Abstract
A few different exposure prediction tools were evaluated for use in the new in vitro-based safety assessment paradigm using di-2-ethylhexyl phthalate (DEHP) and dibutyl phthalate (DnBP) as case compounds. Daily intake of each phthalate was estimated using both high-throughput (HT) prediction models such as the HT Stochastic Human Exposure and Dose Simulation model (SHEDS-HT) and the ExpoCast heuristic model and non-HT approaches based on chemical specific exposure estimations in the environment in conjunction with human exposure factors. Reverse dosimetry was performed using a published physiologically based pharmacokinetic (PBPK) model for phthalates and their metabolites to provide a comparison point. Daily intakes of DEHP and DnBP were estimated based on the urinary concentrations of their respective monoesters, mono-2-ethylhexyl phthalate (MEHP) and monobutyl phthalate (MnBP), reported in NHANES (2011e2012). The PBPK-reverse dosimetry estimated daily in-takes at the 50th and 95th percentiles were 0.68 and 9.58 μg/kg/d and 0.089 and 0.68 μg/kg/d for DEHP and DnBP, respectively. For DEHP, the estimated median from PBPK-reverse dosimetry was about 3.6-fold higher than the ExpoCast estimate (0.68 and 0.18 mg/kg/d, respectively). For DnBP, the estimated median was similar to that predicted by ExpoCast (0.089 and 0.094 mg/kg/d, respectively). The SHEDS-HT prediction of DnBP intake from consumer product pathways alone was higher at 0.67 mg/kg/d. The PBPK-reverse dosimetry-estimated median intake of DEHP and DnBP was comparable to values previously reported for US populations. These comparisons provide insights into establishing criteria for selecting appropriate exposure prediction tools for use in an integrated modeling platform to link exposure to health effects.
Keywords: Risk assessment, Exposure, Prediction, Reverse dosimetry, PBPK modeling, Plethem
1. Introduction
Under the new direction in toxicity testing, in vitro and in silico tools are recommended for assessing potential risks of chemical exposure to humans, with an aim to increase human relevancy and efficiency (NRC, 2007). High-throughput (HT) in vitro screening, toxicokinetic and human exposure data have been used in developing metrics of margin of exposure for prioritization of chemicals for risk assessment (Rotroff et al., 2010; Wetmore et al., 2012; Wambaugh et al., 2015; US U.S. EPA, 2014). Margin of exposure analysis can be used to support chemical-specific risk assessment when combined with equivalent exposures inferred via toxicokinetic modeling from fit-for-purpose in vitro assays. However, obtaining sufficient data to support human chemical exposure is a major challenge. Several HT exposure estimation/prediction tools are available for use in chemical prioritization to estimate human exposure and intake, each possessing different strengths and uncertainties (Isaacs et al., 2014 Wambaugh et al., 2014). Other chemical-specific data-based approaches are also used, including human biomarker- or environmental level-based estimation methods.
An integrated simulation platform could bridge human exposure and health outcomes efficiently given the availability of information for determining a safe exposure level for humans derives from diverse data sources, especially from in vitro and in silico methods. The Population Life Course Exposure to Health Effects Modeling (PLETHEM) platform provides a tool that links results from emerging toxicity testing applications to exposure estimates for humans. Exposure and dosimetry simulation models are essential components of such a platform. This study compares pharmacokinetic reverse dosimetry results with exposure predictions from existing exposure models that could potentially be used in such an integrated modeling platform, using phthalates as a case study.
Di-2(ethylhexyl) phthalate (DEHP) and di-butyl phthalate (DnBP) were selected, as they each have different uses and exposure trends (CDC, 2016). Both phthalates are used in a wide variety of consumer products, household articles and building materials, in addition to being contaminants in foodstuffs. DEHP is a ubiquitous compound that is used as a PVC plasticizer in coatings, adhesives and resins, and it can also be found in cosmetics, liquid soap, and detergents (David et al., 2001; Hauser and Calafat, 2005). DnBP is also used as a plasticizer, but is more often used in a variety of industrial applications such as surface coatings, polymer emulsion for adhesives, and textiles (Wittassek et al., 2011; Hauser et al., 2004; Kamrin, 2009 Otake et al., 2004). The primary route of human exposure to DEHP and DnBP in the general population is through oral consumption, which is mainly due to the transfer of substances from food packaging onto food products as well as dust and soil ingestion (Biryol et al., 2017). After absorption, DEHP is rapidly metabolized to its monoester, mono(2-ethylhexyl) phthalate (MEHP), which is further metabolized by various oxidation reactions to a number of secondary hydrolytic and oxidative metabolites (Fig. 1S, supplemental 2) that are conjugated via glucuronidation and other processes before being eliminated. The three oxidative metabolites of interest here are mono(2-ethyl-5-hydroxyhexyl) phthalate (5OH-MEHP), mono(2-ethyl-5-oxo-hexyl) phthalate (5OXO-MEHP) and mono(2-ethyl-5-carboxy-pentyl) phthalate (5CX-MEPP) (Choi et al., 2013). DnBP is metabolized to its monoester, mono-butylphthalate (MnBP) (Koch et al., 2005; Clewell et al., 2009) (Fig. 2S, supplemental 2). Exposure to phthalate metabolites has been associated with observed infertility and developmental toxicity occurring through testicular effects, malformations, and defects in sexual differentiation in animals (Leung et al. 2015). These metabolites are distributed in all tissues with no evidence of accumulation (Rusyn et al., 2006).
This case study used predictions from two currently available exposure models for population exposure predictions: 1.) the HT Stochastic Human Exposure and Dose Simulation (SHEDS-HT) and; 2.) an empirical heuristics-based model developed under the US Environmental Protection Agency’s (US EPA) Exposure Forecasting (ExpoCast) project (Wambaugh et al., 2014). SHEDS-HT is a probabilistic model that estimates the range of chemical exposure in a population from different consumer product pathways over the course of a 24-h period. These 1-day exposure estimates are calculated based on census data, consumer product use patterns, reported compositions of consumer products and activity diaries (Isaacs et al., 2014), which are appropriate for screening-level assessment. The ExpoCast model is designed to screen and classify thousands of chemicals based on their potential for human exposure (Wambaugh et al., 2014). To provide a point of comparison for those estimates from the two exposure prediction models, the daily exposure to these two phthalates was also estimated by reverse dosimetry using previously developed PBPK models. As exposure to a chemical can vary widely due to inherent properties, product usage and other environmental factors, an additional non-HT approach involving the assemblage of exposure data for the two phthalates from literature was also taken, to compare with values generated by the two exposure models and using reverse dosimetry.
