Abstract
In this review we address the relationship between cytochromes P450 (P450) and H2O2. This association can affect biology in three distinct ways. First, P450s produce H2O2 as a byproduct either during catalysis or when no substrate is present. This reaction, known as uncoupling, releases reactive oxygen species that may have implications in disease. Second, H2O2 is used as an oxygen-donating co-substrate in peroxygenase and peroxidase reactions catalyzed by P450s. This activity has proven to be important mainly in reactions involving prokaryotic P450s, and investigators have harnessed this reaction with the aim of adaptation for industrial use. Third, H2O2-dependent inhibition of human P450s has been studied in our laboratory, demonstrating heme destruction and also the inactivating oxidation of the hemethiolate ligand to a sulfenic acid (-SOH). This reversible oxidative modification of P450s may have implications in the prevention of uncoupling and may give new insights into the oxidative regulation of these enzymes. Research has elucidated many of the chemical mechanisms involved in the relationship between P450 and H2O2, but the application to biology is difficult to evaluate. Further studies are needed reveal both the harmful and protective natures of reactive oxygen species in an organismal context.
Keywords: Cytochrome P450, reactive oxygen species, thiol, oxidative damage
Graphical Abstract
INTRODUCTION
Gillette et al. [1] first observed the NADPH-dependent production of H2O2 in microsomes, and the chemical, biophysical, and biological factors that govern the reactivity of H2O2 and oxygen radicals with cytochrome P450 (P450) have been studied extensively since then. However, there are still many unanswered questions regarding the P450 catalytic cycle and its context in biology. Are P450s as inefficient in vivo as they are in reconstituted systems? How are P450s regulated when no substrate is present to prevent futile cycling with NADPH and oxygen? These questions become much more difficult to answer in the context of cells, tissues, and organisms.
P450s play two major biological roles: (1) xenobiotic metabolism, with the goal being a decrease in the hydrophobicity of compound for ease of excretion and for further metabolism by enzymes such as sulfotransferases and UDP-glucuronyltransferases, and (2) biosynthesis of bioactive molecules including steroids, vitamins, and oxidized fatty acids [2]. A subset of the latter role is the deactivation and turnover of bioactive molecules, e.g. vitamins A and D [3, 4]. Many diseases are associated with specific P450 variants, and other diseases result from a lack of genes or the substitution of functionally inactive mutants [5–7]. Extensive reviews of endogenous and exogenous substrates and metabolites of P450s have been published elsewhere [2, 8] and this area is beyond the scope of this review.
Iron reacts readily with molecular oxygen and H2O2 to produce species capable of performing a diverse array of oxidation reactions. Known broadly as Fenton reactions [9], when this chemistry is uncontrolled it can generate a mixture of nonspecific products with organic reactants and is generally unwanted in most biochemical systems [10]. These are generally controlled in vitro by the addition of iron chelating reagents such as EDTA. P450s, as well as some other iron-centered enzymes [11], control the reaction of oxygen with iron in a stereospecific and regiospecific manner. These enzymes have varying efficiencies and are dependent on numerous factors. The iron-oxo reaction also allows for action on a varied number of substrates, because a general role in much of xenobiotic metabolism is to produce more hydrophilic compounds for facile excretion.
The various P450s are very diverse despite sharing common structural features. P450s are also some of the most promiscuous enzymes, with human P450 3A4 having thousands of reported substrates [12, 13]. Plants have greater numbers of P450-encoded genes than any other kingdom of organisms (e.g., wheat has 1476). These are extensively involved in the synthesis of secondary metabolites and defense molecules [14]. Prokaryotic P450s synthesize important secondary metabolites such as antibiotics and have also been used as model enzymes for the study of all aspects of the general P450 catalytic cycle [15]. The use of prokaryotic P450s to catalyze diverse chemical reactions that are difficult to perform synthetically has proved to be promising as well [16]. This includes the use of H2O2 and high-valent oxygen compounds as oxygen surrogates (e.g., peracids, hydroperoxides, iodosylbenzene) for chemical reactions [17]. In this review we discuss the known interactions of P450s with H2O2. This oxidative chemistry has implications important to the understanding of P450s in a biological context.
H2O2 in Signaling
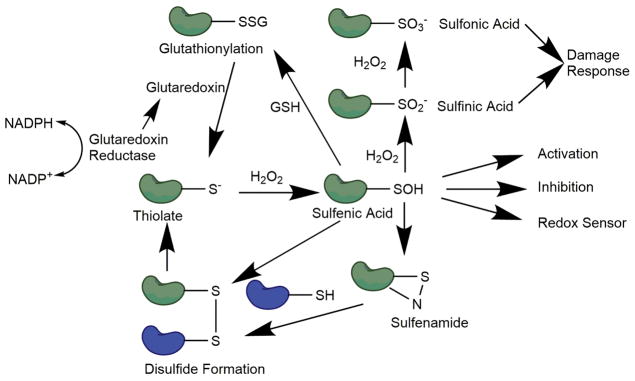
In recent years H2O2 has been recognized as an important secondary signaling molecule, and several laboratories have characterized redox sensitive enzymes [18, 19]. Reactive oxygen species (ROS) have been shown to react specifically with several amino acids, but the sulfur-containing residues cysteine and methionine are the most susceptible to oxidation. The first step of cysteine oxidation by H2O2 is formation of a sulfenic acid (-SOH), initially characterized as an anthraquinone-sulfenic acid by Fries [20] and later by Bruice [21]. This oxidation can occur at rates between 10−1 M−1 s−1 (glutathione, GSH) and 108 M−1 s−1 (peroxiredoxin) [19] (Fig. 1). This large variation in reactivity is due at least in part to the pKa of the particular oxidized cysteine. Sulfenic acids are reactive species and readily react with free thiols to form disulfide bonds through a dehydration reaction or through a sulfenamide intermediate [22]. This is thought to be the general mechanism of physiological disulfide bond formation (Fig. 1). The reaction can occur in an intra- or intermolecular fashion with free thiols (including GSH) and can be reversed by an NADPH-dependent reaction catalyzed by glutaredoxin [23]. Sulfenic acids can be further oxidized to dioxidation (sulfinic acid, SO2−) and trioxidation products (sulfonic acid, SO3−), which are mostly irreversible and induce protein degradation and cellular stress responses (Fig. 1) [24].
FIG. 1. General redox cycle of protein thiols.
In the presence of H2O2, thiols can be oxidized to sulfenic acids, which may elicit a physiological response. Sulfenic acids can then form disulfide bonds, which can be reduced to free thiols. Sulfenic acids can also be further oxidized to sulfinic or sulfonic acids, which can trigger cellular damage responses.
Oxidative regulation of cysteines in proteins have been known for quite some time. Evidence of oxidative inhibition of glyceraldehyde phosphate dehydrogenase [25] and papain [26] led to an interest in the field [27]. After researchers determined conditions to promote the stability of sulfenic acids, they could be studied in a more systematic fashion [28–30]. Recently mechanisms of stability and function have been elucidated. In the case of epidermal growth factor receptor (EGFR), a sulfenic acid is formed in the kinase domain of the protein (Cys-797) in an H2O2-dependent fashion, causing autophosphorylation and activating the EGFR signaling cascade. This sulfenic acid is stabilized by a hydrogen bond with Arg-841, which, when mutated confers resistance to oxidative activation [31]. Additionally, tyrosine phosphoprotein phosphatase 1B (PTP1B) [32], glyceraldehyde phosphate dehydrogenase (GAPDH) [33], Kelch-like ECH-associated protein-1 (Keap1) [34, 35], P450s [36, 37], and many others [38] have been found to be regulated by sulfenic acid formation. Methods for detecting and analyzing cysteine oxidation have remained challenging, but recent advances in chemical trapping methods and in our understanding of redox biology provide promising new ways to elucidate redox functions of cysteines [39, 40].
