Abstract
Background:
Determination of secular trends in cognitive aging is important for prioritization of resources, services, and research in aging populations. Prior studies have identified declining dementia incidence associated with changes in cardiovascular risk factors and increased educational attainment. However, few studies have examined these factors in multi-ethnic cohorts.
Objective:
To identify secular trends in the incidence rate of dementia in an elderly population.
Methods:
Participants in this study were drawn from the Washington Heights-Inwood Columbia Aging Project, a multiethnic cohort study of northern Manhattan residents aged 65 years and older. Cox proportional hazards models were used to examine differences in the incidence of dementia in cohorts recruited in 1992 and 1999, with age at dementia or age at last follow-up visit as the “time-to-event” variable.
Results:
Overall, there was a 41% reduction in the hazard ratio for dementia among participants in the 1999 cohort compared with those in the 1992 cohort, adjusting for age, sex, race, and baseline memory complaints (HR = 0.59). The reduction in incidence was greatest among non-Hispanic Whites and African-Americans and lowest among Hispanic participants (HRs = 0.60, 0.52 and 0.64, respectively), and was associated with increases in level of educational attainment, especially among African-Americans. Reduction in incidence of dementia was also greater among persons 75 years or older than among younger participants (HR = 0.52 versus HR = 0.69).
Conclusions:
Our results support previous findings that secular trends in dementia incidence are changing, including in aging minority populations.
Keywords: Cohorts, dementia, incidence, race/ethnicity, secular trends
INTRODUCTION
Secular trends in cognitive aging have important implications in prioritization of resources, services, and research in aging populations. Multiple studies have explored changes in the prevalence and incidence of dementia and cognitive impairment utilizing separately constructed aging cohorts repeated over time within the same geographic area. Studies drawn from Europe [1–8], Asia [9–11], Africa [12], and North America [12–25] have generated conflicting findings with respect to dementia prevalence, but have shown generally stable or lower dementia incidence rates. Overall, these studies have suggested a static dementia burden, with declining dementia incidence offset by increasing disease duration, leaving stable or even greater prevalence over time [26]. When found, the reasons for declining prevalence or incidence rates in some of the studies are not well understood but are thought to be related to secular changes associated with better control of cardiovascular risk factors and higher attained educational levels in successive aging cohorts [3, 5, 15, 16, 20, 23, 24].
Clarifying the factors associated with a decline in the incidence rate of dementia is important given the opportunity to identify potentially modifiable medical and social factors influencing disease expression [21]. A recent study of 5-year incidence rates in the Framingham Cohort showed declining incidence of dementia over four epochs of study from 1975 to the present in those with at least a high school education [24]. In the Health and Retirement Study, dementia prevalence declined in 2012 compared with 2000 and coincided with increased levels of education; notably, these changes occurred despite an increased burden of diabetes, heart disease, and hypertension in the 2012 survey [23].
Given race-ethnic health disparities in US populations, clarifying factors associated with change in dementia incidence rates may have even greater relevance given the anticipated relative population growth in African-American and Hispanic US populations between 2014 and 2060 [27]. Multi-ethnic epidemiologic studies of US elderly have consistently shown higher prevalence and incidence rates of dementia in African-Americans and Hispanics compared with non-Hispanic Whites [14, 28–31]. The reasons for these epidemiological findings are unclear, but have been proposed to relate to the disproportionately high frequency of vascular risk factors among minority populations [32, 33], including diabetes mellitus, hypertension, cardiovascular disease [33], and cerebrovascular disease [33, 34]. Several of these potentially modifiable vascular risk factors including diabetes [30, 35–37], hyperlipidemia [38], hypertension [39], and smoking [35, 40, 41], have been associated with increased risk of dementia, including Alzheimer’s disease and vascular dementia.
Although several American studies have explored changes in the prevalence of dementia or impaired cognitive test performance, few have explored the changes in incidence rates in a multiethnic United States cohort, including non-Hispanic Whites, non-Hispanic Blacks, and Hispanics. In this study, we examined changes in the incidence rates of dementia in multi-ethnic cohorts of elderly from the Washington Heights-Inwood Columbia Aging Project (WHICHAP) study, enrolled in two time periods.
METHODS
Study population
Participants in the Washington Heights-Inwood Columbia Aging Project (WHICAP) are a representative sample of Medicare recipients, 65 years and older, living in northern Manhattan between 145th Street as its Southern boundary and the Hudson, East, and Harlem Rivers as the Western, Eastern, and Northern boundaries. The sampling strategies and recruitment outcomes of this cohort study have been described in detail elsewhere [30]. The population from which participants were drawn comprises individuals from three broadly defined ethnic categories (i.e., Hispanic, African American, and non-Hispanic White). Ethnic group was determined by self-report using the format of the 1990 US Census [42]. All individuals were first asked to report their race (i.e., American Indian/Alaska Native, Asian, Native Hawaiian, or other Pacific Islander, Black or African American, or White). Then in a second question, they were asked whether or not they were Hispanic.