2. Methods
2.1. Estimation of phthalates daily dose by reverse dosimetry
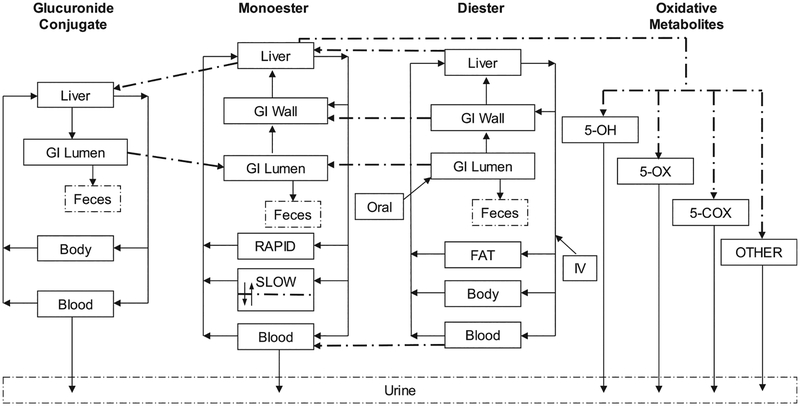
The structure of the PBPK model for DEHP and DnBP and their metabolites was adapted from Gentry et al. (2011). The current model (Fig. 1) includes four interconnected sub-models for the diester, the hydrolytic monoester, the oxidative metabolites and the glucuronide conjugate. The three oxidative metabolites of MEHP (i.e., 5OXO-MEHP, 5OH-MEHP and 5CX-MEPP) which had human data available to parameterize their description, were incorporated as a fraction of the total MEHP oxidative metabolism with a volume of distribution approximating body water distribution. The model was manually optimized to simulate kinetic time-course data in human plasma (DEHP and total MEHP) and urine (MEHP, 5OXOMEHP, 5OH-MEHP and 5CX-MEPP) after oral consumption, based on controlled dosing studies in humans (Kessler et al., 2012; Anderson et al., 2011).
Fig. 1.
Representation of the physiologically based pharmacokinetic model for 2 phthalates (DEHP and DnBP) and their metabolites adapted from Gentry et al. (2011).
At low exposure concentrations, relevant to human exposure in the environment, kinetics are linear and metabolite concentrations in urine can be used to estimate parent exposure levels. Our reverse dosimetry approach combined the simple PBPK model described above with Monte Carlo simulations and calculations of probability to convert distributions of urine concentration measurements from NHANES into probability distributions of exposure doses. Distributions of daily intake of DEHP and DnBP in a population were generated based on the urine concentrations of their mono-ester metabolites as measured in NHANES, 2011e2012 (CDC, 2016) using a Monte Carlo (MC) analysis to incorporate variability in pharmacokinetics and uncertainty in exposure patterns. All model parameters were randomly sampled, and a distribution of phthalate urine concentrations was generated by running the PBPK model for several iterations. All parameters in the MC analysis either were distributed lognormally (e.g., body weight, cardiac output, metabolism constant, metabolites clearance, partition coefficient and oral absorption constants) or were distributed normally (e.g., blood flow, tissue volumes and fraction of metabolites). To exclude physiologically implausible values, CV was assumed to be 50%. with parameters truncated at ± 1.95 SD (Table 1S, supplemental 2). The “Exposure Conversion Factor” (ECF) approach from Tan et al. (2006) was used to estimate the population’s distribution of exposure. Briefly, one thousand urine concentrations were generated for MEHP and its three oxidative metabolites, as well as for MnBP, following different doses of DEHP and DnBP exposure, respectively. These metabolite urine concentrations were grouped with a geometric increment of 100.35. The number of individuals belonging to each group of urine concentrations was computed for each simulated dose. Then, the probabilities of being exposed to the doses set in the first step were calculated for each groups of urine concentrations defined previously. The urine concentrations measured in NHANES were also grouped with a geometric increment of 100.35 and were considered as an adjusted weighting factor that was used in combination with the probability table to derive the estimated population’s distribution of exposure.
2.2. Estimation of DnBP daily intake using SHEDS-HT
The SHEDS-HT model is a cross-sectional mechanistic model that uses exposure scenarios to estimate aggregate exposures over multiple exposure pathways and routes. The model aggregates these exposures over the course of a day and has previously been used to estimate exposures to over 2500 organic chemicals in consumer products and pesticides (Isaacs et al., 2014). SHEDS-HT inform prioritization of chemicals to undergo screening, given its ability to aggregate exposures for large numbers of chemicals very rapidly. SHEDS-HT depends on reported weight fraction data of chemicals in products as well as activity diaries to estimate intake doses for its simulated population. SHEDS-HT presently includes seven exposure scenarios: direct vapor inhalation from product; direct aerosol inhalation from product; direct dermal application of product; indirect exposure from emission; indirect exposure from bolus application of a product to the residence; and incidental direct ingestion. These scenarios are mapped to the dermal, ingestion, and inhalation exposure routes. The exposure estimate for each scenario is determined by calculating the exposure estimate for each chemical-scenario-route combination. After summing each of these exposure estimate for the dermal route, dermal removal is considered by accounting for hand washing/bathing, hand-to-mouth transfer, and brush-off. Finally, the exposures for each route are summed giving the total daily intake dose.
SHEDS-HT models exposure to consumer product formulation (e.g., personal care, cleaners, and home improvement) pathways; articles and food contact pathways are not currently included. DnBP daily intake was estimated using the SHEDS-HT model, which utilizes chemical weight fraction data from Material Data Safety Sheets (MSDS) sheets for compound daily intake estimation (Goldsmith et al., 2014; Isaacs et al., 2016). As the MSDS database contained within SHEDS-HT has no reported weight fraction data for DEHP, no exposure estimates were obtained for DEHP. As more information becomes available for articles in the home, SHEDS-HT could be modified to accommodate exposures via this pathway and thus refined intake doses for both phthalates could be obtained. In addition, methods are being explored to incorporate the food contact pathway in SHEDS, which would allow for further refinement.
