Abstract
This longitudinal pilot study examined whether baseline resting frontal electroencephalographic (EEG) asymmetry correlates with depressive symptoms during the most impaired two-week period in the following year. Current-source-density (CSD) transformed resting frontal EEG asymmetry, severity of depression symptoms (Beck Depression Inventory – II), and stress (indexed by negative life events; NLE) were recorded in never-depressed young adults with no current DSM-IV diagnosis (38 women, 16 men) at baseline. One year later, depression symptoms and NLEs experienced during the interim were assessed. Individuals who reported greater interim NLEs also endorsed interim higher depression symptoms, a pattern that replicated when first accounting for baseline stress and depression. For women, higher depression reported at follow-up was linked to lower left than right frontal EEG activity at baseline, a pattern that replicated when first accounting for depressive symptoms at baseline. Despite the modest sample size of the present analysis, findings are consistent with prior reports of sex differences in patterns of brain laterality and support the idea that CSD-referenced EEG asymmetry may be a risk marker for future depression in previously healthy young women.
Keywords: electroencephalography, frontal asymmetry, depression, stress, longitudinal design
Introduction
Given that major depressive disorder (MDD) is linked to severe impairment and chronic symptom recurrence, creating substantial burden both economically and personally (Burcusa & Iacono, 2007; Greenberg et al., 2003; Judd, Akiskal et al., 2000; Judd, Paulus et al., 2000; Michaud et al., 2006), scientific research focused on the pathways from risk to symptom expression is a priority. With respect to candidate biological markers of MDD, a growing literature demonstrates that a pattern of relatively lower left than right frontal electroencephalographic (EEG) activity at rest (indexed by relatively greater right-than-left frontal alpha-band activity) differentiates individuals with a lifetime history of depression (MDD+) from non-depressed individuals (MDD−) (e.g., Allen, Urry, Hitt, & Coan, 2004; Bruder et al., 2005; Jaworska, Blier, Fusee, & Knott, 2012; Kemp et al., 2010; Stewart, Bismark, Towers, Coan, & Allen, 2010; Thibodeau, Jorgensen, & Kim, 2006). Moreover, this EEG asymmetry is modestly stable over time in both MDD+ and MDD− (e.g., Allen, Urry, et al., 2004; Hagemann, Naumann, Thayer, & Bartussek, 2002; Hagemann, Hewig, Seifert, Naumann, & Bartussek, 2005). Although these findings are consistent with the hypothesis that resting EEG asymmetry may index risk for future depression, definitive prospective studies remain to be conducted.
Frontal asymmetry findings as a function of depression status have proven somewhat inconsistent (e.g., Reid et al., 2008; Segrave et al., 2011), although the inconsistencies are thought to be at least partly attributable to methodological differences in EEG recording, depression assessments, and presence of comorbid psychopathology, thereby complicating interpretation (see Davidson, 1998, Hagemann, 2004, and Stewart et al., 2010 for discussion). Moreover, although the neural differentiation of those with a history of MDD (MDD+) versus with no history (MDD−) groups is a promising start in the search for markers of depression risk, viable risk indicators should also be able to provide clinical utility in sharing variance with future depressive symptoms and/or MDD onset/relapse within those who are vulnerable. A further complication is that risk markers correlating with first-episode MDD might be different from risk indicators of recurrent or past MDD after one has already experienced a period of depressive symptoms (Burcusa & Iacono, 2007). For instance, prior work suggests that life stress is a stronger predictor of first episodes than recurrent episodes of MDD (e.g., Lewinsohn, Allen, Seeley, & Gotlib, 1999; Monroe & Harkness, 2005). The question arises, then, whether resting frontal asymmetry not only distinguishes depressed from non-depressed individuals, but also is prospectively associated with future depressive symptoms and/or episodes of MDD in MDD− individuals as well as identifying those who have already experienced MDD.
Few studies address the prospective utility of prefrontal brain asymmetry relating to future depression, and available longitudinal findings provide somewhat conflicting results. For instance, within a twin sample that was not assessed for MDD status, lower left than right frontal brain activity was associated with future risk of depression, but only in women (Smit, Posthuma, Boomsma, & De Geus, 2007). With respect to resting EEG activity forecasting recurrent depressive symptoms in MDD+, extant research indicates that frontal asymmetry is not related to MDD status or number of depressive symptoms within two (Allen, Urry et al., 2004) or six (McFarland, Shankman, Tenke, Bruder, & Klein, 2006) month follow-up periods in MDD+ individuals. Unlike null findings for MDD+, however, results for MDD− are more promising and warrant further examination. Although one study suggests that frontal asymmetry is not correlated with depressive symptoms in MDD− college students one year later (Blackhart, Minnix, & Kline, 2006), no clinical interview was performed to determine presence or absence of DSM-IV (American Psychiatric Association, 1994) disorders at baseline, so it is possible that participants had symptoms that could have influenced null results. In contrast, lower left than right frontal activity at rest is linked to future depression symptoms one year later in two adolescent MDD− samples after controlling for baseline depressive symptoms (Mitchell & Pössel, 2012; Pössel, Lo, Fritz, & Seemann, 2008), and prospectively is related to self-reported freshman-year home-sickness (Steiner & Coan, 2011). Furthermore, lower left than right resting frontal activity is associated with first-episode MDD onset within three years in 40 MDD− adults thought to be at risk for developing mood disorders: 3 participants subsequently met criteria for a major depressive episode and 10 met criteria for a minor depressive episode during this period (Nusslock et al., 2011). Within this sample, lower relative left frontal activity also correlates with higher depressive symptoms at three year follow-up when accounting for depressive symptoms at baseline. Collectively, these prospective studies differ in number of EEG visits, length of EEG recording, type of EEG reference and follow-up assessment of depressive symptoms, any of which may have contributed to the production of differential results. The research to date thus indicates that frontal EEG asymmetry could be a risk indicator for first- onset escalation of depressive symptoms in MDD−, although additional studies are warranted to investigate this issue.