This study combined longitudinal data from two recruitment efforts in this community, one beginning in 1992 and the other in 1999. Re-evaluations occurred during follow-up waves that were spaced approximately 18 to 30 months apart. Names and addresses of participants were provided for each cohort by Center for Medicare and Medicaid Services [30, 43]. Overall, demographic characteristics of those recruited in 1992 and 1999 are similar to aggregate data from the US Census data of older adults living in northern Manhattan with respect to age, years of education, race/ethnicity, and sex. Recruitment in 1992 was focused on enrolling a cohort that was representative of northern Manhattan for prevalence and incidence studies. A total of 4,865 individuals were sent letters in the recruitment of this cohort in 1992. Of these, attempts at follow-up by telephone or in-person visit indicated that 470 (9.7%) had died, 896 (18.4%) no longer lived in the region, 50 (1.0%) were ineligible, and 1,324 (27.2%) did not wish to participate. The total number recruited in 1992 was 2,125 (43.7%). Recruitment in 1999 was focused on forming a cohort of ethnically and educationally diverse, non-demented elders. Recruitment letters were sent to a total of 7,120 persons living in households with a known phone number. Of these, 265 (3.7%) were found to have died, 1,541 (21.6%) no longer lived in the region, 662 (9.3%) were ineligible, and 2,810 (39.5%) refused to participate. The total number of individuals with known phone numbers recruited was 1,842. The overall recruitment rate for eligible individuals living in the study area was therefore 39.6%. An additional 341 individuals without phone numbers were recruited by paying in-person visits (in many cases multiple visits) to the person at his/her address. The total number recruited therefore was 2,183. The 1992 and 1999 cohorts differed on demographic factors such as ethnicity and education, consistent with changes in the demographic composition of our catchment area. Despite our attempts to exclude dementia at the time of enrollment specifically in the cohort recruited in 1999, 10.2% of the participants were ultimately diagnosed with dementia at baseline. For these analyses, we excluded participants with prevalent dementia (i.e., individuals diagnosed as demented at the baseline evaluation) as well as those with insufficient data to determine dementia status, and those with ethnicities other than non-Hispanic White, African-American, or Hispanic.
We compared baseline cognitive factor scores on language, memory, visual spatial measures, adjusting for age, sex, ethnicity, and education, for the members of the 1992 and 1999 cohorts studied in this analysis. Mean cognitive function in the 1999 cohort did not differ on measures of memory or visual spatial performance, but was significantly better on measures of language than mean cognitive function in the 1992 cohort. We also compared the 1992 and 1999 cohorts in the likelihood of being in the lowest quartile of cognitive performance on cognitive factor scores, adjusting for covariates as above. The two cohorts did not differ in the likelihood of being in the lowest quartile of the cognitive factors versus being in the other three quartiles. In addition, we have adjusted for baseline memory complaints in the analysis to take any remaining recruitment differences into account.
We also compared the 1992 and 1999 cohorts in the likelihood of being in the lowest quartile of cognitive performance on cognitive factor scores, adjusting for covariates as above. The two cohorts did not differ in the likelihood of being in the lowest quartile of the cognitive factors versus being in the other three quartiles. Figure 1A and 1B show that rates of those who were evaluated, refused, unable to locate, moved, and died were comparable across the two cohorts in successive follow-up waves.
Fig. 1.
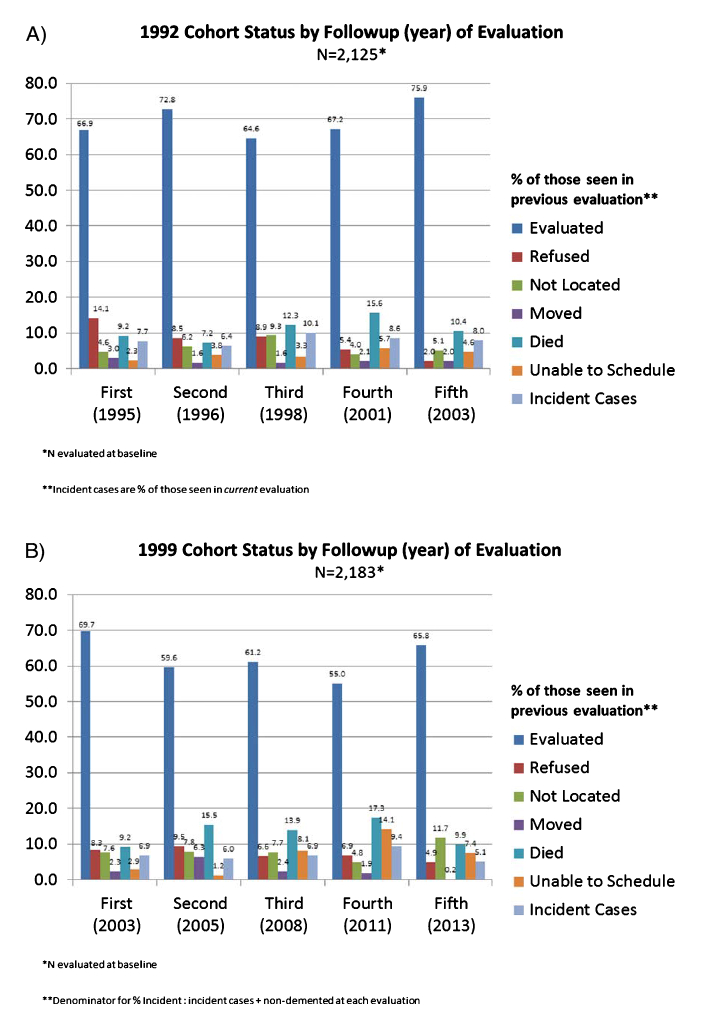
A) 1992 cohort and B) 1999 cohort. Proportions of those who were evaluated, refused to continue participation, could not be located, moved, could not be scheduled for follow-up visit, died, or were new incident dementia across the two cohorts in successive follow-up waves.