H2O2 Production Through Pathway Uncoupling
Since the report of Gillette et al. [1] on the NADPH-dependent production of H2O2 in liver microsomes, there has been interest in this area of study. Several years later, a stoichiometric anomaly observed between NADPH, oxygen consumption, and product formation in liver microsomes [41] was accounted for when H2O2 and H2O production were measured as side products in P450 reactions [42, 43] . H2O2 production, plus the generation of superoxide anion by NADPH-P450 reductase [44] and P450 [45], led to the hypothesis that P450 induction may be related to hepatic disfunctions such as ethanol-induced liver damage [46–48]. Uncoupling has been proposed to have potentially damaging circumstances by contributing to ROS production and to accelerating the aging process [49].
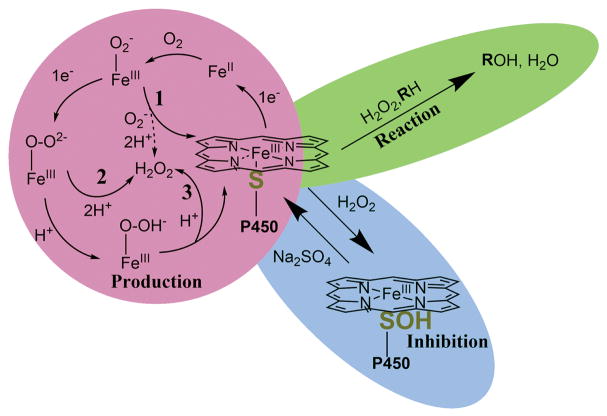
ROS production can, at least in principle, occur at three intermediate stages during the normal P450 catalytic cycle (Fig. 2). The first is directly after molecular oxygen binding to the ferrous heme (Fig. 2, Reaction 1, dashed line). This has been described as the ferric superoxide complex, FeIII-O2− [50]. This state is only 1 kcal mol−1 above the heme resting state (FeIII), and the oxygen-iron bond can easily be broken to form superoxide anion (O2• −) and iron (III) heme [51]. Superoxide is quickly dismutated (non-enzymatically) to H2O2. The rate of this process (FeII → > FeIII + O2•−), termed autoxidation, is related to the stability of the FeII-O2 complex and varies among P450s [52], and the rate of autooxidation is dependent on temperature [53, 54]. Structural studies with P450cam have elucidated a coordination sphere surrounding the heme-thiolate ligand, which reduces the sulfur charge and allows for reduction to ferrous heme [55]. This hydrogen bonding network appears to fine-tune the positioning and electron donating ability of cysteine sulfur [56].
FIG. 2. P450 Production, Reaction, and Inhibition with H2O2.
P450s can produce H2O2 in three separate reactions after molecular oxygen binds to the ferrous heme (red circle, numbered reactions). H2O2 can also be used as a co-substrate and oxygen donor in peroxygenase and peroxidase reactions (green oval). Additionally, H2O2 can inhibit catalysis through sulfenylation of the heme-thiolate ligand (blue oval).
The second and third stages at which ROS can be produced, following the second reduction step, are from the P450 peroxo (FeIII-O-O2−) and hydroperoxo (FeIII-O-OH−) complexes (“Compound 0”). The Fe-O bond of the peroxo complex can be broken, and the oxygen species can be doubly protonated to form H2O2 (Fig. 2, Reaction 2). In a similar fashion, after protonation the hydroperoxo complex can either be further protonated, forming Compound I (FeIV=O3+) and H2O, or the Fe-O bond can break, once again forming H2O2 (Fig. 2, Reaction 3).
This uncoupling appears to be dependent on several factors, including pH, substrate positioning in the active site, and a disturbed substrate binding pocket. The heme thiolate allows for the correct amount of “push” and “pull” of electrons for the successful completion of the P450 catalytic cycle [57]. Bacterial enzymes generally have very high coupling efficiencies for native substrates compared to mammalian P450s (Table 1). This difference in coupling efficiency may be due to the number of substrates mammalian P450s can accommodate compared to bacterial enzymes.
Table 1.
Human P450: Coupling efficiency (Product/NADPH ratio)
| P450 | Substrate | % coupling efficiency | Reference |
|---|---|---|---|
|
| |||
| 1A1 | Phenacetin | 2.5 | [58] |
|
| |||
| 1A2 | Methanol | 7.5 | [58, 59] |
| 7-Ethoxycoumarin | 1.2 | ||
| Phenacetin | 5.1 | ||
|
| |||
| 2A6 | Coumarin | 25 | [60] |
|
| |||
| 2B6 | 17-α-ethynylestradiol | 48 | [61] |
| Efavirenz | 42 | ||
|
| |||
| 2C9 | (S)-flurbiprofen | 21 | [62] |
| (S)-Warfarin | 4 | ||
|
| |||
| 2D6 | Bufuralol | 39 | [63, 64] |
| 3-Methoxyphenylethylamine | 43 | ||
| 4-Methoxyphenylethylamine | 42 | ||
|
| |||
| 2E1 | N-Nitrosodimethylamine | 5.6 and 59 (± b5) | [65] |
|
| |||
| 2J2 | Ebastine | 2–17 | [66] |
|
| |||
| 3A4 | Testosterone | 10–16 | [67] |
|
| |||
| 4A11 | Lauric acid | 31 | [68] |
|
| |||
| 17A1 | Progesterone | 22, 41 | [69, 70] |
| 17α-Hydroxyprogesterone | 1.3, 10 | ||
| Pregnenolone | 97, 61 | ||
| 17α-Hydroxypregnenolone | 4, 44 | ||
|
| |||
| 19A1 | Androstenedione | 5 | [71] |
| 19-Hydroxy androstenedione | 34 | ||
| 19-Aldehyde androstenedione | 33 | ||
ROS generated by P450 from inefficient reaction cycles can, in principle, oxidize cellular proteins, lipids, and DNA. This alteration in cellular redox balance can lead to signaling involved in antioxidant responses, create an oxidatively stressed environment, and potentially lead to disease [72]. Evidence for potential ROS-dependent toxicity of P450s in the CYP Subfamilies 1, 2, 3, and 4 has been reviewed recently [49, 73].
ROS-dependent toxicity originating from P450-mediated uncoupling has been difficult to establish in vivo. Many in vitro reconstituted, microsomal, mitochondrial, and cellular studies have provided evidence that induction of P450s can cause elevated ROS production [67, 74–80]. However, in vivo studies in rodents indicate that toxicity may stem from depletion of reducing pools found in cells, such as GSH and reduced pyridine nucleotides (NADPH and NADH) [81, 82]. Conversely, other studies indicate that P450s may have protective effects in the case of the P450 1A subfamily [83, 84]. A major unanswered question in this field is how much do P450s contribute to ROS production. Although much has been written about both topics, there are major issues. One is that much of the experimental work has been done in cell culture, often with the use of inappropriate cellular models (e.g., that do not normally express P450s), or else ROS has been measured using inadequate methods (e.g., several fluorescent dyes [85–87].) A number of papers have touted P450s as a major source of ROS [88–90] although others do not consider this to be as important as mitochondrial leakage, NADPH oxidase, and other sources [91]. In vivo work with both rats and mice, using F2-isoprostane formation [92] (still accepted as the “gold standard” [87]) showed that P450 induction elevated total tissue or urinary ROS only in the case of barbiturate induction, and that was at least in part due to an alteration in levels of pyridine nucleotides due to altered methyl transferase activity [81, 82]. However, other work done has shown that some localized changes (e.g., translocation of P450 2E1 to mitochondria and uncoupling there) may occur and be detrimental [74] (and these ROS changes were confirmed with isoprostane analysis). More in vivo studies will be needed to determine the contribution of P450s to proposed ROS-related toxicities [72].
