Abstract
In social mammals, kinship is an important factor that often affects the interactions among individuals within groups. In primates that live in a multilevel society, kinship may affect affiliative patterns between individuals at different scales within the larger group. For this study, we use field observations and molecular methods to reveal the profiles of how kinship affects affiliative behaviors between individuals in a breeding band of wild golden snub-nosed monkeys (Rhinopithecus roxellana). We use a novel nonparametric test, the partition Mantel test, to measure independently the correlation between kinship and each of three affiliative behaviors. Our results show that more closely related females are more likely to groom each other. Average relatedness between adult females within the same one-male unit (OMU) is higher than that between adult females from different OMUs. We suggest that closely related females may reside in the same OMU in order to attain inclusive fitness benefits, and that kinship plays an important role in maintaining the social structure of this species.
Keywords: golden snub-nosed monkey, kinship, affiliative behavior, partition mantel test
High kinship among individuals is known to facilitate positive social interactions in a variety of animal taxa such as insects (Foster et al. 2006), amphibians (Blaustein and Waldman 1992), reptiles (Davis 2012), birds (Nam et al. 2010), and mammals (Mateo 2002). According to kin selection theory, positive kin bias among individuals can evolve via inclusive fitness (Hamilton 1964). Many nonhuman primate species have complex social structures based on variable hierarchies within each group, which are often established through social ties via the implementation of different strategies and behaviors (Morin and Goldberg 2004). Such species are thus ideal model systems for investigating the role of relatedness among individuals, and how this helps maintain a stable social structure within groups.
Within nonhuman primates positive social interactions, which are typically regarded as affiliative behaviors, are generally classified as grooming, close proximity distances, food sharing, and agonistic support (Strayer and Harris 1979; Sussman et al. 2005). Affiliative behaviors account for >80% of the time that primates spend on social activities (60 primate species from 28 genera) (Sussman et al. 2005). This suggests that affiliative behaviors play an important role in the formation of social alliances among individuals, and are important for maintaining complex primate social systems. Other studies have found that behaviors associated with affiliation also reduce competition within groups by increasing the likelihood of groups accessing resources, ease social tensions after fighting with each other and other groups (Mitani and Watts 2010), and maintain or improve social status within a group (Surbeck and Hohmann 2011), each of which may increase the reproductive rates of some individuals (Pope 2000; Surbeck and Hohmann 2011).
Previous studies on kinship in primates were often conducted via long-term recording of individual specific behaviors in the field and focused mainly on terrestrial species, rather than those that are primarily arboreal such as colobine monkeys (Silk 2002). The disadvantage of such an approach is that kinship determined solely from field observation may be inaccurate, due to environmental variations and vegetation barriers, and a lack of standardization in the methods used among researchers. Thus, molecular methods have been used in addition to the data obtained from ‘traditional’ field work in order to efficiently and correctly measure kinship between individuals (Widdig et al. 2002). For instance, Widdig et al. (2002) measured the pairwise kinships between 91 female rhesus macaques Macaca mulatta based on data from 15 microsatellite loci and field records of patterns of several social behaviors. Gagneux et al. (1999) genotyped 11 microsatellite loci from a total of 21 chimpanzees Pan troglodytes to identify which female chimpanzee had offspring fathered by a male from a different group. Silk et al. (2006) amplified 14 microsatellite loci from baboons Papio cynocephalus to successfully identify the paternities of 286 individuals.
The golden snub-nosed monkey Rhinopithecus roxellana is a colobine monkey species endemic to the broadleaved and mixed temperate mountain forests of central and southwestern China (Li et al. 2003). The social structure of R. roxellana is characterized by the multilevel society, which is organized into four nested levels of social associations: unit, band, herd, and troop. More specifically, units can be further divided into: (1) one-male units (OMU), consisting of a single adult male and 1–7 adult females with their immature ones including infants, and (2) all male units (AMU), which consist of multiple young ‘bachelor’ males yet to secure reproductive opportunities and some adult males that have been usurped from OMUs. Bands can also be divided into either: (1) breeding bands (BB) (each an association of several OMUs), and all-male bands (AMB) (each an association of several AMUs). An AMB will usually be closely associated with a BB. Collectively, an AMB, a BB and several solitary males aggregate to form a herd (Qi et al. 2014), the size of which commonly exceeds 100 individuals (Zhang et al. 2006). Neighboring herds periodically fuse to form a troop (Qi et al. 2014). Males leave their natal OMU before reaching sexual maturity and join an all-male band. Some females stay in their natal OMU and produce offspring. However, most females disperse into other OMUs within the same breeding band, or occasionally, into a neighboring breeding band (Qi et al. 2009). Most social activities occur among individuals within the same unit. The behavioral patterns of the individuals from different OMUs include playing among juveniles, and aggression among adults (Zhang et al. 2008).
Golden snub-nosed monkeys are mainly arboreal, making it difficult to measure kinship between individuals via behaviors associated with kinship through field observations alone. Therefore, direct genetic evidence is also required to obtain kinship measurements between pairs of individuals. Furthermore, due to the long-term provisioning of food to the study breeding band, different behavioral patterns and associations among individuals can be relatively easily quantified.
