennsylvania, Philadelphia, PA, USA.
Robert T. Schultz, Center for Autism Research, Children’s Hospital of Philadelphia and the University of Pennsylvania, Philadelphia, PA, USA
Lauren Kenworthy, Children’s National Medical Center, 15245 Shady Grove Road, Suite 350, Rockville, MD 20850, USA.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: American Psychiatric Association; 2000. text rev. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. fifth. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- Anderson V, Jacobs R, Anderson PJ. Executive functions and the frontal lobe. New York, NY: Taylor & Francis Group LLC; 2008. [Google Scholar]
- Bernstein J, Waber DP. Developmental neuropsychological assessment: The systemic approach. In: Boulton AA, Baker GB, Hiscock M, editors. Neuromethods: Volume 17, neuropsychology. Vol. 17. Clifton, NJ: Humana; 1990. pp. 311–371. [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: Diagnostic validity. British Journal of Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Burgess PW. Theory and methodology in executive function and research. In: Rabbitt P, editor. Methodology of frontal and executive function. Hove, UK: Taylor and Francis; 1997. pp. 81–116. [Google Scholar]
- Cannon L, Kenworthy L, Alexander K, Werner M, Anthony L. Unstuck and on target! An executive function curriculum to improve flexibility for children with autism spectrum disorders (research ed) Baltimore, MD: Pail H. Brookes Publishing Co; 2011. [Google Scholar]
- Corbett BA, Constantine LJ, Hendren R, Rocke D, Ozonoff S. Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder, and typical development. Psychiatry Research. 2009;166:210–222. doi: 10.1016/j.psychres.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsello C, Hus V, Pickles A, Risi S, Cook EH, Leventhal BL, et al. Between a ROC and a hard place: Decision making and making decisions about using the SCQ. Journal of Child Psychology and Psychiatry. 2007;48(9):932–940. doi: 10.1111/j.1469-7610.2007.01762.x. [DOI] [PubMed] [Google Scholar]
- D’Cruz AM, Ragozzino ME, Mosconi MW, Shrestha S, Cook EH, Sweeney JA. Reduced behavioral flexibility in autism spectrum disorders. Neuropsychology. 2013;27(2):152–160. doi: 10.1037/a0031721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano C. The impact of categorization with confirmatory factor analysis. Structural Equation Modeling. 2002;9(3):327–346. [Google Scholar]
- Flora DB, Curran PJ. An empirical evaluation of alternative methods of estimation for confirmatory factor analysis with ordinal data. Psychological Methods. 2004;9(4):466–491. doi: 10.1037/1082-989X.9.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilotty L, Kenworthy L, Sirian L, Black DO, Wagner AE. Adaptive skills and executive function in autism spectrum disorders. Child Neuropsychology. 2002;8(4):241–248. doi: 10.1076/chin.8.4.241.13504. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK. Ecological assessment of executive function in traumatic brain injury. Developmental Neuropsychology. 2004;25(1–2):135–158. doi: 10.1080/87565641.2004.9651925. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy S, Kenworthy L. BRIEF: Behavior Rating Inventory of Executive Function. Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- Gioia GA, Isquith PK, Kenworthy L, Barton RM. Profiles of everyday executive function in acquired and developmental disorders. Child Neuropsychology. 2002a;8:121–137. doi: 10.1076/chin.8.2.121.8727. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Retzlaff PD, Espy KA. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function (BRIEF) in a clinical sample. Child Neuropsychology. 2002b;8(4):249–257. doi: 10.1076/chin.8.4.249.13513. [DOI] [PubMed] [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends in Cognitive Sciences. 2004;8(1):26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indices in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Hume K, Loftin R, Lantz J. Increasing independence in autism spectrum disorders: A review of three focused interventions. Journal of Autism and Developmental Disorders. 2009;39(9):1329–1338. doi: 10.1007/s10803-009-0751-2. [DOI] [PubMed] [Google Scholar]
- Kenworthy L*, Anthony LG*, Naiman DQ, Cannon L, Wills MC, Werner MA, Alexander K, Strang J, Bal E, Sokoloff JL, Wallace GL. Executive function versus social skills interventions for children on the autism spectrum: An effectiveness trial. Journal of Child Psychology & Psychiatry. 2014;55:374–383. doi: 10.1111/jcpp.12161. *Joint first authors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy L, Black DO, Harrison B, Della Rosa A, Wallace GL. Are executive control functions related to autism symptoms in high-functioning children? Child Neuropsychology. 2009;15(5):425–440. doi: 10.1080/09297040802646983. [DOI] [PubMed] [Google Scholar]
- Kenworthy LE, Black DO, Wallace GL, Ahluvalia T, Wagner AE, Sirian LM. Disorganization: The forgotten executive dysfunction in high-functioning autism (HFA) spectrum disorders. Developmental Neuropsychology. 2005;28:809–827. doi: 10.1207/s15326942dn2803_4. [DOI] [PubMed] [Google Scholar]