The present investigation attempted to replicate and extend prior work on the prospective value of frontal asymmetry at baseline, examining whether it relates to future depressive symptoms over the next year within a sizeable sample of MDD− adults, incorporating several methodological improvements including: (1) use of the current source density (CSD) transformation (Kayser & Tenke, 2006; Perrin, Pernier, Bertrand, & Echallier, 1989, 1990) to reduce contributions of non-frontal sources to frontal asymmetry scores; (2) aggregation across several sessions and minutes of EEG recording to derive reliable estimates of trait asymmetry; and, (3) inclusion of life stress as a potential moderator of the prospective utility of EEG asymmetry. The CSD transformation is advantageous as a reference-free algorithm that greatly diminishes volume conduction contributions to EEG alpha power and, in contrast to conventional scalp EEG reference measures, results in unambiguous indices of current sources underlying EEG topography (Tenke & Kayser, 2012). Furthermore, findings indicate that CSD- transformed resting frontal asymmetry differentiates MDD+ and MDD− more robustly than average reference, Cz-reference, and linked-mastoid reference montages traditionally used in the EEG asymmetry literature (Stewart et al., 2010; Stewart, Coan, Towers, & Allen, 2014).
Given that relating frontal asymmetry to categorical outcomes such as future MDD+ versus MDD− status would require large sample sizes, extended follow-up periods, and preidentified high-risk samples, one alternative to MDD categorization is to examine continuous measures of depressive symptom severity as the primary outcome measure. In line with this rationale, research demonstrates that higher depressive symptoms measured dimensionally are correlated with first-onset MDD+ (Horwath, Johnson, Klerman, & Weissman, 1992; Lewinsohn et al., 1999).
On the basis of three studies demonstrating the association of frontal EEG asymmetry with future depressive symptoms (Mitchell & Pössel, 2012; Nusslock et al., 2011; Pössel et al., 2008), it was hypothesized relatively less left than right frontal activity in young adults with no history of MDD and free of any current DSM disorders (MDD−) at baseline would be associated with higher depressive symptoms for the most impairing two-week period within the following year, even after controlling for depressive symptoms at baseline. Given that the relationship between depression and frontal EEG asymmetry appears to be stronger in women than men (e.g., Smit et al., 2007), biological sex was included as a relevant variable in analyses. In addition, exploratory analyses examined relationships between baseline frontal EEG asymmetry, depressive symptoms over the next year, and number of stressful life events experienced between baseline and one-year follow-up, given that prior work shows that stress is linked to first-onset MDD+ (Lewinsohn et al., 1999; Monroe & Harkness, 2005).
Materials and Methods
Participants
The study protocol was approved by the local Human Research Protections Program. All participants provided verbal and written informed consent in accordance with the Declaration of Helsinki. The sample for this study comprises the 163 never-depressed participants from among the 306 participants reported in Stewart et al. (2010). Right-handed participants were recruited from a pool of over 10,000 individuals on the basis of their scores on the Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961). Individuals either completed the BDI online after learning about the study from campus fliers or during pre-testing in a large introductory psychology class. Participants were recruited to span the full dimensional range of depressive severity for a larger grant study investigating state and trait EEG asymmetry. Selected individuals were then phone screened to query exclusion criteria, which included no history of: head injury with loss of consciousness greater than 10 minutes, concussion, epilepsy, electroshock therapy, use of current psychotropic medications, and active suicidal potential necessitating immediate treatment.
Individuals who passed the phone screen were then invited to the lab for a baseline session wherein they completed the BDI-II (Beck, Steer, & Brown, 1996) as well as the Structured Clinical Interview for DSM-IV (SCID, First, Spitzer, Gibbon, & Williams, 1997) administered by a trained graduate clinical rater. The 163 participants in this study met criteria for no current DSM-IV Axis I disorder and no lifetime diagnosis of major depression (MDD−). Participants passing the screening were invited to participate in four additional EEG visits. The lifetime history negative (MDD−) participants were compared to a group (N=143) of lifetime history positive (MDD+) participants on patterns of frontal brain asymmetry in Stewart et al. (2010) and Stewart et al. (2014), but no MDD+ participants were included in the present analysis.