2.3. ExpoCast HT exposure predictions for phthalates
The intake estimates for DEHP and DnBP were taken from Wambaugh et al. (2014), which describes a heuristic model developed as part of the US EPA’s ExpoCast project. It was built by performing a joint Bayesian regression of exposure ‘heuristics” to intakes exposures inferred from NHANES biomarker data. The model incorporated binary industrial use descriptors (e.g., whether a chemical is used in personal care products, or as an inactive ingredient in pesticides etc.) as well as physicochemical properties (e.g., vapor pressure, molecular weight) of the compounds. The best subset descriptors, as determined by lower Akaike information criterion scores, were included in the construction of a linear regression model predicting exposures. This “best subset” was composed of five descriptors: a.) pesticide inert use; b.) production volume; c.) consumer and industrial use; d.) pesticide active use; and e.) industrial no consumer use (Wambaugh et al., 2014). The ExpoCast heuristic model differs from SHEDS-HT in that, rather than requiring detailed information on activities of a person and information about the food eaten and the products used, it was constructed to require minimal input from the user (only these 5 binary descriptors pertaining to the use of a chemical).
2.4. Estimation of phthalates daily intake from literature
The review of the literature was as comprehensive as possible. Databases searched included PubMed, ProQuest Environmental Science Collection, and Science Direct. The search terms used were ([“substance”] OR [“synonym”] OR [“synonym] …), where substance referred to the known phthalate of interest. In this case substance was “diethylhexyl phthalate” and “dibutyl phthalate”. Synonyms included “bis(2-ethylhexl) phthalate”, “Di(2-ethylhexyl) phthalate”, and “DEHP” for the former substance and “Di-n-butyl phthalate” and “DnBP” for the latter substance. In addition, because dibutyl phthalate can have multiple isomers (e.g., diisobutyl phthalate or DiBP), care was taken during literature review to ensure that only intake of DnBP was considered. Finally, each of the two parent phthalates were included in search terms for multiple media which included the string stated above for substance or synonym in addition to the terms:
AND (air OR water OR soil OR dust OR dirt OR sediment OR atmosphere OR plants OR vegetation) for environmental media
AND (consumer OR products OR toy OR “building materials” OR “personal care” OR cosmetics OR “medical devices” OR PVC OR food OR beverages OR water OR vegetables) for consumer and personal care products
AND (blood OR urine OR feces OR tissue OR bone OR hair OR “breast milk” OR semen OR biomarker OR bioaccumulation OR bioconcentration OR bioavailability) for biological media
Numerous studies in literature that were conducted from 1998 until present were reviewed to summarize estimated intakes of phthalate doses. Many of these works provided intake estimates derived through consideration of multiple routes (e.g., oral, dermal, and inhalation exposures). The media in which the two parent phthalates and their monoester metabolites were measured included those of concern in indoor environments such as air and house dust, household articles, building materials, toys, and clothing, in addition to dietary media such as drinking water and food. This method of external exposure modeling used by the authors of these aggregated works to obtain intake doses considered the chemical concentration in media, along with the various mechanistic information regarding exposure factor parameters such as food and drinking water consumption, alveolar ventilation rate, duration and frequency of exposure, absorption factors, and surface area of skin.
Literature references included approaches that not only involved measurements of external exposure, but also measurements of urinary metabolite biomarkers present in voided urine that were used to extrapolate the amount of chemical entering the body. The two common methods used to estimate intake through biomarker measurements involved creatinine corrected spot urinary metabolite concentrations and creatinine excretion rates, as well as urinary voided volume over 24 h and absolute urinary metabolite concentration (i.e., not adjusted for creatinine concentration in urine) (David, 2000; Wittassek et al., 2007). The supplementary information provides details of daily intake estimation based on these two approaches.
3. Results
Tables 1 and 2S (supplemental 2) present the estimates of DEHP exposure dose given measured DEHP metabolite concentrations in urine. The estimated oral doses varied and were dependent on the model used and on which of the individual metabolites was used to conduct the reverse dosimetry. Using the reverse dosimetry PBPK model, the estimates corresponding to use of the 50th and 95th urine concentration in NHANES were similar for 5OXO-MEHP, 5OHMEHP and 5CX-MEPP (1.193, 1.261 and 2.285 mg/kg/d for the 50th percentile, respectively and 12.211, 13.868 and 20.161 mg/kg/d for the 95th percentile, respectively), and higher than MEHP (0.684 and 9.576 mg/kg/d for the 50th percentile and 95th percentile, respectively). SHEDS-HT was not used to model DEHP as no reported weight fraction data for DEHP is currently available in the MSDS data. The ExpoCast model estimate for median population exposure for DEHP was 0.1785 mg/kg/d (Wambaugh et al., 2014) which is lower than the prediction from the reverse dosimetry. Furthermore, the upper limit of the 95% credible interval for this prediction was 12.0055 mg/kg/d. As Wambaugh et al. (2014) only reports median population exposures, only these values for DEHP and DnBP are summarized in the ExpoCast column of Tables 1 and 2, respectively.
Table 1.
Estimates of DEHP exposure dose given the measured DEHP metabolites concentrations in urine (NHANES, 2011–2012).
| Percentile | Reverse dosimetry | ExpoCast | |||
|---|---|---|---|---|---|
| MEHP | 5OH-MEHP | 5CX-MEPP | 5OXO-MEHP | ||
| μg/kg/d | μg/kg/d | μg/kg/d | μg/kg/d | μg/kg/d | |
| 5th | 0.058 | 0.102 | 0.211 | 0.101 | |
| 10th | 0.102 | 0.191 | 0.377 | 0.182 | |
| 25th | 0.247 | 0.487 | 0.911 | 0.457 | |
| 50th | 0.684 | 1.261 | 2.285 | 1.193 | 0.179a |
| 75th | 2.026 | 3.200 | 5.557 | 3.048 | |
| 90th | 5.352 | 7.715 | 12.484 | 7.140 | |
| 95th | 9.576 | 13.868 | 20.161 | 12.211 | |
| 99th | 27.251 | 42.805 | 51.133 | 36.935 | |
Median from Wambaugh et al. (2014)
Table 2.