There were two main aims in this study: (1) to determine the role of kinship among adult females in maintaining social cohesion within golden snub-nosed monkey OMUs; and (2) to determine if kinship among females is positively associated with the likelihood of occurrence of social affiliative behaviors.
Materials and Methods
Study site
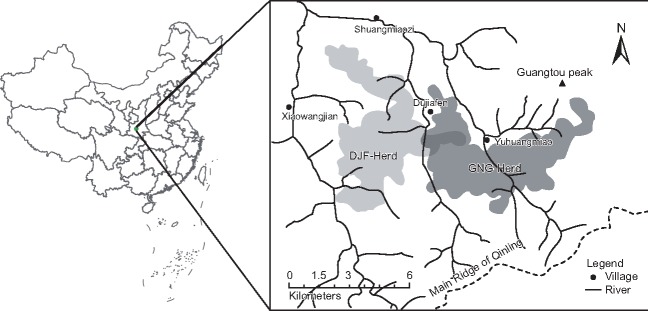
Our study site is located near to YuHuang Miao Village, in the ZhouZhi National Nature Reserve (ZNNR, 108°14’ – 108°18’E, 33°45’ – 33°50’N), on the northern slope of the Qinling Mountains. This region has a temperate climate and ranges in altitude from 1400 to 2890 m above sea level (Figure 1). The annual average temperature is 10.7 °C, and its annual average rainfall is 894 mm (Li et al. 2000).
Figure 1.
Map of the study site: Yuhuangmiao Village, Zhouzhi National Nature Reserve, Shaanxi Province, China. The shadows show the home ranges of the GNG and DJF herds.
The Nancha River separates the two monkey troops present in our study site: the East Ridge Troop (ERT), which is comprised of the HSG and GTS herds, and the West Ridge Troop (WRT), which is consisted of the GNG and DJF herds. This study only involved the GNG herd, which has been studied for the last 16 years using partial food provision in order to enable close observation. In the summer and in the autumn, the WRT mainly occupies areas characterized by high densities of trees, which sometimes makes behavioral observations difficult. Our field work was thus mainly conducted in winter and spring, when temperatures are often low and the ground is covered with snow. The study was conducted from October 2012 to June 2013, when the breeding band of the GNG herd comprised of 13 OMUs and 71 adult individuals. We focused on the adult females in four OMUs because their compositions were more stable during the observation period than the other OMUs. The unit compositions and sampling information are presented in Table 1. The adult individuals from other OMUs were also sampled so as to accurately estimate the allele frequencies and relatedness coefficients among OMUs.
Table 1.
Unit composition and sampling information of the GNG herd
| Age–sex classes | OMU |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PK | HB | SH | RX | JB | BX | FZ | WX | SQ | LD | ZB | HT | SX | |
| Adult male | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 |
| Adult female | 7/7 | 6/6 | 5/5 | 3/3 | 2/3 | 4/4 | 3/3 | 4/4 | 2/3 | 3/4 | 7/7 | 5/5 | 4/4 |
| Juvenile | 4 | 4 | 3 | 2 | 2 | 2 | 1 | 3 | 2 | 3 | 4 | 3 | 2 |
The first numbers in the cells of adult male and adult female are the number of individuals sampled and the second numbers are the number of individuals within OMU.
Behavioral observations
Adult females were observed with both focal animal sampling and scan sampling methods (Altmann 1974), and using both continuous recording (all-occurrence recording) and instantaneous sampling methods (Martin and Patrick 2006). Each of twenty-one females from four OMUs (PK, HB, SH, RX) were randomly selected and observed continuously for a period of three hours. Affiliative behavior patterns of the females, such as grooming another female, proximity to another female and approaching or being approached by another female, were recorded. Two focal females were monitored during each observation day. If the target female moved out of view, or if most of the OMU members of the target female moved away, a new target female was chosen to follow for 3 h. Both focal-animal behavioral sampling and continuous (all-occurrence) recording methods were used to record grooming and approach behaviors. The initiator and receiver of each interaction, the duration of each interaction and the behaviors exhibited after the initial approach, were recorded. The scan sampling and instantaneous recording methods were used for recording proximity behavior, which involved the recorded individuals near to the focal adult female. The definitions of grooming, approach, and proximity are defined as follows.
Grooming one adult female grooms another adult female, including picking out small objects (e.g., dirt or parasites) from the hair or the skin of the individual being groomed. Any parasites that were removed were either put into the mouth of the groomer if removed by the hands of the groomer or were directly removed by the groomer with its mouth (Li et al. 2002).
Approach one focal female moved towards another female, from a distance of >1 m to a distance of ≤0.5 m. The individual to which the focal individual moved did not move its location within 10 s of the event.
Proximity the distance between the two female individuals, not including their tails, is less than two adult-individual female body lengths.
Molecular methods
Hair and fecal samples were collected noninvasively for genetic analysis. A stick with adhesive at each end and food bait in the center was used to collect hair, which was then stored in the laboratory at room temperature after being dried with silica gel. Fresh fecal samples were collected and stored in DETs solution (20% DMSO, 0.25 m sodium–EDTA, 100 mm Tris–HCl, pH 7.5, and NaCl to saturation) at −20 °C.