The original grant-funded study for which the baseline visits were collected did not originally have a follow-up phase planned. Given recently published work demonstrating a link between baseline asymmetry and future depression symptoms in MDD− (e.g., Mitchell & Pössel, 2012; Nusslock et al., 2011; Pössel et al., 2008), we realized that longitudinal data for this project could enable possible replication and extension. After obtaining Institutional Review Board approval to re-contact participants, we called them approximately one year following their last EEG visit and asked if they would be willing to participate in a short follow-up session in exchange for $20 remuneration. As a result, we were able to assess 54 (38 women) of the 163 participants (33.13%) on depressive symptoms (via BDI-II questionnaire and readministration of the SCID MDD module) as well as stressful life events they had experienced since their last EEG visit. No additional EEG data were collected during the follow-up session.
Participants who were reassessed did not differ from those who were not interested with respect to age, socioeconomic status (Hollingshead, 1975), sex, and BDI-II scores at baseline (see Table 1) so it is plausible that they are a representative sample of the larger cohort. With respect to EEG asymmetry data, however, Table 1 indicates that participants who agreed to be followed up exhibited lower relative left frontal EEG activity than those who were not interested, although this was a small effect (Cohen’s d = .04). Among these 54 participants with follow-up SCID MDD symptom data, 3 (6%) met criteria for a current minor (subthreshold) depressive episode (defined as endorsement of 4 symptoms instead of the required 5 for a DSM-IV diagnosis of MDD; see Nusslock et al., 2011). No participants met criteria for a major depressive episode at follow-up. Since the sampling strategy of the parent study from which these individuals were selected (see Stewart et al., 2010) was to identify those with a wide range of depression severity, participants possessed a broad range of baseline BDI-II scores (range = 022; 39% endorsing score > 5).
Table 1.
Demographic information at intake for participants as a function of one year follow-up status.
| Followed Up (n = 54) | Not Followed Up (n = 109) | |||
|---|---|---|---|---|
| M (SD) | M (SD) | Statistic | P | |
| Age (Years) | 18.78 (0.77) | 19.19 (2.01) | t(161)=1.46 | .15 |
| BDI-II | 6.22 (6.45) | 5.96 (5.87) | t(161)= −0.26 | .80 |
| SES | 42.85 (13.78) | 44.52 (13.74) | t(160)=0.73 | .47 |
| Frontal Asymmetry1 | .07 (.08) | .10 (.08) | F(1,2535)=5.09 | .02 |
| Frequency | Frequency | Statistic | P | |
| Sex | 16M, 38F | 40M, 69F | χ2(l)=0.80 | .37 |
Note: BDI-II = Beck Depression Inventory II. SES = socioeconomic status as measured by Hollingshead (1975).
Results from a factorial linear mixed effects model with day (1–4), region (F8-F7, F6-F5, F4-F3, F2-F1), and follow-up group (yes, no) as independent variables and current source density referenced asymmetry score as the dependent variable. This shows the main significant effect for follow-up group; all other interactions included with group were nonsignificant.
Baseline EEG Data Collection and Reduction
Two resting EEG sessions were completed each visit, on four separate days with no fewer than 24 hours between visits, and with all four visits completed within a two-week period. Participants were seated in a sound-attenuated room, separate from the experimenter. Resting EEG was recorded for 8 one minute baselines, in blocks including periods of eyes-open (O) and eyes-closed (C), in one of two counterbalanced orders (OCCOCOOC or COOCOCCO) for 8 minutes per block.
EEG data were collected using a 64-channel NeuroScan Synamps2 amplifier (Charlotte, NC) and acquisition system, utilizing the international 10–20 system for electrode placement. Two electrooculogram (EOG) channels (vertical: superior and inferior orbit of the left eye; lateral: outer canthi) were collected for ocular artifact rejection of resting EEG data. Impedances were maintained under 10K Ohms. Data were collected using 1000 Hz sampling rate, amplified 2816 times, and filtered with a 200 Hz low pass filter prior to digitization.
CSD-Transformed Frontal Asymmetry
EEG data were acquired with an online reference site immediately posterior to Cz and subsequently transformed offline using the reference-free CSD transformation (using algorithms from Kayser & Tenke, 2006, and based on the spherical spline approach summarized by Perrin et al., 1989, 1990). After acquisition, and before CSD transformation, each data file was visually inspected to remove epochs with movement and signal discontinuities. Data reduction was implemented using custom scripts in Matlab (release 2007b, The Mathworks Inc., Natick, MA) and an artifact rejection algorithm rejected segments with large fast deviations in amplitude in any channel (e.g., direct current shifts and spikes) that may have been missed by human inspection. As per convention, a blink rejection algorithm rejected any data segments in the resting EEG data where vertical EOG activity exceeded +/−75 microvolts. Resting data were collected in one-minute EEG blocks, each of which were then epoched into 117 2.048 epochs, overlapping by 1.5 seconds to compensate for the minimal weight applied to the end of the epoch by the use of the Hamming window function. After applying the CSD transform and then the Hamming window, a Fast Fourier Transform (FFT) was applied to all artifact-free epochs. For all 8 minutes of each resting session, total alpha power (8-13 Hz) was extracted from the power spectrum. An asymmetry score was then calculated for total alpha power by subtracting the natural log transformed scores (i.e., ln[Right] – ln[Left]) for each homologous left and right frontal channel pair (F7 & F8, F5 & F6, F3 & F4, F1 & F2). Higher asymmetry score values are commonly believed to reflect relatively greater left activity (i.e., relatively greater right alpha; cf. Allen, Coan, & Nazarian, 2004). Asymmetry scores for each frontal channel pair were computed for each of the four days of recording, averaged across both sessions within day.