Estimates of DnBP exposure dose given the measured MBP concentrations in urine (NHANES).
| Percentile | NHANES Urine Concentration (2011–2012) | Reverse dosimetry | SHEDS-HT | ExpoCast Model |
|---|---|---|---|---|
| (μg/L) | (μg/kg/d) | (μg/kg/d) | (μg/kg/d) | |
| 5th | 0.004 | 0.014 | ||
| 10th | 0.010 | 0.039 | ||
| 25th | 0.033 | 0.158 | ||
| 50th | 9.20 | 0.089 | 0.674 | 0.094b |
| 75th | 20.0 | 0.207 | 2.903 | |
| 90th | 35.5 | 0.430 | 11.685 | |
| 95th | 55.0 | 0.685 | 27.992 | |
| 99th | 1.864 | 174.820 | ||
| Mean | 7.61a | 12.276 |
Geometric mean.
Median from Wambaugh et al. (2014)
The estimates of DnBP exposure dose given the measured MnBP concentrations in urine are provided in Table 2. The estimated oral doses varied and were dependent on the model used. From the reverse dosimetry, the oral doses corresponding to the 50th and 95th percentile urine concentration in NHANES were 0.089 and 0.685 mg/kg/d, respectively. The ExpoCast model estimate for median population exposure for DnBP was 0.09411 mg/kg/d (Wambaugh et al., 2014) which is on the same order as the results from the reverse dosimetry. Furthermore, the upper limit of the 95% credible interval for this prediction was 6.129. From the MSDS database within SHEDS-HT, DnBP was found in fourteen different exposure sources (i.e., consumer products), including adhesives, spray paints, nail polishes, floor sealers, polishes, and primers. There were a variety of exposure scenarios (e.g., indoor, direct dermal, direct aerosol inhalation, direct vapor inhalation, and indirect) considered for DnBP because of the variety of products listing it as an ingredient. Since DnBP was present in a range of products, a large portion of the simulation population experienced exposures to DnBP (99.14% was exposed). The daily mean intake for DnBP was determined to be 12.276 mg/kg/d (the 50th and 95th percentiles were found to be 0.675 and 27.992 mg/kg/d, respectively). The 50th percentile of the estimated oral dose from SHEDS-HT is approximately 10 times higher than that of the two other models.
Estimation of phthalates daily dose from literature is provided in supplemental 3 and 4. In the studies of phthalate exposure throughout the European population, over half of DnBP and DEHP exposure was shown to occur through consumption of foodstuffs, followed by, to a lesser degree, inhalation, contact with dust, use of consumer products, and mouthing of soft plastic toys in the case of exposure to infants and toddlers (Wormuth et al., 2006). In the study by Wormuth et al. (2006), the scenario-based risk assessment (SceBRA) method (Schringer et al., 2001) was implemented to estimate exposures indirectly for eight phthalates, including DEHP and DBP, based on estimated doses derived from oral, dermal, and inhalation exposure pathways, followed by mechanistic equations for determining exposure through different use scenarios, human activity, and chemical concentration. Data used for the study by Wormuth et al. (2006) included collections from European food surveys, measured amounts in human feces, measurements in European house dust, infant mouthing surveys, and measured concentrations of phthalates in European consumer products.”
Four of the 22 references describing biomarker measurements for DnBP did not distinguish between different metabolite isomers due to analytical limitations. Thus, DnBP intake predictions using biomarker measurements may be overestimated. Forty-five references described intake for DnBP in 23 European, North American, and Indo-Asian countries, with nearly an identical number using either scenario-based or biomarker-based methods to provide estimates (Supplemental 3). Nearly 84 references were found that contained intake estimates for DEHP in 22 North American, European, and Indo-Asian countries (Supplemental 4). Again, the number of references using either scenario-based or biomarker-based methods to estimate intake was nearly identical, with at least 8 references providing intake estimates using both methods. Of these references, those investigating multiple media types were found to have similar values as those estimates derived from biomarker measurements. For more detail on these intake estimations, refer to the supplementary section.
4. Discussion
Risk-based decisions based on in vitro results and human exposure information must envision a continuum from exposure to health effects. An integrated simulation platform can serve to bridge human exposure and health outcomes coherently based on diverse data sources, especially from in vitro assays and in silico predictions. This platform includes three critical capabilities: 1.) to estimate human exposure; 2.) to simulate kinetics and estimate internal exposure in the body; and 3.) to describe the doseresponse at biological target that may lead to potential harmful effects. The utility or purpose of this platform can vary, depending on which stage or tier a safety or risk assessment is at (e.g., prioritization or risk-assessment for a specific chemical); therefore, the prediction tool must be sufficiently flexible to provide robust predictions. The Population Life-course Exposure to Health Effects Modeling (PLETHEM) is a modular, open-source modeling platform that will provide such flexibility. It is being developed to meet the challenge of translating in vitro effects data in the context of safety levels for human exposure (Pendse et al., 2017). PLETHEM will allow users to select and integrate existing or new exposure estimation tools and PK or PBPK models from different sources, depending on the user’s purpose. This case study was conducted to determine the most appropriate approach for incorporating exposure and dosimetry tools into such a platform.
PBPK-reverse dosimetry modeling was conducted in this case study to demonstrate the utility of PLETHEM in linking human exposure and internal exposure. In addition, the estimated human exposure to phthalates from reverse dosimetry is used to as a point of reference for margin of exposure (MoE) analysis compared to those estimated from the other two HT-prediction methods. The HT-prediction tools are more generic compared to reverse dosimetry-based exposure estimation, which is specific to the compound of interest and to the population of interest during the period of exposure that is relevant for analysis. The three models (Reverse dosimetry PBPK modeling, SHEDS-HT and ExpoCast) were used to determine human exposure levels to phthalates using different input and different methods (Table 3). The comparison among exposure estimates based on reverse dosimetry, stochastic and empirical prediction models, and reported literature data for the two phthalates revealed several similarities and differences as stated in the results (Tables 1 and 2), suggesting that it is important to consider the strengths and weaknesses of each method when considering their utility for different PLETHEM applications.
Table 3.