The DNA samples extracted from hairs were processed with proteinase K digestion in a PCR compatible buffer (Allen et al. 1998); those from feces were extracted with QIAamp DNA Stool Mini Kits (Qiagen, Germany). Nineteen highly polymorphic microsatellites were amplified from each sample (Huang et al. 2016a), which were sent to Shanghai Sangon Biotech for genotyping. In order to prevent genotyping errors, such as false alleles and/or allelic dropouts (Taberlet et al. 1996), homozygote genotypes were clarified by replication at least seven times, while all heterozygotes were clarified by at least three separate reactions (Taberlet et al. 1996). Alleles were segregated with an ABI PRISM 3100 Genetic Analyser, and their sizes, relative to an internal standard (ROX-labeled HD400), were determined with GeneMapper V3.7 (Applied Biosystems). micro-checker V2.2.3 (van Oosterhout et al. 2004) was used to check microsatellite data for scoring errors, allelic dropouts, and null alleles.
We calculated the genetic diversity parameters for each locus using cervus V3.0 (Kalinowski et al. 2007). A Hardy–Weinberg equilibrium test was performed with genepop V4.3 (Rousset 2008), and sequential Bonferroni correction was used to adjust each P-value for multiple tests. Loci in Hardy–Weinberg disequilibrium were excluded from further analysis. The relatedness coefficient was estimated with Lynch and Ritland’s (1999) estimator with null allele correction (Huang et al. 2016b). The null allele frequency was estimated by Kalinowski and Taper’s (2006) estimator in polyrelatedness V1.6 (Huang et al. 2016 b). A linkage disequilibrium test was performed with genepop V4.3 (Rousset 2008) to avoid inference of linked loci, and each locus was weighted conservatively for relatedness estimation by 1/(n + 1), where n is the number of linked loci (determined by FDR corrected Q < 0.05).
The relatedness between each pair of individuals was calculated, and each dyad was classified into one of the five categories: male–male (MM), female–female within the same unit (FFW), female–female between units (FFB), female–male within the same unit (FMW), and female–male between units (FMB). To test if kinship affects the likelihood of association between dyads, we assessed the relatedness between female–female dyads (FFW versus FFB) and female–male dyads (FMW versus FMB) with a matrix permutation test because dyadic data are not independent (Guo et al. 2015). In order to determine the correlation between kinship and each behavior, we selected female dyads from the four observed units to generate a 21 × 21 pairwise relatedness matrix.
Behavioral analyses
We calculated the proximity index (PI) (Matsumura and Okamoto (1997) for the three affiliative behavior patterns measured. This index is defined as the ratio of the numbers scanned between the two individuals (A and B) to the total number of the scans involving A or B. To standardize all behavioral data, we extended the proximity index to an all-occurrence recording method. For example, the PI for grooming is the ratio of twice the total time that A and B groomed each other to the total time that A or B initiated and received grooming. Similarly, the PI for approaching is the ratio of twice number of approaches between A and B to the total approaches that either A or B initiated and received.
The PI is also dependent on the size of the unit, for this study the OMU. For example, assuming a certain behavior occurs randomly among individuals, and each individual has the same probability of exhibiting the behavior, thus, the expected PI between any two individuals is 1/(n − 1), where n is the total number of individuals (adult females) within the unit. Therefore, this value between individuals in a larger unit would be smaller, and a larger PI may not imply a closer relationship. Thus, we had to standardize PI for each of the OMUs by subtracting the mean and then divided by the standard deviation (the Z-score) of the PI for each unit.
Because affiliative behaviors mainly happened within OMUs, we excluded those interactions between individuals from different OMUs. Three 21 × 21 Z-score matrices were then obtained. The corresponding elements for individuals from different OMUs and those on diagonals within each matrix were left blank.
Statistics
Our dyadic data are not independent because some dyads share the same individuals. Taking geographic distance as an example, following the change in the coordinates of one location, all distances of other locations relative to the original location will change. Therefore, linear regression cannot be applied to these matrices—this method assumes all observations are independent. In this case, the Mantel test (Mantel 1976) is usually used to measure the degree of association between two distance matrices. This randomly permutes one of the two matrices, and calculates the probability of the correlation coefficient between the two matrices after permutation is greater than the original value with a Monte-Carlo algorithm.
Although we can estimate the pairwise relatedness between individuals within or between OMUs based on molecular data, the proximity index can only be calculated for individuals within a same OMU. Therefore, for dyads between individuals in different OMUs, the corresponding proximity index is invalid. Based on the Mantel test, we overcame this problem by developing a novel nonparametric test, the partition Mantel test, to test the correlation between the relatedness matrix and each Z-score matrix. The individuals within the same unit are randomly permuted, and the blank elements (i.e., the diagonal elements and the elements of the individuals from different OMUs) are not used in the calculation for correlation between matrices. Similarly, the probability of the correlation between two matrices after permutation is greater than original value is calculated. Significance values are thus one-tailed.