We compared differences in the incidence rates for dementia in the cohort enrolled between 1992 and 1994 (1992 cohort) and the cohort enrolled between 1999 and 2001 (1999 cohort). Members of the 1992 cohort were born between 1905 and 1928 while members of the 1999 cohort were born between 1914 and 1934. In order to ensure comparable age and duration of follow-up for the two cohorts, we selected people who were between 65 and 86 years of age at baseline, had 16 years of follow-up or less and who were 95 years or younger at their final visit: this included 1129 participants from the 1992 cohort and 1728 participants from the 1999 cohort. Follow-up time was calculated as time in years between the first and last visit. Figure 2 shows how the two cohorts were selected. We analyzed secular changes in the incidence of dementia both for the total group and within each race/ethnicity group. Participants selected for the current study (n = 2,857) had an average age of 75.0 years (SD = 5.0) and an average of 9.9 years of school (SD = 4.8). They were significantly younger and better educated than those excluded because of missing data or lack of follow-up, whose average age was 76.6 years (SD = 6.8) and who had an average of 9.2 years of school (SD = 4.4). Those selected for the current study did not differ from those excluded with respect to racial composition, but there was a higher percentage of women (66.1%) in the final sample than among those who were excluded (64.9%). Using a summary measure of medical burden, we found that those who were excluded did not have more medical illnesses than those in the final sample. Recruitment, informed consent and study procedures were approved by the Institutional Review Boards of Columbia University Medical Center and Columbia University Health Sciences and the New York State Psychiatric Institute.
Fig. 2.
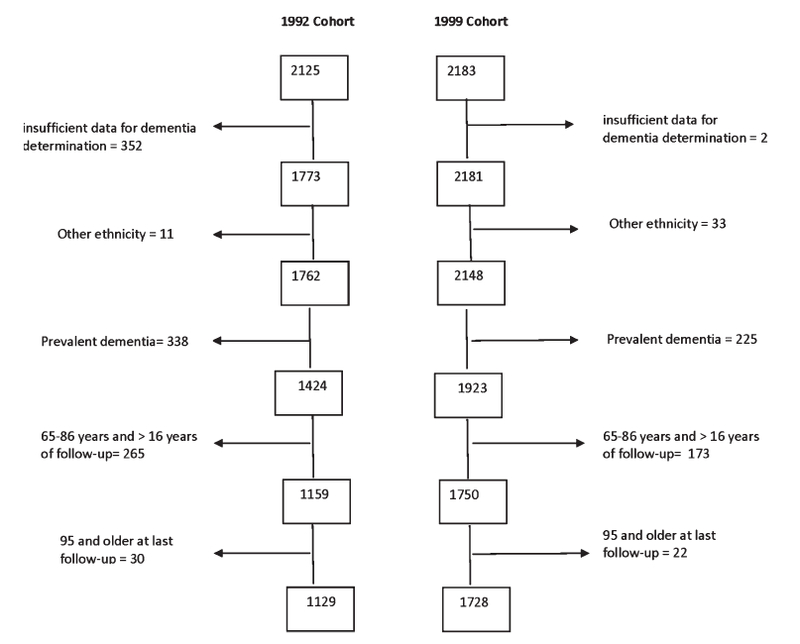
Flow diagram for how the two participants for study were identified from within each cohort. For both the 1992 and 1999 cohorts, successive steps for exclusion included: 1) insufficient data for determination of dementia diagnosis; 2) ethnicity other than non-Hispanic White, African-American, or Caribbean Hispanic; 3) dementia prevalent at baseline; 4) those who were aged older than 86 at baseline and had more than 16 years of follow-up; and 5) aged 95 years or older at last follow-up.
Clinical evaluation
At each assessment, each participant underwent an in-person interview of general health and functional ability followed by a semi-structured standardized assessment, including medical history, physical and neurological examination, and a neuropsychological battery that included measures of memory, orientation, language, abstract reasoning, and visuospatial ability [44]. The neuropsychological test battery and its validity in the diagnosis of dementia has been described in detail in a previous publication [44].
Memory complaints were assessed using methods as previously described [45]. In brief, participants were asked whether they had memory difficulties in general, as well as difficulties in specific areas such as memory for names, lists, and coming up with words.
All participants received structured neurologic, medical, and functional assessments. The diagnosis of dementia was based on standard research criteria [46] and was established using all available information gathered from the initial and follow-up assessments and medical records at a consensus conference of physicians, neurologists, neuropsychologists, and psychiatrists. Onset of dementia was classified as occurring at the first visit at which the participant met criteria for dementia. Measures and methods for determining medical and neurological history, examination, neuropsychological testing, and cognitive status diagnosis, did not change over the course of the study.
Vascular disease history and antecedent risk factors
History of diabetes, hypertension, heart disease, and stroke was ascertained by self-report [36]. History of heart disease included arrhythmias (e.g., atrial fibrillation), coronary artery disease, and congestive heart failure. Stroke was defined according to the WHO criteria [47], based on self-report, supplemented by a neurological examination. Current smoking was ascertained by self-report. Information on vascular disease history and current smoking was collected at baseline and at each follow-up. Participants were classified as positive for a history of vascular disease or as a smoker if the condition was reported at any visit.
Statistical analysis
Distribution and differences between cohorts in demographic data and vascular disease history variables (i.e., hypertension, diabetes, heart disease, stroke) and other covariates were determined with Chi-squared analysis and general linear models. We used Cox-proportional hazards regression models to examine differences in the incidence rates for dementia between the 1992 and 1999 cohorts, with age at dementia or age at last follow-up visit among those remaining non-demented as the “time-to-event” variable. Cohort was the principal predictor of interest, using the 1992 cohort as the reference group. Covariates were examined in three models: Model 1 included age, sex, and race/ethnicity and baseline memory complaints. Model 2 included Model 1 variables plus diabetes, heart disease, stroke, hypertension, and smoking history; Model 3 included Model 2 variables plus education. Risk factors were considered as confounders (e.g., education), or mediators (e.g., vascular risk factors) of dementia. All models were repeated within strata defined by race/ethnicity. Using Cox analyses, we dichotomized age as below and above 75 years at baseline, to allow for sufficient numbers among those in the oldest age group and thus at highest risk for dementia. Incidence rates per 1,000 person years were calculated by birth cohort and by race/ethnicity within each cohort. All analyses were performed using SPSS v. 21.