Models used to determine human exposure levels to phthalates but using different input and different methods.
| Models | PBPK Model | SHEDS | ExpoCast from Wambaugh et al. (2014),b |
|---|---|---|---|
| Model form | Reverse dosimetry | Monte Carlo-based | Heuristic |
| Mechanistic (e.g., human behavior, exposure equations) High-Throughput | High-Throughput | ||
| Input data | NHANES data | Consumer product chemical weight fractions | Has industrial and consumer use |
| Physiological and Physicochemical properties | Consumer product use patterns | Is a pesticide inert | |
| Chemical properties | Is a pesticide active | ||
| Food and drinking water concentrations | Has industrial use but no consumer | ||
| U.S. Census data | use | ||
| Food, drinking, and activity diariesa | |||
| Output data | Estimation of population exposure intakes | ||
| Regulatory Use | Screening chemicals | ||
| Evaluating chemicals | |||
| Classifying chemicals for more toxicity testing | |||
| Metabolites analyzed | MEHP | MnBP | MEHP |
| 5OH-MEHP | MnBP | ||
| 5CX-MEPP | |||
| 5OXO-MEHP | |||
| MnBP |
Information adapted from Isaacs et al., (2014) (Fig. 1).
Values were taken from Wambaugh et al. (2014)
Although it uses more compound- and population specific biomonitoring data to estimate human exposure to chemicals, there are several uncertainties inherent in reverse dosimetry-based exposure estimations that should be considered when using this method. For example, the short half-lives of phthalates may result in uncertainties in estimated exposure from reverse dosimetry given that biomonitoring data only provides a measure of concentration at the time of sample collection. With the half-life around 12 h in humans for DEHP and DnBP (Schmid and Schlatter, 1985), the measured urine concentrations in NHANES might only reflect recent exposures to these chemicals rather than long-term exposures (Albertini et al., 2006; Meeker et al., 2009). Yang et al. (2012) showed that cumulative urine samples provide more precise intake estimates than a spot urine sample. In addition, exposure to phthalates can also vary among individuals as well as among populations, depending on the types and levels of exposure sources such as specific food categories and consumer products. It should be noted that urine concentrations of these phthalates, measured at different periods of time in NHANES (2005e2012), are decreasing, as shown in the supplemental tables (TableS3 and S4) reflecting the changes in their levels in the environment. In the current study, metabolites were used for reverse dosimetry to estimate the exposure to their respective parent chemicals, which may have resulted in additional uncertainties in the predicted exposure for those parents. Inter-individual variability in pharmacokinetics, most importantly for metabolism parameters, was simulated by varying those metabolism parameters when conducting Monte Carlo simulations. Distributions used for each parameter are listed in Table S1 5OH-MEHP, 5OXO-MEHP and 5CXMEPP have urinary levels 5e10-fold higher than levels of MEHP (From NHANES, the 50 t h percentile urine concentrations are 1.40, 5.30, 8.30 and 13.5 μg/L for MEHP, 5OXO-MEHP, 5OH-MEHP and 5CX-MEPP respectively). MEHP is considered to be the more relevant metabolite because it is more likely to be linked to DEHP effects (Gray, 1986; Chauvigné et al., 2009). However, 5OH-MEHP, 5OXO-MEHP, and 5CX-MEPP are more sensitive urinary biomarkers with lower detection limits (Kato et al., 2004), indicating the importance of using multiple metabolites to increase confidence in the estimated exposure from reverse dosimetry. Furthermore, environmental degradation of DEHP can partly explain the differences observed in the results derived using reverse dosimetry with concentrations of MEHP or 5OXO-MEHP, 5OH-MEHP and 5CXMEPP to estimate intakes of DEHP. DEHP can be degraded in the environment under various conditions by microorganisms to MEHP and phthalic acid (Staples et al., 1997). It is also important to consider that some metabolites may originate from different parent compounds, as is the case for DnBP and butylbenzyl phthalate (BBzP) that share the common metabolite MnBP. Anderson et al. (2001) showed that after exposure to BBzP, 6% of the dose was excreted as MnBP in urine and 68e78% as MBzP. Thus, measurements of MnBP in urine may result in overestimation of exposure to DnBP in instances where exposure to BzBP is also possible.
The laboratory environment in which urine analysis is conducted can also influence measurements, especially when accounting for possible contamination from sources such as labware and reagents that might contain monoester or diester phthalates (Blount et al., 2000). The hydrolytic monoesters, which are more lipophilic, are generally found in lower concentrations within urine compared to the more polar oxidized monoesters (Blount et al., 2000). This observation suggests low exposure to their parent phthalates, accumulation in fatty tissue, or elimination of the hydrolytic monoesters through other routes (e.g., feces). Additionally, the levels of detection for the hydrolytic monoesters within the NHANES dataset were higher (0.4e0.5 ng/ml analyte) compared to those of the three additional monoesters for DEHP (0.2 ng/ml analyte for each) (CDC, 2016), suggesting possible technical limitations in the analysis for certain metabolite subsets. It has been shown that DEHP is partially excreted in feces (Schmid and Schattler, 1985), in addition to evidence of bioaccumulation and MEHP levels twice as high in sweat than in urine (Genuis et al., 2012). Therefore, other routes of elimination and limited excretion in urine should certainly be considered when conducting reverse dosimetry for lipophilic chemicals like phthalates.