Results
Behavioral data
We studied a total of 21 females from four OMUs, the compositions of which were stable during the observation period. We made 877 grooming events measurements, 2,127 for of proximity and 431 for “approach”. The proximity indices (PI) of these three affiliative behaviors (grooming time, and frequencies for both proximity and “approach”’) for 49 female–male dyads within the same units were calculated and are presented in Table 2.
Table 2.
The descriptive statistics of kinship and affiliative behaviors within OMUs
| Behavior | Count | Time | Unit | #dyads | Mean | Std | Min | Max |
|---|---|---|---|---|---|---|---|---|
| Grooming | 248 | 12.10 h | PK | 21 | 0.132 | 0.121 | 0.000 | 0.423 |
| 255 | 13.54 h | HB | 15 | 0.189 | 0.113 | 0.000 | 0.372 | |
| 259 | 13.12 h | SH | 10 | 0.244 | 0.085 | 0.092 | 0.378 | |
| 90 | 4.92 h | RX | 3 | 0.465 | 0.283 | 0.176 | 0.740 | |
| Proximity | 862 | PK | 21 | 0.159 | 0.076 | 0.027 | 0.328 | |
| 600 | HB | 15 | 0.188 | 0.095 | 0.023 | 0.329 | ||
| 538 | SH | 10 | 0.250 | 0.079 | 0.113 | 0.370 | ||
| 115 | RX | 3 | 0.492 | 0.133 | 0.357 | 0.623 | ||
| Approach | 115 | PK | 21 | 0.159 | 0.072 | 0.034 | 0.343 | |
| 145 | HB | 15 | 0.191 | 0.121 | 0.016 | 0.415 | ||
| 116 | SH | 10 | 0.246 | 0.076 | 0.143 | 0.372 | ||
| 42 | RX | 3 | 0.522 | 0.247 | 0.276 | 0.840 |
Genetic diversity
We used 68 independent genetic samples (17 hair and 51 feces) for microsatellite analysis (the sampling ratio was 95.8%). In order to reliably estimate allele frequency, we sampled and genotyped more adult individuals in the breeding band, which contained a total of 71 adults. DNA extracts were amplified at 19 microsatellite loci. The characteristics of these loci are presented in Table 3. The number of alleles per locus ranged from 3 to 5, averaging 3.95. The observed heterozygosity ranged from 0.226 to 0.776, with an average of 0.578. The expected heterozygosity ranged from 0.245 to 0.765, 0.582 on average. The polymorphic information content (PIC) ranged from 0.229 to 0.729, 0.518 on average. Allelic richness ranged from 1.324 to 4.246, 2.562 on average Table 3.
Table 3.
Characteristics of the 19 microsatellite loci used to assess the genetic structure of 68 individuals of R. roxellana
| Locus | k | T% | HO | HE | PIC | Ar | FIS | P |
|---|---|---|---|---|---|---|---|---|
| D10s1432 | 5 | 98.5 | 0.567 | 0.534 | 0.476 | 2.147 | −0.062 | 0.483 |
| D10s2483 | 4 | 100 | 0.544 | 0.617 | 0.544 | 2.609 | 0.118 | 0.251 |
| D10s676 | 3 | 91.2 | 0.484 | 0.402 | 0.328 | 1.673 | −0.203 | 0.342 |
| D12s375 | 3 | 92.6 | 0.540 | 0.551 | 0.449 | 2.227 | 0.020 | 0.898 |
| D14s306 | 4 | 98.5 | 0.776 | 0.680 | 0.621 | 3.128 | −0.141 | 0.199 |
| D16s540 | 3 | 98.5 | 0.433 | 0.499 | 0.425 | 1.996 | 0.132 | 0.431 |
| D19s1034 | 4 | 97.1 | 0.606 | 0.605 | 0.523 | 2.529 | −0.003 | 0.958 |
| D19s248 | 3 | 100 | 0.677 | 0.607 | 0.538 | 2.546 | −0.114 | 0.507 |
| D19s582 | 4 | 92.6 | 0.619 | 0.577 | 0.531 | 2.361 | −0.074 | 0.469 |
| D21s2054 | 3 | 98.5 | 0.508 | 0.529 | 0.423 | 2.123 | 0.041 | 0.055 |
| D3s1766 | 4 | 100 | 0.632 | 0.627 | 0.556 | 2.681 | −0.008 | 0.580 |
| D6s1050 | 4 | 98.5 | 0.642 | 0.654 | 0.581 | 2.888 | 0.018 | 0.424 |
| D6s493 | 5 | 97.1 | 0.712 | 0.765 | 0.729 | 4.246 | 0.069 | 0.347 |
| D6s501 | 4 | 98.5 | 0.612 | 0.597 | 0.512 | 2.478 | −0.026 | 0.671 |
| D7s1804 | 4 | 91.2 | 0.226 | 0.245 | 0.229 | 1.324 | 0.078 | 0.371 |
| D7s2204 | 5 | 100 | 0.677 | 0.714 | 0.669 | 3.496 | 0.053 | 0.333 |
| D7s820 | 5 | 98.5 | 0.672 | 0.719 | 0.672 | 3.560 | 0.066 | 0.721 |
| D9s252 | 4 | 92.6 | 0.508 | 0.518 | 0.475 | 2.074 | 0.019 | 0.179 |
| TPOX | 4 | 95.6 | 0.569 | 0.610 | 0.542 | 2.565 | 0.067 | 0.498 |
| Average | 3.95 | 96.8 | 0.578 | 0.582 | 0.518 | 2.562 | 0.009 |
Header row description: k is the number of alleles, T% is the genotyped percentage, HO and HE are the observed and expected heterozygosities, PIC and AR are the polymorphic information content and allelic richness, respectively, P is the significance of a HWE test.