RESULTS
Cohorts
As shown in Table 1, compared with the 1992 cohort, participants in the 1999 cohort were slightly younger at baseline, with slightly longer follow-up. The 1999 cohort also had higher educational attainment, a slightly lower unadjusted number of memory complaints at baseline and a lower unadjusted rate of incident dementia cases. The distribution of cardiovascular risk factors also differed between cohorts. Compared with the 1992 cohort, the 1999 cohort had a higher proportion of participants with hypertension, heart disease and higher average body mass index (BMI) but a lower rate of smoking. The cohorts did not differ by sex, the proportion with diabetes (27.0% versus 24.5%) or stroke (17.0% versus 19.2%) (Table 1).
Table 1.
Demographic characteristics and cardiovascular risk factors by cohort and race/ethnicity
| Characteristic Cohort | Total | White | African-American | Hispanic | ||||
|---|---|---|---|---|---|---|---|---|
| 1992 | 1999 | 1992 | 1999 | 1992 | 1999 | 1992 | 1999 | |
| N | 1129 | 1728 | 245 | 528 | 393 | 569 | 491 | 631 |
| Baseline Age (M, SD) | 75.4 | 74.8* | 75.5 (5.0) | 75.1 (5.2) | 75.7 (4.6) | 75.0 (5.2)* | 75.1 (4.7) | 74.4 (5.1)* |
| Follow-up (M, SD) | 4.9 (4.4) | 5.5 (4.7)* | 4.5 (4.1) | 5.9 (4.7)* | 4.7 (4.2) | 5.2 (4.4) | 5.2 (4.6) | 5.5 (4.9) |
| Female (n, %) | 754 (66.8) | 1136 (65.7) | 153 (62.4) | 318 (60.2) | 269 (68.4) | 396 (69.6) | 332 (67.6) | 422 (66.9) |
| Education (M, SD) | 8.7 (4.6) | 10.6 (4.8)* | 12.0 (4.2) | 13.8 (3.2)* | 9.8 (3.5) | 12.0 (3.5)* | 6.2 (4.2) | 6.8 (4.3*) |
| Memory Complaints (M, S.D.) | 1.53 (1.5) | 1.34 (1.58)* | 1.2 (1.3) | 1.2 (1.4) | 1.5 (1.5) | 1.2 (1.5)* | 1.7 (1.6) | 1.5 (1.7)* |
| Incident Dementia (n, %) | 222 (19.7) | 194 (11.2)* | 20 (8.2) | 30 (5.7) | 76 (19.3) | 57 (10.0)* | 126 (25.7) | 107 (17.0)* |
| Diabetes (n, %) | 251 (24.5) | 462 (27.0) | 30 (13.9) | 99 (18.9) | 90 (24.6) | 162 (28.6) | 131 (29.5) | 201 (32.2) |
| Hypertension (n, %) | 719 (70.4) | 1413 (82.5)* | 132 (61.1) | 405 (77.4)* | 256 (70.1) | 488 (86.2)* | 331 (75.1) | 520 (83.3)* |
| Heart Disease (n, %) | 307 (29.9) | 738 (43.1)* | 61 (28.2) | 258 (49.2)* | 103 (28.1) | 245 (43.3)* | 143 (32.2) | 235 (37.7) |
| Stroke (n, %) | 147 (19.2) | 291 (17.0) | 24 (14.8) | 76 (14.5) | 50 (18.8) | 100 (17.7) | 73 (21.6) | 115 (18.4) |
| Current Smoking (n, %) | 159 (14.1) | 166 (9.6)* | 30 (13.9) | 42 (8.5) | 71 (19.9) | 77 (14.7)* | 58 (13.6) | 47 (8.2)* |
| BMI (M,SD) | 27.2 (5.2) | 28.1 (5.9)* | 26.5 (4.9) | 27.0 (5.3) | 27.4 (5.6) | 28.9 (6.8)* | 27.5 (5.0) | 28.4 (5.1)* |
Chi square and t tests were used to compare group means
p < 0.05.
Percentages can vary because of missing data.
Table 1 also shows the distribution of these factors by race/ethnicity group. In both the 1992 and 1999 cohorts, Hispanics had the highest proportion of participants who developed dementia, followed by African-Americans and then non-Hispanic Whites (Table 1). The proportion of participants developing dementia was lower in the 1999 cohort compared with the 1992 cohort among African-Americans and Hispanics, but the difference was not statistically significant for non-Hispanic Whites. Among non-Hispanic Whites the proportion of those with hypertension and heart disease were significantly higher in the 1999 cohort compared with the 1992 cohort. Among African Americans, those in the 1999 cohort were younger at baseline, had fewer memory complaints, had a higher proportion with hypertension and heart disease, had higher average BMI, and had a lower proportion of smoking than those in the 1992 cohort. Among Hispanics, those in the 1999 cohort were younger at baseline, had fewer memory complaints, a higher proportion of hypertension, higher average BMI and a lower frequency of smoking than those in the 1992 cohort. Among all race/ethnicity groups, the proportion of those with diabetes and stroke did not differ by cohort.
