Abstract
Objective
Persistent pain causes untold misery worldwide and is a leading cause of disability. Despite its astonishing prevalence, pain is undertreated, at least in part because existing therapeutics are ineffective or cause intolerable side effects. In this review, we cover new findings about the neurobiology of pain and argue that all but the most transient forms of pain needed to avoid tissue damage should be approached as a disease where a cure can be the goal of all treatment plans, even if attaining this goal is not yet always possible.
Design
We reviewed the literature to highlight recent advances in the area of the neurobiology of pain.
Results
We discuss barriers that are currently hindering the achievement of this goal, as well as the development of new therapeutic strategies. We also discuss innovations in the field that are creating new opportunities to treat and even reverse persistent pain, some of which are in late-phase clinical trials.
Conclusion
We conclude that the confluence of new basic science discoveries and development of new technologies are creating a path toward pain therapeutics that should offer significant hope of a cure for patients and practitioners alike.
Classification of Evidence. Our review points to new areas of inquiry for the pain field to advance the goal of developing new therapeutics to treat chronic pain.
Keywords: Neurobiology of Pain, Pain Cure, Peripheral Sensitization, Pain Centralization, Central Sensitization, Nociceptor
Introduction
Persistent pain affects as many as 100 million Americans and is equally prevalent in most of the developed world [1]. The cost of treatment of pain that fails to follow a normal healing process is more than expenses for diabetes, heart disease, and cancer combined in the United States, and such persistent pain is the leading cause of disability [2]. The most commonly used drugs to treat this type of pain are opioids, and opioid overdose is now a leading cause of death among young Americans [3]. Opioids are the most widely prescribed drugs for pain, with current estimates at nearly one opioid prescription per living American [4,5]. While opioids are not the only options for moderate to severe pain, other drugs are no more effective. In fact, for the gabapentinoids, which are top-line treatments for neuropathic pain, the number needed to treat in most meta-analyses is between 7 and 10 [6]. These issues present a devastating problem for patients, health care systems, and society.
One potential solution to this critical medical problem is a refocusing on the mechanisms that drive pain in patients. This can be achieved through basic research using preclinical models and by pushing forward with the development of human-based molecular neuroscience tools that can provide meaningful insight into mechanisms of pain in patients. We propose that this approach will lead to the generation of new therapeutic strategies. Such strategies could redefine pain treatment, reducing the burden that pain places on patients, health care workers, and society.
Do We Know Enough Already?
Pain patients are heterogeneous, presenting with a various combination of pain qualities, sensory symptoms, and other comorbidities. This heterogeneity contributes to the difficulty in the development of effective management strategies. It has been argued that this heterogeneity is a major, if not the primary cause of so many failed clinical trials [7]. Historically, pain patients have been grouped for treatment and clinical trials based on assumptions about the underlying cause of the pain (i.e., diabetes or a shingles outbreak) or the inclusion and exclusion criteria used to define a syndrome. Admittedly, even further subgrouping has been employed for the most general of pain syndromes, such as low back pain. But there remains a considerable amount of heterogeneity in patients with relatively narrowly defined pain syndromes such as trigeminal neuralgia: approximately 30% of patients with “classic trigeminal neuralgia” are unresponsive to microvascular decompression surgery, one of the most effective interventions for this particularly debilitating neuropathic pain syndrome [8]. Thus, neither underlying disease nor rigid inclusion and exclusion criteria appears to be particularly useful in guiding treatment decisions or designing better clinical trials [9].
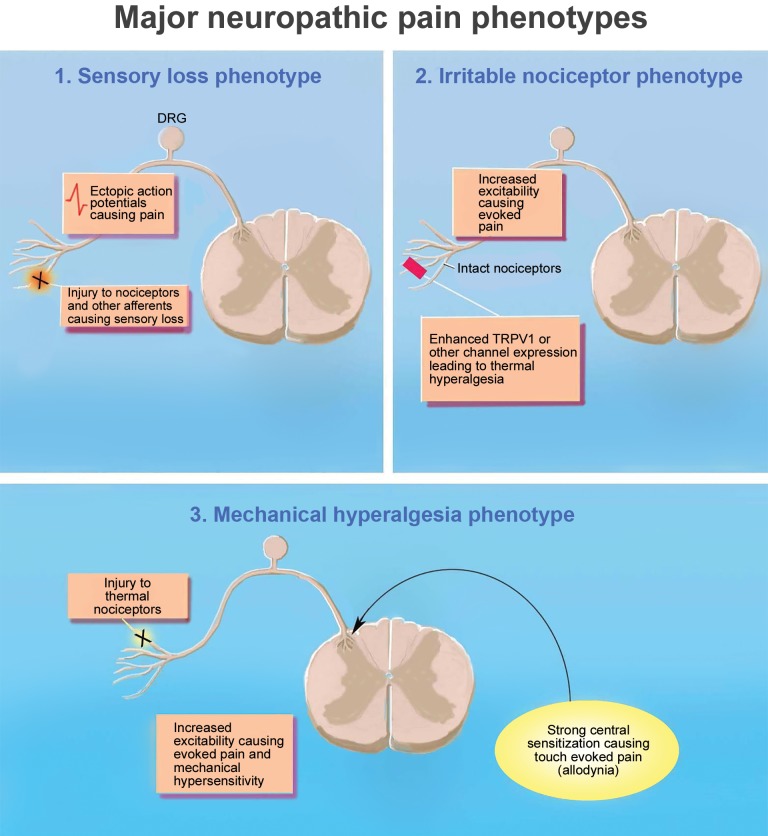
Baron and colleagues suggested a solution to this problem based on the premise that sensory symptoms and pain qualities are likely to reflect an underlying mechanism [10]. They suggested, and subsequently demonstrated, that it was possible to identify subgroups of pain patients based on symptoms, regardless of the underlying disease [7,10–13]. Based on the symptomology of 2,100 patients with painful diabetic neuropathy (DPN) and postherpetic neuralgia (PHN) gleaned from a cross-sectional survey (painDETECT), the investigators were able to identify five distinct subgroups of patients [13]. The pattern of symptoms was then used to suggest underlying mechanisms. For example, the prominent symptoms of subgroup 1 were spontaneous burning pain with only slight to moderate dynamic mechanical allodynia (DMA) and little, if any, evidence of numbness. This suggested a relatively intact peripheral nervous system characterized by the presence of “irritable nociceptors” that both contributed to the pain directly and maintained a state of central sensitization [13]. Based on these potential mechanisms, the authors suggested that compounds that mitigate sensitization could be used to treat these patients. Similarly, the authors suggested that drugs that reduce ectopic neuronal firing such as sodium channel blockers could be used in patients who fell into subgroup 2 because their prominent symptom was severe pain attacks. Interestingly, when a similar statistical approach was used to identify subgroups of neuropathic pain patients pooled from three multinational pain networks in which quantitative sensory testing was used as the primary means of assessing sensory symptoms, only three subgroups emerged [11]. The authors referred to these as clusters defined by the dominant sensory feature—sensory loss (cluster 1), thermal hyperalgesia/irritable nociceptor (cluster 2), or mechanical hyperalgesia (cluster 3)—but went on to describe a relatively complex combination of sensory features and potential mechanisms for each cluster (Figure 1). For example, patients in cluster 1 not only demonstrated clear signs of both small and large fiber loss, but also reported paradoxical heat sensations and mild dynamic mechanical allodynia in a few patients [11]. The mechanism implicated was a loss of central inhibitory tone, with spontaneous pain driven by ectopic activity arising proximal to sites of injury. The authors arguing for this more objective approach to the identification of patient subpopulations have been appropriately cautious, with a focus on the use of this approach for patient enrichment in clinical trials, rather than treatment per se. But the goal is the same where new drugs approved for the treatment of pain would cover a cluster rather than a disease state or syndrome [7,11,13].
Figure 1.
Clustering of neuropathic pain patients into three major subtypes. The EuroPain consortium identified three major types of neuropathic pain patients using a clustering analysis algorithm. These are defined by their dominant sensory feature, sensory loss (cluster 1), irritable nociceptor/thermal hyperalgesia (cluster 2), and mechanical hyperalgesia (cluster 3), but there are other dominant features found in these clusters that give further clues into mechanisms involved in neuropathic pain in these patients. PAG = periaqueductal grey.
Not Even Close…
Implicit in the assumption that approaching pain as a heterogeneous problem will lead to better management is that it is or ultimately will be possible to target the “mechanism(s)” responsible for the pain qualities and sensory symptoms that define a cluster. And while the authors make a very compelling case for classifying patients based on signs and symptoms rather than underlying disease or syndrome, the problem with this assumption is that the gap between pain qualities and sensory symptoms and mechanism is still too wide for this detailed assessment to be of much use for trials or treatment (Figure 2 highlights some divergent mechanisms that can lead to pain). That is, if the available preclinical and more mechanistic clinical data have taught us anything, it is that the approaches currently available to define subpopulations/clusters of patients do not enable identification of underlying mechanisms at a level of resolution that will be clinically meaningful [14]. This is because there are multiple ways of generating the same phenotype [14–16] and very compelling evidence that the specific mechanisms responsible for the same phenotype depend on a variety of factors, including time after injury, previous history, type of injury, site of injury, sex, and genetics.
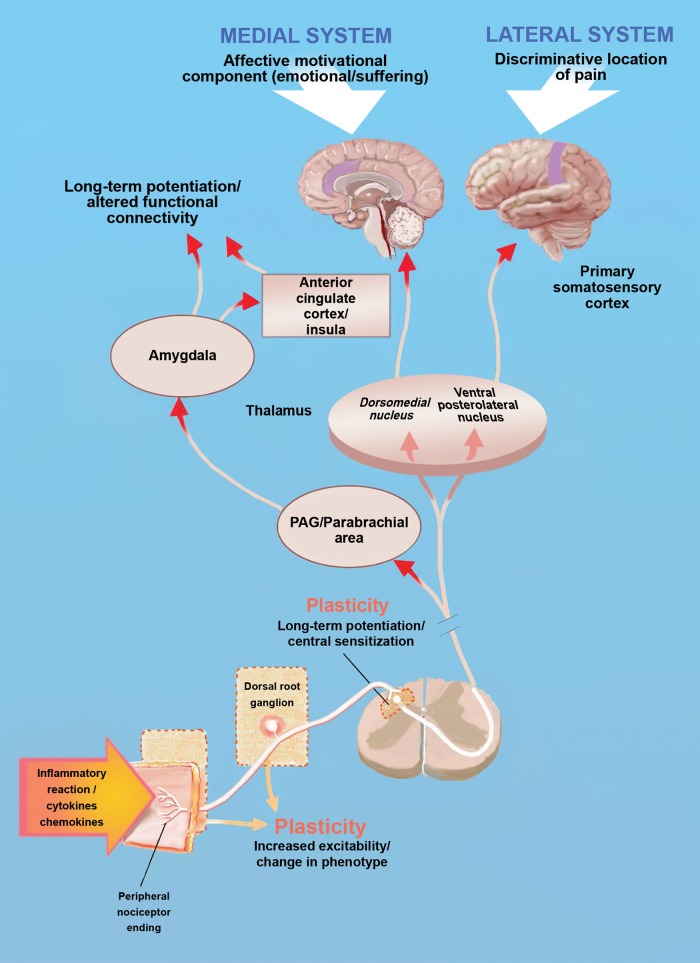
Figure 2.
Major areas of the ascending pain system and locations of plasticity. The diagram shows the basic anatomy of the ascending pain pathway with examples of locations where plasticity can occur, driving persistent pain. For instance, inflammation or injury to nociceptors can cause changes in the excitability and/or phenotype of these cells, causing them to fire action potentials to low-threshold stimuli and/or in the absence of any apparent stimulus (ectopic activity).
To illustrate the complexity of the problem, one need only consider subgroup 1 in the Baron study [13], which aligns with cluster 2 in the more recent clustering analysis [11]. This patient phenotype is characterized by so-called “irritable nociceptors” where the peripheral C-fibers have become hyperexcitable, causing the patient to experience thermal hyperalgesia and ongoing pain as a result of the sensitization and aberrant activity, respectively, in nociceptive afferents. From a basic mechanism perspective, this is an area where preclinical research has excelled in establishing a scientific foundation to help us understand this phenotype [17]. One of the features of this patient subgroup is heat hyperalgesia. This also happens to be one of the best understood pain phenotypes from a basic mechanism perspective. At its core, heat hyperalgesia can be linked, in almost every known case, to increased activity in the TRPV1 ion channel, mediated by phosphorylation of the channel, leading to increased probability of channel opening or a decrease in the channel’s temperature threshold, increased channel trafficking to the membrane, and/or increased expression of the TRPV1 channel [18]. But this simplicity rapidly devolves into a convoluted labyrinth of mechanisms that can achieve enhanced TRPV1 activity. The list of mediators capable of enhancing TRPV1 functional activity would create a table at least a page long, and the number of kinases capable of phosphorylating TRPV1 or regulating another kinase that can then phosphorylate TRPV1 would be equally long [18]. But you may be thinking, it need not be this complex, we need simply to create antagonists of TRPV1, and these will then at least solve the issue of thermal hyperalgesia. Unfortunately, this has proven to be an exceptionally challenging task. Although the list of TRPV1 antagonists that have been developed is quite long, most of these compounds cause hyperthermia in animals and humans, creating a serious safety concern for the use of these compounds in the clinic [19]. Other approaches may well prove to be useful. These include the use of agonists of TRPV1 that desensitize irritable nociceptors and the use of inhibitors of enzymes that create endogenous mediators that act on TRPV1 [20] to produce pain in patients with (or potentially without) irritable nociceptors. Potentially more problematic, however, is that thermal hyperalgesia ranks a distant fourth, behind ongoing pain, mechanical sensitivity, and cool sensitivity in the overall prevalence of complaints across all neuropathic pain patients [21,22], and even in the subpopulation in which it is a prominent symptom, there is good reason to think that addressing heat hyperalgesia would leave another major complaint of this patient intact, ongoing or spontaneous pain. To make matters worse, the complexity of TRPV1 signaling pales in comparison with that associated with potential mechanisms underlying ongoing pain.