H2O2 as a P450 Co-substrate
Reactions with H2O2 as the oxygen donor for P450 peroxygenase and peroxidase reactions are known [93, 94]. In these reactions, the ferric heme reacts directly with an oxygen of H2O2 or other hydroperoxide species and proceeds with heterolytic cleavage of the oxygen-oxygen bond to form Compound I [95]. Other oxygen donating molecules (“oxygen surrogates”) have been noted to have oxidation activity including iodosylbenzene [96] and sodium chlorite [97]. Additionally, Rittle and Green used m-chloroperbenzoic acid as an oxygen surrogate for CYP119 to successfully isolate Compound I [98].
In the case of the peroxidase function of P450s, once Compound I is formed by either H2O2 or an organic hydroperoxide, a one-electron oxidation is performed on a substrate, reducing the porphyrin radical of Compound I to form Compound II (FeIV=O) and a radical product. Compound II performs a subsequent one-electron reduction on another substrate generating ferric heme, water, and a second radical product [17]. Mammalian P450s are known to act on endogenous hydroperoxide species, reducing them to their corresponding alcohols [99, 100]. This may be one of the metabolic mechanisms to reduce the levels of reactive hydroperoxides in cells, although the overall contribution is unknown.
P450s can also perform peroxygenase reactions in which the enzyme can catalyze monooxygenase reactions without the requirement for ferric iron reduction or redox partner proteins. Peroxygenase reactions are thought to react in a chemically similar way to the monooxygenase activity. Various hydroperoxide substrates have been explored in the oxidation of P450 substrates [101–104]. This provides further evidence that P450s may utilize endogenous hydroperoxides as co-substrates in vivo. However, there are many technological challenges to study this hypothesis including the need for highly sensitive detection methods and the inherent instability of hydroperoxides [105].
In mammalian P450s the reaction with H2O2 is generally very inefficient and is dependent on high concentrations of H2O2 that are well above estimated physiological concentrations, suggesting that this reaction does not occur in vivo [106]. However, some bacterial enzymes are known to have fast catalytic rates and highly specific hydroxylation products of saturated fatty acids, e.g. P450SPα, P450BSβ [107], and OleT [108–110]. These enzymes and other bacterial P450s are highly stable in the presence of H2O2. P450 BM3 (102A1) has been an important enzyme in the study of peroxygenases, especially after engineering an increase in stability [111–113]. Other bacterial enzymes have also shown high stability in the presence H2O2 [114, 115].
More recently, scientists have recognized the utility of peroxygenase reactions in the development of P450s as industrial biocatalysts [116, 117]. This industrial role has potential, and ongoing discovery and characterization of novel peroxygenase- and peroxidase-catalyzing P450s may lead to novel enzymes that are useful products, e.g., biofuels and molecules that are difficult or expensive to synthesize.
H2O2 as a P450 Inhibitor
It has been known that H2O2 and other peroxides can inhibit P450 activity as well through heme degradation [118, 119]. Furthermore, it has been shown that incubation with H2O2 can also inhibit P450 by oxidizing the heme thiolate ligand to a sulfenic acid, thus inhibiting P450 catalysis [36, 37]. This phenomenon, first identified in human recombinant P450 4A11 [37], can affect other human P450 enzymes, as well as other drug metabolizing enzymes. Spectral studies indicated that loss of the proximal heme ligand inhibited carbon monoxide binding and/or ferric heme iron reduction by NADPH-P450 reductase but can be reversed using a reducing agent, e.g., dithiothreitol, tris-carboxyethylphosphine (TCEP), or sodium dithionite (Na2S2O4). In these studies, human P450s 2D6, 2C8, 3A4, and 4A11 exhibited redox sensitivity and P450 1A2 was redox insensitive, suggesting that there is differential redox regulation among P450s [36]. P450 1A2 was found to undergo extensive oxidation of one ancillary cysteine (Cys-159) that had no effect on catalysis. This was contrary to the case of P450 3A4, which showed an irreversible inhibition related to hyperoxidation of ancillary Cys-468. This may be reasonable, as Sevrioukova has recently reported a cysteine-depleted P450 3A4 enzyme with higher catalytic activity [120].
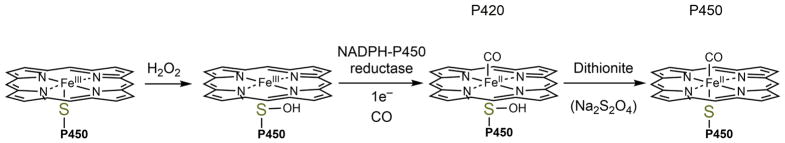
P450s 2D6, 2C8, and 4A11 seemed to behave similarly both in inhibitory and spectral aspects [37]. In anaerobic spectral studies, P450s in the presence of CO, NADPH-P450 reductase, and NADPH exhibited a maximal absorbance of 420 nm, indicating a 5-coordinate heme center. After the addition of dithionite, the typical 450 nm absorbance was observed. This change was interpreted as H2O2-dependent oxidation of the heme-thiolate ligand to a sulfenic acid which lost iron coordination. Dithionite was then able to reduce the sulfenic acid, allowing for re-liganding of the thiolate to the iron (Figure 3) [37 16183].
FIG. 3. Mechanism of loss of activity due to sulfenylation.
In the presence of H2O2, the heme-thiolate ligand becomes oxidized and loses its coordination with the heme iron. The sulfenic acid is not reduced by NADPH-P450 reductase but can be reduced by dithionite, re-forming the heme coordination.
There have been multiple reports of variation in pharmacokinetics (PK) of drugs due to disease and/or inflammation [121]. PK differences have been observed in celiac patients (which were reversed with treatment [122]) in which ROS levels are elevated, in untreated rheumatoid arthritis patients with extended verapamil half-lives compared to treated patients [123], and in P450 1A2 activity in patients with heart failure [124]. Other reports highlighting a two- to fivefold change increase in the area under the curve (AUC) in P450 substrates has been reviewed in detail recently by Coutant and Hall [125]. There is a strong link between autoimmune and inflammatory diseases to increased ROS production and also transcriptional downregulation of P450s [125, 126]. The redox sensitivity observed with some P450s may explain this variability, or at least contribute to it. Further testing in cellular and animal models is needed to confirm this, in that an alternate explanation is that the inflammation lowers overall levels of P450s at a pre-translational level or through other phenomena [127, 128].
This inhibition may function as a sensor where P450s are switched off when there is a high oxidizing environment and a low amount of NADPH may be present. The reducing equivalents of NADPH and/or NADH may be required to perform functions critical for life such as reversing glutathionylated GAPDH [33] or maintaining general redox homeostasis [129–131]. This may also be a negative feedback loop in place to limit uncoupling and further H2O2 production. Interestingly, in 1971 Hrycay and O’Brien hypothesized that heme-thiolate sulfenylation could occur and that modified P450s would preferentially catalyze peroxidase reactions over monooxygenase reactions [119]. This hypothesis requires further testing.