Incidence
The incidence rates of dementia were 44.9/1,000 persons years (95% CI: 39.1–50.7) in the 1992 cohort and 21.1/1,000 person years (95% CI: 18.1–24.0) in the 1999 cohort, a decline of 53%. Incidence rates of dementia in the 1999 cohort compared with the 1992 cohort declined 48.1% among non-Hispanic Whites, 56.2% among African-Americans, and 42% among Hispanics (Table 2).
Table 2.
Cox proportional hazards model: all participants and by race/ethnicity
| Model 1 |
Model 2 |
Model 3 |
|||
|---|---|---|---|---|---|
| Incidence/1000 Person Years | No. (%) with Dementia | HR (95%CI) | HR (95%CI) | HR (95%CI) | |
| All Participants | |||||
| 1999 | 21.1 | 194 (11.2) | 0.59 (0.49–0.70) | 0.62 (0.50–0.77) | 0.69 (0.55–0.86) |
| 1992 | 44.9 | 222 (19.7) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Non-Hispanic White | |||||
| 1999 | 9.6 | 30 (5.7) | 0.60 (0.34–1.05) | 0.72 (0.35–1.47) | 0.80 (0.37–1.71) |
| 1992 | 18.5 | 20 (8.2) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| African-American | |||||
| 1999 | 19.7 | 57 (10.0) | 0.52 (0.36–0.73) | 0.65 (0.44–0.97) | 0.87 (0.57–1.34) |
| 1992 | 45.0 | 76 (19.3) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Hispanic | |||||
| 1999 | 33.6 | 107 (17.0) | 0.64 (0.49–0.83) | 0.60 (0.45–0.79) | 0.62 (0.47–0.83) |
| 1992 | 57.9 | 126 (25.7) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
Model 1: Including cohort as predictor, adjusted for age, sex, race/ethnicity, baseline memory complaints. Model 2: Model 1 plus diabetes, heart disease, stroke, hypertension, current smoking, and BMI. Model 3: Model 2 plus education.
Cox-proportional hazards regression models were used to explore the contribution of sociodemographic and antecedent vascular risk factors and education to changes in dementia incidence between the 1992 and 1999 cohorts (Table 2). Among all participants, the hazard rate for incident dementia was 41% lower in the 1999 cohort than in the 1992 cohort, adjusting for age, sex, and race/ethnicity and baseline memory complaints (Table 2). Inclusion of vascular risk factors and education in Models 2 and 3 attenuated the difference in hazard rate of dementia found for the 1999 cohort compared with the 1992 cohort in the total group (Table 2). Race-ethnicity-specific Cox models indicated that compared with the 1992 cohorts, all race/ethnicity groups in the 1999 cohort showed a reduced hazard ratio of dementia, although this difference did not reach statistical significance among non-Hispanic Whites (Table 2). The largest reduction in dementia incidence was found among African-Americans. Inclusion of vascular risk factors and education in the models attenuated the hazard ratio for dementia for non-Hispanic Whites and for African-Americans, but not for Hispanics (Table 2). In additional models, we further stratified by baseline age (65–74, ≥ 75) (Table 3). Among those younger than 75 years at baseline, there was a significant reduction in the hazard ratio for dementia incidence for participants in the 1999 cohort compared with the 1992 cohort, which was attenuated when cardiovascular risk factors and education were added to the models (Table 3). This pattern was also observed among all race/ethnicity groups, although the small sample sizes reduced power to detect a significant change (Table 3). Among those older than 75 years at baseline, there was a significant reduction in the hazard ratio for dementia among participants of the 1999 cohort compared with the 1992 cohort which was attenuated but remained significant with inclusion of vascular risk factors and educational attainment (Table 3). When these models were repeated within strata defined by race/ethnicity, inclusion of vascular risk factors and educational attainment attenuated the reduction in the hazard ratio for non-Hispanic Whites and African-Americans, which were no longer significant, but not for Hispanics, which remained significant (Table 3).
Table 3.
Cox Proportional Hazards Models for comparisons within age strata
| Participants younger than 75 years |
Participants 75 years of age or older |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cohort | n (%) with Dementia | Model 1 HR (95%CI) | Model 2 HR (95%CI) | Model 3 HR (95%CI) | n with Dementia | Model 1 HR (95%CI) | Model 2 HR (95%CI) | Model 3 HR (95%CI) |
| Total Group | ||||||||
| 1999 | 80 (8.5) | 0.69 (0.50–0.95) | 0.81 (0.56–1.16) | 0.88 (0.60–1.27) | 114 (14.5) | 0.52 (0.40–0.66) | 0.54 (0.41–0.71) | 0.63 (0.48–0.84) |
| 1992 | 77 (13.3) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 145 (26.3) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Non-Hispanic Whites | ||||||||
| 1999 | 15 (5.2) | 0.75 (0.27–2.07) | 1.02 (0.31–3.34) | 1.07 (0.30–3.80) | 15 (6.2) | 0.41 (0.20–0.86) | 0.50 (0.18–1.39) | 0.63 (0.21–1.86) |
| 1992 | 5 (4.1) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 15 (12.4) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| African-American | ||||||||
| 1999 | 22 (7.3) | 0.56 (0.31–1.00) | 0.95 (0.46–1.98) | 1.52 (0.66–3.49) | 35 (13.1) | 0.45 (0.29–0.69) | 0.50 (0.30–0.83) | 0.65 (0.38–1.11) |
| 1992 | 24 (12.6) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 52 (25.6) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Hispanic | ||||||||
| 1999 | 43 (12.2) | 0.76 (0.50–1.17) | 0.76 (0.48–1.12) | 0.76 (0.48–1.22) | 64 (23.0) | 0.57 (0.41–0.79) | 0.55 (0.38–0.79) | 0.60 (0.41–0.87) |
| 1992 | 48 (18.2) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 78 (34.4) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
Model 1: Including birth cohort as predictor, adjusted for age, sex and ethnicity, and baseline memory complaints. Model 2: Model 1 plus diabetes, heart disease, stroke, hypertension and current smoking, and BMI. Model 3: Model 2 plus education.