Multiple Roads Lead to Irritable Nociceptors
What mechanisms drive ongoing pain in patients with irritable nociceptors (Figure 3)? Here, preclinical models have also led to the generation of a broad variety of mechanisms that can change the excitability of nociceptors, causing them to generate action potentials more readily or even without any detectable stimulus (ectopic activity) [17,23]. One of the best described mechanisms for this form of plasticity is a shift in the balance of ion channels that are expressed in the nociceptor, or even a certain area of the nociceptor, that causes the neuron to change its excitability profile. One of the first molecular descriptions of such a change was an increase in the expression of a voltage-gated Na+ channel, NaV1.3 [24], that was subsequently demonstrated to have biophysical properties consistent with observed increases in excitability [25]. This channel is developmentally regulated in sensory neurons, where it is expressed at high levels during development but is normally absent in the adult [24]. The dramatic upregulation of this channel in injured neurons was exactly the direction of change expected for a channel contributing to the emergence of ongoing pain following nerve injury, accounting for a shift in the balance of inhibitory and excitatory ion channels toward excitation. However, while a shift in the balance of inhibitory and excitatory ion channels appears to be a common mechanism underlying hyperexcitability, the increase in NaV1.3 is far from the only channel implicated. Other excitatory channels include the NaV1.6 [26,27], 1.7 [28–30], 1.8 [31–35], and 1.9 [36] subtypes of voltage-gated Na+ channels, T-type voltage-gated Ca2+ channels [37], and HCN channels [38–41]. Decreases in a variety of inhibitory, primarily K+ channels, have also been described, including those gated by voltage [42], Ca2+ [43], and ATP [44,45], as well as those mediating resting or leak currents [46,47] (see [48] for a recent comprehensive review of all of these mechanisms). Adding to this complexity is the observation that changes in expression are just one of the many mechanisms contributing to the shift in the balance of excitation and inhibition, where changes in channel properties [48–50] and distribution [26,31,51,52], as well as the relative localization with respect to other cellular processes such as Ca2+ release sites from the endoplasmic reticulum [53,54], may be just as, if not more important than, changes in expression.
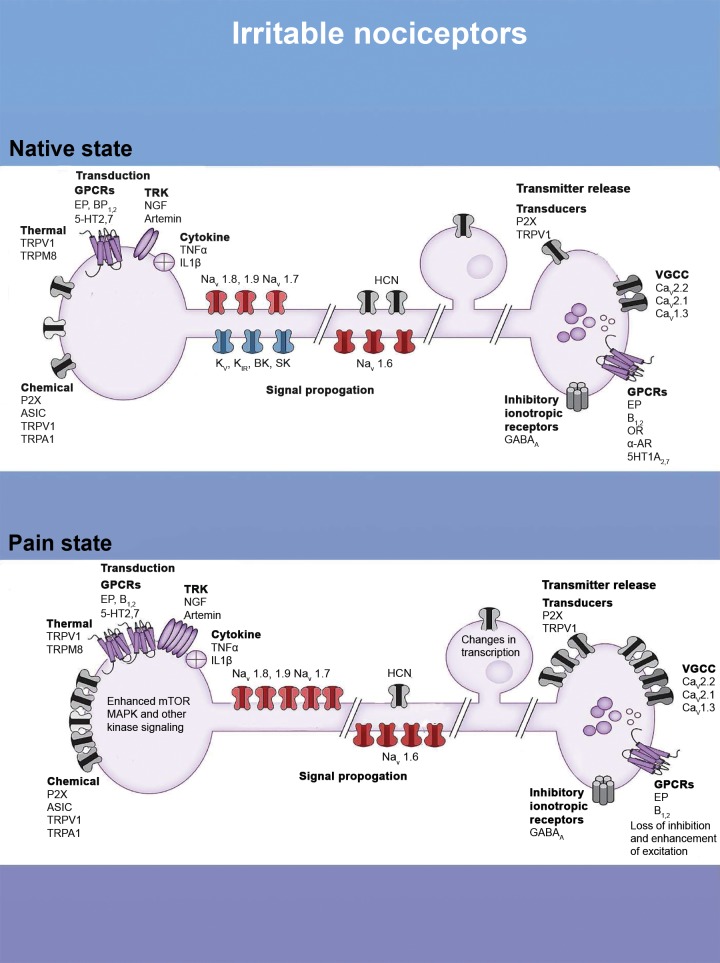
Figure 3.
The irritable nociceptor, molecular mechanisms. The diagram shows channels and modulatory proteins involved in pain transduction, signal propagation, and transmitter release in the spinal dorsal horn. The top panel shows these in the normal state, and the bottom panel shows how changes in these proteins can lead to an irritable nociceptor phenotype. Such changes include increased expression or activity in transducers like TRPV1 or P2X channels, increases in G-protein coupled receptor (GPCRs) like EP receptors, and enhanced signaling in nociceptor terminals. Increases in the expression of voltage-gated sodium channels (Navs) and decreased expression of potassium channels can also shift the balance toward excitation in these nociceptors. Finally, changes in expression of inhibitory and excitatory proteins in the central terminals of nociceptors can also enhance the irritability of these cells. CNS = central nervous system.
Of course, a consistent pattern of changes has also been described in excitatory and inhibitory ligand gated ion channels such as glutamate [55–58] and GABAA receptors [59,60]. The bulk of the data on excitatory ionotropic receptors has focused on the increase in N-methyl-D-aspartate (NMDA) receptors and their role in facilitating transmitter release from the central terminals of nociceptive afferents following nerve injury [55–58]. Similarly, the decrease in GABAergic inhibition of afferent terminals has also been implicated in the pain associated with nerve injury [59,60]. The result of both of these changes is the amplification of afferent input to the central nervous system (CNS). This form of a shift in the balance of excitation and inhibition is further complicated by the fact that changes in the machinery regulating the synthesis, storage, release, and re-uptake of transmitters may contribute as much to the shift in balance as the changes in receptor function. And of course, GABA signaling is also strongly influenced by factors that regulate the concentration of intracellular Cl− [61,62], including neuronal activity [63] and expression of NKCC1 [64] in primary afferents and KCC2 activity and expression in dorsal horn neurons, as described below.
In addition to ion channels, similar shifts in the balance of excitatory and inhibitory metabotropic receptor signaling have been described. The loss of inhibition, in the form of decreases in the expression of inhibitory receptors [65–67] and their second messenger machinery [68], has been most extensively documented. However, in the context of neuropathic pain, the emergence of excitatory receptor signaling has also been described, where adrenergic receptors have probably received the most attention [69–71]. What is particularly important about this shift in the balance of metabotropic receptor signaling is that these receptors are able to influence an array of other channels, and thereby have a cumulative effect on the net change in the balance of excitation and inhibition, as exemplified by expressing exogenous receptors that profoundly shift this balance by their simple presence in DRG neurons [72].
Finally, while not relevant to the manifestation of the irritable nociceptor, the “numbness” so commonly associated with neuropathy reflects a loss of low threshold mechanoreceptor input to the injured tissue [73,74]. The result is a shift in the balance between the low threshold input to the spinal cord that normally inhibits nociceptive input [75]. Given that the mechanical threshold of many polymodal nociceptive afferents is well within the range of innocuous stimulation [76], such a shift in the balance of excitation and inhibition of afferent input to the spinal cord may result in the emergence of burning pain. A standard nerve-cuff block can be used to demonstrate how profound the loss of low threshold afferent inhibition of high threshold input can be [75].
Potentially more problematic still is evidence that the relative contribution of mechanisms responsible for the shift in the balance of excitation and inhibition that may contribute to the manifestation of the irritable nociceptor may change over time and may be influenced by the site of injury as well as the type of injury. For example, NaV1.8 appears to be necessary for the initial maintenance of the excitability of uninjured afferents following a traumatic nerve injury [31], whereas the same channel may contribute to ongoing activity in injured afferents with time [77]. The result in this case is the same therapeutic target that may require different access routes over time. The observation that the Na+ blocking compounds carbamazepine and oxcarbamazepine may be relatively effective for the treatment of trigeminal neuralgia [78], yet far less so for the treatment of other types of neuropathic pain [79,80], argues that the site of injury may also influence underlying mechanisms. Another example of the impact of target of innervation on the mechanisms underlying the manifestation of the irritable nociceptor phenotype comes from a model of chemotherapeutic-induced peripheral neuropathy. The burning pain associated with this neuropathy is primarily restricted to the glabrous skin of the hands and feet [81]. Recent evidence suggests that differences in gene expression between nociceptors that innervate glabrous and hairy skin may determine the way that nociceptors respond to damage-associated molecular patterns (DAMPs) that promote nociceptor irritability [82,83]. These DAMPs are signaling proteins that are released by cells, including neurons, that act on other receptors to induce cellular signaling. Strong evidence supports a role of DAMP signaling via toll-like receptors (TLRs) to promote pain in chemotherapeutic-induced peripheral neuropathy.
With respect to the type of injury, traumatic nerve injury may lead to activation of immune cells, like macrophages, that profoundly alter the excitability of the nociceptor [16] while chemotherapeutics might lead to intrinsic changes in nociceptor excitability (possibly mediated by DAMPs) that create a similar neuropathic pain phenotype with different underlying mechanisms [84,85]. Similarly, ongoing burning pain is reported in patients suffering from traumatic nerve injuries as well as chemotherapeutic-induced peripheral neuropathy [21,22]. However, the cellular changes observed in the neurons that appear to be responsible for the pain associated with these different types of nerve injury are very different. For example, one set of Ca2+ regulatory proteins appears to be important for the manifestation of pain associated with traumatic nerve injury [53,54], while a different set of Ca2+ regulatory proteins has been implicated in chemotherapeutic-induced peripheral neuropathy [83].
One of the better examples of the impact of previous history on mechanisms that may contribute to the irritable nociceptor phenotype has been described in an experimental paradigm called “hyperalgesic priming.” This phenomenon refers to the impact of a previous injury on the response to a subsequent injury to the same tissue. Available evidence indicates that when nociceptors are exposed to factors like cytokines (e.g., interleukin 6 [IL6]) or growth factors (e.g., nerve growth factor [NGF]) released with the “priming” injury, they undergo a very long-lasting, if not permanent, change, even though the tissue appears to heal normally following the initial insult. Importantly, this change manifests when the tissue is challenged a second time as the neurons are not only more responsive to lower concentrations of inflammatory mediators, but they remain irritable in response to even a brief exposure to a single inflammatory mediator for 10 to 24 hours, compared with the normal 30 to 45 minutes [86–88]. This can lead to ongoing pain that appears to have no cause but may, in fact, be driven by inflammation that is below the normal detection threshold. The mechanisms that drive this change in the nociceptor phenotype involve many of the same signaling cascades that regulate acute changes in excitability via the phosphorylation of channels (e.g., mitogen-activated protein kinase signaling [MAPK]) [89], but the downstream targets are different. One of the more intriguing of these is signaling factors that lead to changes in local gene expression that are required to induce a primed state in these nociceptors [90,91]. This means that mechanisms driving augmented excitability acutely also lead to changes in gene expression that alter the phenotype of the nociceptor over the much longer term. An implication of this work is that the mechanisms underlying pain associated with these “memories” of prior injury may be very different from those underlying the pain associated with the initial injury. The nociceptor is irritable in both instances, but different therapeutic approaches may be needed to achieve the same degree of pain relief [86,88].
And as if all of this was not enough, sex and genetic background may influence the specific mechanisms underlying the irritable nociceptor phenotype. A recent example of the impact of sex is the effect of prolactin on nociceptors: Prolactin apparently has little, if any, impact on male nociceptors, but it robustly excites female nociceptors and causes pain specifically in female mice [92–94]. Similarly, the list of gain of function and loss of function genetic mutations in ion channels associated with the pain phenotypes, in particular those associated with burning pain, continues to grow [48,95]. Furthermore, it is now becoming clear that some other mutations in these channels do not cause a pain phenotype by themselves, but, in the context of injury, can lead to development of long-lasting neuropathic pain that is likely driven by development of nociceptor irritability [96]. And, of course, there is a range of polymorphisms in genes associated with pain signaling that can influence the balance of excitation and inhibition, tipping it toward the irritable nociceptor phenotype [97].