One Year Follow-Up Assessment
Participants were asked to identify their worst two-week period, in terms of their mood, between the time they finished EEG visits and the current time (at least one year after those assessments). With that time-frame in mind, participants completed the BDI-II (Beck et al., 1996) using a web-based form that reminded them to focus on the worst two-week period since their last EEG visit, and also indicated whether they had experienced each of 25 stressors, also known as negative life events (NLE), listed in the Life History Calendar (Caspi et al., 1996) since their last EEG lab visit. A graduate clinical rater also readministered the SCID MDD module, querying participants on whether they had met symptom criteria for at least two weeks during the past year.
Statistical Analyses
Depression and stress over time
Two repeated measures analyses of variance (ANOVAs) were computed in SPSS Version 20 (SPSS IBM, New York) to compare BDI-II scores and number of NLEs as a function of biological sex and time (baseline and follow up). Pearson correlations were also computed between baseline and follow-up scores to determine shared variance across reports. Partial q2 and R2 estimates of effect size are reported for ANOVA and correlation results, respectively.
Depression and frontal asymmetry
Three factorial linear mixed models were computed in SPSS Version 20 (SPSS IBM, New York) with baseline CSD-transformed frontal EEG asymmetry as the dependent variable1. Day of EEG visit (1-4) and frontal region (F2-F1, F4-F3, F6-F5, F8-F7) were within-subjects factors, biological sex was the between-subjects factor, and each of the following z-scored continuous variables was included in separate models: (1) baseline BDI-II score; (2) follow-up BDI-II score; and (3) follow-up BDI-II score residualized on baseline BDI-II score (so that scores would reflect whether participants had more or less severe depression than would be expected based on their depressive symptoms at the time they participated in baseline EEG visits). The main effect of BDI-II and the sex by BDI-II interaction were the primary asymmetry effects of interest. Effect size was estimated by aggregating frontal asymmetry sessions across days and regions and then correlating aggregate asymmetry with the relevant BDI-II score, reporting R2 values.
Depression and stress
Total NLEs experienced during the one-year follow-up period were calculated for 53 participants (n = 1 did not complete the NLE assessment). Pearson correlations were computed between BDI-II and NLEs: (1) endorsed at baseline; (2) endorsed at follow-up; and (3) follow-up scores residualized on baseline scores, reflecting whether participants had higher or lower scores than would be expected based on what they reported at baseline. R2 estimates of effect size are presented. Potential sex differences in correlations were examined using r-to-z transformations.
Results
Depression over Time
A time main effect showed that BDI-II scores were higher during the worst two-week period in the next year (M = 13.97, SE = 1.63, range = 0-45) compared to baseline (M = 5.74, SE = 0.96, range=0-22), F(1, 52) = 30.72, p < .001, partial η2 = .37. No sex main effect or interaction with time emerged (both p> .35 and partial η2 < .02). Symptoms were higher for 78%, were the same for 5%, and the worst two-week period was less severe than baseline for 17%. BDI-II scores at baseline and follow-up were moderately correlated, r(54) = .45, p < .001, R2 = .20.
Stress over Time
A time main effect demonstrated that NLEs decreased from baseline (M=5.82, SE=.40, range = 0-13) to follow-up (M = 3.59, SE = .33, range = 0-9), F(1,51) = 30.72,p < .001. No sex main effect or interaction with time emerged (both p > .35). NLEs at baseline and follow-up were moderately correlated, r(53) = .40, p < .01, R2 = .16.
Depression and Frontal Asymmetry
A significant sex by BDI-II interaction emerged for all three models: (1) baseline BDI-II, F(1, 774) = 4.41, p = .04; (2) follow-up BDI-II, F(1, 774) = 4.12, p = .04; and (3) residualized BDI-II, F(1, 776) = 8.31, p < .01. Figure 1A illustrates that for participants endorsing high baseline BDI-II scores, men exhibited lower left than right activity compared to women; however, the overall relationship between asymmetry and BDI-II was relatively weak within men (r = -.21, R2 = .04), and absent in women (r = .09, R2 = .01). In contrast, Figure 1B shows that higher follow-up BDI-II scores in women were linked to lower left than right frontal activity (r = -.27, R2 = .07); this pattern was absent in men (r = .01, R2 = 0). Figure 1C demonstrates that when accounting for baseline BDI-II scores, findings replicate Figure 1B, such that higher BDI- II scores at follow-up related to lower left than right frontal activity in women (r = -.33, R2 = .11) but not men (r = .12, R2 = .01). Main effects of BDI-II and sex were not significant.
Figure 1.