Relatedness analyses
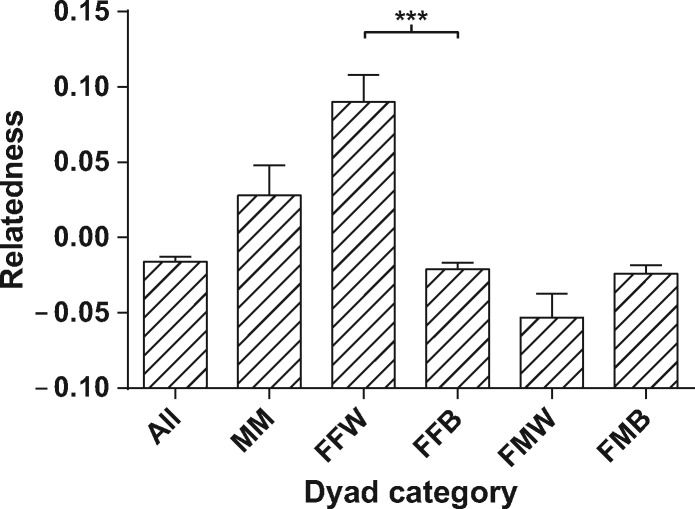
For the genotypes at 19 microsatellite loci from 68 individuals, relatedness coefficients (r) were estimated using Lynch and Ritland’s (1999) estimator. The means and standard errors of this coefficient for each of the five categories are shown in Figure 2. The relatedness coefficient between females within the same OMU ( = 0.045, n = 106) is significantly greater than that between different OMUs ( = −0.010, n = 1,379, P < 0.001), while the difference in the relatedness coefficients between female–male dyads within the same OMU ( = −0.026, n = 55) and female–male dyads between OMUs is not significant ( = −0.012, n = 660, P = 0.098). This shows that females within an OMU are more closely related to each other than the overall level of relatedness between females within the breeding band Figure 2.
Figure 2.
Mean value and standard error of relatedness for each dyad category. The description of dyad categories are as follows: All denotes dyads between all kinds of individuals; MM denotes male–male dyads; FFW and FFB denote female–female dyads within a same unit and between different units; FMW and FMB denote female–male dyads within the same unit and between different units, respectively.
Kinship and behavior
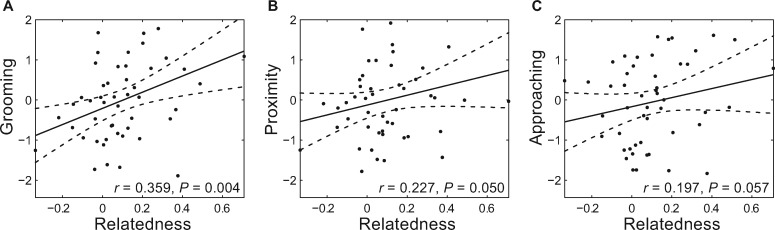
We found significant correlations between relatedness and affiliative behaviors (Table 4). For grooming there was a significant positive correlation with the relatedness coefficient (partition Mantel test, r = 0.359, P = 0.004, Figure 3A). We also found a significant but weaker positive trend between the relatedness coefficient and proximity (partition Mantel test r = 0.227, P < 0.05, Figure 3B). There was a marginally nonsignificant positive correlation between r and approach in adult female dyads (r = 0.197, P = 0.057, Table 4, Figure 3A-3C).
Table 4.
The correlation coefficients between relatedness and affiliative behaviors in adult female dyads
| Behavior | r | R2 | P | β1 | β0 |
|---|---|---|---|---|---|
| Grooming | 0.359 | 0.129 | 0.004 | 1.832 | −0.196 |
| Proximity | 0.227 | 0.052 | 0.050 | 1.159 | −0.124 |
| Approach | 0.197 | 0.039 | 0.057 | 1.030 | −0.161 |
Figure 3.
The relationship between r and each affiliative behavior in adult female dyads. The plots of the Z-scores of each of three affiliative behaviors, grooming, proximity and approach, versus the relatedness coefficients are shown in the three subfigures. Each dot in the scatter plots denotes a dyad, and regression analyses of those Z-scores on relatedness coefficients were performed. The correlation coefficient between independent and dependent variables and their significance is shown in the bottom of each subfigure. The line in each subfigure shows the regression equation and the two curves denote the 95% confidence interval of the estimated Z-score.