Conclusions
In summary, P450s and H2O2 have an intricate intertwined relationship which has both potentially harmful and beneficial results. P450 uncoupling seems to have the potential to be harmful and cause toxicity, but this uncoupling inefficiency may be a cost paid by P450s to allow for reactivity with many substrates. Hence, more in vivo studies are needed to fully understand the contributions P450s have to ROS-dependent toxicities and to determine if prevention of uncoupling is a reasonable defense against these diseases. Peroxidase and peroxygenase activities of P450s most likely do not occur in mammals, due to the amount of H2O2 required for catalysis, but are important for some prokaryotes in metabolism of specific substrates. Discovery and characterization of novel P450s that catalyze reactions with H2O2 as a co-substrate is a promising field and should be pursued further. Reversible H2O2-dependent inhibition of P450s is also an interesting interaction that needs further evaluation. Both in vivo and in vitro studies are required to fully understand if the sulfenylated heme-thiolate ligand have implications regarding uncoupling, peroxidase activity, and protein stability.
HIGHLIGHTS (Bullet Points).
P450s produce H2O2 during the reaction cycle, a process known as uncoupling, thus releasing reactive oxygen species that may have implications in disease.
H2O2 is used as an oxygen-donating co-substrate in peroxygenase and peroxidase reactions catalyzed by P450 and has implications in industrial chemical synthesis.
H2O2-dependent inhibition of human P450s occurs through the inactivating oxidation of the heme-thiolate ligand to a sulfenic acid (-SOH), which may have physiological implications.
Acknowledgments
This article is dedicated to the memory of Prof. Dr. Klaus Ruckpaul, who passed away recently. He worked in the P450 field for many years, often under difficult conditions. Prof. Ruckpaul worked on some aspects of ROS and P450. He is fondly remembered for starting the International Conferences on Cytochrome P450 in 1976, which continues today. The poster awards are named in his honor.
This work was supported by the National Institutes of Health [R01 GM118122, F31 F31HL136133] (M.E.A.) and an American Heart Association Predoctoral Fellowship [PRE33410007] (M.E.A.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gillette JR, Brodie BB, La Du BN. The oxidation of drugs by liver microsomes: on the role of TPNH and oxygen. J Pharmacol Exp Ther. 1957;119:532–540. [PubMed] [Google Scholar]
- 2.Guengerich FP. Human cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. 4. Springer; New York: 2015. pp. 523–785. [Google Scholar]
- 3.Ohyama Y, Yamasaki T. Eight cytochrome P450s catalyze vitamin D metabolism. Front Biosci. 2004;9:3007–3018. doi: 10.2741/1455. [DOI] [PubMed] [Google Scholar]
- 4.Raner GM, Vaz AD, Coon MJ. Metabolism of all-trans, 9-cis, and 13-cis isomers of retinal by purified isozymes of microsomal cytochrome P450 and mechanism-based inhibition of retinoid oxidation by citral. Mol Pharmacol. 1996;49:515–522. [PubMed] [Google Scholar]
- 5.Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C. Influence of cytochrome P450 polymorphisms on drug therapies: Pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther. 2007;116:496–526. doi: 10.1016/j.pharmthera.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Lee CR, Goldstein JA, Pieper JA. Cytochrome P450 2C9 polymorphisms: A comprehensive review of the in-vitro and human data. Pharmacogenetics. 2002;12:251–263. doi: 10.1097/00008571-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Zordoky BN, El-Kadi AO. Effect of cytochrome P450 polymorphism on arachidonic acid metabolism and their impact on cardiovascular diseases. Pharmacol Ther. 2010;125:446–463. doi: 10.1016/j.pharmthera.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Denisov IG, Makris TM, Sligar SG, Schlichting I. Structure and chemistry of cytochrome P450. Chem Rev. 2005;105:2253–2277. doi: 10.1021/cr0307143. [DOI] [PubMed] [Google Scholar]
- 9.Fenton HJH. LXXIII.— Oxidation of tartaric acid in presence of iron. J Chem Soc Transactions. 1894;65:899–910. [Google Scholar]
- 10.Walling C. Fenton's reagent revisited. Acc Chem Res. 1975;8:125–131. [Google Scholar]
- 11.Yoshimoto FK, Guengerich FP. Formation and cleavage of C-C bonds by enzymatic oxidation-reduction reactions. Chem Rev. 2018 doi: 10.1021/acs.chemrev.8b00031. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rendic S, Guengerich FP. Survey of human oxidoreductases and cytochrome P450 enzymes involved in the metabolism of xenobiotic and natural chemicals. Chem Res Toxicol. 2015;28:38–42. doi: 10.1021/tx500444e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saravanakumar A, Sadighi A, Ryu R, Akhlaghi F. Contribution of cytochrome P450 and other enzymes to the metabolism of FDA approved drugs between 2005–2016. Drug Metab Pharmacokinet. 2018;33:S36. [Google Scholar]
- 14.Schuler MA. P450s in plants, insects, and their fungal pathogens. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. 4. Springer; New York: 2015. pp. 409–449. [Google Scholar]
- 15.Khmelevtsova LE, Sazykin IS, Sazykina MA, Seliverstova EY. Prokaryotic cytochromes P450. Appl Biochem Microbiol. 2017;53:401–409. [Google Scholar]
- 16.Girvan HM, Munro AW. Applications of microbial cytochrome P450 enzymes in biotechnology and synthetic biology. Curr Opin Chem Biol. 2016;31:136–145. doi: 10.1016/j.cbpa.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Hrycay EG, Bandiera SM. Monooxygenase, peroxidase and peroxygenase properties and reaction mechanisms of cytochrome P450 enzymes. In: Hrycay EG, Bandiera SM, editors. Monooxygenase, peroxidase and peroxygenase properties and mechanisms of cytochrome P450. Springer; New York: 2015. pp. 1–61. [DOI] [PubMed] [Google Scholar]
- 18.Gupta V, Yang J, Liebler DC, Carroll KS. Diverse redoxome reactivity profiles of carbon nucleophiles. J Am Chem Soc. 2017;139:5588–5595. doi: 10.1021/jacs.7b01791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trujillo M, Alvarez B, Radi R. One- and two-electron oxidation of thiols: Mechanisms, kinetics and biological fates. Free Radic Res. 2016;50:150–171. doi: 10.3109/10715762.2015.1089988. [DOI] [PubMed] [Google Scholar]
- 20.Fries K. Über α-anthrachinon-sulfensäure. Eur J Inorg Chem. 1912;45:2965–2973. [Google Scholar]
- 21.Bruice TC, Markiw R. The synthesis of a disulfenic acid. anthraquinone-1,4-disulfenic acid. J Am Chem Soc. 1957;79:3150–3153. [Google Scholar]