And Multiple Roads Lead to Central Sensitization
Yet another level of complexity is introduced when one considers changes within the CNS that may contribute to the manifestation of pain. Because many, if not all of the changes within the CNS are driven by aberrant activity in nociceptive afferents, the first level of complexity in this context reflects the variety of mechanisms summarized above that not only contribute to the emergence of aberrant afferent activity, but to aberrant activity in specific subpopulations of afferents. This is further compounded by the number of different neurons, local circuits, and distinct regions that serve as a substrate for afferent-driven changes, sources of amplification of afferent input, and changes in perception, which are most dramatically illustrated by the phenomenon of dynamic mechanical allodynia (DMA). DMA is the second most common symptom across all neuropathic pain patients [21,22] and is particularly troubling because it reflects the perception of pain in response to normally innocuous stimuli such as a gentle breeze on exposed skin.
The “mechanism” thought to account for the emergence of DMA is referred to as “central sensitization.” As DMA was one of the dominant features of patients who fell into subgroup 5 [13] or the “mechanical hyperalgesia” cluster 3 [11], it was suggested that this patient population might be most responsive to therapies targeting this “mechanism.” On the surface, this sounds as reasonable as targeting voltage-gated Na+ channels for the irritable nociceptor patient because they have been implicated in the bursts of afferent activity that underlie the pain attacks common to that patient subgroup [11,17]. Unfortunately, there are at least three related limitations stemming from this suggestion. The first is that the term central sensitization is now used so commonly by both basic pain researchers and clinicians to refer to any and all CNS processes implicated in an increase in the perception of pain [98–100] that it is of limited utility. Like many terms that have lost their meaning, this was not always so, as it was originally used to describe the long-lasting NMDA receptor–dependent increase in the response of dorsal horn neurons to afferent activity following tissue injury or intense noxious stimulation [101,102]. Second, a variety of other mechanisms have been implicated in the maintenance of the injury-induced increased response of dorsal horn neurons, and the available evidence suggests that the specific dorsal horn neurons that have been altered depend on the type of injury [15,103–105], and by definition, DMA must reflect more than a simple increase in the stimulus-response function of a dorsal horn neuron. That is, it must reflect a change in circuitry such that normally innocuous stimuli are able to engage a “pain” circuit [61]. Third, as noted above, cells and circuits throughout the CNS have been implicated in the amplification of nociceptive signaling.
With respect to spinal mechanisms, the majority of the early work in this area focused on stimulus-response functions, where potentiated responses can be short or long lasting. The shortest form of potentiation is most easily explained by a simple relief of Mg2+ block of NMDA receptors leading to recruitment of an additional ligand gated channel in response to presynaptically released glutamate. This process, referred to as “wind-up” [106,107], requires relatively high stimulation frequencies (i.e., 0.5 Hz), is observed during the delivery of repeated stimuli, and decays relatively rapidly following stimulus termination. Central sensitization, as originally defined, was induced with a single high-intensity burst of afferent activity and was associated with an increase in the response to afferent input that lasted anywhere from 45 to 180 minutes depending on the nerves stimulated [98,108,109]. Based on similarities between synaptic mechanisms of learning and memory observed in the hippocampus and the changes in the spinal cord dorsal horn, pain researchers adopted the learning and memory term “long-term potentiation” or “LTP” to refer to the long-term increase in synaptic responses observed in the dorsal horn [110–112]. LTP in projection neurons is mediated by activation of NMDA receptors, Ca2+ influx through these receptors, or from release from intracellular stores and engagement of signaling pathways that ultimately lead to increased trafficking of AMPA receptors to the postsynaptic membrane. It is perhaps unfortunate that this term is still used today to refer to plasticity in the spinal cord dorsal horn because data from subsequent research indicated that the changes in the dorsal horn are distinct from those observed in the hippocampus and other structures implicated in learning and memory. Plasticity in the dorsal horn can be induced by low-frequency stimulation [112], which would induce long-term depression at most of the CNS synapses. It appears to require at least some ongoing afferent input for its maintenance, as can be readily demonstrated in patients [113]. And it can be induced in a heterosynaptic fashion by recruitment of glial cells [114]. This may be a key process for the development of referred hyperalgesia after injury. Regardless, it is clear that additional processes are required for the manifestation of DMA because the above mechanisms still fail to explain a basic feature of DMA.
It has long been appreciated that the emergence of DMA must involve a change in spinal cord circuitry [115,116], and a number of different lines of evidence point to a decrease in inhibitory tone, primarily mediated by ionotropic GABA (GABAA) and glycine receptors, as a key mechanism underlying the change in circuitry. However, one of the more surprising findings to arise from the study of this process has been the discovery that glial cells may be involved. Both astrocytes and microglial cells are quite robustly activated by nerve injury and/or inflammation, and both of these cell types secrete mediators that alter synaptic transmission in the spinal dorsal horn [117]. While many glial-dependent mechanisms for this have been proposed, one that has gained particular prominence involves a relatively complex sequence of events. The process is initiated by a nerve injury–induced upregulation of CSF1 [118], interferon γ [119], or some other signaling molecule in primary afferents. These mediators drive an increase in the ionotropic purinergic receptor P2X4 in microglia [120]. P2X4 activation then results in the release of brain-derived neurotrophic factor (BDNF) from microglia that acts on dorsal horn neurons to, among other things [120], decrease the activity of the Cl− transporter KCC2 [62]. The decrease in KCC2 results in an increase in intracellular Cl− and a decrease in the efficacy of GABAergic and glycinergic inhibition in the dorsal horn [121,122]. This decrease in inhibition is thought to be one way in which low-threshold afferents may gain access to pain circuitry, resulting in DMA [61].
While the glial hypothesis has led to exciting research in the field, it has so far failed to lead to a clinical breakthrough. In fact, microglial inhibitors have failed to show efficacy in several clinical trials [123,124]. Available evidence suggests a number of potential reasons for this failure. In contrast to the robust activation of microglia in response to traumatic nerve injury, there is far less microglial activation in association with other forms of peripheral neuropathy [125–127]. Furthermore, even in models of traumatic nerve injury, microglial activation appears to be relatively transient, with evidence for astrocytes contributing to the hypersensitivity with time [128,129]. There are also recent data suggesting that microglia may only play a major role in promoting neuropathic pain in male mice [130,131]. Nevertheless, recent evidence suggesting that the specific circuit changes contributing to the emergence of DMA depend on the type of injury argues that the widespread activation of microglia and astrocytes is only part of the story.
An additional mechanism implicated in the emergence of DMA is changes in descending pain modulation. While descending inhibitory and facilitatory mechanisms have long been known to be important controllers of nociceptive thresholds and are targets for many clinically utilized drugs (e.g., opioids, norepinephrine reuptake inhibitors, and likely even cannabinoids), it has only recently been recognized that these systems are fundamentally involved in controlling the persistence of pain after injury [132]. For instance, descending facilitatory mechanisms are required for the persistence of neuropathic pain in the spinal nerve ligation model [133]. This apparent shift in the contribution of CNS circuitry relative to that of aberrant afferent activity has been used as an example of the “centralization” of pain, despite evidence for an essential, if not mandatory, role for ongoing afferent activity in the production of this type of pain [134,135]. Interestingly, there is evidence to suggest that glial cell activation at higher brain centers may also contribute to a shift in the influence of descending input to the spinal cord dorsal horn [136]. Even more interestingly, and potentially important from a clinical perspective, there is evidence that animals that fail to develop persistent neuropathic pain are protected by strong descending inhibitory controls that are able to actively suppress ongoing peripheral input from the injury as this nociceptive information enters the spinal dorsal horn [137]. Indeed, clinical evidence suggests that people who lack strong conditioned pain modulation (CPM), thought to be a reflection of the descending inhibition described in preclinical studies, are more likely to develop persistent pain after injury, such as surgery [138,139]. The therapeutic efficacy of serotonin/norepinephrine re-uptake inhibitors may reflect, at least in part, the facilitation of this descending, antinociceptive circuitry. And while the narrow efficacy of these drugs may reflect the limited involvement of these changes in the manifestation of neuropathic pain, and/or a shift toward facilitatory processes that are not sufficiently counterbalanced by an increase in descending inhibition, the predictive utility of CPM may enable the identification of strategies that mitigate the potential poor prognosis for pain after surgery. To the extent that CPM is engaged with cognitive interventions such as distraction, virtual reality, and mindfulness meditation [140,141], it is being exploited with some of the most effective pain management strategies available. [139].
While the brainstem periaqueductal grey, rostral ventral medulla (RVM), and adrenergic nuclei have received the most attention in the context of descending modulation of nociceptive signaling, there is also now strong evidence for synaptic plasticity throughout the brain in response to persistent activation of peripheral nociceptors. One of the best examples of this is the central nucleus of the amygdala (CeA). The CeA receives abundant nociceptive inputs, and stimulation of nociceptors produces synaptic plasticity in the CeA [142]. The CeA sends outputs to the basolateral amygdala, which then projects to the prefrontal cortex (PFC), where important processes involved in cognition are performed. Plasticity in the CeA drives altered inputs to the PFC, via the basolateral amygdala, which then changes inhibitory tone in the PFC [143]. The consequences of this are a change in network activity in the PFC and a negative impact on cognition. Therefore, persistent activation of nociceptors not only changes areas of the brain involved in pain perception but can also drives changes in the brain that alter basic functions, such as cognition, creating major comorbidities for patients [143].
There is also emerging evidence for changing circuitry in the brain as pain becomes persistent. One such example is the descending dopaminergic projections that come from the hypothalamus. While these projections normally are capable of producing analgesia that depends on D2 receptors, in hyperalgesic priming models, once animals become primed, this system plays a dominant role in promoting pain, now through the activation of D1/D5 receptors. Interestingly, this change happens as the dorsal horn seems to switch from transmitting pain signals through superficial projection neurons toward deeper dorsal horn neurons that project to many of the same areas of the brain but receive much stronger low-threshold inputs [144]. Another example also involves circuitry controlled by dopamine, the ventral tegmental area (VTA)—nucleus accumbens—PFC circuit. Here the Apkarian lab has shown through a series of elegant studies that high functional connectivity between nucleus accumbens and PFC predicts the transition to persistent chronic low back pain with up to 80% accuracy [145,146]. This suggests that connections between these two brain regions play a key role in amplifying pain information in the brain. Using preclinical neuropathic pain models, these researchers also showed that nerve injury increases excitability of cells in the nucleus accumbens and that inhibiting the activity of nucleus accumbens neurons reduces neuropathic pain [147,148].
Mechanisms Beyond Neuropathic Pain
Of course, this whole discussion has been focused on signs and symptoms of neuropathic pain, while additional mechanisms contribute to the far broader complexity of pain as reflected in muscular, joint, visceral, organ-specific, cancer, and other types of pain. These pain types may reflect dependence on different cells types [149], different populations of neurons [150], different circuits [15], etc., that might also be influenced by distinct factors depending on the condition. Pharmacological evidence in support of this is the relatively narrow therapeutic efficacy of triptans for migraine, carbamazepine for trigeminal neuralgia, and bisphosphonates for bone cancer pain, despite the fact that potential targets for these drugs are not only distributed throughout the body, but implicated in other pain syndromes. While it is not possible to go into all of these conditions here, we will focus on three that allow us to highlight important developments that give insight into mechanisms.
Headache, in particular migraine headache, is a debilitating neurological disorder that is approximately three times more prevalent in women than men. With mounting evidence against the vascular hypothesis of migraine, the field is currently split between those who argue that migraine is essentially a CNS disorder [151] and those who argue that at the least the pain of migraine must reflect the sensitization/activation of nociceptors that innervate the meninges [152,153]. Promising results with the calcitonin gene-related peptide (CGRP)/receptor antibodies in phase II trials may put this debate to bed once and for all (at least with respect to the subpopulation of migraineurs responsive to this new therapy) [154–157] as there is little evidence that there is sufficient antibody penetration of the blood-brain barrier to influence CGRP signaling within the CNS. The implication is that the initiation of a migraine attack in this subpopulation of patients involves peripheral CGRP signaling. Nevertheless, with respect to the latter hypothesis, there is not only evidence that the 5HT1B/D receptors, the primary targets for triptans, are differentially distributed [158,159], but that their therapeutic effects reflect a unique coupling between the receptors on dural afferents and the channels underlying the regulation of dural afferent excitability [160,161]. Interestingly, the ion channels mediating the sensitization of dural afferents also appear to be unique relative to those channels underlying the sensitization of afferents innervating other targets, including other craniofacial structures [153,162–166]. These differences underscore the impact target of innervation on both mechanisms responsible for pain, as well as the potential efficacy of therapeutic interventions.