Discussion
We examined the effects of kinship on grooming, proximity and approach behaviors in golden snub-nosed monkeys, and found that adult females within an OMU are more closely related than those of the same age-sex class between OMUs (Figure 2). This suggests that females with higher kinship are more likely to reside within the same OMU. Indeed, conflicts between the members of different OMUs in R. roxellana are common (Tan et al. 2003) and female alliances are known to play an important role during such conflicts (Guo et al. 2007; Xi et al. 2008). This implies that kinship among females makes a significant contribution to OMU cohesion, resulting in female kin alliances in R. roxellana. This may allow kinship-based alliances to compete more effectively for limited resources (Guo et al. 2007) and defend a territory from other OMUs (Zhang et al. 2006). Similar female–female alliances in other primate species with similar social systems have been shown to be important for access to food and in conflicts with other OMUs, such as in geladas (Dunbar 1993; Dunbar and Dunbar 1975; Kawai et al. 1983), alliances that may have evolved via kin selection (Iwamoto 1993).
We also found that grooming and proximity behaviors occupied most of the times spent on social activities by adult females within an OMU, and these two types of affiliative behaviors occurred more frequently between closely related females than other females (Figure 3). These results are consistent with the predictions of kin-selection; the closer the genetic relationship between individuals, the higher the likelihood that affiliative behaviors will be exhibited. Similar patterns of the behavior are also present in ring-tailed lemurs Lemur catta (Sbeglia et al. 2010), white-faced capuchin monkeys (Cebus capucinus) (Perry et al. 2008), and yellow baboons Papio cynocephalus (Silk et al. 2004, 2006).
However, our data show that approach behavior was only weakly associated with kinship among female R. roxellana (Figure 3). Kinship may thus be less important in determining approach behaviour than for the other two affiliative behaviors that we measured.
Although grooming and proximity between individuals are both significantly positively correlated with kinship, their coefficients of determination are small (Table 4), and the grooming times and proximity frequencies of two individuals varies greatly despite similar levels of kinship (Figure 3). Additionally, the relatedness coefficients between individuals also vary, even though they exhibit similar affiliative behaviors.
These inconsistencies may be associated with at least three factors: 1) relatedness estimators are able to determine the degree of kinship but cannot identify maternal or paternal relatives. The social structure of the golden snub-nosed monkey is based on a loose maternal, one-male and multiple-female multilevel society (Zhang et al. 2006). Maternal relatives tend to be involved in more social activities than nonmaternal relatives, and therefore have a more important social function than paternal relatives in a maternal society (Silk 2002); 2) age differences exists among adult females, and in primates social ties among individuals within the same age class are tighter than those between different age classes (Mitani et al. 2002; Widdig et al. 2002); and 3) a biological market may have influenced partner selection and affected social interactions among females (Wei et al. 2012). For example, females without infants prefer to groom the females with infants to gain access to infants (Wei et al. 2013). Thus, even though kin selection plays an important role in social evolution, close kinship is not always necessary to explain social behavior (de Vladar and Szathmáry 2017). A multitude of factors may are likely to affect social behavior in species such as R. roxellana in addition to those we mentioned previously, e.g., social structure (Silk 2002), rank (Bentley-Condit and Smith 1999), and physiological condition (Guy et al. 2008). It is thus necessary to carry out further studies on kinship and affiliative behaviors in this species.
In conclusion, we show that closely related R. roxellana females are more likely to reside in the same OMU than less related females. Females with higher genetic relatedness between groomed each other more frequently, were in closer proximity, than more distantly related females. We suggest that female kinship plays an important role in the maintenance and organization of the R. roxellana social system. Additional studies are needed to measure the benefits to adult females that reside in the same OMU and preferentially make social affiliations with close kin, and to clarify if kinship-based social associations result in increased inclusive fitness for adult females.
Acknowledgments
We thank the Northwest University students W. Wei, L.L. Wu, and H.Y. Zhang for their fieldwork and experimental assistance. We also thank the management of Zhouzhi National Nature Reserve for permission to conduct this study, which was supported by the National Nature Science Foundation of China (31672301, 31501872, 31572278 31472014), National Key Program of Research and Development, Ministry of Science and Technology of China (2016YFC0503202), Science and Technology Foundation of Shaanxi Academy of Sciences, China (2013 K-04, 2017 K-06 2016K-20; 2017K-09), Science and technology development project of Shaanxi Province, China (2017NY-181; 2016NY-126) and Natural Science Basic Research Plan in Shaanxi Province of China (2016JZ009 2016JM3016).