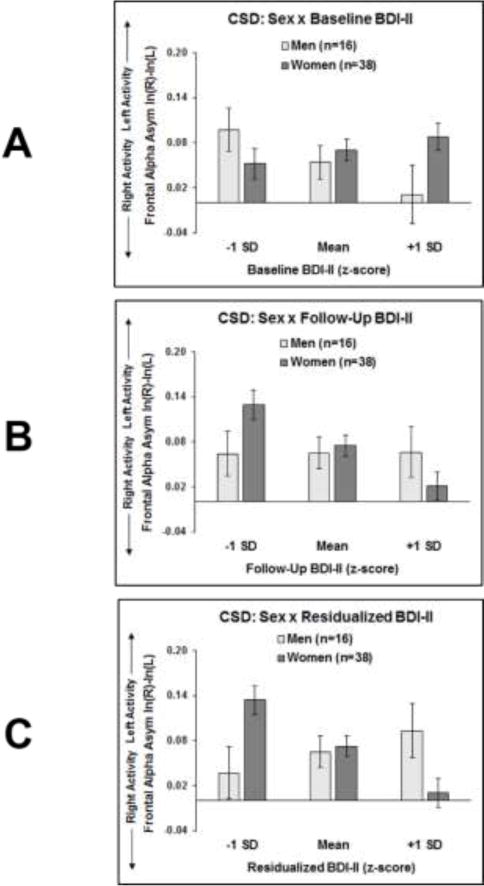
Frontal EEG asymmetry plotted as a function of sex and range of Beck Depression Inventory II (BDI-II) scores: (A) at baseline; (B) at follow-up; and (C) at follow-up residualized on baseline. Higher BDI-II scores at follow-up (B and C) were associated with lower left than right frontal EEG activity at baseline in women, but not men.
Depression and Stress
Although BDI-II scores were unrelated to NLEs at baseline (R2 = .06; see Figure 2A), at higher BDI-II scores were associated with a greater number of NLEs endorsed at follow-up (R2 = .18; see Figure 2B). Moreover, when accounting for baseline BDI-II and NLEs, this positive relationship between follow-up BDI-II and NLEs persisted (R2 = .19; see Figure 2C). No correlations differed significantly as a function of sex (all p > .42).
Figure 2.
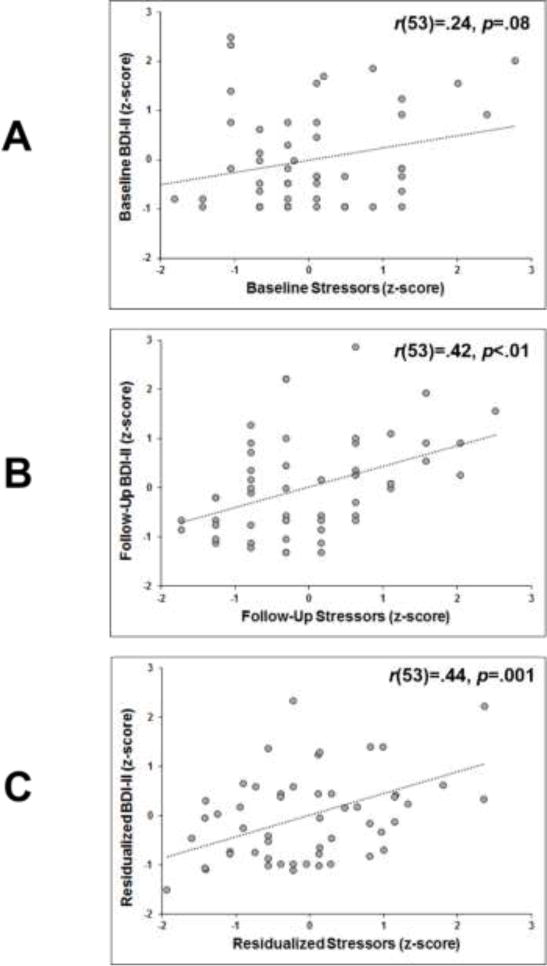
Stressors (quantified as number of Negative Life Events) plotted against Beck Depression Inventory II (BDI-II) scores: (A) at baseline; (B) at follow-up; and (C) at follow-up residualized on baseline. Higher BDI-II scores at follow-up (B and C) were associated with greater frequency of stressors at follow-up.
Moderation of Depression and Frontal Asymmetry by Stress
As lower left than right frontal EEG activity at baseline was related to greater BDI-II scores in women but not men at follow-up, moderation analyses focused on women only. To determine whether the relationship between BDI-II symptoms and frontal asymmetry was moderated by stress, a factorial linear mixed model was run, with day of EEG visit and frontal region as repeated factors, residualized BDI-II scores and residualized NLEs as continuous factors, and frontal asymmetry at baseline as the dependent variable. Results indicated that although a main effect of residualized BDI-II remained significant, F(1, 524) = 22.28, p < .001, R2 = .11, the NLE main effect and interaction with BDI-II were not significant (both p > .24).