- Kenworthy L, Yerys BE, Anthony LG, Wallace GL. Understanding executive control in autism spectrum disorders in the lab and in the real world. Neuropsychology Review. 2008;18(4):320–338. doi: 10.1007/s11065-008-9077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, et al. Head circumference and height in autism: A study by the collaborative program of excellence in autism. American Journal of Medical Genetics Part A. 2006;140(21):2257–2274. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, et al. Autism Diagnostic Interview: A standardized investigator-based instrument. Journal of Autism and Developmental Disorders. 1989;19(3):363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Leung RC, Zakzanis KK. Brief Report: Cognitive Flexibility in Autism Spectrum Disorders: A Quantitative Review. Journal of Autism and Developmental Disorders. 2014 doi: 10.1007/s10803-014-2136-4. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. New York, NY: Oxford University Press; 1995. [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of autistic disorder. Journal of Autism and Developmental Disorders. 2005;35(4):445–460. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mackinlay R, Charman T, Karmiloff-Smith A. High functioning children with autism spectrum disorder: A novel test of multitasking. Brain and Cognition. 2006;61:14–24. doi: 10.1016/j.bandc.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37(1):51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Powell KB, Voeller KK. Prefrontal executive function syndromes in children. Journal of Child Neurology. 2004;19:785–797. doi: 10.1177/08830738040190100801. [DOI] [PubMed] [Google Scholar]
- Reed P, Watts H, Truzoli R. Flexibility in young people with autism spectrum disorders on a card sort task. Autism. 2011;17(2):162–171. doi: 10.1177/1362361311409599. [DOI] [PubMed] [Google Scholar]
- Reed P, Watts H, Truzoli R. Flexibility in young people with autism spectrum disorders on a card sort task. Autism: The International Journal of Research and Practice. 2013;17(2):162–171. doi: 10.1177/1362361311409599. [DOI] [PubMed] [Google Scholar]
- Rosenthal M, Wallace GL, Lawson R, Wills MC, Dixon E, Yerys BE, et al. Impairments in real-world executive function increase from childhood to adolescence in autism spectrum disorders. Neuropsychology. 2013;27(1):13–18. doi: 10.1037/a0031299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RM, Lance CE, Isquith PK, Fischer AS, Giancola PR. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function-Adult Version in healthy adults and application to attention-deficit/hyperactivity disorder. Archive of Clinical Neuropsychology. 2013;28(5):425–434. doi: 10.1093/arclin/act031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Schreiber JB, Nora A, Stage FK, Barlow EA, King J. Reporting structural equation modeling and confirmatory factor analysis results: A review. The Journal of Educational Research. 2006;99(6):323–338. [Google Scholar]
- Sergeant JA, Geurts H, Oosterlaan J. How specific is a deficit of executive functioning for attention-deficit/hyperactivity disorder? Behavioral and Brain Research. 2002;130:3–28. doi: 10.1016/s0166-4328(01)00430-2. [DOI] [PubMed] [Google Scholar]
- Silver CH. Ecological validity of neuropsychological assessment in childhood traumatic brain injury. Journal of head trauma rehabilitation. 2000;15(4):973–988. doi: 10.1097/00001199-200008000-00002. [DOI] [PubMed] [Google Scholar]
- Sinzig J, Morsch D, Bruning N, Schmidt MH, Lehmkuhl G. Inhibition, flexibility, working memory and planning in autism spectrum disorders with and without comorbid ADHD-symptoms. Child and Adolescent Psychiatry and Mental Health. 2008;2(1):4. doi: 10.1186/1753-2000-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Psychological Corporation. Differential Abilities Scales. San Antonio, TX: The Psychological Corporation; 1990. [Google Scholar]
- The Psychological Corporation. Wechsler Abbreviated Scale of intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- The Psychological Corporation. Wechsler Preschool and Primary Scale of intelligence-third edition. San Antonio, TX: The Psychological Corporation; 2002. [Google Scholar]
- The Psychological Corporation. Wechsler Intelligence Scale for children-fourth edition. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Tunc B, Ghanbari Y, Smith AR, Pandey J, Browne A, Schultz RT, Verma R. PUNCH: Population Characterization of Heterogeneity. NeuroImage. 2014 doi: 10.1016/j.neuroimage.2014.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh MC, Pennington BF. Assessing frontal lobe functioning in children: Views from developmental psychology. Developmental Neuropsychology. 1988;4:199–230. [Google Scholar]
- Winsler A, Abar B, Feder MA, Schunn CD, Rubio DA. Private speech and executive functioning among high functioning children with autistic spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37:1617–1635. doi: 10.1007/s10803-006-0294-8. [DOI] [PubMed] [Google Scholar]
- Yerys BE, Wallace GL, Harrison B, Celano MJ, Giedd JN, Kenworthy L. Set-shifting in children with autism spectrum disorders: Reversal shifting deficits on the Intradimensional/Extradimensional Shift Test correlate with repetitive behaviors. Autism. 2009a;13(5):523–538. doi: 10.1177/1362361309335716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys BE, Wallace GL, Sokoloff JL, Shook DA, James JD, Kenworthy L. Attention deficit/hyperactivity disorder symptoms moderate cognition and behavior in children with autism spectrum disorders. Autism Research. 2009b;2(6):322–333. doi: 10.1002/aur.103. [DOI] [PMC free article] [PubMed] [Google Scholar]