References
- Allen M, Engström AS, Meyers S, Handt O, Saldeen T. et al. 1998. Mitochondrial DNA sequencing of shed hairs and saliva on robbery caps: sensitivity and matching probabilities. J. Forensic Sci 43:453–466. [PubMed] [Google Scholar]
- Altmann J, 1974. Observational study of behavior: sampling methods. Behaviour 49:227–267. [DOI] [PubMed] [Google Scholar]
- Bentley-Condit VK, Smith EO, 1999. Female dominance and female social relationships among yellow baboons Papio hamadryas cynocephalus. Am J Primatol 47:321–334. [DOI] [PubMed] [Google Scholar]
- Blaustein AR, Waldman B, 1992. Kin recognition in anuran amphibians. Anim Behav 44:207–221. [Google Scholar]
- Davis AR, 2012. Kin presence drives philopatry and social aggregation in juvenile desert night lizards Xantusia vigilis. Behav Ecol 23:18–24. [Google Scholar]
- de Vladar HP, Szathmáry E, 2017. Beyond Hamilton’s rule. Science 356:485–486. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM, 1993. Social organization of the gelada In: Jablonski NG, editor. Theropithecus: The Rise and Fall of A Primate Genus. Cambridge: Cambridge University Press; 425–439. [Google Scholar]
- Dunbar RIM, Dunbar EP, 1975. Social Dynamics of Gelada Baboons. Basel: Karger. [PubMed] [Google Scholar]
- Foster KR, Wenseleers T, Ratnieks FLW, 2006. Kin selection is the key to altruism. Trends Ecol Evol 21:57–60. [DOI] [PubMed] [Google Scholar]
- Gagneux P, Boesch C, Woodruff DS, 1999. Female reproductive strategies, paternity and community structure in wild West African chimpanzees. Anim. Behav 57:19–32. [DOI] [PubMed] [Google Scholar]
- Guo ST, Huang K, Ji WH, Garber PA, Li BG, 2015. The role of kinship in the formation of a primate multilevel society. Am J Phys Anthropol 156:606–613. [DOI] [PubMed] [Google Scholar]
- Guo ST, Li BG, Watanabe K, 2007. Diet and activity budget of Rhinopithecus roxellana in the Qinling Mountains, China. Primates 48:268–276. [DOI] [PubMed] [Google Scholar]
- Guy AJ, Schuerch FS, Heffernan S, Thomson PC, O’brien JK. et al. 2008. The effect of medroxyprogesterone acetate on behavioural responses of captive female hamadryas baboons Papio hamadryas. Anim Reprod Sci 108:412–424. [DOI] [PubMed] [Google Scholar]
- Hamilton WD, 1964. The genetical evolution of social behaviour. II. J Theor Biol 7:1–16. [DOI] [PubMed] [Google Scholar]
- Huang K, Guo ST, Cushman SA, Dunn DW, Qi XG. et al. 2016a. Population structure of the golden snub-nosed monkey Rhinopithecus roxellana in the Qinling Mountains, central China. Integr Zool 11:350–360. [DOI] [PubMed] [Google Scholar]
- Huang K, Ritland K, Dunn DW, Qi X, Guo S. et al. 2016b. Estimating relatedness in the presence of null alleles. Genetics 202:247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T, 1993. The ecology of Theropithecus gelada In: Jablonski NG, editor. Theropithecus: The Rise and Fall of A Primate Genus. Cambridge: Cambridge University Press; 441–453. [Google Scholar]
- Kalinowski ST, Taper ML, 2006. Maximum likelihood estimation of the frequency of null alleles at microsatellite loci. Conserv Genet 7:991–995. [Google Scholar]
- Kalinowski ST, Taper ML, Marshall TC, 2007. Revising how the computer program cervus accommodates genotyping error increases success in paternity assignment. Mol Ecol 16:1099–1106. [DOI] [PubMed] [Google Scholar]
- Kawai M, Dunbar RIM, Ohsawa H, Mori U, 1983. Social organization of gelada baboons: social units and definitions. Primates 24:13–24. [Google Scholar]
- Li BG, Chen C, Ji WH, Ren BP, 2000. Seasonal home range changes of the Sichuan snub-nosed monkey Rhinopithecus roxellana in the Qinling Mountains of China. Folia Primatol 71:375–386. [DOI] [PubMed] [Google Scholar]
- Li BG, Jia ZY, Pan RL, Ren BP, 2003. Changes in distribution of the snub-nosed monkey in China In: Marsh LK, editor. Primates in Fragments: Ecology and Conservation. New York: Kluwer Academic/Plenum Press; 29–51. [Google Scholar]
- Li BG, Pan RL, Oxnard CE, 2002. Extinction of snub-nosed monkeys in China during the past 400 years. Int J Primatol 23:1227–1244. [Google Scholar]
- Lynch M, Ritland K, 1999. Estimation of pairwise relatedness with molecular markers. Genetics 152:1753–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel N, 1976. Fundamental carcinogenic processes and their implications for low-dose risk assessment. Cancer Res 36:1835–1838. [PubMed] [Google Scholar]
- Martin P, Patrick B, 2006. Measuring Behaviour: An introductory Guide. 2nd edn.Cambridge: Cambridge University Press. [Google Scholar]
- Mateo JM, 2002. Kin-recognition abilities and nepotism as a function of sociality. Proc R Soc Lond B Biol Sci 269:323–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura S, Okamoto K, 1997. Factors affecting proximity among members of a wild group of moor macaques during feeding, moving, and resting. Int. J. Primatol 18:929–940. [Google Scholar]