- 22.Groitl B, Jakob U. Thiol-based redox switches. Biochim Biophys Acta. 2014;1844:1335–1343. doi: 10.1016/j.bbapap.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandes AP, Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 24.Gupta V, Carroll KS. Sulfenic acid chemistry, detection and cellular lifetime. Biochim Biophys Acta. 2014;1840:847–875. doi: 10.1016/j.bbagen.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pihl A, Lange R. The interaction of oxidized glutathione, cystamine monosulfoxide, and tetrathionate with the -SH groups of rabbit muscle D-glyceraldehyde 3-phosphate dehydrogenase. J Biol Chem. 1962;237:1356–1362. [PubMed] [Google Scholar]
- 26.Sanner T, Pihl A. Studies on the active -SH group of papain and on the mechanism of papain activation by thiols. J Biol Chem. 1963;238:165–171. [PubMed] [Google Scholar]
- 27.Allison WS. Formation and reactions of sulfenic acids in proteins. Acc Chem Res. 1976;9:293–299. [Google Scholar]
- 28.Lin WS, Armstrong DA, Gaucher GM. Formation and repair of papain sulfenic acid. Can J Biochem. 1975;53:298–307. doi: 10.1139/o75-042. [DOI] [PubMed] [Google Scholar]
- 29.Poole LB, Claiborne A. The non-flavin redox center of the streptococcal NADH peroxidase. II. Evidence for a stabilized cysteine-sulfenic acid. J Biol Chem. 1989;264:12330–12338. [PubMed] [Google Scholar]
- 30.Storz G, Tartaglia LA, Ames BN. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science. 1990;248:189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- 31.Truong TH, Ung PM, Palde PB, Paulsen CE, Schlessinger A, Carroll KS. Molecular basis for redox activation of epidermal growth factor receptor kinase. Cell Chem Biol. 2016;23:837–848. doi: 10.1016/j.chembiol.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou H, Singh H, Parsons ZD, Lewis SM, Bhattacharya S, Seiner DR, LaButti JN, Reilly TJ, Tanner JJ, Gates KS. The biological buffer bicarbonate/CO2 potentiates H2O2-mediated inactivation of protein tyrosine phosphatases. J Am Chem Soc. 2011;133:15803–15805. doi: 10.1021/ja2077137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peralta D, Bronowska AK, Morgan B, Doka E, Van Laer K, Nagy P, Grater F, Dick TP. A proton relay enhances H2O2 sensitivity of GAPDH to facilitate metabolic adaptation. Nat Chem Biol. 2015;11:156–163. doi: 10.1038/nchembio.1720. [DOI] [PubMed] [Google Scholar]
- 34.Fourquet S, Guerois R, Biard D, Toledano MB. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J Biol Chem. 2010;285:8463–8471. doi: 10.1074/jbc.M109.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki T, Yamamoto M. Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress. J Biol Chem. 2017;292:16817–16824. doi: 10.1074/jbc.R117.800169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albertolle M, Phan TTN, Pozzi A, Guengerich FP. Sulfenylation of human liver and kidney microsomal cytochromes P450 and other drug metabolizing enzymes as a response to redox alteration. Mol Cell Proteomics. 2018 doi: 10.1074/mcp.RA117.000382. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albertolle ME, Kim D, Nagy LD, Yun CH, Pozzi A, Savas U, Johnson EF, Guengerich FP. Heme-thiolate sulfenylation of human cytochrome P450 4A11 functions as a redox switch for catalytic inhibition. J Biol Chem. 2017;292:11230–11242. doi: 10.1074/jbc.M117.792200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J, Carroll KS, Liebler DC. The expanding landscape of the thiol redox proteome. Mol Cell Proteomics. 2016;15:1–11. doi: 10.1074/mcp.O115.056051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alcock LJ, Perkins MV, Chalker JM. Chemical methods for mapping cysteine oxidation. Chem Soc Rev. 2018;47:231–268. doi: 10.1039/c7cs00607a. [DOI] [PubMed] [Google Scholar]
- 40.Gupta V, Paritala H, Carroll KS. Reactivity, selectivity, and stability in sulfenic acid detection: a comparative study of nucleophilic and electrophilic probes. Bioconjug Chem. 2016;27:1411–1418. doi: 10.1021/acs.bioconjchem.6b00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sasame HA, Mitchell JR, Thorgeirsson S, Gillette JR. Relationship between NADH and NADPH oxidation during drug metabolism. Drug Metab Dispos. 1973;1:150–155. [PubMed] [Google Scholar]
- 42.Nordblom GD, Coon MJ. Hydrogen peroxide formation and stoichiometry of hydroxylation reactions catalyzed by highly purified liver microsomal cytochrome P-450. Arch Biochem Biophys. 1977;180:343–347. doi: 10.1016/0003-9861(77)90047-9. [DOI] [PubMed] [Google Scholar]
- 43.Gorsky LD, Koop DR, Coon MJ. On the stoichiometry of the oxidase and monooxygenase reactions catalyzed by liver microsomal cytochrome P-450: Products of oxygen reduction. J Biol Chem. 1984;259:6812–6817. [PubMed] [Google Scholar]
- 44.Aust SD, Roerig DL, Pederson TC. Evidence for superoxide generation by NADPH-cytochrome c reductase of rat liver microsomes. Biochem and Biophys Res Comm. 1972;47:1133–1137. doi: 10.1016/0006-291x(72)90952-7. [DOI] [PubMed] [Google Scholar]
- 45.Sligar SG, Lipscomb JD, Debrunner PG, Gunsalus IC. Superoxide anion production by the autoxidation of cytochrome P450cam. Biochem Biophys Res Commun. 1974;61:290–296. doi: 10.1016/0006-291x(74)90565-8. [DOI] [PubMed] [Google Scholar]
- 46.Di Luzio NR, Hartman AD. Role of lipid peroxidation in the pathogenesis of the ethanol-induced fatty liver. Fed Proc. 1967;26:1436–1442. [PubMed] [Google Scholar]
- 47.Ekstrom G, Ingelman-Sundberg M. Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1) Biochem Pharmacol. 1989;38:1313–1319. doi: 10.1016/0006-2952(89)90338-9. [DOI] [PubMed] [Google Scholar]
- 48.Ekstrom G, von Bahr C, Ingelman-Sundberg M. Human liver microsomal cytochrome P-450IIE1. Immunological evaluation of its contribution to microsomal ethanol oxidation, carbon tetrachloride reduction and NADPH oxidase activity. Biochem Pharmacol. 1989;38:689–693. doi: 10.1016/0006-2952(89)90217-7. [DOI] [PubMed] [Google Scholar]
- 49.Cederbaum AI. Liver Pathophysiology. Academic Press; Boston: 2017. Chapter 31 - Cytochrome P450 and oxidative stress in the liver; pp. 401–419. [Google Scholar]
- 50.Sharrock M, Debrunner PG, Schulz C, Lipscomb JD, Marshall V, Gunsalus IC. Cytochrome P450cam and its complexes. Mössbauer parameters of the heme iron. Biochim Biophys Acta. 1976;420:8–26. doi: 10.1016/0005-2795(76)90340-8. [DOI] [PubMed] [Google Scholar]
- 51.Harris D, Loew G, Waskell L. Calculation of the electronic structure and spectra of model cytochrome P450 compound I. J Inorg Biochem. 2001;83:309–318. doi: 10.1016/s0162-0134(00)00177-x. [DOI] [PubMed] [Google Scholar]
- 52.Denisov IG, Grinkova YV, Baas BJ, Sligar SG. The ferrous-dioxygen intermediate in human cytochrome P450 3A4. Substrate dependence of formation and decay kinetics. J Biol Chem. 2006;281:23313–23318. doi: 10.1074/jbc.M605511200. [DOI] [PubMed] [Google Scholar]
- 53.Eisenstein L, Debey P, Douzou P. P450cam: Oxygenated complexes stabilized at low temperature. Biochem Biophys Res Commun. 1977;77:1377–1383. doi: 10.1016/s0006-291x(77)80131-9. [DOI] [PubMed] [Google Scholar]