That it may not only be necessary, but possible to selectively treat specific types of pain is illustrated by the extraordinary gains that have been made in understanding bone pain [167], in particular pain generated by cancer infiltration into bone [168]. It is now understood that this type of pain can be mechanistically organized along two principles: osteoclastic and osteoblastic bone pain. While these are both able to create nerve damage due to changes in bone structure, the types of nerve damage that develop are different and can lead to different mechanisms driving pain. In support of this, treatments that preserve bone, such as the bisphosphonates, have efficacy against metastatic bone disease that is primarily osteoclastic in nature [168]. While these treatments are far from a cure from this type of pain, they do suggest that appropriately targeting the mechanism can lead to a significant resolution of pain in patients.
A third example of how a more detailed mechanistic understanding of a pain syndrome may lead to more effective therapeutic interventions comes from the study of fibromyalgia. Because of the apparent absence of a peripheral driver for the widespread pain associated with this syndrome, it is often held up as a prime example of a “centralized” pain syndrome [169,170]. Changes in CNS structure [171,172] and function [170,173,174] have been used as evidence that fibromyalgia is a central pain syndrome. And while several cellular changes have been described in brain areas such as the ACC [175,176], the amygdala [143], and the RVM [136,137], the extent to which any of these changes identified in preclinical models contributes to the clinical manifestation of fibromyalgia remains to be determined. In addition to these central changes, recent findings suggest that at least some fibromyalgia patients may actually have a small fiber neuropathy that was not detectable with previously used methods [177–180]. Even more exciting is the evidence that at least some of this neuropathy may be due to autoimmunity [181–184]. These findings suggest a clear treatment strategy for at least a subpopulation of patients who have been relegated to “management” status. While much more work is needed along these lines, this innovative hypothesis could point to new mechanistic insight that could develop therapeutics that reverse, rather than palliatively treat, these disorders.
Can We Cure Pain? Three Major Barriers to Success
So, while the phenotyping of pain patients is an excellent start, it is clear that the tools currently available to identify subpopulations of pain patients are not sufficient to address the complexity of the problem or the underlying mechanisms. And while we remain convinced that it will ultimately be possible to cure all but the most transient forms of pain that protect us from injury or potential injury, achieving this goal will require overcoming three major barriers.
The first of these is that the concept of pain, and consequently its medical management, is still burdened by a variety of sociological issues. These range from the stigma attached to pain and beliefs about what it means to suffer and ask for help to the medical approach to pain, where pain was historically viewed as a symptom of other underlying pathology. In this latter context, there were concerns that treating the pain would mask the underlying pathology and/or the assessment of the efficacy of the intervention, as well as the fundamental belief that the only really viable strategy to treat pain was to appropriately treat the underlying cause. More recently, discussions over pain have revolved around the concept of chronic pain, which will be defined in the new ICD-11 as pain persisting more than three months but is more generally accepted to reflect pain that persists after the inciting processes have been resolved. The problems with a simple time-dependent definition of chronic pain are myriad but include the simple observations that most major injuries are associated with pain that persists well over three months. There are almost as many problems with the latter definition, not the least of which is that, in the context of the homeostatic system that is the human body, pain that persists following a perturbation such as that associated with an injury may simply be the body functioning at a new set point. It is at the point when it becomes “chronic,” however, that many have argued pain should be considered a disease in its own right, and treated as such. However, given that there are likely many more ways in which the nervous system may establish a new “set point” than there are ways to establish or maintain an “irritable nociceptor,” we suggest that conceptually the term chronic pain does not help with either diagnosis or treatment. As an alternative, we suggest that all but the most transient pain, necessary for the avoidance of tissue damage and that can be resolved with the termination of the stimulus, should be viewed as a disease. Viewed in this way, it is possible to leverage what we already know about the underlying mechanisms of pain to address, or at least weigh, the two issues fundamental to any disease; those are prognosis and treatment. As suggested by the discussion above, progress is needed on both, but viewing pain as a disease provides a framework for a more rapid implementation of knowledge.
The second major barrier to progress has been a shift in the general approach to pain away from even the possibility of a cure toward its management. This is certainly not true of pain patients. Nor is it true of all pain clinicians, with many still convinced that their approach will work for all. However, whether a reflection of the limited number of options available or the deleterious consequences of options recently relied upon as a viable approach to persistent pain (i.e., opioids), there has been a shift in focus from pain per se to quality of life in the presence of pain. The result is pain management strategies rather than pain relief strategies. And while pain management is essential, particularly when all available alternatives have been tried, we simply cannot lose sight of or stop working toward a cure.
The third barrier is the largest scientific challenge: the development of diagnostic tools that give meaningful insight into mechanism. Given all the factors that influence the manifestation of pain, it is essential that clinicians are able to appropriately assess and identify which of the many factors and potential mechanisms are contributing to pain at any given point in its course. Tremendous strides have been made in advanced imaging techniques, as well as high-resolution analysis of very small samples. The latter approach has enabled significant advances in the treatment of cancer where the tumor can almost always be biopsied, extensively characterized, and even genotyped. Unfortunately, even a small biopsy can be a problem for the nervous system. Thus, the development of diagnostic tools remains an area in need of significant investment.
Where Might These New Diagnostic Tools Come from?
One important emerging area is the development of theragnostics, or nanoparticles, that can be used to label cell types and deliver therapeutics to specific cells. Many theragnostics have already been developed that home to specific types of cells in the body. These theragnostics can deliver labels that allow for cellular imaging in a variety of different contexts. For instance, theragnostics that can specifically deliver fluorescent labels to macrophages can be imaged in living animals in a completely noninvasive fashion [185,186]. Imagine a patient with suspected irritable nociceptors driven by macrophage infiltration to a superficial nerve. An appropriate theragnostic could be used to test this diagnosis and then also used to deliver an appropriate drug to this specific cell type to reverse the pathology. In fact, this specific approach has already been used in preclinical models, suggesting that it can also be advantageously employed in the clinic [185].
Another important area of development is in biomarkers. There has been a strong emphasis in the past on developing a general biomarker for pain. We do not think that this is useful for most patients who are perfectly capable of telling the clinician that he or she has pain. The patient is likely even able to explain their pain in exquisite detail, but as suggested by the results of the Baron studies [11,13], pain descriptors alone do not provide sufficient insight into mechanism. Thus, biomarkers reflective of specific mechanisms remain an intriguing but elusive goal. One potentially exciting avenue of exploration has been microRNA, which appear to have unique profiles both locally at the site of injury [187–189] and systemically [190,191]. Interestingly, the patterns of microRNA may not only prove to be useful biomarkers, but because they are able to recapitulate phenotype, they may also reveal underlying mechanisms of complex disorders such as complex regional pain syndrome [190,191]. Similarly, given evidence that the contribution of microglia to neuropathic pain may not only reflect the type of injury [117,125,129] but also the sex of the injured [130,131], it may be possible to develop biomarkers that enable the identification of a subset of patients with microglial involvement. There is at least some evidence that such an approach may be within reach due to the development of positron emission tomography imaging ligands that can image microglial activation in humans [192].
Bio- and health informatics are growing fields where advances in our ability to handle “big data” are leading to novel discoveries that are often completely nonbiased in nature because modeling can be done based on all available variables. Let’s go back to the refined clustering analysis that identified three major subtypes of neuropathic pain patients based on quantitative sensory testing (QST) profiles. The authors of this work predictions about efficacy of drugs for each of these patient phenotypes [11]. Given the large network of clinics and hospitals where this work was based, it seems reasonable to retrospectively, and prospectively, group patients by QST variables, drug prescriptions and response, and all other available medical record variables to look for additional insight into which patients are likely to have a positive response to a specific intervention. While it is not possible to predict what kind of results might come from these types of analyses, the proverbial stars are aligning with technology and electronic health records to finally make large-scale projects like this possible. Another area where this technology could have a huge impact is prevention. It seems almost certain that studies such as the one proposed above will find factors that contribute to the prevention of persistent pain after injury. When such factors are discovered, mitigation strategies should be implemented widely because the best way to treat pain is to prevent it in the first place.
Focusing more on bio-informatics, the genomics revolution has enabled remarkable discovery in the pain field [97,193]. Highlights of this body of work are the identification of genes responsible for congenital insensitivities to pain and inherited pain disorders [95,96]. However, the genetics revolution has so far not had a profound impact on our ability to determine who will develop persistent pain. While there are likely many reasons for this, and the topic has been covered extensively elsewhere (see, for instance, [97,193]), one thing that is almost certainly true is that persistent pain is unlikely to be explained, in most cases, by the presence or absence of simple single gene variations in any given population. The explosion of next-generation sequencing techniques, which allow for identification of new gene variants or for direct, genome-wide assessment of gene expression using RNA sequencing, may give significant insight into this problem, where traditional genomics has failed. While the genome in the nervous system is relatively static, at least at the DNA sequence level, the transcriptome, defined as all of the RNA found in a cell, is remarkably dynamic and can give insight into changes in phenotype and function that genome sequencing cannot resolve [194,195]. The problem here is, again, that nervous system tissues cannot be “sampled” with available technology to enable application of the highly sensitive RNA-sequencing approaches currently available. However, other samples may be very instructive. For instance, as mentioned above, the immune system is thought to be a major driver of many forms of persistent pain [16]. Sampling and sequencing the RNA from immune cells from pain patients may give significant insight into the changes in their immune system that cause these cells to interact with the nociceptors driving pain. Certain immune cells may also tell us interesting things about the nervous system. Along these lines, in a remarkable paper, Laura Stone and colleagues showed that the epigenomic landscape of PFC nervous tissue and peripheral T cells were remarkably similar months after the development of neuropathic pain in mice [196]. While this is a long way from human validation, T cells are readily accessible in human patients and may be able to give us a window into molecular pathology in a tissue that would otherwise not be obtainable until after the patient dies. Finally, returning to the primary afferent, it is now understood that at least some of the RNA that is made in the nucleus of these neurons is transported into their axons [197–199]. As these axons can be sampled by tissue biopsy, it is possible that simple skin biopsies that are routinely taken from neuropathic pain patients for epidermal nerve fiber assessment could also be submitted to RNA sequencing to examine transcriptional changes in RNAs that are found in the axons of DRG neurons in different pain pathologies. As the cost of next-generation sequencing continues to fall and the machines needed to do this become more ubiquitous, it is almost certain that this technology will gain a strong foothold in basic and clinical pain research.
From Mechanism to Cure
We believe that the emphasis on managing pain is useful because patients must have some hope for treatment in the absence of cures. However, we also think that this emphasis, along with understandable disappointment at failed clinical trials, has created a loss of optimism in the possibility of developing new and better therapeutic strategies. As recently highlighted by the director of The National Institute of Drug Abuse, new medicines for pain are desperately needed and the sheer volume of the need will continue to accelerate [200]. But while there remain significant barriers to progress and much work still needs to be done, we also believe there is reason to be optimistic about cures for pain. This optimism comes from recent successes in mechanism-based therapeutics. These include very successful trials for anti–nerve growth factor (NGF) therapies in arthritis, low back pain, and several other pain conditions [201–204], successes of anti-CGRP therapies for migraine pain [154–157], and early but exciting data on Nav1.7 inhibitors [205]. What is distinct about these mediators and their clinical success is that they all have a strong foundation in basic science, where the mechanism has been linked to the pain phenotype in animal models and in humans. This is in contrast to, for example, the fatty acid amide hydrolase inhibitors that were shown to be effective in certain preclinical models and then applied in the clinic in a patient population where there was little preclinical evidence for efficacy (in this case, osteoarthritis), and the therapeutic ultimately failed in clinical trials [206]. As we continue to gain evidence for specific overlapping pain mechanisms in humans and in animal models, this gives increasing confidence that these therapeutics targeting these mechanisms can follow the route of anti-NGF, -CGRP, and -Nav1.7 medicines toward the clinic. While it is always possible that these therapeutics can be derailed by safety issues (see, for instance, the continuous safety issues regarding anti-NGF therapies [207]), the very strong evidence for efficacy that is already building demonstrates that it is possible to have a large impact on pain, including a reversal of pain, by targeting specific pain-promoting mediators that are key to certain pain types (Figure 4).