- Mitani JC, Watts DP, 2010. Why do chimpanzees hunt and share meat? Anim Behav 61:915–924. [Google Scholar]
- Mitani JC, Watts DP, Pepper JW, Merriwether DA, 2002. Demographic and social constraints on male chimpanzee behaviour. Anim Behav 64:727–737. [Google Scholar]
- Morin PA, Goldberg TL, 2004. Determination of genealogical relationships from genetic data: a review of methods and applications In: Chapais B, Berman CM, editors. Kinship and Behavior in Primates. Oxford: Oxford University Press; 15–45. [Google Scholar]
- Nam KB, Simeoni M, Sharp SP, Hatchwell BJ, 2010. Kinship affects investment by helpers in a cooperatively breeding bird. Proc R Soc Lond B Biol Sci 277:3299–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S, Manson JH, Muniz L, Gros-Louis J, Vigilant L, 2008. Kin-biased social behaviour in wild adult female white-faced capuchins, Cebus capucinus. Anim Behav 76:187–199. [Google Scholar]
- Pope TR, 2000. Reproductive success increases with degree of kinship in cooperative coalitions of female red howler monkeys Alouatta seniculus. Behav Ecol Sociobiol 48:253–267. [Google Scholar]
- Qi XG, Garber PA, Ji W, Huang ZP, Huang K. et al. 2014. Satellite telemetry and social modeling offer new insights into the origin of primate multilevel societies. Nat Commun 5:5296–5296.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XG, Li BG, Garber PA, Ji WH, Watanabe K, 2009. Social dynamics of the golden snub-nosed monkey Rhinopithecus roxellana: female transfer and one-male unit succession. Am J Primatol 71:670–679. [DOI] [PubMed] [Google Scholar]
- Rousset F, 2008. GENEPOP ’007: a complete re-implementation of the genepop software for Windows and Linux. Mol Ecol Resour 8:103–106. [DOI] [PubMed] [Google Scholar]
- Sbeglia GC, Tang-Martinez Z, Sussman RW, 2010. Effects of food, proximity, and kinship on social behavior in ringtailed lemurs. Am J Primatol 72:981–991. [DOI] [PubMed] [Google Scholar]
- Silk JB, 2002. Kin selection in primate groups. Int J Primatol 23:849–875. [Google Scholar]
- Silk JB, Alberts SC, Altmann J, 2004. Patterns of coalition formation by adult female baboons in Amboseli, Kenya. Anim Behav 67:573–582. [Google Scholar]
- Silk JB, Altmann J, Alberts SC, 2006. Social relationships among adult female baboons Papio cynocephalus. I. Variation in the strength of social bonds. Behav Ecol Sociobiol 61:183–195. [Google Scholar]
- Strayer FF, Harris PJ, 1979. Social cohesion among captive squirrel monkeys Saimiri sciureus. Behav Ecol Sociobiol 5:93–110. [Google Scholar]
- Surbeck M, Hohmann G, 2011. Mothers matter! Maternal support, dominance status and mating success in male bonobos Pan paniscus. Proc R Soc Lond B Biol Sci 278:590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman RW, Garber PA, Cheverud JM, 2005. Importance of cooperation and affiliation in the evolution of primate sociality. Am J Phys Anthropol 128:84–97. [DOI] [PubMed] [Google Scholar]
- Taberlet P, Griffin S, Goossens B, Questiau S, Manceau V. et al. 1996. Reliable genotyping of samples with very low DNA quantities using PCR. Nucleic Acids Res 24: 3189–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CL, Zhang P, Li BG, Watanabe K, Wada K, 2003. A preliminary study on the social organization of Sichuan snub-nosed monkeys Rhinopithecus roxellana in Qinling, China. Am J Primatol 60:144. [Google Scholar]
- van Oosterhout C, Hutchinson WF, Wills DP, Shipley P, 2004. MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538. [Google Scholar]
- Wei W, Qi XG, Garber PA, Guo ST, Zhang P. et al. 2013. Supply and demand determine the market value of access to infants in the golden snub-nosed monkey Rhinopithecus roxellana. PLoS ONE 8: e65962., [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Qi XG, Guo ST, Zhao DP, Zhang P. et al. 2012. Market powers predict reciprocal grooming in golden snub-nosed monkeys Rhinopithecus roxellana. PLoS ONE 7:e36802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdig A, Nürnberg P, Krawczak M, Streich WJ, Bercovitch F, 2002. Affiliation and aggression among adult female rhesus macaques: a genetic analysis of paternal cohorts. Behaviour 139:371–391. [Google Scholar]
- Xi WZ, Li BG, Zhao DP, Ji WH, Zhang P, 2008. Benefits to female helpers in wild Rhinopithecus roxellana. Int J Primatol 29:593–600. [Google Scholar]
- Zhang P, Watanabe K, Li BG, Qi XG, 2008. Dominance relationships among one-male units in a provisioned free-ranging band of the Sichuan snub-nosed monkeys Rhinopithecus roxellana in the Qinling Mountains, China. Am J Primatol 70:634–641. [DOI] [PubMed] [Google Scholar]
- Zhang P, Watanabe K, Li BG, Tan CL, 2006. Social organization of Sichuan snub-nosed monkeys Rhinopithecus roxellana in the Qinling Mountains, Central China. Primates 47:374–382. [DOI] [PubMed] [Google Scholar]