Discussion
Findings of the present pilot study suggest that prefrontal brain asymmetry may indeed be associated with greater risk for future depression among never depressed women. Compared to baseline, depression symptoms across men and women were more severe during the worst two- week period over the next year, and scores at these two time points were only moderately correlated with each other, consistent with BDI-II indexing state depression during distinct periods of time (e.g., the past two weeks at intake, and the worst two-week period in the past year at follow-up). As hypothesized, relatively lower left frontal activity at baseline was associated with higher depression symptoms during the worst period of the ensuing year, even after accounting for depression symptoms endorsed at baseline; however, this relationship was present in women, not men. These results are consistent with: (1) cross-sectional studies highlighting significant links between frontal EEG asymmetry and depressive symptoms in women (e.g., Jaworska et al., 2012; Nusslock et al., 2018; Stewart et al., 2010); (2) longitudinal studies showing that baseline frontal EEG asymmetry shares variance with future first-episode depression (Mitchell & Pössel, 2012; Nusslock et al., 2011; Pössel et al., 2008) as well as recent work demonstrating a relationship between frontal asymmetry and antidepressant treatment response in women, but not men (Arns et al., 2016). In contrast, across women and men, higher depression symptoms were also linked to a greater number of aversive life stressors experienced over the one-year follow-up period, findings replicating prior work (e.g., Lewinsohn et al., 1999; Monroe & Harkness, 2005).
Prior work has suggested that the CSD transform holds theoretical and empirical advantages for assessing frontal resting EEG asymmetry. The theoretical advantage is that the CSD transformation should produce frontal asymmetry scores that are relatively uncontaminated by volume-conducted alpha activity from distal sources, especially occipital (cf. Velo et al., 2012). Empirically, frontal EEG asymmetry based on CSD-transformed signals is a more robust marker of MDD+ than EEG asymmetry referenced to other montages utilized in the literature such as average, Cz, and linked mastoids (Stewart et al., 2010). The present findings suggest that CSD-transformed frontal EEG asymmetry can forecast heightened depression in young women with no history of MDD at baseline assessment.
Contrary to our prediction, NLEs did not moderate the relationship between baseline prefrontal brain asymmetry and future depression, but non-significant results could feasibly be due to lack of statistical power to detect complex interactions. Additionally, our measure, a simple count of negative events, is admittedly coarse, and neglects each individual’s perception of the impact of each event, and each individual’s means of responding to such events. In fact, the perception of stress may be more strongly associated with depression than NLEs per se, as the link between perceived stress and future depression severity was substantial in a recent large study of depression (r = .58, in Candrian, Farabaugh, Pizzagalli, Baer, & Fava, 2007).
Limitations
Limitations of the present study include potential impact of unassessed anxiety symptoms, sample selection bias, a relatively small sample size, and assessment of just the most impairing two-week period of depressive symptoms over the next year. First, although participants at baseline did not have any lifetime anxiety or depression diagnosis, it is possible that comorbid anxiety symptoms experienced within the one-year follow-up period may be contributing to the heightened depression symptoms endorsed at follow-up. Future longitudinal studies should include measures of anxious apprehension and anxious arousal, types of anxiety that co-occur with depression symptoms and are associated with differential patterns of EEG asymmetry (Stewart, Levin-Silton, Sass, Heller, & Miller, 2008). Second, we were only able to recruit approximately 1/3 of our MDD− sample to participate in the follow-up assessment, and although those who agreed to participate in the follow-up session did not differ in baseline depression scores or demographic characteristics, they exhibited relatively lower left frontal EEG activity at baseline than those who did not participate. Perhaps those motivated to complete the follow-up session were somehow at risk for more severe psychopathology than those who refused to participate, thereby cautioning the generalizability of the present findings. Although the sample in this longitudinal analysis is representative of our larger never-depressed sample at baseline, it is not representative of the population of depressed individuals, given its limited age range, student population, and exclusion of participants with prior MDD.
Third, our modest sample size (38 women, 16 men) limits the power to examine potential sex differences in the relationships between asymmetry, future depression, and NLEs. Despite this weakness, our findings still demonstrated a relationship between baseline asymmetry and future depression symptoms in women; recruitment of larger samples are warranted to more effectively explore how stress may moderate asymmetry-depression relationships, particularly in men. Fourth, our primary follow-up assessment consisted of participants reporting on depression symptoms during the worst two-week period over the past year, which may bias the follow-up assessment toward symptom overreporting by suggesting that individuals report on impairment during a particular time window. However, given that MDD− who agreed to participate in a follow-up session did not differ on baseline BDI-II scores from those who did participate, it is less likely that individuals from the present analysis tended to report more impairment overall.
Conclusions
The first investigation of the prospective ability of CSD-transformed frontal EEG asymmetry suggests the promise of future larger prospective investigations. Should a larger prospective investigation identify a similar relationship, the utility of frontal EEG asyfmmetry as a biomarker of risk for depression would hold several implications for future research and practice, including: (1) promoting premorbid risk assessment by facilitating earlier diagnosis than symptom-based assessments; (2) transcending traditional diagnostic categories (consistent with the Research Domain Criteria (RDoC) initiative; Insel, 2014); and (3) rapidly assessing the potential impacts of preventions by examining whether they alter frontal EEG asymmetry rather than waiting for longitudinal data on whether individuals ultimately show full symptoms of MDD.
Supplementary Material
Highlights.