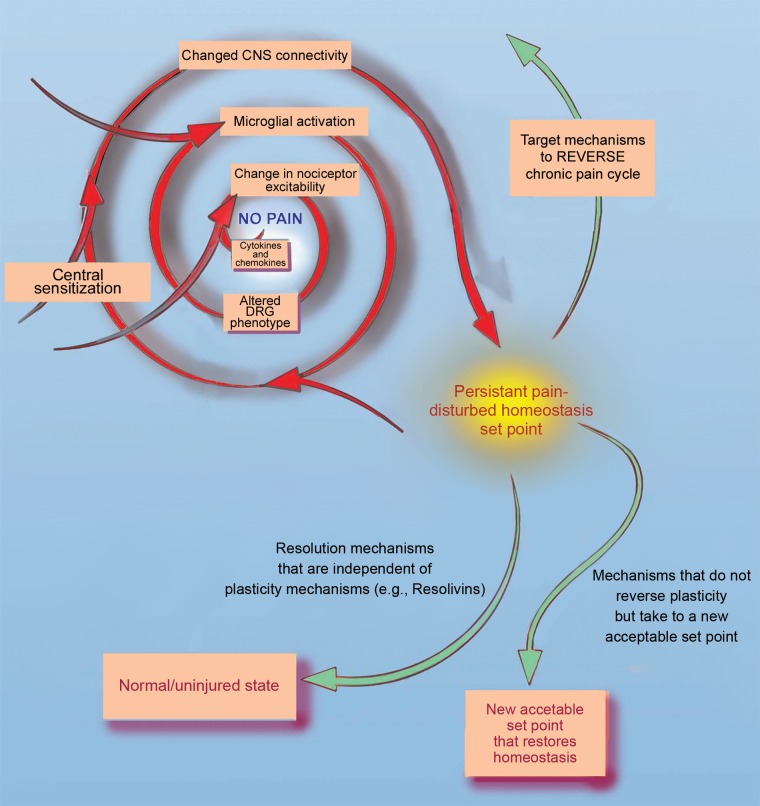
Figure 4.
Mechanisms driving pain and three opportunities to reverse chronic or persistent pain. The cycle at the top left shows many mechanisms that can lead to persistent pain. One way that treatments can reverse persistent pain would be to directly target those mechanisms that caused the pain to become persistent to effectively reverse the cycle. Another way would be to employ endogenous resolution mechanisms, like resolvins, to reverse persistent pain in a manner that is not dependent on its cause. Finally, treatments that can take persistent pain to a new, acceptable set point could also be engineered. These might include employing viral vectors to introduce optogenetic control of nociceptor activity in persistent pain conditions.
Given the very likely possibility that much, if not all, pain reflects a loss of homeostasis and/or the establishment of a new homeostatic set point, another potentially productive strategy for the development of more effective pain treatments is to focus on restoration of “normal” homeostasis. We would argue that the emerging therapeutics do just that by normalizing NGF or CGRP signaling or neuronal excitability. However, emerging technologies suggest even more directed approaches.
One of these is found in our expanding ability to create molecules that have positive or negative allosteric modulatory effects and/or signaling pathway–specific mechanisms on neurotransmitter systems. An important example of this is in the emerging new classes of opioid analgesics that positively modulate the receptors to achieve activation of specific signaling pathways that are involved in analgesia while avoiding others that are involved in addiction, the generation of somnolence, or even the slowing of gastrointestinal transit [208]. The creation of so-called biased ligands would undoubtedly have a huge impact on pain treatment because they could allow for the achievement of a new set point for pain modulation in the brain that may effectively compensate for loss of inhibitory tone or amplification of plasticity in the nucleus accumbens. This is just one of many examples that could be employed from this emerging area of pharmacology.
Another pharmacological approach has emerged from the identification of molecules that appear to function as “master switches” for the regulation of whole processes. This approach is particularly important because it is becoming increasingly clear that the multiple mechanisms that can lead to nociceptor hyperexcitability can likely not be targeted individually to achieve resolution of pain [209]. What is needed is to take advantage of factors that might be able to reverse this process once the system that has been perturbed. Potential factors include the resolvins [210] and anti-inflammatory immune factors like IL-10 [211–214]. In some instances, these endogenous resolution pathways may have failed to turn on or have been inefficient when they did turn on. The strategy then is to provide the mediators exogenously or engineer new ways to turn on these pathways so as to facilitate normal resolution of a pain state. In this way, our increasing knowledge of endogenous mechanisms that mediate the resolution of inflammation and pain may provide opportunities for disease modification that can function independently of the mechanisms that caused the pain to become persistent (Figure 3). In a similar vein, signaling pathways that maintain persistent pain have been discovered that show striking similarity across preclinical models. Two examples of such pathways are the mechanistic target of rapamycin (mTOR) and mitogen-activated protein kinase (MAPK) pathways [215]. While it may be difficult to target these pathways individually, an endogenous negative regulator of these pathways exists and can be activated by a variety of drugs, including one that is widely clinically available. This kinase, called adenosine monophosphate-activated protein kinase (AMPK), profoundly decreases the activity of two other kinases, mTOR and MAPK, in nociceptors and activators of AMPK have disease-modifying properties in neuropathic and postsurgical pain preclinical models [216,217]. These three examples highlight the potential of this relatively new and exciting line of investigation. It is likely that additional pain resolution pathways exist that will create further opportunities for discovery and therapeutic development.
Two other exciting developments are the fields of optogenetics and chemogenetics, which are developing so rapidly and impacting so many aspects of biomedicine that their impact is nothing short of a revolution. Optogenetics refers to the control of neuronal (or other cellular activity) through engineered ion channels or pumps that are activated by light [218]. The power of this approach comes from the fact that these channels and pumps can be used to both excite and inhibit cells and that they do so with very precise temporal parameters in response to very specific wavelengths of light. In this way, it becomes possible to excite and inhibit different cells at the same time, or even the same cell to more precisely control output. Chemogenetics refers to the control of neuronal (or other cellular activity) through engineered receptors that are only activated by exogenous compounds that do not act on any other receptor or protein in the body. These receptors, now commonly referred to as designer receptors exclusively activated by designer drugs, or DREADDs, may also be excitatory or inhibitory. The most widely used of these DREADDs were generated from the G-protein coupled muscarinic acetyl choline receptor [219]. Mutations in the ligand binding domain rendered this receptor unresponsive to any endogenous ligands but responsive to a drug, clozapine-N-oxide, that acts at no other receptors in the body. Mutations in the cell signaling domain enable the receptor to be used to drive either endogenous inhibitory or excitatory signaling cascades. Thus, like optogenetics, chemogenetics can be used to excite or inhibit targeted cells, but with a drug that can be given systemically [219]. And while this is a clear advantage of this technology, enabling the receptors to be activated for longer periods of time, there is far less control over the temporal dynamics or magnitude of receptor activation. Furthermore, we have recently demonstrated that at least one of the GPCRs–based DREADDs is not as benign as originally anticipated, with the mere expression of the receptor enough to drive changes in ion channel density and endogenous cell signaling [72]. Nevertheless, in basic neuroscience, the development of these tools has led to entirely new ways to interrogate neural circuits and new discoveries about the connectivity of the brain. Importantly, for optogenetics, technological advances are keeping pace with the increasing precision with which it is possible to control protein functions with light. For instance, groups led by Robert Gereau, Michael Bruchas, and John Rogers have created fully implantable, miniaturized, wirelessly controlled devices that are biocompatible and operational for up to several months in vivo and that can deliver light pulses continuously to control neuronal activity in virtually any setting [220–222]. These devices have already been tested with inhibitory optogenetic channels in preclinical models and are effective in reducing many different kinds of pain [220–223]. Given the ability to not only precisely control cell activity but to do so in specific populations of cells, when these approaches are finally employed in patient populations, we will wonder why we even bothered with the electrical stimulators still so widely used today. In the context of restoration of homeostasis in the nervous system, this would be close to an ideal approach because of the possibility of restoring “normal” patterns of activity. All that said, despite how tangibly close this novel approach feels, there remains a significant barrier to its implementation, and that is the vehicle for gene delivery. Modified viruses remain the strategy of choice, where a renewed focus on viral vector delivery for use in human populations may be all that is needed. But novel strategies may ultimately enable avoiding this potentially problematic delivery system (Figure 5).
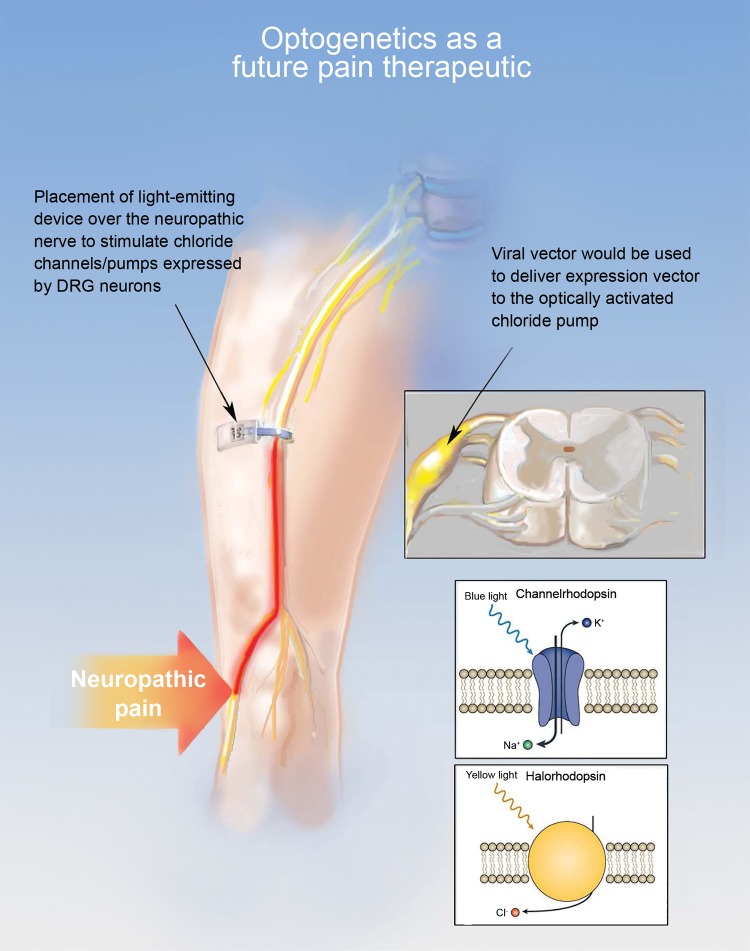
Figure 5.
Optogenetic control of nociceptors in vivo using implantable LED devices. The diagram shows a neuropathic pain patient with the irritable nerve at the site indicated with the large arrow. The implantable LED device is placed along the nerve and the DRG is transduced with a vector to allow for expression of halorhodopsin which causes Cl- influx to the cell in response to light. The combination of the implantable LED and the expressed halorhodopsin allows for termination of the pain signal at the site of the LED through a strong inhibitory current produced by the exogenous channel.
Finally, while stem cell therapy may never enable restoration of CNS function lost to trauma or disease, considerably more promising results have been generated in the context of pain. Basbaum and colleagues have recently demonstrated that a unique population of embryonic stem cells destined to become inhibitory neurons in the brain not only become inhibitory neurons in the spinal cord, but help normalize inhibitory tone in the spinal cord disrupted by neve injury [224–226]. And while access to these cells may be difficult for therapeutic purposes, two groups have obtained promising results with bone marrow–derived stromal stem cells that utilize a TGFβ-dependent signaling mechanism to normalize changes in the spinal cord that arise from nerve injury [227,228].
Conclusion: Cures for Pain Are Attainable
We began this review by considering the potential of using phenotyping to guide both trials and treatment of neuropathic pain patients. However, this consideration served more as a vehicle to not only illustrate the complexity of the picture of pain processing that has emerged from the study of pain over the last several decades, but also illustrate why this approach is doomed to fail if employed only with available technologies. We went on to describe additional barriers to progress in finding a cure for the disease of pain. We ended, however, with all the reasons to be optimistic about the development of diagnostic tools that will enable us to appropriately treat patients, as well as novel approaches for treatment. These are incredibly exciting times to be working for a cure. Our only hope is that society has the will to commit the resources necessary to enable the achievement of this goal.
Acknowledgments
This work was supported by National Institutes of Health grants R01NS065926 (TJP), R01GM102575 (TJP), R01DK107966 (MSG), and R01NS083347 (MSG). We thank Bill Winn (billwinn.com) for assistance with the Figures in this manuscript.
Funding Sources: Work described in the manuscript was supported by the National Institutes of Health R01NS065926 (TJP), R01GM102575 (TJP), R01DK107966 (MSG) and R01NS083347 (MSG) and the Global Pain Foundation.
References
- 1. Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: Institute of Medicine of the National Academies; 2011. [PubMed] [Google Scholar]
- 2. Steglitz J, Buscemi J, Ferguson MJ.. The future of pain research, education, and treatment: A summary of the IOM report “Relieving pain in America: A blueprint for transforming prevention, care, education, and research.” Transl Behav Med 2012;2:6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Case A, Deaton A.. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc Natl Acad Sci U S A 2015;112:15078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rudd RA, Aleshire N, Zibbell JE, Gladden RM.. Increases in drug and opioid overdose deaths—United States, 2000–2014. MMWR Morb Mortal Wkly Rep 2016;64:1378–82. [DOI] [PubMed] [Google Scholar]
- 5. Volkow ND. America’s addiction to opioids: Heroin and prescription drug abuse. 2014. Available at: http://www.drugabuse.gov/National Institutes of Health (accessed September 30, 2017).