DISCUSSION
In this multi-ethnic cohort of elders residing in Northern Manhattan, we found a reduction in incidence rate of dementia in the 1999 cohort compared with the 1992 cohort and these findings were consistent across ethnic groups. The greatest reduction in incidence of dementia was among those over 75 years of age, particularly among non-Hispanic Whites and African-Americans. Both non-Hispanic Whites and African-Americans showed significant increases in educational attainment over the cohort periods, while educational attainment for Hispanics did not improve substantially between cohorts. After adjusting for education, the relative rate estimate was attenuated for non-Hispanic Whites and African-Americans, but not for Hispanics, suggesting that changes in educational achievement may explain more of the reduction in dementia incidence in African-Americans and non-Hispanic Whites than in Hispanics. Furthermore, inclusion of vascular risk factors attenuated the hazard ratio estimates for non-Hispanic whites but not for African-Americans or for Hispanics. Taken together, these findings raise the possibility that the declining incidence rate of dementia in this population reflects reduction or better treatment of antecedent vascular risk factors and increased educational attainment in the more recent cohort.
Our findings are consistent with recent European and American studies suggesting declining dementia prevalence and incidence rates [2, 3, 5, 15, 23]. Although those studies attributed declines in dementia to improvements in managing cardiovascular disease, reduced prevalence of vascular risk factors, and increased educational level, in this study, the frequency of heart disease increased among non-Hispanic whites and African-Americans, and hypertension increased in all race and ethnicity groups. Educational attainment also increased significantly among non-Hispanic Whites and African-Americans, who also showed larger reductions in incidence rates than did Hispanics, among whom educational level did not change substantially over time. Our findings are also consistent with a recent report on secular trends in incidence of dementia from the Framingham Cohort, where a 44% decline in dementia incidence was found among those 60 years and older in the current epoch compared with those 60 and older during the late 1970s and early 1980s [24]. In contrast, no changes in incidence rates were identified in a biracial study of non-Hispanic Whites and African Americans in Chicago [18], although that study did not examine changes in incidence rates by ethnic group. At least four US nationwide studies of aging have identified a lower prevalence of cognitive impairment among cohorts assessed more recently [15, 16, 20, 23], with a suggestion of a greater effect among ethnic minority populations [20]. The Health and Retirement Study [20, 23] examined changes in the prevalence of cognitive impairment from 1993 to 2004 among elders 70 years and older and found that the annual percentage decline in cognitive impairment was greater among African-Americans and Hispanic elders than among non-Hispanic Whites and attributed the greater decline among minority groups in part to improvement in education level [20].
Potentially relevant period effects in this study are changes in cardiovascular disease diagnosis and treatment as they occurred in the community. Within the study itself, the measures for all vascular risk factors were unchanged across the time of the study (e.g., summative criteria for hypertension which includes patient self-report and medication). Changes in the prevalence of hypertension or other vascular risk factors over a life course may reflect increased awareness, diagnosis and treatment of the conditions. However, by the time these persons enrolled in the study in late life, the proportion of those receiving treatment for cardiovascular risk factors, including diabetes and hypertension, were comparable between the two cohorts. It is still possible that differences in diagnosis and treatment of these conditions earlier in adulthood may have contributed to decline in dementia incidence in the 1999 cohort. Importantly, the more recent birth cohort would have been exposed to an era with greater focus on stroke and cardiovascular risk factor reduction, with potential for more treatments being available and prescribed, including both pharmacological and non-pharmacological strategies.
This study has a number of strengths. First, the structure of the WHICAP cohort examination has been consistent over time and across cohorts with regard to medical and neuropsychological assessment. We attempted also to eliminate potential bias associated with age at entry and to allow for comparable periods of follow-up between the two cohorts. This study also has a number of limitations warranting discussion. We identified trends of declining incidence rates within age stratified race-ethnic groups, but the sample size was not sufficiently powered to identify significant differences in many subgroup analyses. A number of residual confounders, such as level of physical activity or social support, may explain these relationships but the inclusion of the sociodemographic variables in our models may capture some of these risk factors indirectly, although the weighting of these confounders may be incorrect as a result. The inclusion criteria for the 1992 and 1999 cohorts were somewhat different. Recruitment in 1992 was focused on enrolling a cohort that was representative of northern Manhattan for prevalence and incidence studies, while recruitment in 1999 was focused on forming a cohort of ethnically and educationally diverse, non-demented elders, although 10.2% of the participants were ultimately diagnosed with dementia at baseline. In addition, refusal rates were higher for the 1999 cohort than for the 1992 cohort (39.5% versus 27.2%) and might include people refusing because of memory concerns or at increased risk. While we cannot know the outcomes of those who refused participation, we took several steps to minimize the possibility that selection bias among those included in the analysis influenced the findings. While we excluded participants from the analyses who were diagnosed with dementia at baseline as well as those with insufficient data to determine dementia status, it is possible that differences in refusal associated with prevalent dementia may have contributed to a lower risk of dementia in the 1999 cohort compared with the 1992 cohort. To evaluate this possibility, we (1) compared baseline cognitive performance for the 1992 and 1999 cohorts and found that mean cognitive function in the 1999 cohort did not differ on measures of memory or visual spatial performance, but was significantly better on measures of language than mean cognitive function in the 1992 cohort; (2) we found that that the two cohorts did not differ in the likelihood of being in the lowest quartile of the cognitive factors versus being in the other three quartiles; and (3) and we adjusted for baseline memory complaints in all analytic models.