The high urine concentrations of the three DEHP oxidized monoesters from the 2011e2012 data sets indicates that exposure to the parent compound may be higher than estimated when using only the hydrolytic monoester. DEHP intake for couples planning pregnancy in Michigan and Texas from 2005 to 2009 was 0.78e1.11 mg/kg/d, as estimated from urine concentrations of 5OHMEHP or 5OXO-MEHP and using the creatinine-adjustment method (Guo et al., 2014), which is comparable to the current reverse dosimetry estimates at the 50th percentile (0.684e2.285 μg/kg/d when using urine concentrations of only one of the four monoesters at a time). In contrast, MEHP levels in pregnant women from New Jersey were nearly 30 times that of the general US population, while 5OXO-MEHP and 5OH-MEHP levels were only 5e6 times that of the general population (Yan et al., 2009), resulting in intake estimates 4 times greater when using MEHP concentrations and which are nearly 70 times greater than intake estimated from reverse dosimetry using MEHP as input. Authors of the New Jersey study attributed the abnormally high phthalate monoester levels to a small sample size of 150 women, in addition to possible contamination from plastic medical devices in the hospital to which the women were exposed prior to delivery (Yan et al., 2009). The highest estimate of DEHP intake (183 μg/kg/d) occurred in children in Seattle after short-term consumption of catered foods in a dietary supplementation trial (Sathyanarayana et al., 2013). Though this study used urine data 1e4 years more recent than NHANES input used here, it is highly unlikely that DEHP intake has increased significantly, and certainly not more than the 2-fold suggested by comparing the two intake amounts. Rather, the authors attributed such high intake to either a rare packing abnormality leading to contamination or systemic contamination of the food supply (Sathyanarayana et al., 2013), which is not indicative of exposure to the general population. Such examples illustrate the influence of confounding factors involved in urine biomarker studies, other than simply age-specific, geographic, or demographic differences. These examples also compared estimated intake using reverse dosimetry to that from specific known sources of exposure.
The similar intake values that are estimated for DnBP in Hartmann et al. (2015) study (2015) using either urine void volume (0.28e0.4 μg/kg/d) or creatinine-adjusted metabolite concentrations in conjunction with creatinine excretion rate (0.24e0.35 μg/kg/d) suggests that which calculations are used is less important than metabolite concentration. Some of the highest intake DnBP intake estimates were associated with sampling of populations from Asian countries, as it is thought that the over 16 million tons of PVC products containing phthalates that are produced in such countries comprised nearly 50% of global production in 2007 (Guo et al., 2011). Only one study describes use of urine biomarkers to estimate DnBP intake in the US, and this refers to NHANES samples taken 6e9 years prior to the NHANES data used as input for PLE-THEM (Shin et al., 2014). Despite the difference in time, intake estimated from this earlier study, at 14.2 μg/d and assuming a body weight of 74.7 kg for females and 88.3 for males, is only 2 times greater than the median DnBP intake using reverse dosimetry (0.16e0.19 μg/kg/d vs. 0.089 mg/kg/d).
SHEDS-HT was primarily developed to aid in prioritizing thousands of chemicals for toxicological testing or further exposure assessment. Knowing the order of magnitude of exposure is helpful in deciding which chemicals to screen more closely without unnecessary use of resources. The higher median intake dose for DnBP in SHEDS-HT compared to other predictions seen here (even though it only covers consumer product pathways) is primarily the result of many conservative model assumptions, some of which are necessary to allow the model to be applied to thousands of chemicals. For example, if a chemical is found in any consumer product MSDS within a category (e.g. spray paints) it is assumed to be in all products used by the population. Such assumptions, as well as the limited media in which SHEDS-HT currently operates, remain inherent limitations of this application despite its utility in providing exposure information rapidly for numerous chemicals. As more data come available, SHEDS-HT is expanding to include other sources, use scenarios, and other inputs (such as the prevalence of chemical within products of a given type) will be refined.
While the median of the exposure predictions of the heuristic ExpoCast model for DnBP (Table 2) is 1.06-fold of the reverse dosimetry estimations for DnBP, Table 1 shows the intake dose estimates for DEHP vary from 9-fold (compared to the 5CX-MEPP estimation) to 3.6-fold (compared to the MEHP estimation). This heuristic model was developed to obtain an estimate of exposures for thousands of chemicals in a short time, similar to the purpose of SHEDS-HT (Wambaugh et al., 2014). Because the ExpoCast heuristic model only requiring 5 binary descriptors as input, it does not provide a mechanism-driven or refined exposure estimates for a chemical, but rather provides a broad enough view that allows for prioritizing of thousands of chemicals for further testing. Indeed, Wambaugh et al. (2014) state that only half of the variance of the exposures predicted from the heuristic model from the inferred exposures from NHANES can be explained. Further explanation of the variance would likely require more complicated models. Because ExpoCast predictions provide only median exposure estimate, there is room for uncertainty in how much both the lower and upper ends of the heuristic model population and the PBPK-reverse dosimetry population differ.
5. Conclusion
By comparing the assumptions and estimation methods used in each exposure estimation tool evaluated in this study, we have evaluated the applicability or utility of reverse dosimetry compared to two HT prediction models, i.e. the HT Stochastic Human Exposure and Dose Simulation model (SHEDS-HT) and the ExpoCast empirical heuristic model, as well as non-HT approaches based on chemical specific exposure estimations in supporting risk assessment at different stages. Each tool can be applied in relation to its unique purposes, and the limitations inherent within each indicate a need for considering how they might be further improved in regards to their development and application, particularly for incorporation into the integrated source-to-outcome modeling platform such as PLETHEM to support different tiers/stages of risk assessment. For reverse-dosimetry with PBPK models, increasing confidence in model parameters, especially those concerning metabolism, is the key. Understanding and acknowledging the limitations that arise from biomarker measurements is also essential. The utility of the HT-prediction/estimation models likely lies in the use of those applications for prioritization and screening. However, understanding the exposure data used to build such predictive/estimation models is still useful for mindful application of those tools to support risk assessment.
Supplementary Material
HIGHLIGHTS.
Evaluation of available exposure prediction tools for source-to-outcome modeling.
Estimation of the human exposure based on PBPK-reverse dosimetry for comparison to predictions.
Discussion of the utility and areas for improvement of each tool.
Implication in risk assessment for prioritization and safe exposure decision.
Disclaimer and acknowledgments
The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.chemosphere.2017.06.098.
References
- Anderson WA, Castle L, Scotter MJ, Massey RC, Springall C, 2001. A biomarker approach to measuring human dietary exposure to certain phthalate diesters. Food Addit. Contam 18, 1068–1074. [DOI] [PubMed] [Google Scholar]
- Albertini R, Bird M, Doerrer N, Needham L, Robison S, Sheldon L, Zenick H, 2006. The use of biomonitoring data in exposure and human health risk assessments. Environ. Health Perspect 114, 1755–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WA, Castle L, Hird S, Jeffery J, Scotter MJ, 2011. A twenty-volunteer study using deuterium labelling to determine the kinetics and fractional excretion of primary and secondary urinary metabolites of di-2-ethylhexylphthalate and di-iso-nonylphthalate. Food Chem. Toxicol 49, 2022–2029. [DOI] [PubMed] [Google Scholar]
- Biryol D, Wambaugh JF, Nicolas CI, Phillips KA, Isaacs KK, 2017. High throughput dietary exposure predictions for chemical migrants from food contact substances for use in chemical prioritization. Environ. Int (In Submission). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount BC, Silva MJ, Caudill SP, Needham LL, Pirkle JL, Sampson EJ, Lucier GW, Jackson RJ, Brock JW, 2000. Levels of seven urinary phthalate metabolites in a human reference population. Environ. Health Perspect 108, 979–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2016. National Report on Human Exposure to Environmental Chemical. Fourth Report, Updated Tables. https://www.cdc.gov/exposurereport/.