- 6. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol 2015;14:162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. von Hehn CA, Baron R, Woolf CJ.. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 2012;73:638–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zakrzewska JM, Coakham HB.. Microvascular decompression for trigeminal neuralgia: Update. Curr Opin Neurol 2012;25:296–301. [DOI] [PubMed] [Google Scholar]
- 9. Helfert SM, Reimer M, Hoper J, Baron R.. Individualized pharmacological treatment of neuropathic pain. Clin Pharmacol Ther 2015;97:135–42. [DOI] [PubMed] [Google Scholar]
- 10. Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain 2006;123:231–43. [DOI] [PubMed] [Google Scholar]
- 11. Baron R, Maier C, Attal N, et al. Peripheral neuropathic pain: A mechanism-related organizing principle based on sensory profiles. 2017;1582:261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reimer M, Helfert SM, Baron R.. Phenotyping neuropathic pain patients: Implications for individual therapy and clinical trials. Curr Opin Support Palliat Care 2014;8:124–9. [DOI] [PubMed] [Google Scholar]
- 13. Baron R, Tolle TR, Gockel U, Brosz M, Freynhagen R.. A cross-sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: Differences in demographic data and sensory symptoms. Pain 2009;146:34–40. [DOI] [PubMed] [Google Scholar]
- 14. Sommer C. Exploring pain pathophysiology in patients. Science 2016;354:588–92. [DOI] [PubMed] [Google Scholar]
- 15. Peirs C, Seal RP.. Neural circuits for pain: Recent advances and current views. Science 2016;354:578–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ji RR, Chamessian A, Zhang YQ.. Pain regulation by non-neuronal cells and inflammation. Science 2016;354:572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gold MS, Gebhart GF.. Nociceptor sensitization in pain pathogenesis. Nat Med 2010;16:1248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Julius D. TRP channels and pain. Annu Rev Cell Dev Biol 2013;29:355–84. [DOI] [PubMed] [Google Scholar]
- 19. Bevan S, Quallo T, Andersson DA.. Trpv1. Handb Exp Pharmacol 2014;222:207–45. [DOI] [PubMed] [Google Scholar]
- 20. Hargreaves KM, Ruparel S.. Role of oxidized lipids and TRP channels in orofacial pain and inflammation. J Dent Res 2016;95:1117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Backonja MM, Attal N, Baron R, et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain 2013;154:1807–19. [DOI] [PubMed] [Google Scholar]
- 22. Backonja MM, Stacey B.. Neuropathic pain symptoms relative to overall pain rating. J Pain 2004;5:491–7. [DOI] [PubMed] [Google Scholar]
- 23. Campbell JN, Meyer RA.. Mechanisms of neuropathic pain. Neuron 2006;52:77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Waxman SG, Kocsis JD, Black JA.. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is reexpressed following axotomy. J Neurophysiol 1994;72:466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cummins TR, Aglieco F, Renganathan M, et al. Nav1.3 sodium channels: Rapid repriming and slow closed-state inactivation display quantitative differences after expression in a mammalian cell line and in spinal sensory neurons. J Neurosci 2001;21:5952–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Henry MA, Freking AR, Johnson LR, Levinson SR.. Sodium channel Nav1.6 accumulates at the site of infraorbital nerve injury. BMC Neurosci 2007;8:56.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xie W, Strong JA, Zhang JM.. Local knockdown of the NaV1.6 sodium channel reduces pain behaviors, sensory neuron excitability, and sympathetic sprouting in rat models of neuropathic pain. Neuroscience 2015;291:317–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Persson AK, Gasser A, Black JA, Waxman SG.. Nav1.7 accumulates and co-localizes with phosphorylated ERK1/2 within transected axons in early experimental neuromas. Exp Neurol 2011;230:273–9. [DOI] [PubMed] [Google Scholar]
- 29. Minett MS, Nassar MA, Clark AK, et al. Distinct Nav1.7-dependent pain sensations require different sets of sensory and sympathetic neurons. Nat Commun 2012;3:791.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee JH, Park CK, Chen G, et al. A monoclonal antibody that targets a NaV1.7 channel voltage sensor for pain and itch relief. Cell 2014;157:1393–404. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31. Gold MS, Weinreich D, Kim CS, et al. Redistribution of Na(V)1.8 in uninjured axons enables neuropathic pain. J Neurosci 2003;23:158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lai J, Gold MS, Kim CS, et al. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. Pain 2002;95:143–52. [DOI] [PubMed] [Google Scholar]
- 33. Roza C, Laird JM, Souslova V, Wood JN, Cervero F.. The tetrodotoxin-resistant Na+ channel Nav1.8 is essential for the expression of spontaneous activity in damaged sensory axons of mice. J Physiol 2003;550:921–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McGaraughty S, Chu KL, Scanio MJ, et al. A selective Nav1.8 sodium channel blocker, A-803467 [5-(4-chlorophenyl-N-(3,5-dimethoxyphenyl)furan-2-carboxamide], attenuates spinal neuronal activity in neuropathic rats. J Pharmacol Exp Ther 2008;324:1204–11. [DOI] [PubMed] [Google Scholar]
- 35. Thakor DK, Lin A, Matsuka Y, et al. Increased peripheral nerve excitability and local NaV1.8 mRNA up-regulation in painful neuropathy. Mol Pain 2009;5:14.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dib-Hajj SD, Black JA, Waxman SG.. NaV1.9: A sodium channel linked to human pain. Nat Rev Neurosci 2015;16:511–9. [DOI] [PubMed] [Google Scholar]
- 37. Todorovic SM, Jevtovic-Todorovic V.. T-type voltage-gated calcium channels as targets for the development of novel pain therapies. Br J Pharmacol 2011;163:484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jiang YQ, Sun Q, Tu HY, Wan Y.. Characteristics of HCN channels and their participation in neuropathic pain. Neurochem Res 2008;33:1979–89. [DOI] [PubMed] [Google Scholar]
- 39. Mazo I, Rivera-Arconada I, Roza C.. Axotomy-induced changes in activity-dependent slowing in peripheral nerve fibres: Role of hyperpolarization-activated/HCN channel current. Eur J Pain 2013;17:1281–90. [DOI] [PubMed] [Google Scholar]
- 40. Emery EC, Young GT, Berrocoso EM, Chen L, McNaughton PA.. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science 2011;333:1462–6. [DOI] [PubMed] [Google Scholar]
- 41. Chaplan SR, Guo HQ, Lee DH, et al. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci 2003;23:1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Laumet G, Garriga J, Chen SR, et al. G9a is essential for epigenetic silencing of K(+) channel genes in acute-to-chronic pain transition. Nat Neurosci 2015;18:1746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cao XH, Chen SR, Li L, Pan HL.. Nerve injury increases brain-derived neurotrophic factor levels to suppress BK channel activity in primary sensory neurons. J Neurochem 2012;121:944–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sarantopoulos C, McCallum B, Sapunar D, Kwok WM, Hogan Q.. ATP-sensitive potassium channels in rat primary afferent neurons: The effect of neuropathic injury and gabapentin. Neurosci Lett 2003;343:185–9. [DOI] [PubMed] [Google Scholar]
- 45. Zoga V, Kawano T, Liang MY, et al. KATP channel subunits in rat dorsal root ganglia: Alterations by painful axotomy. Mol Pain 2010;6:6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Amir R, Liu CN, Kocsis JD, Devor M.. Oscillatory mechanism in primary sensory neurones. Brain 2002;125:421–35. [DOI] [PubMed] [Google Scholar]
- 47. Acosta C, Djouhri L, Watkins R, et al. TREK2 expressed selectively in IB4-binding C-fiber nociceptors hyperpolarizes their membrane potentials and limits spontaneous pain. J Neurosci 2014;34:1494–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Waxman SG, Zamponi GW.. Regulating excitability of peripheral afferents: Emerging ion channel targets. Nat Neurosci 2014;17:153–63. [DOI] [PubMed] [Google Scholar]
- 49. Stamboulian S, Choi JS, Ahn HS, et al. ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel Na(v)1.7 and alters its gating properties. J Neurosci 2010;30:1637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gold MS, Reichling DB, Shuster MJ, Levine JD.. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci U S A 1996;93:1108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brittain JM, Duarte DB, Wilson SM, et al. Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca(2)(+) channel complex. Nat Med 2011;17:822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bauer CS, Nieto-Rostro M, Rahman W, et al. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J Neurosci 2009;29:4076–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rigaud M, Gemes G, Weyker PD, et al. Axotomy depletes intracellular calcium stores in primary sensory neurons. Anesthesiology 2009;111:381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gemes G, Rigaud M, Weyker PD, et al. Depletion of calcium stores in injured sensory neurons: Anatomic and functional correlates. Anesthesiology 2009;111:393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xie JD, Chen SR, Chen H, Zeng WA, Pan HL.. Presynaptic N-Methyl-d-aspartate (NMDA) receptor activity is increased through protein Kinase C in paclitaxel-induced neuropathic pain. J Biol Chem 2016;291:19364–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gong K, Kung LH, Magni G, Bhargava A, Jasmin L.. Increased response to glutamate in small diameter dorsal root ganglion neurons after sciatic nerve injury. PLoS One 2014;9:e95491.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen W, Walwyn W, Ennes HS, et al. BDNF released during neuropathic pain potentiates NMDA receptors in primary afferent terminals. Eur J Neurosci 2014;39:1439–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yan X, Jiang E, Gao M, Weng HR.. Endogenous activation of presynaptic NMDA receptors enhances glutamate release from the primary afferents in the spinal dorsal horn in a rat model of neuropathic pain. J Physiol 2013;591:2001–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fukuoka T, Tokunaga A, Kondo E, et al. Change in mRNAs for neuropeptides and the GABA(A) receptor in dorsal root ganglion neurons in a rat experimental neuropathic pain model. Pain 1998;78:13–26. [DOI] [PubMed] [Google Scholar]
- 60. Chen JT, Guo D, Campanelli D, et al. Presynaptic GABAergic inhibition regulated by BDNF contributes to neuropathic pain induction. Nat Commun 2014;5:5331.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Price TJ, Prescott SA.. Inhibitory regulation of the pain gate and how its failure causes pathological pain. Pain 2015;156:789–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J.. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci 2014;15:637–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhu Y, Zhang XL, Gold MS.. Activity-dependent hyperpolarization of EGABA is absent in cutaneous DRG neurons from inflamed rats. Neuroscience 2014;256:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pitcher MH, Cervero F.. Role of the NKCC1 co-transporter in sensitization of spinal nociceptive neurons. Pain 2010;151:756–62. [DOI] [PubMed] [Google Scholar]
- 65. Lee CY, Perez FM, Wang W, et al. Dynamic temporal and spatial regulation of mu opioid receptor expression in primary afferent neurons following spinal nerve injury. Eur J Pain 2011;15:669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Uchida H, Ma L, Ueda H.. Epigenetic gene silencing underlies C-fiber dysfunctions in neuropathic pain. J Neurosci 2010;30:4806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang Y, Chen SR, Laumet G, Chen H, Pan HL.. Nerve injury diminishes opioid analgesia through lysine methyltransferase-mediated transcriptional repression of mu-opioid receptors in primary sensory neurons. J Biol Chem 2016;291:8475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liu Z, Wang F, Fischer G, Hogan QH, Yu H.. Peripheral nerve injury induces loss of nociceptive neuron-specific Galphai-interacting protein in neuropathic pain rat. Mol Pain 2016;12: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sato J, Perl ER.. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science 1991;251:1608–10. [DOI] [PubMed] [Google Scholar]
- 70. Birder LA, Perl ER.. Expression of alpha2-adrenergic receptors in rat primary afferent neurones after peripheral nerve injury or inflammation. J Physiol 1999;515:533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chen Y, Michaelis M, Janig W, Devor M.. Adrenoreceptor subtype mediating sympathetic-sensory coupling in injured sensory neurons. J Neurophysiol 1996;76:3721–30. [DOI] [PubMed] [Google Scholar]
- 72. Saloman JL, Scheff NN, Snyder LM, et al. Gi-DREADD expression in peripheral nerves produces ligand-dependent analgesia, as well as ligand-independent functional changes in sensory neurons. J Neurosci 2016;36:10769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kinnman E, Wiesenfeld-Hallin Z.. Time course and characteristics of the capacity of sensory nerves to reinnervate skin territories outside their normal innervation zones. Somatosens Mot Res 1993;10:445–54. [DOI] [PubMed] [Google Scholar]
- 74. Nurse CA, Macintyre L, Diamond J.. Reinnervation of the rat touch dome restores the Merkel cell population reduced after denervation. Neuroscience 1984;13:563–71. [DOI] [PubMed] [Google Scholar]
- 75. Mendell LM. Constructing and deconstructing the gate theory of pain. Pain 2014;155:210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bessou P, Burgess PR, Perl ER, Taylor CB.. Dynamic properties of mechanoreceptors with unmyelinated (C) fibers. J Neurophysiol 1971;34:116–31. [DOI] [PubMed] [Google Scholar]
- 77. Coward K, Plumpton C, Facer P, et al. Immunolocalization of SNS/PN3 and NaN/SNS2 sodium channels in human pain states. Pain 2000;85:41–50. [DOI] [PubMed] [Google Scholar]
- 78. Zakrzewska JM. Medical management of trigeminal neuropathic pains. Expert Opin Pharmacother 2010;11:1239–54. [DOI] [PubMed] [Google Scholar]