Overall, our results add to the evidence that secular trends are changing, including in aging minority populations and that that general health and wellbeing in an aging population is likely protective.
ACKNOWLEDGMENTS
The study was supported by NIH/NIA grants P01AG07232 and R01 AG037212, RF1AG054023. Drs. Schupf and Mayeux had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001873. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The authors thank Dr. Ursula Staudinger, PhD, Director, Columbia Aging Center, Columbia University for her contributions to the study design and analysis of this paper.
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/17-0300r1).
REFERENCES
- [1].Lobo A, Saz P, Marcos G, Dia JL, De-la-Camara C, Ventura T, Montanes JA, Lobo-Escolar A, Aznar S (2007) Prevalence of dementia in a southern European population in two different time periods: The ZARADEMP Project. Acta Psychiatr Scand 116, 299–307. [DOI] [PubMed] [Google Scholar]
- [2].Schrijvers EM, Verhaaren BF, Koudstaal PJ, Hofman A, Ikram MA, Breteler MM (2012) Is dementia incidence declining? Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology 78, 1456–1463. [DOI] [PubMed] [Google Scholar]
- [3].Qiu C, von Strauss E, Backman L, Winblad B, Fratiglioni L (2013) Twenty-year changes in dementia occurrence suggest decreasing incidence in central Stockholm, Sweden. Neurology 80, 1888–1894. [DOI] [PubMed] [Google Scholar]
- [4].Wiberg P, Waern M, Billstedt E, Ostling S, Skoog I (2013) Secular trends in the prevalence of dementia and depression in Swedish septuagenarians 1976-2006. Psychol Med 43, 2627–2634. [DOI] [PubMed] [Google Scholar]
- [5].Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, Brayne C (2013) A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: Results of the Cognitive Function and Ageing Study I and II. Lancet 382, 1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Christensen K, Thinggaard M, Oksuzyan A, Steenstrup T, Andersen-Ranberg K, Jeune B, McGue M, Vaupel JW (2013) Physical and cognitive functioning of people older than 90 years: A comparison of two Danish cohorts born 10 years apart. Lancet 382, 1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Abdulrahman GO (2014) Alzheimer’s disease: Current trends in Wales. Oman Med J 29, 280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Grasset L, Brayne C, Joly P, Jacqmin-Gadda H, Peres K, Foubert-Samier A, Dartigues JF, Helmer C (2016) Trends in dementia incidence: Evolution over a 10-year period in France. Alzheimers Dement 12, 272–280. [DOI] [PubMed] [Google Scholar]
- [9].Sekita A, Ninomiya T, Tanizaki Y, Doi Y, Hata J, Yonemoto K, Arima H, Sasaki K, Iida M, Iwaki T, Kanba S, Kiyohara Y (2010) Trends in prevalence of Alzheimer’s disease and vascular dementia in a Japanese community: The Hisayama Study. Acta Psychiatr Scand 122, 319–325. [DOI] [PubMed] [Google Scholar]
- [10].Chan KY, Wang W, Wu JJ, Liu L, Theodoratou E, Car J, Middleton L, Russ TC, Deary IJ, Campbell H, Rudan I (2013) Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990-2010: A systematic review and analysis. Lancet 381, 2016–2023. [DOI] [PubMed] [Google Scholar]
- [11].Wu YT, Lee HY, Norton S, Prina AM, Fleming J, Matthews FE, Brayne C (2014) Period, birth cohort and prevalence of dementia in mainland China, Hong Kong and Taiwan: A meta-analysis. Int J Geriatr Psychiatry 29, 1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gao S, Ogunniyi A, Hall KS, Baiyewu O, Unverzagt FW, Lane KA, Murrell JR, Gureje O, Hake AM, Hendrie HC (2016) Dementia incidence declined in African-Americans but not in Yoruba. Alzheimers Dement 12, 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sauvaget C, Tsuji I, Haan MN, Hisamichi S (1999) Trends in dementia-free life expectancy among elderly members of a large health maintenance organization. Int J Epidemiol 28, 1110–1118. [DOI] [PubMed] [Google Scholar]
- [14].Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA (2003) Design of the Chicago Health and Aging Project (CHAP). J Alzheimers Dis 5, 349–355. [DOI] [PubMed] [Google Scholar]
- [15].Manton KC, Gu XL, Ukraintseva SV (2005) Declining prevalence of dementia in the U.S. elderly population. Adv Gerontol 16, 30–37. [PubMed] [Google Scholar]
- [16].Langa KM, Larson EB, Karlawish JH, Cutler DM, Kabeto MU, Kim SY, Rosen AB (2008) Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity? Alzheimers Dement 4, 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hall KS, Gao S, Baiyewu O, Lane KA, Gureje O, Shen J, Ogunniyi A, Murrell JR, Unverzagt FW, Dickens J, Smith-Gamble V, Hendrie HC (2009) Prevalence rates for dementia and Alzheimer’s disease in African Americans: 1992 versus 2001. Alzheimers Dement 5, 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hebert LE, Bienias JL, Aggarwal NT, Wilson RS, Bennett DA, Shah RC, Evans DA (2010) Change in risk of Alzheimer disease over time. Neurology 75, 786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rocca WA, Petersen RC, Knopman DS, Hebert LE, Evans DA, Hall KS, Gao S, Unverzagt FW, Langa KM, Larson EB, White LR (2011) Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement 7, 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sheffield KM, Peek MK (2011) Changes in the prevalence of cognitive impairment among older Americans, 1993-2004: Overall trends and differences by race/ethnicity. Am J Epidemiol 174, 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Larson EB, Yaffe K, Langa KM (2013) New insights into the dementia epidemic. N Engl J Med 369, 2275–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kosteniuk JG, Morgan DG, O’Connell ME, Kirk A, Crossley M, Teare GF, Stewart NJ, Bello-Haas VD, McBain L, Mou H, Forbes DA, Innes A, Quail JM (2016) Simultaneous temporal trends in dementia incidence and prevalence, 2005-2013: A population-based retrospective cohort study in Saskatchewan, Canada. Int Psychogeriatr 28, 1643–1658. [DOI] [PubMed] [Google Scholar]