- Chauvigné F, Menuet A, Lesne L, Chagnon MC, Chevrier C, Regnier JF, Angerer J, Jegou B, 2009. Time- and dose-related effects of di-(2-ethylhexyl) phthalate and its main metabolites on the function of the rat fetal testis vitro. Environ Health Perspect 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Joo H, Campbell JL Jr., Andersen ME, Clewell HJ 3rd, 2013. In vitro intestinal and hepatic metabolism of Di(2-ethylhexyl) phthalate (DEHP) in human and rat. Toxicol Vitro 27, 1451–1457. [DOI] [PubMed] [Google Scholar]
- Clewell RA, Kremer JJ, Williams CC, Campbell JL, Sochaskib MA, Andersen ME, Borghoff SJ, 2009. Kinetics of selected di-n-butyl phthalate metabolites and fetal testosterone following repeated and single administration in pregnant rats. Toxicol 255, 80–90. [DOI] [PubMed] [Google Scholar]
- David RM, 2000. Exposure to phthalate esters. Environ. Health Perspect 108, A440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David RM, McKee RH, Butala JH, Barter RA, Kayser M, 2001. Esters of aromatic mono-, di-, and tricarboxylic acids, aromatic diacids, and di-, tri-, or poly-alcohols In: Bingham E, Cohrssen B, Powell CH (Eds.), Patty’s Toxicology. John Wiley & Sons, New York, NY, pp. 635–932. [Google Scholar]
- Genuis SJ, Beesoon S, Lobo RA, Birkholz D, 2012. Human elimination of phthalate compounds: blood, urine, and sweat (BUS) study. ScientificWorldJournal 2012, 615068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry PR, Clewell HJ, Clewell R, Campbell J, Van Landingham C, Shipp AM, 2011. Challenges in the application of quantitative approaches in risk assessment: a case study with di-(2-ethylhexyl)phthalate. Crit. Rev. Toxicol 41, 1–72. [DOI] [PubMed] [Google Scholar]
- Goldsmith MR, Grulke CM, Brooks RD, Transue TR, Tan YM, Frame A, Egeghy PP, Edwards R, Chang DT, Tornero-Velez R, Isaacs K, Wang A, Johnson J, Holm K, Reich M, Mitchell J, Vallero DA, Phillips L, Phillips M, Wambaugh JF, Judson RS, Buckley TJ, Dary CC, 2014. Development of a consumer product ingredient database for chemical exposure screening and prioritization. Food Chem. Toxicol 65, 269–279. 10.1016/j.fct.2013.12.029. [DOI] [PubMed] [Google Scholar]
- Gray TJ, 1986. Testicular toxicity in vitro: sertoli-germ cell co-cultures as a model system. Food Chem. Toxicol 24, 601–605. [DOI] [PubMed] [Google Scholar]
- Guo Y, Weck J, Sundaram R, Goldstone AE, Louis GB, Kannan K, 2014. Urinary concentrations of phthalates in couples planning pregnancy and its association with 8-hydroxy-2’-deoxyguanosine, a biomarker of oxidative stress: longitudinal investigation of fertility and the environment study. Environ. Sci. Technol 48, 9804–9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Wu Q, Kannan K, 2011. Phthalate metabolites in urine from China, and implications for human exposures. Environ. Int 37, 893–898. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Uhl M, Weiss S, Koch HM, Scharf S, König J, 2015. Human bio-monitoring of phthalate exposure in Austrian children and adults and cumulative risk assessment. Int. J. Hyg. Environ. Health 218, 489–499. [DOI] [PubMed] [Google Scholar]
- Hauser R, Duty S, Godfrey-Bailey L, Calafat AM, 2004. Medications as a source of human exposure to phthalates. Environ. Health Perspect 112, 751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Calafat AM, 2005. Phthalates and human health. Occup. Environ. Med 62, 806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs KK, Glen WG, Egeghy P, Goldsmith MR, Smith L, Vallero D, Brooks R, Grulke CM, Özkaynak H, 2014. SHEDS-HT: An Integrated Probabilistic Exposure Model for Prioritizing Exposures to Chemicals with Near-Field and Dietary Sources. Environ. Sci. Technol 48, 12750–12759. [DOI] [PubMed] [Google Scholar]
- Isaacs KK, Goldsmith MR, Egeghy P, Phillips K, Brooks R, Hong T, Wambaugh JF, 2016. Characterization and prediction of chemical functions and weight fractions in consumer products. Toxicol. Rep 3, 723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamrin MA, 2009. Phthalate risks, phthalate regulation, and public health: a review. J. Toxicol. Environ. Health 12, 157–174. [DOI] [PubMed] [Google Scholar]
- Kato K, Silva MJ, Reidy JA, Hurtz D 3rd, Malek NA, Needham LL, Nakazawa H, Barr DB, Calafat AM, 2004. Mono(2-ethyl-5-hydroxyhexyl) phthalate and mono-(2-ethyl-5-oxohexyl) phthalate as biomarkers for human exposure assessment to di-(2-ethylhexyl) phthalate. Environ. Health Perspect 112, 327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler W, Numtip W, Völkel W, Seckin E, Csanády GA, Pütz C, Klein D, Fromme H, Filser JG, 2012. Kinetics of di(2-ethylhexyl) phthalate (DEHP) and mono(2-ethylhexyl) phthalate in blood and of DEHP metabolites in urine of male volunteers after single ingestion of ring-deuterated DEHP. Toxicol. Appl. Pharmacol 264, 284–291. [DOI] [PubMed] [Google Scholar]
- Koch HM, Bolt HM, Preuss R, Angerer J, 2005. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch. Toxicol 79, 367–376. [DOI] [PubMed] [Google Scholar]