- 54.Sevrioukova IF, Peterson JA. Reaction of carbon monoxide and molecular oxygen with P450terp (CYP108) and P450BM-3 (CYP102) Arch Biochem Biophys. 1995;317:397–404. doi: 10.1006/abbi.1995.1180. [DOI] [PubMed] [Google Scholar]
- 55.Yoshioka S, Tosha T, Takahashi S, Ishimori K, Hori H, Morishima I. Roles of the proximal hydrogen bonding network in cytochrome P450cam-catalyzed oxygenation. J Am Chem Soc. 2002;124:14571–14579. doi: 10.1021/ja0265409. [DOI] [PubMed] [Google Scholar]
- 56.Galinato MG, Spolitak T, Ballou DP, Lehnert N. Elucidating the role of the proximal cysteine hydrogen-bonding network in ferric cytochrome P450cam and corresponding mutants using magnetic circular dichroism spectroscopy. Biochemistry. 2011;50:1053–1069. doi: 10.1021/bi101911y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yosca TH, Ledray AP, Ngo J, Green MT. A new look at the role of thiolate ligation in cytochrome P450. J Biol Inorg Chem. 2017;22:209–220. doi: 10.1007/s00775-016-1430-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang Q, Deshmukh RS, Ericksen SS, Tu Y, Szklarz GD. Preferred binding orientations of phenacetin in CYP1A1 and CYP1A2 are associated with isoform-selective metabolism. Drug Metab Dispos. 2012;40:2324–2331. doi: 10.1124/dmd.112.047308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mayuzumi H, Sambongi C, Hiroya K, Shimizu T, Tateishi T, Hatano M. Effect of mutations of ionic amino acids of cytochrome P450 1A2 on catalytic activities toward 7-ethoxycoumarin and methanol. Biochemistry. 1993;32:5622–5628. doi: 10.1021/bi00072a018. [DOI] [PubMed] [Google Scholar]
- 60.Yun CH, Kim KH, Calcutt MW, Guengerich FP. Kinetic analysis of oxidation of coumarins by human cytochrome P450 2A6. J Biol Chem. 2005;280:12279–12291. doi: 10.1074/jbc.M411019200. [DOI] [PubMed] [Google Scholar]
- 61.Bumpus NN, Hollenberg PF. Investigation of the mechanisms underlying the differential effects of the K262R mutation of P450 2B6 on catalytic activity. Mol Pharmacol. 2008;74:990–999. doi: 10.1124/mol.108.048637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mosher CM, Hummel MA, Tracy TS, Rettie AE. Functional analysis of phenylalanine residues in the active site of cytochrome P450 2C9. Biochemistry. 2008;47:11725–11734. doi: 10.1021/bi801231m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guengerich FP, Miller GP, Hanna IH, Sato H, Martin MV. Oxidation of methoxyphenethylamines by cytochrome P450 2D6. Analysis of rate-limiting steps. J Biol Chem. 2002;277:33711–33719. doi: 10.1074/jbc.M205146200. [DOI] [PubMed] [Google Scholar]
- 64.Hanna IH, Krauser JA, Cai H, Kim MS, Guengerich FP. Diversity in mechanisms of substrate oxidation by cytochrome P450 2D6. Lack of an allosteric role of NADPH-cytochrome P450 reductase in catalytic regioselectivity. J Biol Chem. 2001;276:39553–39561. doi: 10.1074/jbc.M106841200. [DOI] [PubMed] [Google Scholar]
- 65.Patten CJ, Koch P. Baculovirus expression of human P450 2E1 and cytochrome b5: Spectral and catalytic properties and effect of b5 on the stoichiometry of P450 2E1-catalyzed reactions. Arch Biochem Biophys. 1995;317:504–513. doi: 10.1006/abbi.1995.1194. [DOI] [PubMed] [Google Scholar]
- 66.McDougle DR, Palaria A, Magnetta E, Meling DD, Das A. Functional studies of N-terminally modified CYP2J2 epoxygenase in model lipid bilayers. Protein Sci. 2013;22:964–979. doi: 10.1002/pro.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grinkova YV, Denisov IG, McLean MA, Sligar SG. Oxidase uncoupling in heme monooxygenases: Human cytochrome P450 CYP3A4 in nanodiscs. Biochem Biophys Res Commun. 2013;430:1223–1227. doi: 10.1016/j.bbrc.2012.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim D, Cha GS, Nagy LD, Yun CH, Guengerich FP. Kinetic analysis of lauric acid hydroxylation by human cytochrome P450 4A11. Biochemistry. 2014;53:6161–6172. doi: 10.1021/bi500710e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peng HM, Im SC, Pearl NM, Turcu AF, Rege J, Waskell L, Auchus RJ. Cytochrome b5 activates the 17,20-lyase activity of human cytochrome P450 17A1 by increasing the coupling of NADPH consumption to androgen production. Biochemistry. 2016;55:4356–4365. doi: 10.1021/acs.biochem.6b00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khatri Y, Gregory MC, Grinkova YV, Denisov IG, Sligar SG. Active site proton delivery and the lyase activity of human CYP17A1. Biochem Biophys Res Commun. 2014;443:179–184. doi: 10.1016/j.bbrc.2013.11.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sohl CD, Guengerich FP. Kinetic analysis of the three-step steroid aromatase reaction of human cytochrome P450 19A1. J Biol Chem. 2010;285:17734–17743. doi: 10.1074/jbc.M110.123711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 73.Veith A, Moorthy B. Role of cytochrome P450s in the generation and metabolism of reactive oxygen species. Curr Opin Toxicol. 2018;7:44–51. doi: 10.1016/j.cotox.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bansal S, Anandatheerthavarada HK, Prabu GK, Milne GL, Martin MV, Guengerich FP, Avadhani NG. Human cytochrome P450 2E1 mutations that alter mitochondrial targeting efficiency and susceptibility to ethanol-induced toxicity in cellular models. J Biol Chem. 2013;288:12627–12644. doi: 10.1074/jbc.M113.452367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bansal S, Liu CP, Sepuri NB, Anandatheerthavarada HK, Selvaraj V, Hoek J, Milne GL, Guengerich FP, Avadhani NG. Mitochondria-targeted cytochrome P450 2E1 induces oxidative damage and augments alcohol-mediated oxidative stress. J Biol Chem. 2010;285:24609–24619. doi: 10.1074/jbc.M110.121822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Imaoka S, Osada M, Minamiyama Y, Yukimura T, Toyokuni S, Takemura S, Hiroi T, Funae Y. Role of phenobarbital-inducible cytochrome P450s as a source of active oxygen species in DNA-oxidation. Cancer Lett. 2004;203:117–125. doi: 10.1016/j.canlet.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 77.Puntarulo S, Cederbaum AI. Production of reactive oxygen species by microsomes enriched in specific human cytochrome P450 enzymes. Free Radic Biol Med. 1998;24:1324–1330. doi: 10.1016/s0891-5849(97)00463-2. [DOI] [PubMed] [Google Scholar]
- 78.Roberts BJ, Song BJ, Soh Y, Park SS, Shoaf SE. Ethanol induces CYP2E1 by protein stabilization. Role of ubiquitin conjugation in the rapid degradation of CYP2E1. J Biol Chem. 1995;270:29632–29635. doi: 10.1074/jbc.270.50.29632. [DOI] [PubMed] [Google Scholar]