Less left than right frontal activity in women predicts future depression
Baseline and future depression are positively correlated
Future negative life events and future depression are positively correlated
Acknowledgments
Funding Source: This research was supported in part by grants from the National Institute of Mental Health (R01–MH066902 and R21-MH101398) and from the National Alliance for Research on Schizophrenia and Depression (NARSAD).
The authors wish to thank Jamie R. Velo, James A. Coan, David N. Towers, Andrew W. Bismark, Craig Santerre, Eynav E. Accortt, Amanda Brody, Jay Hegde and myriad research assistants for their help on this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Two sets of analogous analyses were computed, with average- and linked mastoid-referenced frontal EEG asymmetry as the dependent variables, respectively; findings are reported in Supplemental Materials and replicate follow-up/residualized CSD asymmetry results.
Declaration of Interest
J. L. Stewart – Conflicts of interest: none
J. J. B. Allen – Conflicts of interest: none
References
- Allen JJ, Coan JA, Nazarian M. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology. 2004;67:183218. doi: 10.1016/j.biopsycho.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Allen JJB, Urry HL, Hitt SK, Coan JA. The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology. 2004;41:269–280. doi: 10.1111/j.1469-8986.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Arns M, Bruder G, Hegerl U, Spooner C, Palmer DM, Etkin A, Gordon E. EEG alpha asymmetry as a gender-specific predictor of outcome to acute treatment with different antidepressant medications in the randomized iSPOT-D study. Clinical Neurophysiology. 2016;127:509–519. doi: 10.1016/j.clinph.2015.05.032. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Blackhart GC, Minnix JA, Kline JP. Can EEG asymmetry patterns predict future development of anxiety and depression?: A preliminary study. Biological Psychology. 2006;72:46–50. doi: 10.1016/j.biopsycho.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Warner V, Nomura Y, Grillon C, Hille J, Weissman MM. Electroencephalographic measures of regional hemispheric activity in offspring at risk for depressive disorders. Biological Psychiatry. 2005;57:328–335. doi: 10.1016/j.biopsych.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Burcusa SL, Iacono WG. Risk for recurrence in depression. Clinical Psychology Review. 2007;27:959–985. doi: 10.1016/j.cpr.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candrian M, Farabaugh A, Pizzagalli DA, Baer L, Fava M. Perceived stress and cognitive vulnerability mediate the effects of personality disorder comorbidity on treatment outcome in major depressive disorder: a path analysis study. Journal of Nervous and Mental Disorders. 2007;195:729–737. doi: 10.1097/NMD.0b013e318142cbd5. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Thornton A, Freedman D, Amell JW, Harrington H, Silva PA. The life history calendar: a research and clinical assessment method for collecting retrospective event-history data. International Journal of Methods in Psychiatric Research. 1996;6:101–114. doi: 10.1002/(SICI)1234-988X(199607)6:2%3C101::AID-MPR156%3E3.3.CO;2-E. [DOI] [Google Scholar]
- Davidson RJ. Anterior electrophysiological asymmetries, emotion, and depression: Conceptual and methodological conundrums. Psychophysiology. 1998;35:607–614. doi: 10.1017/S0048577298000134. [DOI] [PubMed] [Google Scholar]
- First MG, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV Axis I disorder—clinical version, administration booklet. New York, NY: Biometrics Research Department; 1997. http://cpmcnet.columbia.edu/dept/scid/ [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK. The economic burden of depression in the United States: how did it change between 1990 and 2000? Journal of Clinical Psychiatry. 2003;64:1465–1475. doi: 10.4088/JCP.v64n1211. [DOI] [PubMed] [Google Scholar]
- Hagemann D. Individual differences in anterior EEG asymmetry: methodological problems and solutions. Biological Psychology. 2004;67:157–182. doi: 10.1016/j.biopsycho.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Hewig J, Seifert J, Naumann E, Bartussek D. The latent state-trait structure of resting EEG asymmetry: Replication and extension. Psychophysiology. 2005;42:740–752. doi: 10.1111/j.1469-8986.2005.00367.x. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Naumann E, Thayer JF, Bartussek D. Does resting electroencephalograph asymmetry reflect a trait? an application of latent state-trait theory. Journal of Personality and Social Psychology. 2002;82:619–641. doi: 10.1037/0022-3514.82.4.619. [DOI] [PubMed] [Google Scholar]
- Hollingshead AA. Four-factor index of social status. Yale University; New Haven, CT: 1975. Unpublished manuscript. [Google Scholar]
- Horwath E, Johnson J, Klerman GL, Weissman MM. Depressive symptoms as relative and attributable risk factors for first-onset major depression. Archives of General Psychiatry. 1992;49:817–823. doi: 10.1001/archpsyc.1992.01820100061011. [DOI] [PubMed] [Google Scholar]