- 79. Grosskopf J, Mazzola J, Wan Y, Hopwood M.. A randomized, placebo-controlled study of oxcarbazepine in painful diabetic neuropathy. Acta Neurol Scand 2006;114:177–80. [DOI] [PubMed] [Google Scholar]
- 80. Wiffen PJ, Derry S, Moore RA, Kalso EA.. Carbamazepine for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev 2014;CD005451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cata JP, Weng HR, Lee BN, Reuben JM, Dougherty PM.. Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anestesiol 2006;72:151–69. [PubMed] [Google Scholar]
- 82. Yilmaz E, Gold MS.. Paclitaxel-induced increase in NCX activity in subpopulations of nociceptive afferents: A protective mechanism against chemotherapy-induced peripheral neuropathy? Cell Calcium 2016;60:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yilmaz E, Gold MS.. Sensory neuron subpopulation-specific dysregulation of intracellular calcium in a rat model of chemotherapy-induced peripheral neuropathy. Neuroscience 2015;300:210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Li Y, Zhang H, Kosturakis AK, et al. MAPK signaling downstream to TLR4 contributes to paclitaxel-induced peripheral neuropathy. Brain Behav Immun 2015;49:255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li Y, Zhang H, Zhang H, et al. Toll-like receptor 4 signaling contributes to Paclitaxel-induced peripheral neuropathy. J Pain 2014;15:712–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Price TJ, Inyang KE.. Commonalities between pain and memory mechanisms and their meaning for understanding chronic pain. Prog Mol Biol Transl Sci 2015;131:409–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kandasamy R, Price TJ.. The pharmacology of nociceptor priming. Handb Exp Pharmacol 2015;227:15–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Reichling DB, Levine JD.. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci 2009;32:611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ji RR, Gereau RWt, Malcangio M, Strichartz GR.. MAP kinase and pain. Brain Res Rev 2009;60:135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Melemedjian OK, Tillu DV, Moy JK, et al. Local translation and retrograde axonal transport of CREB regulates IL-6-induced nociceptive plasticity. Mol Pain 2014;10:45.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bogen O, Alessandri-Haber N, Chu C, Gear RW, Levine JD.. Generation of a pain memory in the primary afferent nociceptor triggered by PKCepsilon activation of CPEB. J Neurosci 2012;32:2018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Patil MJ, Ruparel SB, Henry MA, Akopian AN.. Prolactin regulates TRPV1, TRPA1, and TRPM8 in sensory neurons in a sex-dependent manner: Contribution of prolactin receptor to inflammatory pain. Am J Physiol Endocrinol Metab 2013;305:E1154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Patil MJ, Green DP, Henry MA, Akopian AN.. Sex-dependent roles of prolactin and prolactin receptor in postoperative pain and hyperalgesia in mice. Neuroscience 2013;253:132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Belugin S, Diogenes AR, Patil MJ, et al. Mechanisms of transient signaling via short and long prolactin receptor isoforms in female and male sensory neurons. J Biol Chem 2013;288:34943–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Dib-Hajj SD, Cummins TR, Black JA, Waxman SG.. Sodium channels in normal and pathological pain. Annu Rev Neurosci 2010;33:325–47. [DOI] [PubMed] [Google Scholar]
- 96. Theile JW, Cummins TR.. Recent developments regarding voltage-gated sodium channel blockers for the treatment of inherited and acquired neuropathic pain syndromes. Front Pharmacol 2011;2:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zorina-Lichtenwalter K, Meloto CB, Khoury S, Diatchenko L.. Genetic predictors of human chronic pain conditions. Neuroscience 2016;338:36–62. [DOI] [PubMed] [Google Scholar]
- 98. Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. 2011;152(3 Suppl):S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sandkuhler J. Central sensitization versus synaptic long-term potentiation (LTP): A critical comment. J Pain 2010;11:798–800. [DOI] [PubMed] [Google Scholar]
- 100. Latremoliere A, Woolf CJ.. Synaptic plasticity and central sensitization: Author reply. J Pain 2010;11:801–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ma QP, Woolf CJ.. Noxious stimuli induce an N-methyl-D-aspartate receptor-dependent hypersensitivity of the flexion withdrawal reflex to touch: Implications for the treatment of mechanical allodynia. Pain 1995;61:383–90. [DOI] [PubMed] [Google Scholar]
- 102. Woolf CJ, Thompson SW.. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain 1991;44:293–9. [DOI] [PubMed] [Google Scholar]
- 103. Cui L, Miao X, Liang L, et al. Identification of early RET+ deep dorsal spinal cord interneurons in gating pain. Neuron 2016;91:1137–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Petitjean H, Pawlowski SA, Fraine SL, et al. Dorsal horn parvalbumin neurons are gate-keepers of touch-evoked pain after nerve injury. Cell Rep 2015;13:1246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Peirs C, Williams SP, Zhao X, et al. Dorsal horn circuits for persistent mechanical pain. Neuron 2015;87:797–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Herrero JF, Laird JM, Lopez-Garcia JA.. Wind-up of spinal cord neurones and pain sensation: Much ado about something? Prog Neurobiol 2000;61:169–203. [DOI] [PubMed] [Google Scholar]
- 107. Mendell LM, Wall PD.. Responses of single dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature 1965;206:97–9. [DOI] [PubMed] [Google Scholar]
- 108. Woolf CJ, Thompson SW, King AE.. Prolonged primary afferent induced alterations in dorsal horn neurones, an intracellular analysis in vivo and in vitro. J Physiol (Paris) 1988;83:255–66. [PubMed] [Google Scholar]
- 109. Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature 1983;306:686–8. [DOI] [PubMed] [Google Scholar]
- 110. Ruscheweyh R, Wilder-Smith O, Drdla R, Liu XG, Sandkuhler J.. Long-term potentiation in spinal nociceptive pathways as a novel target for pain therapy. Mol Pain 2011;7:20.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sandkuhler J. Understanding LTP in pain pathways. Mol Pain 2007;3:9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ikeda H, Stark J, Fischer H, et al. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science 2006;312:1659–62. [DOI] [PubMed] [Google Scholar]
- 113. Gracely RH, Lynch SA, Bennett GJ.. Painful neuropathy: Altered central processing maintained dynamically by peripheral input. Pain 1992;51:175–94. [DOI] [PubMed] [Google Scholar]
- 114. Kronschlager MT, Drdla-Schutting R, Gassner M, et al. Gliogenic LTP spreads widely in nociceptive pathways. Science 2016;354:1144–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Schmelz M, Schmid R, Handwerker HO, Torebjork HE.. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain 2000;123(pt 3): 560–71. [DOI] [PubMed] [Google Scholar]
- 116. Torebjork E. Human microneurography and intraneural microstimulation in the study of neuropathic pain. Muscle Nerve 1993;16:1063–5. [DOI] [PubMed] [Google Scholar]
- 117. Milligan ED, Watkins LR.. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci 2009;10:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Guan Z, Kuhn JA, Wang X, et al. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci 2016;19:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tsuda M, Masuda T, Kitano J, et al. IFN-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc Natl Acad Sci U S A 2009;106:8032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Beggs S, Trang T, Salter MW.. P2X4R+ microglia drive neuropathic pain. Nat Neurosci 2012;15:1068–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ferrini F, Trang T, Mattioli TA, et al. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl(-) homeostasis. Nat Neurosci 2013;16:183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Coull JA, Beggs S, Boudreau D, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005;438:1017–21. [DOI] [PubMed] [Google Scholar]
- 123. Landry RP, Jacobs VL, Romero-Sandoval EA, DeLeo JA.. Propentofylline, a CNS glial modulator does not decrease pain in post-herpetic neuralgia patients: In vitro evidence for differential responses in human and rodent microglia and macrophages. Exp Neurol 2012;234:340–50. [DOI] [PubMed] [Google Scholar]
- 124. Kwok YH, Swift JE, Gazerani P, Rolan P.. A double-blind, randomized, placebo-controlled pilot trial to determine the efficacy and safety of ibudilast, a potential glial attenuator, in chronic migraine. J Pain Res 2016;9:899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Robinson CR, Zhang H, Dougherty PM.. Astrocytes, but not microglia, are activated in oxaliplatin and bortezomib-induced peripheral neuropathy in the rat. Neuroscience 2014;274:308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Di Cesare Mannelli L, Pacini A, Micheli L, et al. Glial role in oxaliplatin-induced neuropathic pain. Exp Neurol 2014;261:22–33. [DOI] [PubMed] [Google Scholar]
- 127. Yoon SY, Robinson CR, Zhang H, Dougherty PM.. Spinal astrocyte gap junctions contribute to oxaliplatin-induced mechanical hypersensitivity. J Pain 2013;14:205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gwak YS, Kang J, Unabia GC, Hulsebosch CE.. Spatial and temporal activation of spinal glial cells: Role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp Neurol 2012;234:362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhang J, De Koninck Y.. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem 2006;97:772–83. [DOI] [PubMed] [Google Scholar]
- 130. Sorge RE, Mapplebeck JC, Rosen S, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 2015;18:1081–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Taves S, Berta T, Liu DL, et al. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain Behav Immun 2016;55:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ossipov MH, Dussor GO, Porreca F.. Central modulation of pain. J Clin Invest 2010;120:3779–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Gardell LR, Vanderah TW, Gardell SE, et al. Enhanced evoked excitatory transmitter release in experimental neuropathy requires descending facilitation. J Neurosci 2003;23:8370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Vaso A, Adahan HM, Gjika A, et al. Peripheral nervous system origin of phantom limb pain. Pain 2014;155:1384–91. [DOI] [PubMed] [Google Scholar]
- 135. Haroutounian S, Nikolajsen L, Bendtsen TF, et al. Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy. Pain 2014;155:1272–9. [DOI] [PubMed] [Google Scholar]
- 136. Wei F, Guo W, Zou S, Ren K, Dubner R.. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J Neurosci 2008;28:10482–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. De Felice M, Sanoja R, Wang R, et al. Engagement of descending inhibition from the rostral ventromedial medulla protects against chronic neuropathic pain. Pain 2011;15212:2701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yarnitsky D. Role of endogenous pain modulation in chronic pain mechanisms and treatment. Pain 2015;156(suppl 1):S24–31. [DOI] [PubMed] [Google Scholar]
- 139. Nir RR, Yarnitsky D.. Conditioned pain modulation. Curr Opin Support Palliat Care 2015;9:131–7. [DOI] [PubMed] [Google Scholar]
- 140. Zeidan F, Emerson NM, Farris SR, et al. Mindfulness meditation-based pain relief employs different neural mechanisms than Placebo and Sham mindfulness meditation-induced analgesia. J Neurosci 2015;35:15307–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zeidan F, Grant JA, Brown CA, McHaffie JG, Coghill RC.. Mindfulness meditation-related pain relief: Evidence for unique brain mechanisms in the regulation of pain. Neurosci Lett 2012;520:165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Cheng SJ, Chen CC, Yang HW, et al. Role of extracellular signal-regulated kinase in synaptic transmission and plasticity of a nociceptive input on capsular central amygdaloid neurons in normal and acid-induced muscle pain mice. J Neurosci 2011;31:2258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Neugebauer V. Amygdala pain mechanisms. Handb Exp Pharmacol 2015;227:261–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kim JY, Tillu DV, Quinn TL, et al. Spinal dopaminergic projections control the transition to pathological pain plasticity via a D1/D5-mediated mechanism. J Neurosci 2015;35:6307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Apkarian AV, Baliki MN, Farmer MA.. Predicting transition to chronic pain. Curr Opin Neurol 2013;26:360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Baliki MN, Petre B, Torbey S, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 2012;15:1117–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ren W, Centeno MV, Berger S, et al. The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nat Neurosci 2016;19:220–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Chang PC, Pollema-Mays SL, Centeno MV, et al. Role of nucleus accumbens in neuropathic pain: Linked multi-scale evidence in the rat transitioning to neuropathic pain. Pain 2014;155:1128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Birder LA. More than just a barrier: Urothelium as a drug target for urinary bladder pain. Am J Physiol Renal Physiol 2005;289:F489–F95. [DOI] [PubMed] [Google Scholar]
- 150. Feng B, La JH, Schwartz ES, Gebhart GF.. Irritable bowel syndrome: Methods, mechanisms, and pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2012;302:G1085–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Akerman S, Holland PR, Goadsby PJ.. Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci 2011;12:570–84. [DOI] [PubMed] [Google Scholar]
- 152. Jacobs B, Dussor G.. Neurovascular contributions to migraine: Moving beyond vasodilation. Neuroscience 2016;338:130–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Yan J, Dussor G.. Ion channels and migraine. Headache 2014;54:619–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Voss T, Lipton RB, Dodick DW, et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia 2016;36:887–98. [DOI] [PubMed] [Google Scholar]