- 79.Sun X, Ai M, Wang Y, Shen S, Gu Y, Jin Y, Zhou Z, Long Y, Yu Q. Selective induction of tumor cell apoptosis by a novel P450-mediated reactive oxygen species (ROS) inducer methyl 3-(4-nitrophenyl) propiolate. J Biol Chem. 2013;288:8826–8837. doi: 10.1074/jbc.M112.429316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tang Y, Scheef EA, Gurel Z, Sorenson CM, Jefcoate CR, Sheibani N. CYP1B1 and endothelial nitric oxide synthase combine to sustain proangiogenic functions of endothelial cells under hyperoxic stress. Am J Physiol Cell Physiol. 2010;298:C665–678. doi: 10.1152/ajpcell.00153.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dostalek M, Brooks JD, Hardy KD, Milne GL, Moore MM, Sharma S, Morrow JD, Guengerich FP. In vivo oxidative damage in rats is associated with barbiturate response but not other cytochrome P450 inducers. Mol Pharmacol. 2007;72:1419–1424. doi: 10.1124/mol.107.040238. [DOI] [PubMed] [Google Scholar]
- 82.Dostalek M, Hardy KD, Milne GL, Morrow JD, Chen C, Gonzalez FJ, Gu J, Ding X, Johnson DA, Johnson JA, Martin MV, Guengerich FP. Development of oxidative stress by cytochrome P450 induction in rodents is selective for barbiturates and related to loss of pyridine nucleotide-dependent protective systems. J Biol Chem. 2008;283:17147–17157. doi: 10.1074/jbc.M802447200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Couroucli XI, Liang YH, Jiang W, Wang L, Barrios R, Yang P, Moorthy B. Prenatal administration of the cytochrome P4501A inducer, β-naphthoflavone (BNF), attenuates hyperoxic lung injury in newborn mice: implications for bronchopulmonary dysplasia (BPD) in premature infants. Toxicol Appl Pharmacol. 2011;256:83–94. doi: 10.1016/j.taap.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maturu P, Wei-Liang Y, Jiang W, Wang L, Lingappan K, Barrios R, Liang Y, Moorthy B, Couroucli XI. Newborn mice lacking the gene for cyp1a1 are more susceptible to oxygen-mediated lung injury, and are rescued by postnatal β-naphthoflavone administration: implications for bronchopulmonary dysplasia in premature infants. Toxicol Sci. 2017;157:260–271. doi: 10.1093/toxsci/kfx036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, 2nd, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kalyanaraman B, Hardy M, Podsiadly R, Cheng G, Zielonka J. Recent developments in detection of superoxide radical anion and hydrogen peroxide: opportunities, challenges, and implications in redox signaling. Arch Biochem Biophys. 2017;617:38–47. doi: 10.1016/j.abb.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kadiiska MB, Gladen BC, Baird DD, Graham LB, Parker CE, Ames BN, Basu S, Fitzgerald GA, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ, 2nd, Rokach J, Shigenaga MK, Sun J, Walter PB, Tomer KB, Barrett JC, Mason RP. Biomarkers of oxidative stress study III. Effects of the nonsteroidal anti-inflammatory agents indomethacin and meclofenamic acid on measurements of oxidative products of lipids in CCl4 poisoning. Free Radic Biol Med. 2005;38:711–718. doi: 10.1016/j.freeradbiomed.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 88.Persson JO, Terelius Y, Ingelman-Sundberg M. Cytochrome P-450-dependent formation of reactive oxygen radicals: isozyme-specific inhibition of P-450-mediated reduction of oxygen and carbon tetrachloride. Xenobiotica. 1990;20:887–900. doi: 10.3109/00498259009046904. [DOI] [PubMed] [Google Scholar]
- 89.Cederbaum AI. Role of CYP2E1 in ethanol-induced oxidant stress, fatty liver and hepatotoxicity. Dig Dis. 2010;28:802–811. doi: 10.1159/000324289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- 91.Emanuele S, D’Anneo A, Calvaruso G, Cernigliaro C, Giuliano M, Lauricella M. The double-edged sword profile of redox signaling: oxidative events as molecular switches in the balance between cell physiology and cancer. Chem Res Toxicol. 2018;31:201–210. doi: 10.1021/acs.chemrestox.7b00311. [DOI] [PubMed] [Google Scholar]
- 92.Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts LJ., II Non-cyclooxygenase- derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc Natl Acad Sci USA. 1992;89:10721–10725. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hrycay EG, Gustafsson JA, Ingelman-Sundberg M, Ernster L. Sodium periodate, sodium chlorite, and organic hydroperoxides as hydroxylating agents in hepatic microsomal steroid hydroxylation reactions catalyzed by cytochrome P-450. FEBS Lett. 1975;56:161–165. doi: 10.1016/0014-5793(75)80132-3. [DOI] [PubMed] [Google Scholar]
- 94.Nordblom GD, White RE, Coon MJ. Studies on hydroperoxide-dependent substrate hydroxylation by purified liver microsomal cytochrome P-450. Arch Biochem Biophys. 1976;175:524–533. doi: 10.1016/0003-9861(76)90541-5. [DOI] [PubMed] [Google Scholar]
- 95.Shoji O, Watanabe Y. Peroxygenase reactions catalyzed by cytochromes P450. J Biol Inorg Chem. 2014;19:529–539. doi: 10.1007/s00775-014-1106-9. [DOI] [PubMed] [Google Scholar]
- 96.Lichtenberger F, Nastainczyk W, Ullrich V. Cytochrome P450 as an oxene transferase. Biochem Biophys Res Commun. 1976;70:939–946. doi: 10.1016/0006-291x(76)90682-3. [DOI] [PubMed] [Google Scholar]
- 97.Hrycay EG, Gustafsson JA, Ingelman-Sundberg M, Ernster L. Sodium periodate, sodium chloride, organic hydroperoxides, and H2O2 as hydroxylating agents in steroid hydroxylation reactions catalyzed by partially purified cytochrome P-450. Biochem Biophys Res Commun. 1975;66:209–216. doi: 10.1016/s0006-291x(75)80315-9. [DOI] [PubMed] [Google Scholar]
- 98.Rittle J, Green MT. Cytochrome P450 Compound I: capture, characterization, and C-H bond activation kinetics. Science. 2010;330:933–937. doi: 10.1126/science.1193478. [DOI] [PubMed] [Google Scholar]
- 99.O'Brien JP, Rahimtula A. Involvement of cytochrome P-450 in the intracellular formation of lipid peroxides. J Agric Food Chem. 1975;23:154–158. doi: 10.1021/jf60198a045. [DOI] [PubMed] [Google Scholar]
- 100.Plastaras JP, Guengerich FP, Nebert DW, Marnett LJ. Xenobiotic-metabolizing cytochromes P450 convert prostaglandin endoperoxide to hydroxyheptadecatrienoic acid and the mutagen, malondialdehyde. J Biol Chem. 2000;275:11784–11790. doi: 10.1074/jbc.275.16.11784. [DOI] [PubMed] [Google Scholar]
- 101.Chefson A, Zhao J, Auclair K. Replacement of natural cofactors by selected hydrogen peroxide donors or organic peroxides results in improved activity for CYP3A4 and CYP2D6. Chembiochem. 2006;7:916–919. doi: 10.1002/cbic.200600006. [DOI] [PubMed] [Google Scholar]
- 102.Muindi JF, Young CW. Lipid hydroperoxides greatly increase the rate of oxidative catabolism of all-trans-retinoic acid by human cell culture microsomes genetically enriched in specified cytochrome P-450 isoforms. Cancer Res. 1993;53:1226–1229. [PubMed] [Google Scholar]
- 103.Tan L, Wang HM, Falardeau P. 17α-Hydroperoxypregnenes. 3. Studies on their role as possible precursors in the biosynthesis of adrenosteroid hormones. Biochim Biophys Acta. 1972;260:731–740. doi: 10.1016/0005-2760(72)90022-7. [DOI] [PubMed] [Google Scholar]