- Insel TR. The NIMH research domain criteria (RDoC) project: precision medicine for psychiatry. American Journal of Psychiatry. 2014;171:395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- Jaworska N, Blier P, Fusee W, Knott V. Alpha power, alpha asymmetry and anterior cingulate cortex activity in depressed males and females. Journal of Psychiatric Research. 2012;46:1483–1491. doi: 10.1016/j.jpsychires.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Zeller PJ, Paulus M, Leon AC, Maser JD, Keller MB. Psychosocial disability during the long-term course of unipolar major depressive disorder. Archives of General Psychiatry. 2000;57:375–380. doi: 10.1001/archpsyc.57.4.375. [DOI] [PubMed] [Google Scholar]
- Judd LL, Paulus MJ, Schettler PJ, Akiskal HS, Endicott J, Leon AC, Keller MB. Does incomplete recovery from first lifetime major depressive episode herald a chronic course of illness? American Journal of Psychiatry. 2000;157:1501–1504. doi: 10.1176/appi.ajp.157.9.1501. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clinical Neurophysiology. 2006;117:348–368. doi: 10.1016/j.clinph.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Griffiths K, Felmingham KL, Shankman SA, Drinkenburg W, Arns M, Bryant RA. Disorder specificity despite comorbidity: Resting EEG alpha asymmetry in major depressive disorder and post-traumatic stress disorder. Biological Psychology. 2010;85:350–354. doi: 10.1016/j.biopsycho.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Allen NB, Seeley JR, Gotlib IH. First onset versus recurrence of depression: differential processes of psychosocial risk. Journal of Abnormal Psychology. 1999;108:483. doi: 10.1037/0021-843X.108.3.483. [DOI] [PubMed] [Google Scholar]
- McFarland BR, Shankman SA, Tenke CE, Bruder GE, Klein DN. Behavioral activation system deficits predict the six-month course of depression. Journal of Affective Disorders. 2006;91:229–234. doi: 10.1016/j.jad.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Michaud CM, McKenna MT, Begg S, Tomijima N, Majmudar M, Bulzacchelli MT, Murray CJ. The burden of disease and injury in the United States 1996. Population Health Metrics. 2006;4:11. doi: 10.1186/1478-7954-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AM, Pössel P. Frontal brain activity pattern predicts depression in adolescent boys. Biological Psychology. 2012;89:525–527. doi: 10.1016/j.biopsycho.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Harkness KL. Life stress, the “kindling” hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychological Review. 2005;112:417–445. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Shackman AJ, Harmon-Jones E, Alloy LB, Coan JA, Abramson LY. Cognitive vulnerability and frontal brain asymmetry: Common predictors of first prospective depressive episode. Journal of Abnormal Psychology. 2011;120:497–503. doi: 10.1037/a0022940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Shackman AJ, McMenamin BW, Greischar LL, Davidson RJ, Kovacs M. Comorbid anxiety moderates the relationship between depression history and prefrontal EEG asymmetry. Psychophysiology. 2018;55:e12953. doi: 10.1111/psyp.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Corrigenda. Electroencephalography and Clinical Neurophysiology. 1990;76:565–566. doi: 10.1016/0013-4694(90)90009-9. [DOI] [PubMed] [Google Scholar]
- Pössel P, Lo H, Fritz A, Seemann S. A longitudinal study of cortical EEG activity in adolescents. Biological Psychology. 2008;78:173–178. doi: 10.1016/j.biopsycho.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Reid SA, Duke LM, Allen JJ. Resting frontal electroencephalographic asymmetry in depression: Inconsistencies suggest the need to identify mediating factors. Psychophysiology. 1998;35:389–404. doi: 10.1111/1469-8986.3540389. [DOI] [PubMed] [Google Scholar]
- Segrave RA, Cooper NR, Thomson RH, Croft RJ, Sheppard DM, Fitzgerald PB. Individualized alpha activity and frontal asymmetry in major depression. Clinical EEG and Neuroscience. 2011;42:45–52. doi: 10.1177/155005941104200110. [DOI] [PubMed] [Google Scholar]
- Smit DJA, Posthuma D, Boomsma DI, De Geus EJC. The relation between frontal EEG asymmetry and the risk for anxiety and depression. Biological Psychology. 2007;74:26–33. doi: 10.1016/j.biopsycho.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Solomon DA, Keller MB, Leon AC, Mueller TI, Lavori PW, Shea MT, Endicott J. Multiple recurrences of major depressive disorder. American Journal of Psychiatry. 2000;157:229–233. doi: 10.1176/appi.ajp.157.2.229. [DOI] [PubMed] [Google Scholar]
- Steiner ARW, Coan JA. Prefrontal asymmetry predicts affect, but not beliefs about affect. Biological Psychology. 2011;88:65–71. doi: 10.1016/j.biopsycho.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJ. Resting frontal EEG asymmetry as an endophenotype for depression risk: sex-specific patterns of frontal brain asymmetry. Journal of Abnormal Psychology. 2010;119:502–512. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Coan JA, Towers DN, Allen JJ. Resting and task-elicited prefrontal EEG alpha asymmetry in depression: Support for the capability model. Psychophysiology. 2014;51:446–455. doi: 10.1111/psyp.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Levin-Silton R, Sass SM, Heller W, Miller GA. Anger style, psychopathology, and regional brain activity. Emotion. 2008;8:701–713. doi: 10.1037/a0013447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. Generator localization by current source density (CSD): Implications of volume conduction and field closure at intracranial and scalp resolutions. Clinical Neurophysiology. 2012;123:2328–2345. doi: 10.1016/j.clinph.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. Journal of Abnormal Psychology. 2006;115:715–729. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