- 155. Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 2016;15:382–90. [DOI] [PubMed] [Google Scholar]
- 156. Dodick DW, Goadsby PJ, Silberstein SD, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: A randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol 2014;13:1100–7. [DOI] [PubMed] [Google Scholar]
- 157. Hewitt DJ, Aurora SK, Dodick DW, et al. Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine. Cephalalgia 2011;31:712–22. [DOI] [PubMed] [Google Scholar]
- 158. Longmore J, Shaw D, Smith D, et al. Differential distribution of 5HT1D- and 5HT1B-immunoreactivity within the human trigemino-cerebrovascular system: Implications for the discovery of new antimigraine drugs. Cephalalgia 1997;17:833–42. [DOI] [PubMed] [Google Scholar]
- 159. Harriott AM, Gold MS.. Serotonin type 1D receptors (5HTR) are differentially distributed in nerve fibres innervating craniofacial tissues. Cephalalgia 2008;28:933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Harriott AM, Scheff NN, Gold MS.. The complex actions of sumatriptan on rat dural afferents. Cephalalgia 2012;32:738–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Donaldson C, Boers PM, Hoskin KL, Zagami AS, Lambert GA.. The role of 5-HT1B and 5-HT1D receptors in the selective inhibitory effect of naratriptan on trigeminovascular neurons. Neuropharmacology 2002;42:374–85. [DOI] [PubMed] [Google Scholar]
- 162. Yan J, Edelmayer RM, Wei X, et al. Dural afferents express acid-sensing ion channels: A role for decreased meningeal pH in migraine headache. Pain 2011;152:106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Vaughn AH, Gold MS.. Ionic mechanisms underlying inflammatory mediator-induced sensitization of dural afferents. J Neurosci 2010;30:7878–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Harriott AM, Gold MS.. Electrophysiological properties of dural afferents in the absence and presence of inflammatory mediators. J Neurophysiol 2009;101:3126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Levy D, Strassman AM.. Mechanical response properties of A and C primary afferent neurons innervating the rat intracranial dura. J Neurophysiol 2002;88:3021–31. [DOI] [PubMed] [Google Scholar]
- 166. Strassman AM, Raymond SA, Burstein R.. Sensitization of meningeal sensory neurons and the origin of headaches. Nature 1996;384:560–4. [DOI] [PubMed] [Google Scholar]
- 167. Mantyh PW. The neurobiology of skeletal pain. Eur J Neurosci 2014;39:508–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Mantyh PW. Bone cancer pain: From mechanism to therapy. Curr Opin Support Palliat Care 2014;8:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Schweinhardt P, Sauro KM, Bushnell MC.. Fibromyalgia: A disorder of the brain? Neuroscientist 2008;14:415–21. [DOI] [PubMed] [Google Scholar]
- 170. Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci 2007;25:3576–82. [DOI] [PubMed] [Google Scholar]
- 171. Jensen KB, Srinivasan P, Spaeth R, et al. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis Rheum 2013;65:3293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Ceko M, Bushnell MC, Fitzcharles MA, Schweinhardt P.. Fibromyalgia interacts with age to change the brain. Neuroimage Clin 2013;3:249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Ichesco E, Schmidt-Wilcke T, Bhavsar R, et al. Altered resting state connectivity of the insular cortex in individuals with fibromyalgia. J Pain 2014;15:815–26.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Gracely RH, Petzke F, Wolf JM, Clauw DJ.. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum 2002;46:1333–43. [DOI] [PubMed] [Google Scholar]
- 175. Bliss TV, Collingridge GL, Kaang BK, Zhuo M.. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci 2016;17:485–96. [DOI] [PubMed] [Google Scholar]
- 176. Neugebauer V, Galhardo V, Maione S, Mackey SC.. Forebrain pain mechanisms. Brain Res Rev 2009;60:226–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Doppler K, Rittner HL, Deckart M, Sommer C.. Reduced dermal nerve fiber diameter in skin biopsies of patients with fibromyalgia. Pain 2015;156:2319–25. [DOI] [PubMed] [Google Scholar]
- 178. Oaklander AL, Herzog ZD, Downs HM, Klein MM.. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 2013;154:2310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Ramirez M, Martinez-Martinez LA, Hernandez-Quintela E, et al. Small fiber neuropathy in women with fibromyalgia. An in vivo assessment using corneal confocal bio-microscopy. Semin Arthritis Rheum 2015;45:214–9. [DOI] [PubMed] [Google Scholar]
- 180. Serra J, Collado A, Sola R, et al. Hyperexcitable C nociceptors in fibromyalgia. Ann Neurol 2014;75:196–208. [DOI] [PubMed] [Google Scholar]
- 181. Goebel A, Blaes F.. Complex regional pain syndrome, prototype of a novel kind of autoimmune disease. Autoimmun Rev 2013;12:682–6. [DOI] [PubMed] [Google Scholar]
- 182. Kohr D, Tschernatsch M, Schmitz K, et al. Autoantibodies in complex regional pain syndrome bind to a differentiation-dependent neuronal surface autoantigen. Pain 2009;143:246–51. [DOI] [PubMed] [Google Scholar]
- 183. Sommer C. Anti-autonomic nervous system antibodies in CRPS. Pain 2011;152:2675–6. [DOI] [PubMed] [Google Scholar]
- 184. Oaklander AL. Immunotherapy prospects for painful small-fiber sensory neuropathies and ganglionopathies. Neurotherapeutics 2016;13:108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Patel SK, Beaino W, Anderson CJ, Janjic JM.. Theranostic nanoemulsions for macrophage COX-2 inhibition in a murine inflammation model. Clin Immunol 2015;160:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Vasudeva K, Andersen K, Zeyzus-Johns B, et al. Imaging neuroinflammation in vivo in a neuropathic pain rat model with near-infrared fluorescence and (1)(9)F magnetic resonance. PLoS One 2014;9:e90589.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Leinders M, Uceyler N, Pritchard RA, Sommer C, Sorkin LS.. Increased miR-132-3p expression is associated with chronic neuropathic pain. Exp Neurol 2016;283:276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Leinders M, Doppler K, Klein T, et al. Increased cutaneous miR-let-7d expression correlates with small nerve fiber pathology in patients with fibromyalgia syndrome. Pain 2016;157:2493–503. [DOI] [PubMed] [Google Scholar]
- 189. Kress M, Huttenhofer A, Landry M, et al. MicroRNAs in nociceptive circuits as predictors of future clinical applications. Front Mol Neurosci 2013;6:33.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. McDonald MK, Ajit SK.. MicroRNA biology and pain. Prog Mol Biol Transl Sci 2015;131:215–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. McDonald MK, Tian Y, Qureshi RA, et al. Functional significance of macrophage-derived exosomes in inflammation and pain. Pain 2014;155:1527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Loggia ML, Chonde DB, Akeju O, et al. Evidence for brain glial activation in chronic pain patients. Brain 2015;138:604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. van Hecke O, Kamerman PR, Attal N, et al. Neuropathic pain phenotyping by international consensus (NeuroPPIC) for genetic studies: A NeuPSIG systematic review, Delphi survey, and expert panel recommendations. Pain 2015;156:2337–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Mele M, Ferreira PG, Reverter F, et al. Human genomics. The human transcriptome across tissues and individuals. Science 2015;348:660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Consortium GT. Human genomics. The genotype-tissue expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 2015;348:648–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Massart R, Dymov S, Millecamps M, et al. Overlapping signatures of chronic pain in the DNA methylation landscape of prefrontal cortex and peripheral T cells. Sci Rep 2016;6:19615.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Minis A, Dahary D, Manor O, et al. Subcellular transcriptomics-dissection of the mRNA composition in the axonal compartment of sensory neurons. Dev Neurobiol 2014;74:365–81. [DOI] [PubMed] [Google Scholar]
- 198. Obara I, Geranton SM, Hunt SP.. Axonal protein synthesis: A potential target for pain relief? Curr Opin Pharmacol 2012;12:42–8. [DOI] [PubMed] [Google Scholar]
- 199. Price TJ, Geranton SM.. Translating nociceptor sensitivity: The role of axonal protein synthesis in nociceptor physiology. Eur J Neurosci 2009;29:2253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Skolnick P, Volkow ND.. Re-energizing the development of pain therapeutics in light of the opioid epidemic. Neuron 2016;92:294–7. [DOI] [PubMed] [Google Scholar]
- 201. Kivitz AJ, Gimbel JS, Bramson C, et al. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain 2013;1547:1009–21. [DOI] [PubMed] [Google Scholar]
- 202. Brown MT, Murphy FT, Radin DM, et al. Tanezumab reduces osteoarthritic hip pain: Results of a randomized, double-blind, placebo-controlled phase 3 trial. Arthritis Rheum 2013;657:1795–803. [DOI] [PubMed] [Google Scholar]
- 203. Brown MT, Murphy FT, Radin DM, et al. Tanezumab reduces osteoarthritic knee pain: Results of a randomized, double-blind, placebo-controlled phase III trial. J Pain 2012;13:790–8. [DOI] [PubMed] [Google Scholar]
- 204. Katz N, Borenstein DG, Birbara C, et al. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain 2011;152:2248–58. [DOI] [PubMed] [Google Scholar]
- 205. Cao L, McDonnell A, Nitzsche A, et al. Pharmacological reversal of a pain phenotype in iPSC-derived sensory neurons and patients with inherited erythromelalgia. Sci Transl Med 2016;8:335ra56.. [DOI] [PubMed] [Google Scholar]
- 206. Huggins JP, Smart TS, Langman S, Taylor L, Young T.. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain 2012;153:1837–46. [DOI] [PubMed] [Google Scholar]
- 207. Kumar V, Mahal BA.. NGF—the TrkA to successful pain treatment. J Pain Res 2012;5:279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Manglik A, Lin H, Aryal DK, et al. Structure-based discovery of opioid analgesics with reduced side effects. Nature 2016;537:185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Ratte S, Prescott SA.. Afferent hyperexcitability in neuropathic pain and the inconvenient truth about its degeneracy. Curr Opin Neurobiol 2016;36:31–7. [DOI] [PubMed] [Google Scholar]
- 210. Ji RR, Xu ZZ, Strichartz G, Serhan CN.. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci 2011;34:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Milligan ED, Sloane EM, Langer SJ, et al. Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain 2006;126:294–308. [DOI] [PubMed] [Google Scholar]
- 212. Milligan ED, Sloane EM, Langer SJ, et al. Controlling neuropathic pain by adeno-associated virus driven production of the anti-inflammatory cytokine, interleukin-10. Mol Pain 2005;1:9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Milligan ED, Langer SJ, Sloane EM, et al. Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci 2005;21:2136–48. [DOI] [PubMed] [Google Scholar]
- 214. Eijkelkamp N, Steen-Louws C, Hartgring SA, et al. IL4-10 fusion protein is a novel drug to treat persistent inflammatory pain. J Neurosci 2016;36:7353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Melemedjian OK, Asiedu MN, Tillu DV, et al. Targeting adenosine monophosphate-activated protein kinase (AMPK) in preclinical models reveals a potential mechanism for the treatment of neuropathic pain. Mol Pain 2011;7:70.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Price TJ, Das V, Dussor G.. Adenosine monophosphate-activated protein kinase (AMPK) activators for the prevention, treatment and potential reversal of pathological pain. Curr Drug Targets 2016;178:908–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Price TJ, Dussor G.. AMPK: An emerging target for modification of injury-induced pain plasticity. Neurosci Lett 2013;557(pt A):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Rajasethupathy P, Ferenczi E, Deisseroth K.. Targeting neural circuits. Cell 2016;165:524–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Roth BL. DREADDs for neuroscientists. Neuron 2016;89:683–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Park SI, Shin G, McCall JG, et al. Stretchable multichannel antennas in soft wireless optoelectronic implants for optogenetics. Proc Natl Acad Sci U S A 2016;113:E8169–E77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Copits BA, Pullen MY, Gereau RWt.. Spotlight on pain: Optogenetic approaches for interrogating somatosensory circuits. Pain 2016;157:2424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Park SI, Brenner DS, Shin G, et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat Biotechnol 2015;33:1280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Daou I, Beaudry H, Ase AR, et al. Optogenetic silencing of Nav1.8-positive afferents alleviates inflammatory and neuropathic pain. eNeuro 2016;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Etlin A, Braz JM, Kuhn JA, et al. Functional synaptic integration of forebrain GABAergic precursors into the adult spinal cord. J Neurosci 2016;36:11634–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Braz JM, Wang X, Guan Z, Rubenstein JL, Basbaum AI.. Transplant-mediated enhancement of spinal cord GABAergic inhibition reverses paclitaxel-induced mechanical and heat hypersensitivity. Pain 2015;156:1084–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Braz JM, Sharif-Naeini R, Vogt D, et al. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron 2012;74:663–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Chen G, Park CK, Xie RG, Ji RR.. Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-beta secretion. J Clin Invest 2015;125:3226–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Schafer S, Berger JV, Deumens R, et al. Influence of intrathecal delivery of bone marrow-derived mesenchymal stem cells on spinal inflammation and pain hypersensitivity in a rat model of peripheral nerve injury. J Neuroinflammation 2014;11:157. [DOI] [PMC free article] [PubMed] [Google Scholar]