- 104.van Lier JE, Mast N, Pikuleva IA. Cholesterol hydroperoxides as substrates for cholesterol-metabolizing cytochrome P450 enzymes and alternative sources of 25-hydroxycholesterol and other oxysterols. Angew Chem Int Ed. 2015;54:11138–11142. doi: 10.1002/anie.201505002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ademowo OS, Dias HKI, Burton DGA, Griffiths HR. Lipid (per) oxidation in mitochondria: an emerging target in the ageing process? Biogerontology. 2017;18:859–879. doi: 10.1007/s10522-017-9710-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Renneberg R, Scheller F, Ruckpaul K, Pirrwitz J, Mohr P. NADPH and H2O2-dependent reactions of cytochrome P-450LM compared with peroxidase catalysis. FEBS Lett. 1978;96:349–353. doi: 10.1016/0014-5793(78)80434-7. [DOI] [PubMed] [Google Scholar]
- 107.Fujishiro T, Shoji O, Nagano S, Sugimoto H, Shiro Y, Watanabe Y. Crystal structure of H2O2-dependent cytochrome P450SPα with its bound fatty acid substrate: insight into the regioselective hydroxylation of fatty acids at the alpha position. J Biol Chem. 2011;286:29941–29950. doi: 10.1074/jbc.M111.245225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Belcher J, McLean KJ, Matthews S, Woodward LS, Fisher K, Rigby SE, Nelson DR, Potts D, Baynham MT, Parker DA, Leys D, Munro AW. Structure and biochemical properties of the alkene producing cytochrome P450 OleTJE (CYP152L1) from the Jeotgalicoccus sp. 8456 bacterium. J Biol Chem. 2014;289:6535–6550. doi: 10.1074/jbc.M113.527325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hsieh CH, Makris TM. Expanding the substrate scope and reactivity of cytochrome P450 OleT. Biochem Biophys Res Commun. 2016;476:462–466. doi: 10.1016/j.bbrc.2016.05.145. [DOI] [PubMed] [Google Scholar]
- 110.Rude MA, Baron TS, Brubaker S, Alibhai M, Del Cardayre SB, Schirmer A. Terminal olefin (1-alkene) biosynthesis by a novel P450 fatty acid decarboxylase from Jeotgalicoccus species. Appl Environ Microbiol. 2011;77:1718–1727. doi: 10.1128/AEM.02580-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cirino PC, Arnold FH. A self-sufficient peroxide-driven hydroxylation biocatalyst. Angew Chem Int Ed. 2003;42:3299–3301. doi: 10.1002/anie.200351434. [DOI] [PubMed] [Google Scholar]
- 112.Lundemo MT, Woodley JM. Guidelines for development and implementation of biocatalytic P450 processes. Appl Microbiol Biotechnol. 2015;99:2465–2483. doi: 10.1007/s00253-015-6403-x. [DOI] [PubMed] [Google Scholar]
- 113.Ma N, Chen Z, Chen J, Chen J, Wang C, Zhou H, Yao L, Shoji O, Watanabe Y, Cong Z. Dual-functional small molecules for generating an efficient cytochrome P450BM3 peroxygenase. Angew Chem Int Ed. 2018 doi: 10.1002/anie.201801592. [DOI] [PubMed] [Google Scholar]
- 114.Girhard M, Kunigk E, Tihovsky S, Shumyantseva VV, Urlacher VB. Light-driven biocatalysis with cytochrome P450 peroxygenases. Biotechnol Appl Biochem. 2013;60:111–118. doi: 10.1002/bab.1063. [DOI] [PubMed] [Google Scholar]
- 115.Wang Y, Lan D, Durrani R, Hollmann F. Peroxygenases en route to becoming dream catalysts. What are the opportunities and challenges? Curr Opin Chem Biol. 2017;37:1–9. doi: 10.1016/j.cbpa.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 116.Joo H, Lin Z, Arnold FH. Laboratory evolution of peroxide-mediated cytochrome P450 hydroxylation. Nature. 1999;399:670–673. doi: 10.1038/21395. [DOI] [PubMed] [Google Scholar]
- 117.Wei Y, Ang EL, Zhao H. Recent developments in the application of P450 based biocatalysts. Curr Opin Chem Biol. 2017;43:1–7. doi: 10.1016/j.cbpa.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 118.Guengerich FP. Destruction of heme and hemoproteins mediated by liver microsomal reduced nicotinamide adenine dinucleotide phosphate-cytochrome P-450 reductase. Biochemistry. 1978;17:3633–3639. doi: 10.1021/bi00610a033. [DOI] [PubMed] [Google Scholar]
- 119.Hrycay EG, O'Brien PJ. Cytochrome P-450 as a microsomal peroxidase utilizing a lipid peroxide substrate. Arch Biochem Biophys. 1971;147:14–27. doi: 10.1016/0003-9861(71)90304-3. [DOI] [PubMed] [Google Scholar]
- 120.Sevrioukova IF. High-level production and properties of the cysteine-depleted cytochrome P450 3A4. Biochemistry. 2017;56:3058–3067. doi: 10.1021/acs.biochem.7b00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schmith VD, Foss JF. Inflammation: planning for a source of pharmacokinetic/pharmacodynamic variability in translational studies. Clin Pharmacol Ther. 2010;87:488–491. doi: 10.1038/clpt.2009.258. [DOI] [PubMed] [Google Scholar]
- 122.Lang CC, Brown RM, Kinirons MT, Deathridge MA, Guengerich FP, Kelleher D, O'Briain DS, Ghishan FK, Wood AJ. Decreased intestinal CYP3A in celiac disease: reversal after successful gluten-free diet: a potential source of interindividual variability in first-pass drug metabolism. Clin Pharmacol Ther. 1996;59:41–46. doi: 10.1016/S0009-9236(96)90022-3. [DOI] [PubMed] [Google Scholar]
- 123.Ling S, Lewanczuk RZ, Russell AS, Ihejirika B, Jamali F. Influence of controlled rheumatoid arthritis on the action and disposition of verapamil: focus on infliximab. J Clin Pharmacol. 2009;49:301–311. doi: 10.1177/0091270008328099. [DOI] [PubMed] [Google Scholar]
- 124.Frye RF, Schneider VM, Frye CS, Feldman AM. Plasma levels of TNF-α and IL-6 are inversely related to cytochrome P450-dependent drug metabolism in patients with congestive heart failure. J Card Fail. 2002;8:315–319. doi: 10.1054/jcaf.2002.127773. [DOI] [PubMed] [Google Scholar]
- 125.Coutant DE, Hall SD. disease-drug interactions in inflammatory states via effects on CYP-mediated drug clearance. J Clin Pharmacol. 2018 doi: 10.1002/jcph.1093. in press. [DOI] [PubMed] [Google Scholar]
- 126.Hoffmann MH, Griffiths HR. The dual role of ROS in autoimmune and inflammatory diseases: Evidence from preclinical models. Free Radic Biol Med. 2018 doi: 10.1016/j.freeradbiomed.2018.03.016. in press. [DOI] [PubMed] [Google Scholar]
- 127.Lee CM, Pohl J, Morgan ET. Dual mechanisms of CYP3A protein regulation by proinflammatory cytokine stimulation in primary hepatocyte cultures. Drug Metab Dispos. 2009;37:865–872. doi: 10.1124/dmd.108.026187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee CM, Tripathi S, Morgan ET. Nitric oxide-regulated proteolysis of human CYP2B6 via the ubiquitin-proteasome system. Free Radic Biol Med. 2017;108:478–486. doi: 10.1016/j.freeradbiomed.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Holmgren A, Johansson C, Berndt C, Lonn ME, Hudemann C, Lillig CH. Thiol redox control via thioredoxin and glutaredoxin systems. Biochem Soc Trans. 2005;33:1375–1377. doi: 10.1042/BST0331375. [DOI] [PubMed] [Google Scholar]
- 130.Johansson C, Lillig CH, Holmgren A. Human mitochondrial glutaredoxin reduces S-glutathionylated proteins with high affinity accepting electrons from either glutathione or thioredoxin reductase. J Biol Chem. 2004;279:7537–7543. doi: 10.1074/jbc.M312719200. [DOI] [PubMed] [Google Scholar]
- 131.Mailloux RJ, Treberg JR. Protein S-glutathionlyation links energy metabolism to redox signaling in mitochondria. Redox Biol. 2016;8:110–118. doi: 10.1016/j.redox.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]