- Leung MCK, Phuong J, Baker NC, Sipes NS, Klinefelter GR, Martin MT, McLaurin KW, Setzer W, Darney SP, Judson RS, Knudsen TB, 2015. Systems toxicology of male reproductive development: profiling 774 chemicals for molecular targets and adverse outcomes. Environ. Health Perspect 10.1289/ehp.1510385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Sathyanarayana S, Swan SH, 2009. Phthalates and other additives in plastics: human exposure and associated health outcomes. Philos. Trans. R. Soc. Lond B Biol. Sci 364, 2097–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (NRC), 2007. Toxicity Testing in the 21st Century: a Vision and a Strategy. DCNational Academy Press, Washington. [Google Scholar]
- Otake T, Yoshinaga J, Yanagisawa Y, 2004. Exposure to phthalate esters from indoor environment. J. Expo. Anal. Environ. Epidemiol 14, 524–528. [DOI] [PubMed] [Google Scholar]
- Pendse SN, Efremenko A, McMullen PD, Yoon M, Clewell HJ, 2017. PLETHEM e an Interactive Open-source Platform for Bridging the Source-to-outcome Continuum. Abstract SOT2017. Baltimore, Maryland, USA. [Google Scholar]
- Rotroff DM, Wetmore BA, Dix DJ, Ferguson SS, Clewell HJ, Houck KA, Lecluyse EL, Andersen ME, Judson RS, Smith CM, Sochaski MA, Kavlock RJ, Boellmann F, Martin MT, Reif DM, Wambaugh JF, Thomas RS, 2010. Incorporating human dosimetry and exposure into high throughput in vitro toxicity screening. Toxicol. Sci 117, 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusyn I, Peters JM, Cunningham ML, 2006. Effects of DEHP in the liver: modes of action and species-specific differences. Crit. Rev. Toxicol 36, 459e479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Alcedo G, Saelens BE, Zhou C, Dills RL, Yu J, Lanphear B,2013. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures. J. Expo. Sci. Environ. Epidemiol 23, 378–384. [DOI] [PubMed] [Google Scholar]
- Scheringer M, Vögel T, Vongrote J, Capaul B, Schubert R, Hungerbüler K, 2001. Scenario-based risk assessment of multi-use chemicals: application to solvents. J. Exp. Ana Env. Epi 21, 481–497. [DOI] [PubMed] [Google Scholar]
- Schmid P, Schlatter C, 1985. Excretion and metabolism of di(2-ethylhexyl) phthalate in man. Xenobiotica 15, 251–256. [DOI] [PubMed] [Google Scholar]
- Shin HM, McKone TE, Bennett DH, 2014. Attributing population-scale human exposure to various source categories: merging exposure models and bio-monitoring data. Environ. Int 70, 183–191. [DOI] [PubMed] [Google Scholar]
- Staples CA, Peterson DR, Parkerton TF, Adams WJ, 1997. The environmental fate of phthalate esters: a literature review. Chemosphere 35, 667–749. [Google Scholar]
- Tan YM, Liao KH, Conolly RB, Blount BC, Mason AM, Clewell HJ, 2006. Use of a physiologically based pharmacokinetic model to identify exposures consistent with human biomonitoring data for chloroform. J. Toxicol. Environ. Health, Part A 69, 1727–1756. [DOI] [PubMed] [Google Scholar]
- U.S. EPA, 2014. Integrated Bioactivity and Exposure Ranking: a Computational Approach for the Prioritization and Screening of Chemicals in the Endocrine Disruptor Screening Program. https://www.regulations.gov/#!documentDetail; D EPA-HQ-OPP-2014-0614-0003.
- Wambaugh JF, Wang A, Dionisio KL, Frame A, Egeghy P, Judson R, Woodrow Setzer R, 2014. High throughput heuristics for prioritizing human exposure to environmental chemicals. J. Env. Sci. Tech 48, 12760–12767. [DOI] [PubMed] [Google Scholar]
- Wambaugh JF, Wetmore BA, Pearce R, Strope C, Goldsmith R, Sluka JP, Sedykh A, Tropsha A, Bosgra S, Shah I, Judson R, Thomas RS, Setzer RW, 2015. Toxicokinetic triage for environmental chemicals. Toxicol. Sci 147, 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmore B, Wambaugh J, Ferguson S, Sochaski M, Rotroff D, Freeman K, Clewell H, Dix D, Andersen M, Houck K, Allen B, Judson R, Singh R, Kavlock R, Richard A, Thomas R, 2012. Integration of dosimetry, exposure and high-throughput screening data in chemical toxicity assessment. Toxicol. Sci 125, 157–174. [DOI] [PubMed] [Google Scholar]
- Wittassek M, Hegera W, Koch HM, Beckera K, Angerer J, Kolossa-Gehring M, 2007. Daily intake of di(2-ethylhexyl)phthalate (DEHP) by German children e a comparison of two estimation models based on urinary DEHP metabolite levels. Int. J. Hyg. Environ. Health 210, 35–42. [DOI] [PubMed] [Google Scholar]
- Wittassek M, Koch HM, Angerer J, Bruning T, 2011. Assessing exposure to phthalates e the human biomonitoring approach. Mol. Nutr. Food Res 55, 7e31. [DOI] [PubMed] [Google Scholar]
- Wormuth M, Scheringer M, Vollenweider M, Hungerbüler K, 2006. What are the sources of exposure to eight frequently used phthalic acid esters in europeans? Risk Anal. 26, 803–824. [DOI] [PubMed] [Google Scholar]
- Yan X, Calafat A, Lashley S, Smulian J, Ananth C, Barr D, Silva M, Ledoux T, Hore P, Robson MG, 2009. Phthalates biomarker identification and exposure estimates in a population of pregnant women. Hum. Ecol. Risk Assess 15, 565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Tan YM, Blount B, Murray C, Egan S, Bolger M, Clewell H, 2012. Using a physiologically based pharmacokinetic model to link urinary biomarker concentrations to dietary exposure of perchlorate. Chemosphere 88, 1019–1027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.