- [23].Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU, Weir DR (2017) A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med 177, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S (2016) Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med 374, 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dodge HH, Zhu J, Hughes TF, Snitz BE, Chang CH, Jacobsen EP, Ganguli M (2017) Cohort effects in verbal memory function and practice effects: A population-based study. Int Psychogeriatr 29, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Prince M, Ali GC, Guerchet M, Prina AM, Albanese E, Wu YT (2016) Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther 8, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Colby S, Ortman J (2015) Projections of the Size and Composition of the US Population: 2014 to 2060. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- [28].Gurland BJ, Wilder DE, Lantigua R, Stern Y, Chen J, Killeffer EH, Mayeux R (1999) Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry 14, 481–493. [PubMed] [Google Scholar]
- [29].Perkins P, Annegers JF, Doody RS, Cooke N, Aday L, Vernon SW (1997) Incidence and prevalence of dementia in a multiethnic cohort of municipal retirees. Neurology 49, 44–50. [DOI] [PubMed] [Google Scholar]
- [30].Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R (2001) Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol 154, 635–641. [DOI] [PubMed] [Google Scholar]
- [31].Prineas RJ, Demirovic J, Bean JA, Duara R, Gomez-Marin O, Loewenstein D, Sevush S, Stitt F, Szapocznik J (1995) South Florida Program on Aging and Health. Assessing the prevalence of Alzheimer’s disease in three ethnic groups. J Fla Med Assoc 82, 805–810. [PubMed] [Google Scholar]
- [32].Hutchinson RG, Watson RL, Davis CE, Barnes R, Brown S, Romm F, Spencer JM, Tyroler HA, Wu K (1997) Racial differences in risk factors for atherosclerosis. The ARIC Study. Atherosclerosis Risk in Communities. Angiology 48, 279–290. [DOI] [PubMed] [Google Scholar]
- [33].Sundquist J, Winkleby MA, Pudaric S (2001) Cardiovascular disease risk factors among older black, Mexican-American, and white women and men: An analysis of NHANES III, 1988-1994. Third National Health and Nutrition Examination Survey. J Am Geriatr Soc 49, 109–116. [DOI] [PubMed] [Google Scholar]
- [34].LaRosa JC, Brown CD (2005) Cardiovascular risk factors in minorities. Am J Med 118, 1314–1322. [DOI] [PubMed] [Google Scholar]
- [35].Luchsinger JA, Mayeux R (2004) Cardiovascular risk factors and Alzheimer’s disease. Curr Atheroscler Rep 6, 261–266. [DOI] [PubMed] [Google Scholar]
- [36].Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R (2005) Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology 65, 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM (1999) Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 53, 1937–1942. [DOI] [PubMed] [Google Scholar]
- [38].Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA (2000) Statins and the risk of dementia. Lancet 356, 1627–1631. [DOI] [PubMed] [Google Scholar]
- [39].Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, Persson G, Oden A, Svanborg A (1996) 15-year longitudinal study of blood pressure and dementia. Lancet 347, 1141–1145. [DOI] [PubMed] [Google Scholar]
- [40].Merchant C, Tang MX, Albert S, Manly J, Stern Y, Mayeux R (1999) The influence of smoking on the risk of Alzheimer’s disease. Neurology 52, 1408–1412. [DOI] [PubMed] [Google Scholar]
- [41].Ott A, Slooter AJ, Hofman A, van Harskamp F, Witteman JC, Van Broeckhoven C, van Duijn CM, Breteler MM (1998) Smoking and risk of dementia and Alzheimer’s disease in a population-based cohort study: The Rotterdam Study. Lancet 351, 1840–1843. [DOI] [PubMed] [Google Scholar]
- [42].Population and Housing Census (1991) Bureau of the Census, Washington, DC. [Google Scholar]
- [43].Tang MX, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, Andrews H, Feng L, Tycko B, Mayeux R (1998) The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA 279, 751–755. [DOI] [PubMed] [Google Scholar]
- [44].Stern Y, Andrews H, Pittman J, Sano M, Tatemichi T, Lantigua R, Mayeux R (1992) Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol 49, 453–460. [DOI] [PubMed] [Google Scholar]
- [45].Manly JJ, Bell-McGinty S, Tang MX, Schupf N, Stern Y, Mayeux R (2005) Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Arch Neurol 62, 1739–1746. [DOI] [PubMed] [Google Scholar]
- [46].Frances A (1994) Diagnostic and statistical manual of mental disorders: DSM-IV, American Psychiatric Association. [Google Scholar]
- [47].Hatano S (1976) Plans for prevention of stroke formulated by WHO and practice in Japan. Nihon Rinsho 34, 131–136. [PubMed] [Google Scholar]


